Development and Evaluation of a Quantitative Systems Pharmacology Model for Mechanism Interpretation and Efficacy Prediction of Atezolizumab in Combination with Carboplatin and Nab-Paclitaxel in Patients with Non-Small-Cell Lung Cancer
Abstract
1. Introduction
2. Results
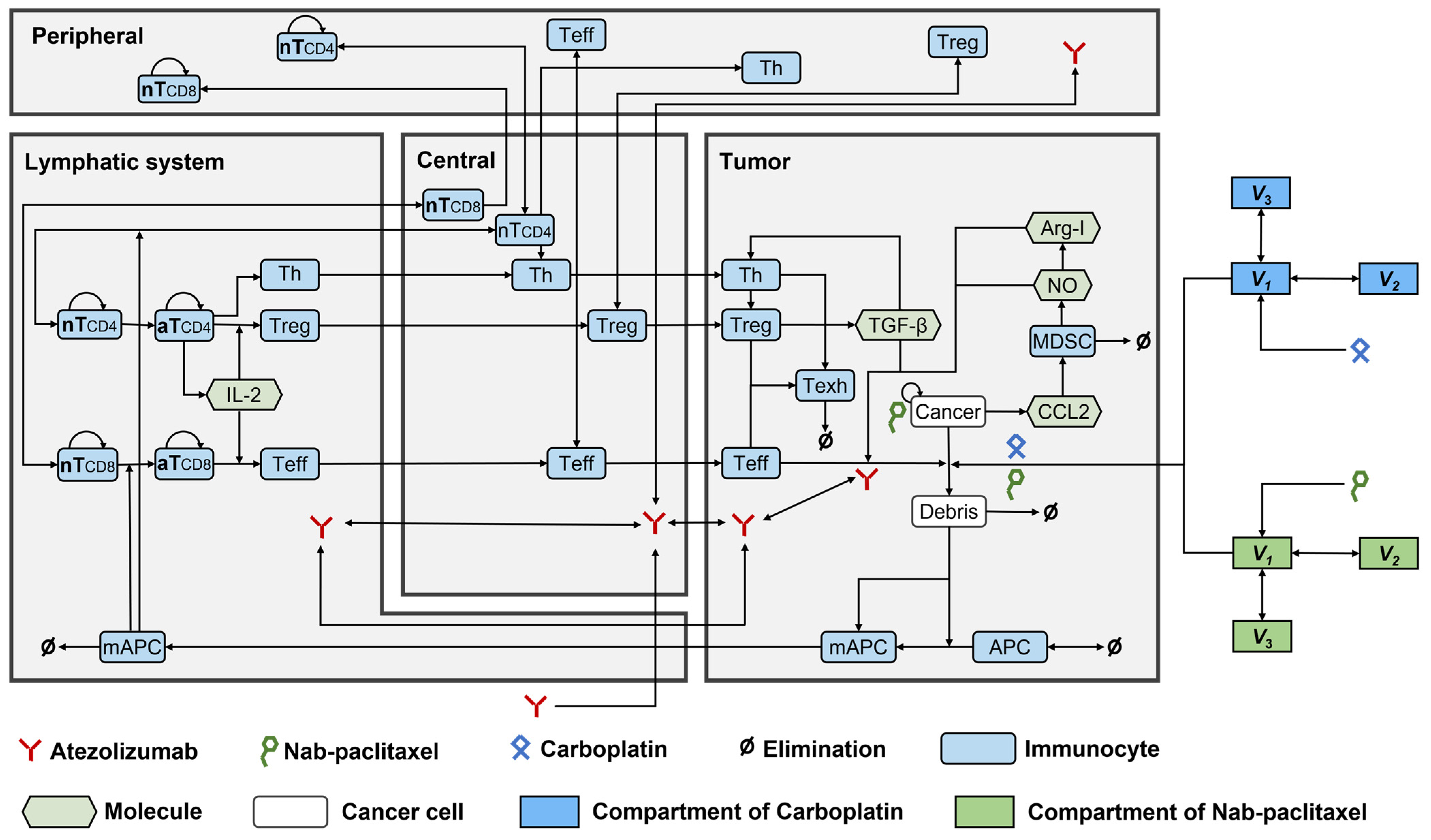
2.1. Model Development
2.1.1. Model Structure
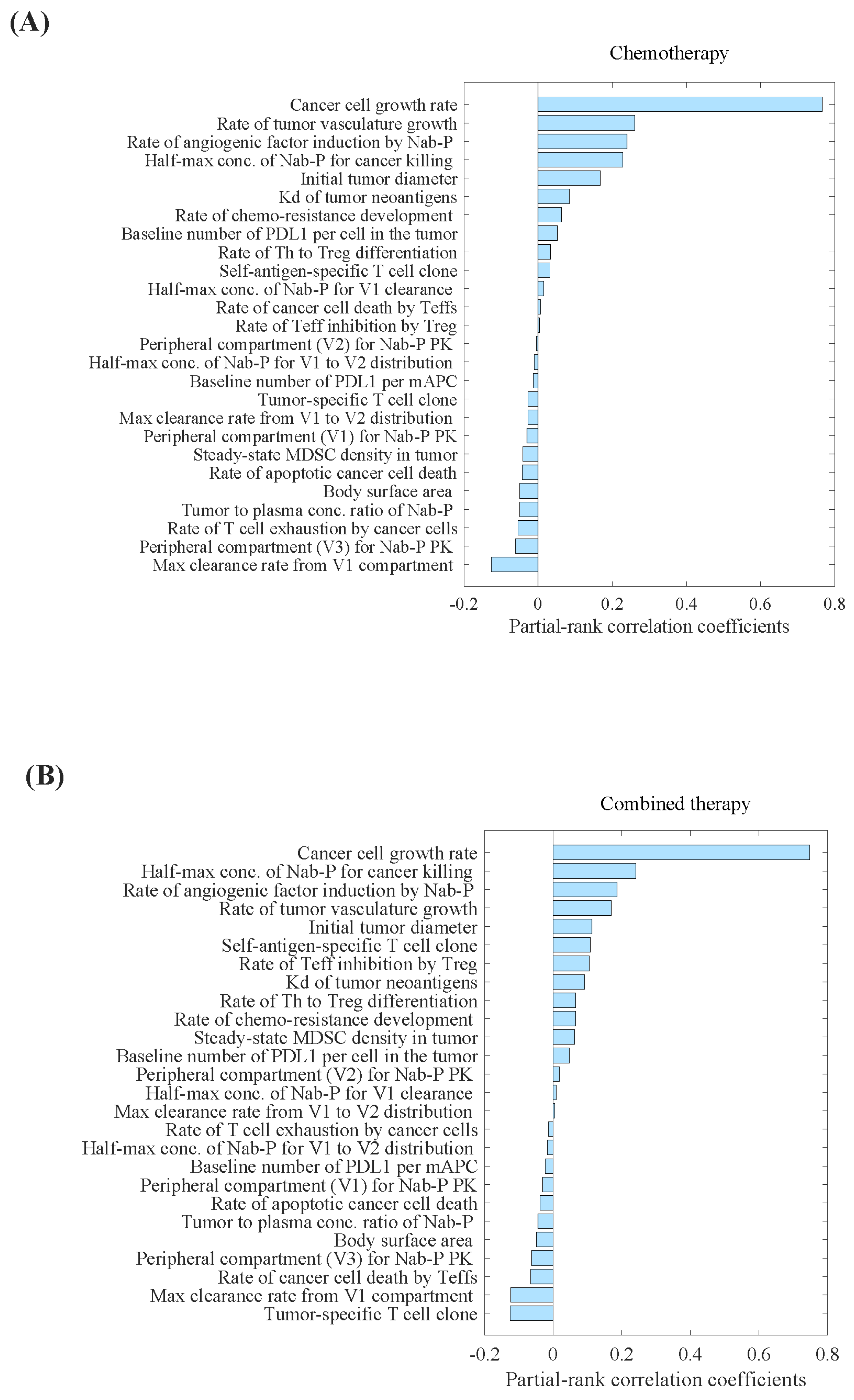
2.1.2. Model Parameters
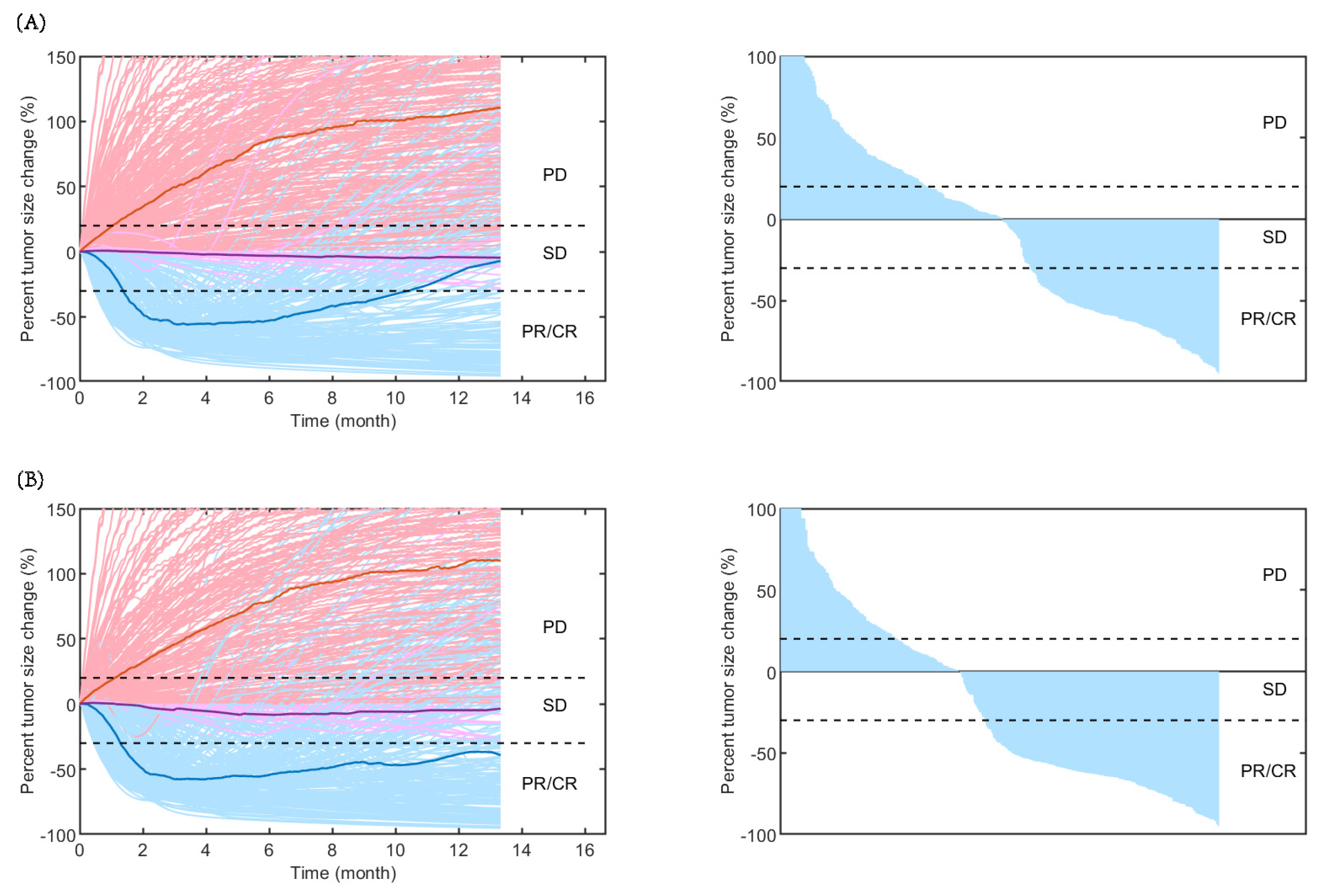
2.2. Model Evaluation
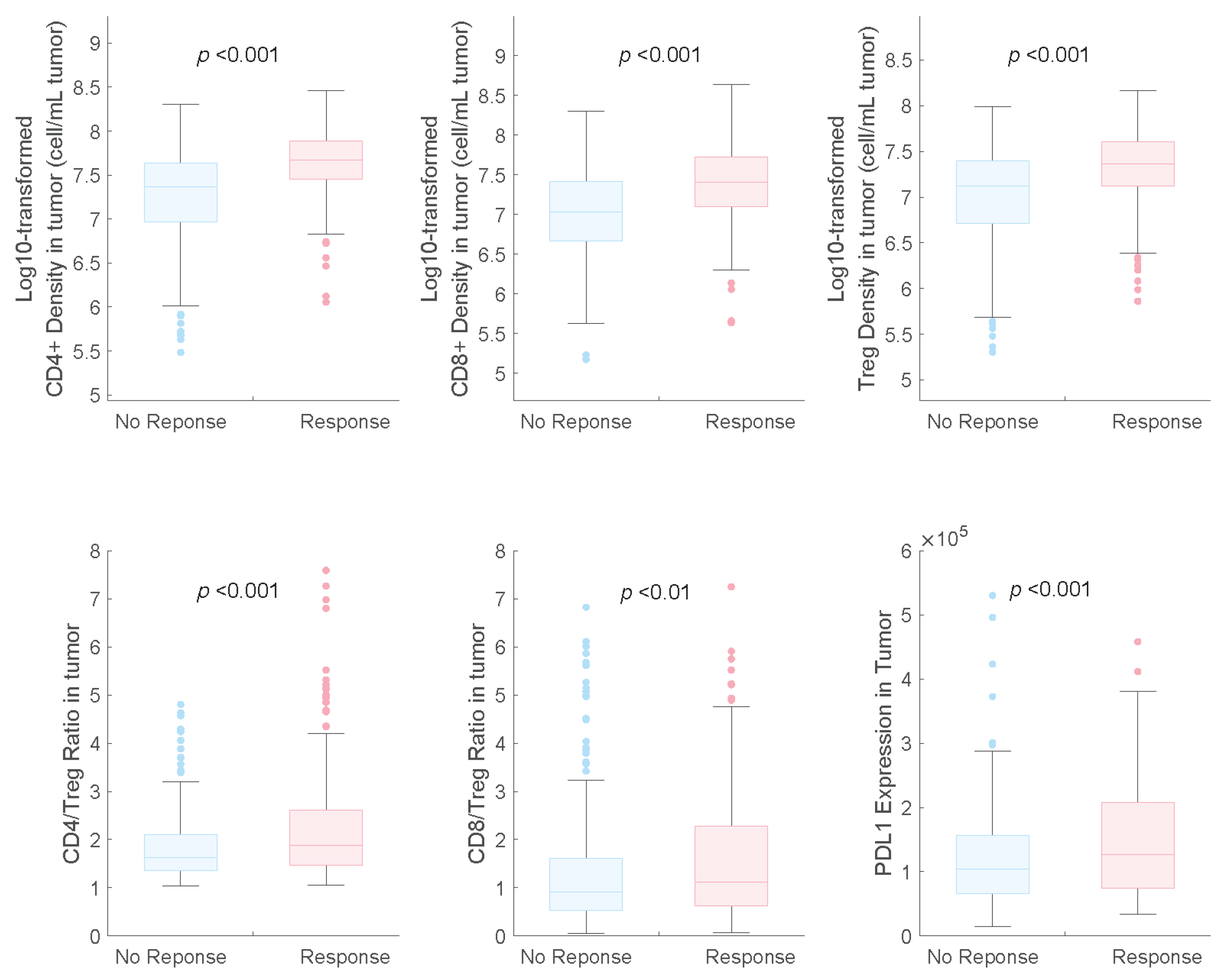
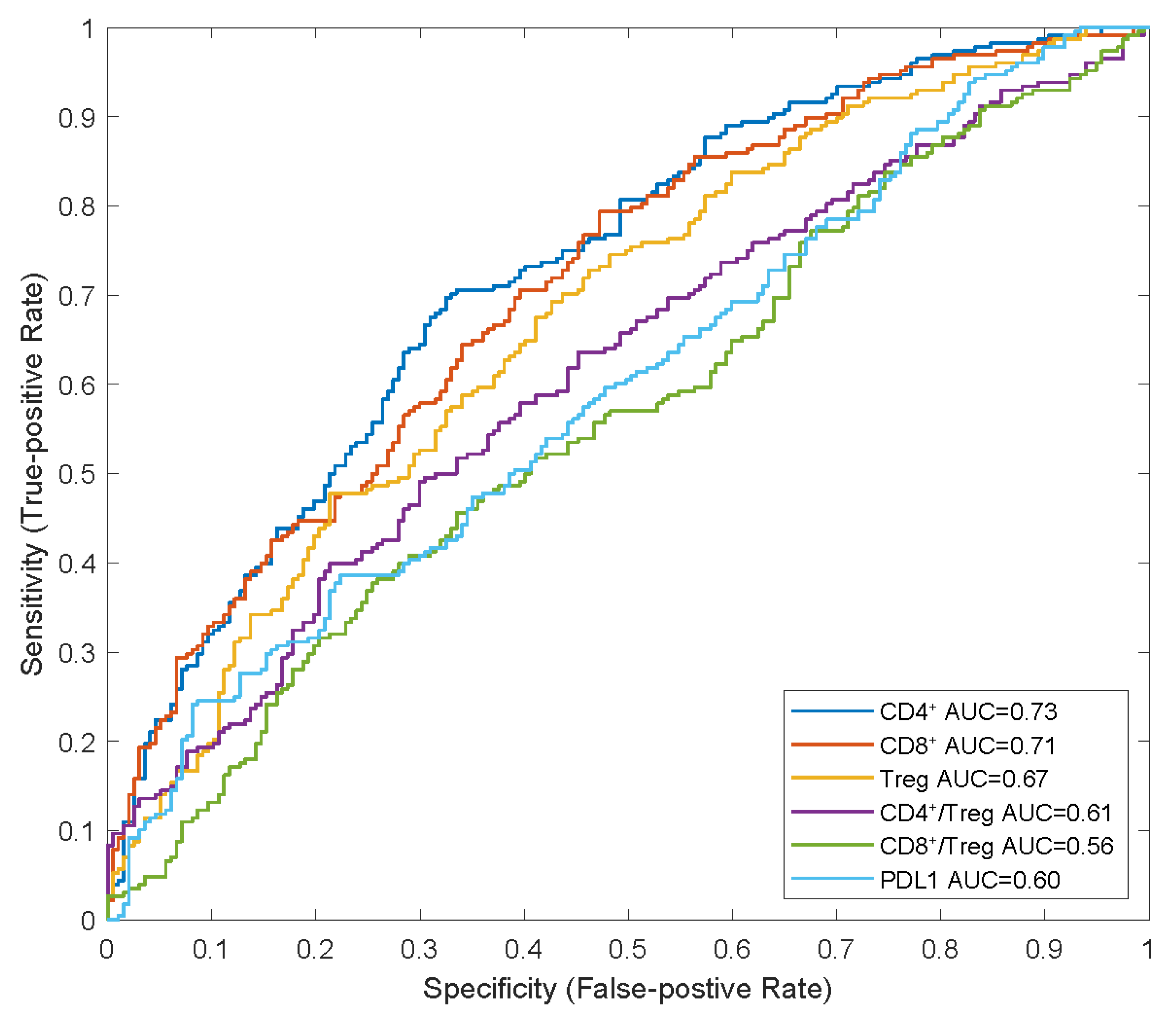
2.3. Model Application
3. Discussion
4. Materials and Methods
4.1. Model Development
4.1.1. Model Structure
4.1.2. Model Parameters
4.2. Model Evaluation
4.3. Model Application
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- De Mello, R.A.B.; Voscaboinik, R.; Luciano, J.V.P.; Cremonese, R.V.; Amaral, G.A.; Castelo-Branco, P.; Antoniou, G. Immunotherapy in Patients with Advanced Non-Small Cell Lung Cancer Lacking Driver Mutations and Future Perspectives. Cancers 2021, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Melosky, B.; Chu, Q.; Juergens, R.A.; Leighl, N.; Ionescu, D.; Tsao, M.S.; McLeod, D.; Hirsh, V. Breaking the biomarker code: PD-L1 expression and checkpoint inhibition in advanced NSCLC. Cancer Treat. Rev. 2018, 65, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Azer, K.; Kaddi, C.D.; Barrett, J.S.; Bai, J.P.F.; McQuade, S.T.; Merrill, N.J.; Piccoli, B.; Neves-Zaph, S.; Marchetti, L.; Lombardo, R.; et al. History and Future Perspectives on the Discipline of Quantitative Systems Pharmacology Modeling and Its Applications. Front. Physiol. 2021, 12, 637999. [Google Scholar] [CrossRef] [PubMed]
- Sove, R.J.; Jafarnejad, M.; Zhao, C.; Wang, H.; Ma, H.; Popel, A.S. QSP-IO: A Quantitative Systems Pharmacology Toolbox for Mechanistic Multiscale Modeling for Immuno-Oncology Applications. CPT Pharmacomet. Syst. Pharmacol. 2020, 9, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Arulraj, T.; Kimko, H.; Popel, A.S. Generating immunogenomic data-guided virtual patients using a QSP model to predict response of advanced NSCLC to PD-L1 inhibition. NPJ Precis. Oncol. 2023, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, C.; Santa-Maria, C.A.; Emens, L.A.; Popel, A.S. Dynamics of tumor-associated macrophages in a quantitative systems pharmacology model of immunotherapy in triple-negative breast cancer. iScience 2022, 25, 104702. [Google Scholar] [CrossRef]
- Wang, H.; Ma, H.; Sove, R.J.; Emens, L.A.; Popel, A.S. Quantitative systems pharmacology model predictions for efficacy of atezolizumab and nab-paclitaxel in triple-negative breast cancer. J. Immunother. Cancer 2021, 9, e002100. [Google Scholar] [CrossRef]
- Wang, H.; Sove, R.J.; Jafarnejad, M.; Rahmeh, S.; Jaffee, E.M.; Stearns, V.; Roussos Torres, E.T.; Connolly, R.M.; Popel, A.S. Conducting a Virtual Clinical Trial in HER2-Negative Breast Cancer Using a Quantitative Systems Pharmacology Model with an Epigenetic Modulator and Immune Checkpoint Inhibitors. Front. Bioeng. Biotechnol. 2020, 8, 141. [Google Scholar] [CrossRef]
- Wang, H.; Milberg, O.; Bartelink, I.H.; Vicini, P.; Wang, B.; Narwal, R.; Roskos, L.; Santa-Maria, C.A.; Popel, A.S. In silico simulation of a clinical trial with anti-CTLA-4 and anti-PD-L1 immunotherapies in metastatic breast cancer using a systems pharmacology model. R. Soc. Open Sci. 2019, 6, 190366. [Google Scholar] [CrossRef]
- Jotte, R.; Cappuzzo, F.; Vynnychenko, I.; Stroyakovskiy, D.; Rodriguez-Abreu, D.; Hussein, M.; Soo, R.; Conter, H.J.; Kozuki, T.; Huang, K.C.; et al. Atezolizumab in Combination With Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results From a Randomized Phase III Trial. J. Thorac. Oncol. 2020, 15, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gumus, M.; Mazieres, J.; Hermes, B.; Cay Senler, F.; Csoszi, T.; Fulop, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Jafarnejad, M.; Gong, C.; Gabrielson, E.; Bartelink, I.H.; Vicini, P.; Wang, B.; Narwal, R.; Roskos, L.; Popel, A.S. A Computational Model of Neoadjuvant PD-1 Inhibition in Non-Small Cell Lung Cancer. AAPS J. 2019, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hickling, T.P.; Vicini, P. A mechanistic, multiscale mathematical model of immunogenicity for therapeutic proteins: Part 1-theoretical model. CPT Pharmacomet. Syst. Pharmacol. 2014, 3, e133. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Veverka, V.; Radhakrishnan, A.; Waters, L.C.; Muskett, F.W.; Morgan, S.H.; Huo, J.; Yu, C.; Evans, E.J.; Leslie, A.J.; et al. Structure and interactions of the human programmed cell death 1 receptor. J. Biol. Chem. 2013, 288, 11771–11785. [Google Scholar] [CrossRef] [PubMed]
- Kay, K.; Dolcy, K.; Bies, R.; Shah, D.K. Estimation of Solid Tumor Doubling Times from Progression-Free Survival Plots Using a Novel Statistical Approach. AAPS J. 2019, 21, 27. [Google Scholar] [CrossRef]
- Hosokawa, M.; Kenmotsu, H.; Koh, Y.; Yoshino, T.; Yoshikawa, T.; Naito, T.; Takahashi, T.; Murakami, H.; Nakamura, Y.; Tsuya, A.; et al. Size-based isolation of circulating tumor cells in lung cancer patients using a microcavity array system. PLoS ONE 2013, 8, e67466. [Google Scholar] [CrossRef]
- Kowanetz, M.; Zou, W.; Gettinger, S.N.; Koeppen, H.; Kockx, M.; Schmid, P.; Kadel, E.E., 3rd; Wistuba, I.; Chaft, J.; Rizvi, N.A.; et al. Differential regulation of PD-L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti-PD-L1). Proc. Natl. Acad. Sci. USA 2018, 115, E10119–E10126. [Google Scholar] [CrossRef]
- Zeng, D.Q.; Yu, Y.F.; Ou, Q.Y.; Li, X.Y.; Zhong, R.Z.; Xie, C.M.; Hu, Q.G. Prognostic and predictive value of tumor-infiltrating lymphocytes for clinical therapeutic research in patients with non-small cell lung cancer. Oncotarget 2016, 7, 13765–13781. [Google Scholar] [CrossRef]
- Linette, G.P.; Carreno, B.M. Tumor-Infiltrating Lymphocytes in the Checkpoint Inhibitor Era. Curr. Hematol. Malig. Rep. 2019, 14, 286–291. [Google Scholar] [CrossRef]
- Bodor, J.N.; Boumber, Y.; Borghaei, H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer 2020, 126, 260–270. [Google Scholar] [CrossRef]
- Yi, M.; Jiao, D.; Xu, H.; Liu, Q.; Zhao, W.; Han, X.; Wu, K. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol. Cancer 2018, 17, 129. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, I.; Yamamoto, N.; Kubota, K.; Ohe, Y.; Nogami, N.; Murakami, H.; Yamaya, H.; Ono, K.; Nakagawa, K. Safety and pharmacokinetic study of nab-paclitaxel plus carboplatin in chemotherapy-naive patients with advanced non-small cell lung cancer. Investig. New Drugs 2012, 30, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xue, J.; Yu, Z.; Wang, Z.; Chen, R.; Zhou, T. Interpretation of pharmacokinetic-based criteria for supporting alternative dosing regimens of programmed cell death receptor-1 (PD-1) or programmed cell death-ligand 1 (PD-L1) blocking antibodies for treatment of patients with cancer guidance for industry. Chin. J. Clin. Pharmacol. Ther. 2022, 27, 86. [Google Scholar]
| Therapy Regimen | Carboplatin Plus Nab-Paclitaxel | Atezolizumab Plus Carboplatin and Nab-Paclitaxel | ||
|---|---|---|---|---|
| Prediction (95% CI) | Observation (95% CI) | Prediction (95% CI) | Observation (95% CI) | |
| ORR, % | 40.5 (37.1–43.9) | 41.0 (35.7–46.6) | 52.0 (45.8–55.4) | 49.7 (44.3–55.1) |
| Median DOR (month) | 5.3 (5.3–7.1) | 5.2 (4.4–5.6) | 7.1 (5.3–8.8) | 7.3 (6.8–9.5) |
| Value | Atezolizumab Regimens | Chemotherapy Regimens | Median | 25th Percentiles | 75th Percentiles |
|---|---|---|---|---|---|
| Change in tumour diameter (%) | 840 mg Q2W | Nab-paclitaxel 100 mg/m2 on days 1, 8, and 15 and carboplatin at an AUC of 6 mg/mL/min on day 1 | −10.97 | −49.40 | 20.58 |
| 1200 mg Q3W | −7.33 | −47.89 | 19.86 | ||
| 1680 mg Q4W | −7.73 | −46.92 | 21.45 | ||
| Tumour Volume (mm3) | 840 mg Q2W | 6.72 | 0.68 | 22.06 | |
| 1200 mg Q3W | 7.23 | 0.74 | 21.93 | ||
| 1680 mg Q4W | 7.14 | 0.80 | 21.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-Y.; Dai, H.-R.; Tan, Y.-P.; Yang, D.-H.; Niu, X.-M.; Han, L.; Wang, W.; Ma, L.-L.; Julku, A.; Jiao, Z. Development and Evaluation of a Quantitative Systems Pharmacology Model for Mechanism Interpretation and Efficacy Prediction of Atezolizumab in Combination with Carboplatin and Nab-Paclitaxel in Patients with Non-Small-Cell Lung Cancer. Pharmaceuticals 2024, 17, 238. https://doi.org/10.3390/ph17020238
Wang C-Y, Dai H-R, Tan Y-P, Yang D-H, Niu X-M, Han L, Wang W, Ma L-L, Julku A, Jiao Z. Development and Evaluation of a Quantitative Systems Pharmacology Model for Mechanism Interpretation and Efficacy Prediction of Atezolizumab in Combination with Carboplatin and Nab-Paclitaxel in Patients with Non-Small-Cell Lung Cancer. Pharmaceuticals. 2024; 17(2):238. https://doi.org/10.3390/ph17020238
Chicago/Turabian StyleWang, Chen-Yu, Hao-Ran Dai, Yu-Ping Tan, Di-Hong Yang, Xiao-Min Niu, Lu Han, Wen Wang, Ling-Ling Ma, Aleksi Julku, and Zheng Jiao. 2024. "Development and Evaluation of a Quantitative Systems Pharmacology Model for Mechanism Interpretation and Efficacy Prediction of Atezolizumab in Combination with Carboplatin and Nab-Paclitaxel in Patients with Non-Small-Cell Lung Cancer" Pharmaceuticals 17, no. 2: 238. https://doi.org/10.3390/ph17020238
APA StyleWang, C.-Y., Dai, H.-R., Tan, Y.-P., Yang, D.-H., Niu, X.-M., Han, L., Wang, W., Ma, L.-L., Julku, A., & Jiao, Z. (2024). Development and Evaluation of a Quantitative Systems Pharmacology Model for Mechanism Interpretation and Efficacy Prediction of Atezolizumab in Combination with Carboplatin and Nab-Paclitaxel in Patients with Non-Small-Cell Lung Cancer. Pharmaceuticals, 17(2), 238. https://doi.org/10.3390/ph17020238









