Small Molecules in Parkinson’s Disease Therapy: From Dopamine Pathways to New Emerging Targets
Abstract
1. Introduction
2. Dopamine Pathway
3. FDA-Approved Therapy (Currently Available Treatments)
3.1. Levodopa with Dopa-Decarboxylase Inhibitor (DDCI)
3.2. Dopamine Agonist
3.3. Catechol-O-Methyltransferase (COMT) Inhibitor
3.4. Monoamine Oxidase Type B (MAO-B) Inhibitor
3.5. Cholinesterase Inhibitor
3.6. Adenosine A2A Receptor Antagonist
4. Potent Targets for Parkinson’s Disease
4.1. α-Synuclein
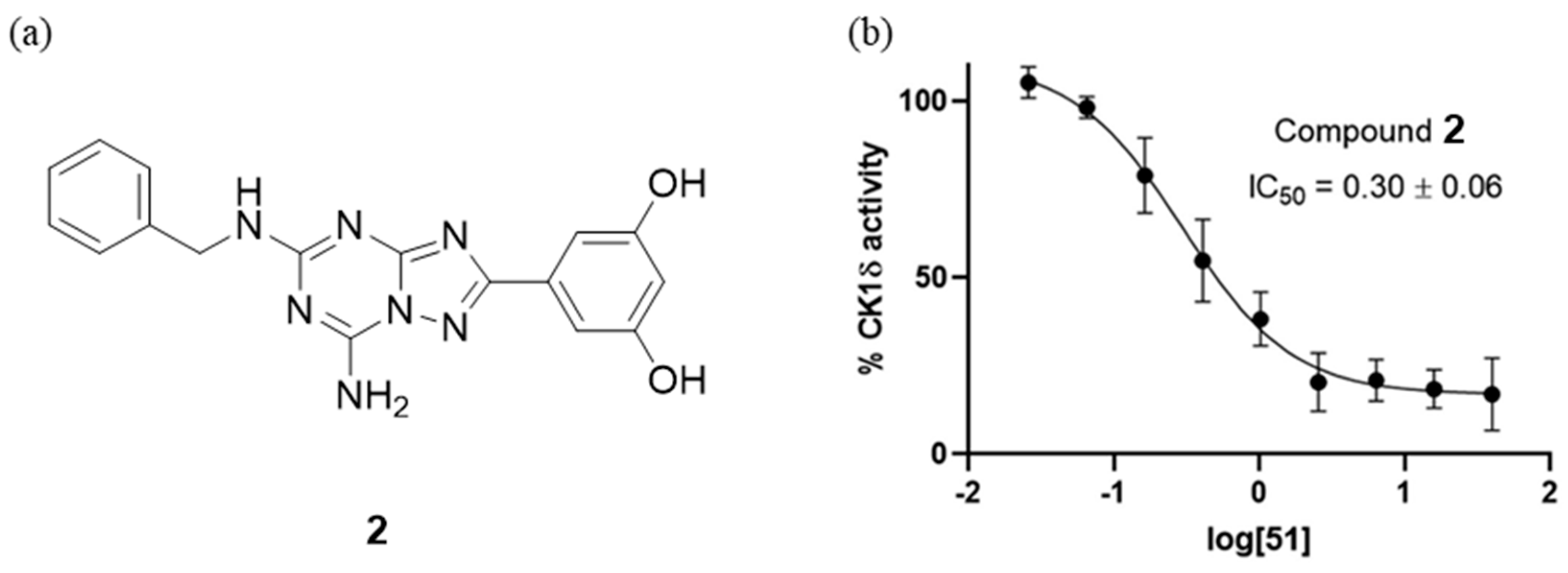
4.2. CK-1δ Inhibitors
4.3. CaV1.3 Calcium Channel Selective Antagonists
4.4. LRRK2 Inhibitors
4.5. Mitochondria
4.6. Glucocerebrosidase (GBA)
5. Conclusions
Funding
Conflicts of Interest
References
- Collaborators, G.N. Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Zhu, J.; Cui, Y.; Zhang, J.; Yan, R.; Su, D.; Zhao, D.; Wang, A.; Feng, T. Temporal trends in the prevalence of Parkinson’s disease from 1980 to 2023: A systematic review and meta-analysis. Lancet Healthy Longev. 2024, 5, e464–e479. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.; Gordon, G.; Hand, A.; Walker, R.W.; Fisher, J.M. 200 Years of Parkinson’s disease: What have we learnt from James Parkinson? Age Ageing 2018, 47, 209–214. [Google Scholar] [CrossRef]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139 (Suppl. S1), 318–324. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Su, C.H. Evidence Supports PA Prescription for Parkinson’s Disease: Motor Symptoms and Non-Motor Features: A Scoping Review. Int. J. Environ. Res. Public Health 2020, 17, 2894. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W. Non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008, 15 (Suppl. S1), 14–20. [Google Scholar] [CrossRef]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef]
- Levey, A.I.; Hersch, S.M.; Rye, D.B.; Sunahara, R.K.; Niznik, H.B.; Kitt, C.A.; Price, D.L.; Maggio, R.; Brann, M.R.; Ciliax, B.J. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc. Natl. Acad. Sci. USA 1993, 90, 8861–8865. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Jahangeer, M.; Maknoon Razia, D.; Ashiq, M.; Ghaffar, A.; Akram, M.; El Allam, A.; Bouyahya, A.; Garipova, L.; Ali Shariati, M.; et al. Dopamine in Parkinson’s disease. Clin. Chim. Acta Int. J. Clin. Chem. 2021, 522, 114–126. [Google Scholar] [CrossRef]
- Salat, D.; Tolosa, E. Levodopa in the treatment of Parkinson’s disease: Current status and new developments. J. Park. Dis. 2013, 3, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Seeberger, L.C.; Hauser, R.A. Levodopa/carbidopa/entacapone in Parkinson’s disease. Expert Rev. Neurother. 2009, 9, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Ferreira, J.J.; Rascol, O. COMT Inhibitors in the Management of Parkinson’s Disease. CNS Drugs 2022, 36, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, I.; Shafi, A.; Haq, E. Pharmacological Treatment of Parkinson’s Disease. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018. [Google Scholar]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Kopin, I.J.; Goldstein, D.S. Catecholamine metabolism: A contemporary view with implications for physiology and medicine. Pharmacol. Rev. 2004, 56, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Tan, E.K. Parkinson’s disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef]
- Pérez-Arancibia, R.; Cisternas-Olmedo, M.; Sepúlveda, D.; Troncoso-Escudero, P.; Vidal, R.L. Small molecules to perform big roles: The search for Parkinson’s and Huntington’s disease therapeutics. Front. Neurosci. 2022, 16, 1084493. [Google Scholar] [CrossRef] [PubMed]
- Reich, S.G.; Savitt, J.M. Parkinson’s Disease. Med. Clin. N. Am. 2019, 103, 337–350. [Google Scholar] [CrossRef]
- Reichmann, H.; Bilsing, A.; Ehret, R.; Greulich, W.; Schulz, J.B.; Schwartz, A.; Rascol, O. Ergoline and non-ergoline derivatives in the treatment of Parkinson’s disease. J. Neurol. 2006, 253 (Suppl. S4), Iv36–Iv38. [Google Scholar] [CrossRef]
- Alonso Cánovas, A.; Luquin Piudo, R.; García Ruiz-Espiga, P.; Burguera, J.A.; Campos Arillo, V.; Castro, A.; Linazasoro, G.; López Del Val, J.; Vela, L.; Martínez Castrillo, J.C. Dopaminergic agonists in Parkinson’s disease. Neurologia 2014, 29, 230–241. [Google Scholar] [CrossRef]
- Carbone, F.; Djamshidian, A.; Seppi, K.; Poewe, W. Apomorphine for Parkinson’s Disease: Efficacy and Safety of Current and New Formulations. CNS Drugs 2019, 33, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Crosby, N.; Deane, K.H.; Clarke, C.E. Amantadine in Parkinson’s disease. Cochrane Database Syst. Rev. 2003, 2003, Cd003468. [Google Scholar] [CrossRef]
- Kornhuber, J.; Weller, M.; Schoppmeyer, K.; Riederer, P. Amantadine and memantine are NMDA receptor antagonists with neuroprotective properties. J. Neural Transm. Suppl. 1994, 43, 91–104. [Google Scholar]
- Takahashi, T.; Yamashita, H.; Zhang, Y.X.; Nakamura, S. Inhibitory effect of MK-801 on amantadine-induced dopamine release in the rat striatum. Brain Res. Bull. 1996, 41, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Dooley, M.; Markham, A. Pramipexole. A review of its use in the management of early and advanced Parkinson’s disease. Drugs Aging 1998, 12, 495–514. [Google Scholar] [CrossRef] [PubMed]
- Scheller, D.; Ullmer, C.; Berkels, R.; Gwarek, M.; Lübbert, H. The in vitro receptor profile of rotigotine: A new agent for the treatment of Parkinson’s disease. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2009, 379, 73–86. [Google Scholar] [CrossRef]
- Frampton, J.E. Rotigotine Transdermal Patch: A Review in Parkinson’s Disease. CNS Drugs 2019, 33, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Connolly, B.S.; Lang, A.E. Pharmacological treatment of Parkinson disease: A review. JAMA 2014, 311, 1670–1683. [Google Scholar] [CrossRef]
- Dezsi, L.; Vecsei, L. Monoamine Oxidase B Inhibitors in Parkinson’s Disease. CNS Neurol. Disord. Drug Targets 2017, 16, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Finberg, J.P.; Gillman, K. Selective inhibitors of monoamine oxidase type B and the “cheese effect”. Int. Rev. Neurobiol. 2011, 100, 169–190. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Jenner, P.; Chen, S.D. Monoamine Oxidase-B Inhibitors for the Treatment of Parkinson’s Disease: Past, Present, and Future. J. Park. Dis. 2022, 12, 477–493. [Google Scholar] [CrossRef]
- Alborghetti, M.; Nicoletti, F. Different Generations of Type-B Monoamine Oxidase Inhibitors in Parkinson’s Disease: From Bench to Bedside. Curr. Neuropharmacol. 2019, 17, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Winter, Y.; von Campenhausen, S.; Arend, M.; Longo, K.; Boetzel, K.; Eggert, K.; Oertel, W.H.; Dodel, R.; Barone, P. Health-related quality of life and its determinants in Parkinson’s disease: Results of an Italian cohort study. Park. Relat. Disord. 2011, 17, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Rengo, G.; Pasqualetti, G.; Femminella, G.D.; Monzani, F.; Ferrara, N.; Tagliati, M. Cholinesterase inhibitors for Parkinson’s disease: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2015, 86, 767–773. [Google Scholar] [CrossRef]
- de Lau, L.M.; Schipper, C.M.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M. Prognosis of Parkinson disease: Risk of dementia and mortality: The Rotterdam Study. Arch. Neurol. 2005, 62, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Lalli, S.; Albanese, A. Rivastigmine in Parkinson’s disease dementia. Expert Rev. Neurother. 2008, 8, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Latini, S.; Pedata, F. Adenosine in the central nervous system: Release mechanisms and extracellular concentrations. J. Neurochem. 2001, 79, 463–484. [Google Scholar] [CrossRef]
- Stiles, G.L. Adenosine receptors. J. Biol. Chem. 1992, 267, 6451–6454. [Google Scholar] [CrossRef] [PubMed]
- Cieślak, M.; Komoszyński, M.; Wojtczak, A. Adenosine A(2A) receptors in Parkinson’s disease treatment. Purinergic Signal. 2008, 4, 305–312. [Google Scholar] [CrossRef]
- Jenner, P. An overview of adenosine A2A receptor antagonists in Parkinson’s disease. Int. Rev. Neurobiol. 2014, 119, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Jenner, P. Can adenosine A2A receptor antagonists modify motor behavior and dyskinesia in experimental models of Parkinson’s disease? Park. Relat. Disord. 2020, 80, S21–S27. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-F.; Cunha, R.A. The belated US FDA approval of the adenosine A2A receptor antagonist istradefylline for treatment of Parkinson’s disease. Purinergic Signal. 2020, 16, 167–174. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, X.; Zhen, X. Development of Adenosine A(2A) Receptor Antagonists for the Treatment of Parkinson’s Disease: A Recent Update and Challenge. ACS Chem. Neurosci. 2019, 10, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Scott, D.A.; Tabarean, I.; Tang, Y.; Cartier, A.; Masliah, E.; Roy, S. A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 8083–8095. [Google Scholar] [CrossRef]
- Borghammer, P. How does parkinson’s disease begin? Perspectives on neuroanatomical pathways, prions, and histology. Mov. Disord. Off. J. Mov. Disord. Soc. 2018, 33, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, M.G. Gastrointestinal Biopsies for the Diagnosis of Alpha-Synuclein Pathology in Parkinson’s Disease. Gastroenterol. Res. Pract. 2015, 2015, 476041. [Google Scholar] [CrossRef]
- Atik, A.; Stewart, T.; Zhang, J. Alpha-Synuclein as a Biomarker for Parkinson’s Disease. Brain Pathol. 2016, 26, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Fanning, S.; Haque, A.; Imberdis, T.; Baru, V.; Barrasa, M.I.; Nuber, S.; Termine, D.; Ramalingam, N.; Ho, G.P.H.; Noble, T.; et al. Lipidomic Analysis of α-Synuclein Neurotoxicity Identifies Stearoyl CoA Desaturase as a Target for Parkinson Treatment. Mol. Cell 2019, 73, 1001–1014.e8. [Google Scholar] [CrossRef] [PubMed]
- Troncoso-Escudero, P.; Sepulveda, D.; Pérez-Arancibia, R.; Parra, A.V.; Arcos, J.; Grunenwald, F.; Vidal, R.L. On the Right Track to Treat Movement Disorders: Promising Therapeutic Approaches for Parkinson’s and Huntington’s Disease. Front. Aging Neurosci. 2020, 12, 571185. [Google Scholar] [CrossRef]
- Angot, E.; Brundin, P. Dissecting the potential molecular mechanisms underlying alpha-synuclein cell-to-cell transfer in Parkinson’s disease. Park. Relat. Disord. 2009, 15 (Suppl. S3), S143–S147. [Google Scholar] [CrossRef] [PubMed]
- Bisaglia, M.; Mammi, S.; Bubacco, L. Structural insights on physiological functions and pathological effects of alpha-synuclein. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 329–340. [Google Scholar] [CrossRef]
- Fares, M.B.; Jagannath, S.; Lashuel, H.A. Reverse engineering Lewy bodies: How far have we come and how far can we go? Nat. Rev. Neurosci. 2021, 22, 111–131. [Google Scholar] [CrossRef]
- García-Sanz, P.; MFG Aerts, J.; Moratalla, R. The Role of Cholesterol in α-Synuclein and Lewy Body Pathology in GBA1 Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2021, 36, 1070–1085. [Google Scholar] [CrossRef]
- Hoyer, W.; Cherny, D.; Subramaniam, V.; Jovin, T.M. Rapid self-assembly of alpha-synuclein observed by in situ atomic force microscopy. J. Mol. Biol. 2004, 340, 127–139. [Google Scholar] [CrossRef][Green Version]
- Mehra, S.; Sahay, S.; Maji, S.K. α-Synuclein misfolding and aggregation: Implications in Parkinson’s disease pathogenesis. Biochim. Biophys. Acta. Proteins Proteom. 2019, 1867, 890–908. [Google Scholar] [CrossRef]
- Savitt, D.; Jankovic, J. Targeting α-Synuclein in Parkinson’s Disease: Progress Towards the Development of Disease-Modifying Therapeutics. Drugs 2019, 79, 797–810. [Google Scholar] [CrossRef]
- Jia, L.; Wang, W.; Yan, Y.; Hu, R.; Sang, J.; Zhao, W.; Wang, Y.; Wei, W.; Cui, W.; Yang, G.; et al. General Aggregation-Induced Emission Probes for Amyloid Inhibitors with Dual Inhibition Capacity against Amyloid β-Protein and α-Synuclein. ACS Appl. Mater. Interfaces 2020, 12, 31182–31194. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, L.; Zhou, F.; Zhang, Y.X.; Xu, J.; Xu, M.; Bai, S.P. Anti-oligomerization sheet molecules: Design, synthesis and evaluation of inhibitory activities against α-synuclein aggregation. Bioorganic Med. Chem. 2019, 27, 3089–3096. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.; Schumacher, N.U.; Pürner, D.; Machetanz, G.; Demleitner, A.F.; Feneberg, E.; Hagemeier, M.; Lingor, P. Parkinson’s disease therapy: What lies ahead? J. Neural. Transm. 2023, 130, 793–820. [Google Scholar] [CrossRef] [PubMed]
- Catarzi, D.; Varano, F.; Vigiani, E.; Lambertucci, C.; Spinaci, A.; Volpini, R.; Colotta, V. Casein Kinase 1δ Inhibitors as Promising Therapeutic Agents for Neurodegenerative Disorders. Curr. Med. Chem. 2022, 29, 4698–4737. [Google Scholar] [CrossRef] [PubMed]
- Morales-Garcia, J.A.; Salado, I.G.; Sanz-San Cristobal, M.; Gil, C.; Pérez-Castillo, A.; Martínez, A.; Pérez, D.I. Biological and Pharmacological Characterization of Benzothiazole-Based CK-1δ Inhibitors in Models of Parkinson’s Disease. ACS Omega 2017, 2, 5215–5220. [Google Scholar] [CrossRef]
- Grieco, I.; Bissaro, M.; Tiz, D.B.; Perez, D.I.; Perez, C.; Martinez, A.; Redenti, S.; Mariotto, E.; Bortolozzi, R.; Viola, G.; et al. Developing novel classes of protein kinase CK1δ inhibitors by fusing [1,2,4]triazole with different bicyclic heteroaromatic systems. Eur. J. Med. Chem. 2021, 216, 113331. [Google Scholar] [CrossRef] [PubMed]
- Striessnig, J.; Pinggera, A.; Kaur, G.; Bock, G.; Tuluc, P. L-type Ca(2+) channels in heart and brain. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2014, 3, 15–38. [Google Scholar] [CrossRef]
- Zaveri, S.; Srivastava, U.; Qu, Y.S.; Chahine, M.; Boutjdir, M. Pathophysiology of Ca(v)1.3 L-type calcium channels in the heart. Front. Physiol. 2023, 14, 1144069. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Schumacker, P.T.; Guzman, J.D.; Ilijic, E.; Yang, B.; Zampese, E. Calcium and Parkinson’s disease. Biochem. Biophys. Res. Commun. 2017, 483, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Ravindranath, V. Ca(V)1.3 L-Type Calcium Channels Increase the Vulnerability of Substantia Nigra Dopaminergic Neurons in MPTP Mouse Model of Parkinson’s Disease. Front. Aging Neurosci. 2019, 11, 382. [Google Scholar] [CrossRef]
- Filippini, L.; Ortner, N.J.; Kaserer, T.; Striessnig, J. Ca(v) 1.3-selective inhibitors of voltage-gated L-type Ca(2+) channels: Fact or (still) fiction? Br. J. Pharmacol. 2023, 180, 1289–1303. [Google Scholar] [CrossRef]
- Kang, S.; Cooper, G.; Dunne, S.F.; Dusel, B.; Luan, C.H.; Surmeier, D.J.; Silverman, R.B. CaV1.3-selective L-type calcium channel antagonists as potential new therapeutics for Parkinson’s disease. Nat. Commun. 2012, 3, 1146. [Google Scholar] [CrossRef]
- Jia, F.; Fellner, A.; Kumar, K.R. Monogenic Parkinson’s Disease: Genotype, Phenotype, Pathophysiology, and Genetic Testing. Genes 2022, 13, 471. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, D.; Tian, K.; Ren, C.; Li, H.; Lin, C.; Huang, X.; Liu, J.; Mao, W.; Zhang, J. Small-molecule LRRK2 inhibitors for PD therapy: Current achievements and future perspectives. Eur. J. Med. Chem. 2023, 256, 115475. [Google Scholar] [CrossRef]
- Tolosa, E.; Vila, M.; Klein, C.; Rascol, O. LRRK2 in Parkinson disease: Challenges of clinical trials. Nat. Rev. Neurol. 2020, 16, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Konno, T.; Siuda, J.; Wszolek, Z.K. Genetics of Parkinson’s disease: A review of SNCA and LRRK2. Wiad. Lek. 2016, 69, 328–332. [Google Scholar]
- Dulski, J.; Ross, O.A.; Wszolek, Z.K. VPS35-Related Parkinson Disease. In GeneReviews(®®); Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Smith, L.; Schapira, A.H.V. GBA Variants and Parkinson Disease: Mechanisms and Treatments. Cells 2022, 11, 1261. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Li, Y.; Nishioka, K.; Daida, K.; Hayashida, A.; Ishiguro, Y.; Yamada, D.; Izawa, N.; Nishi, K.; Nishikawa, N.; et al. Genotype-phenotype correlation of Parkinson’s disease with PRKN variants. Neurobiol. Aging 2022, 114, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K. The PINK1-Parkin axis: An Overview. Neurosci. Res. 2020, 159, 9–15. [Google Scholar] [CrossRef]
- Dolgacheva, L.P.; Berezhnov, A.V.; Fedotova, E.I.; Zinchenko, V.P.; Abramov, A.Y. Role of DJ-1 in the mechanism of pathogenesis of Parkinson’s disease. J. Bioenerg. Biomembr. 2019, 51, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Rui, Q.; Ni, H.; Li, D.; Gao, R.; Chen, G. The Role of LRRK2 in Neurodegeneration of Parkinson Disease. Curr. Neuropharmacol. 2018, 16, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Dawson, T.M.; Dawson, V.L. Models of LRRK2-Associated Parkinson’s Disease. Adv. Neurobiol. 2017, 14, 163–191. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Dzamko, N.; Prescott, A.; Davies, P.; Liu, Q.; Yang, Q.; Lee, J.-D.; Patricelli, M.P.; Nomanbhoy, T.K.; Alessi, D.R.; et al. Characterization of a selective inhibitor of the Parkinson’s disease kinase LRRK2. Nat. Chem. Biol. 2011, 7, 203–205. [Google Scholar] [CrossRef]
- Scott, J.D.; DeMong, D.E.; Greshock, T.J.; Basu, K.; Dai, X.; Harris, J.; Hruza, A.; Li, S.W.; Lin, S.-I.; Liu, H.; et al. Discovery of a 3-(4-Pyrimidinyl) Indazole (MLi-2), an Orally Available and Selective Leucine-Rich Repeat Kinase 2 (LRRK2) Inhibitor that Reduces Brain Kinase Activity. J. Med. Chem. 2017, 60, 2983–2992. [Google Scholar] [CrossRef] [PubMed]
- Estrada, A.A.; Liu, X.; Baker-Glenn, C.; Beresford, A.; Burdick, D.J.; Chambers, M.; Chan, B.K.; Chen, H.; Ding, X.; DiPasquale, A.G.; et al. Discovery of Highly Potent, Selective, and Brain-Penetrable Leucine-Rich Repeat Kinase 2 (LRRK2) Small Molecule Inhibitors. J. Med. Chem. 2012, 55, 9416–9433. [Google Scholar] [CrossRef] [PubMed]
- Estrada, A.A.; Chan, B.K.; Baker-Glenn, C.; Beresford, A.; Burdick, D.J.; Chambers, M.; Chen, H.; Dominguez, S.L.; Dotson, J.; Drummond, J.; et al. Discovery of highly potent, selective, and brain-penetrant aminopyrazole leucine-rich repeat kinase 2 (LRRK2) small molecule inhibitors. J. Med. Chem. 2014, 57, 921–936. [Google Scholar] [CrossRef]
- Jennings, D.; Huntwork-Rodriguez, S.; Henry, A.G.; Sasaki, J.C.; Meisner, R.; Diaz, D.; Solanoy, H.; Wang, X.; Negrou, E.; Bondar, V.V.; et al. Preclinical and clinical evaluation of the LRRK2 inhibitor DNL201 for Parkinson’s disease. Sci. Transl. Med. 2022, 14, eabj2658. [Google Scholar] [CrossRef]
- Jennings, D.; Huntwork-Rodriguez, S.; Vissers, M.; Daryani, V.M.; Diaz, D.; Goo, M.S.; Chen, J.J.; Maciuca, R.; Fraser, K.; Mabrouk, O.S.; et al. LRRK2 Inhibition by BIIB122 in Healthy Participants and Patients with Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2023, 38, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, R.W. 1-Pyrazolyl-5,6-Disubstituted Indazole Derivatives as LRRK2 Inhibitors for Treating Parkinson’s Disease. ACS Med. Chem. Lett. 2021, 12, 310–311. [Google Scholar] [CrossRef]
- Sabnis, R.W. Novel N-Heteroaryl Indazole Derivatives as LRRK2 Inhibitors for Treating Parkinson’s Disease. ACS Med. Chem. Lett. 2021, 12, 530–531. [Google Scholar] [CrossRef]
- Sabnis, R.W. Novel N-Heteroaryl Quinazolin-2-amine Derivatives as LRRK2 Inhibitors for Treating Parkinson’s Disease. ACS Med. Chem. Lett. 2021, 12, 1063–1064. [Google Scholar] [CrossRef]
- Sabnis, R.W. Novel Macrocyclic LRRK2 Inhibitors for Treating Parkinson’s Disease. ACS Med. Chem. Lett. 2022, 13, 25–26. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, R.W. 2-Aminoquinazolines as LRRK2 Inhibitors for Treating Parkinson’s Disease. ACS Med. Chem. Lett. 2022, 13, 775–776. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, R.W. Novel N-Linked Isoquinoline Amides as LRRK2 Inhibitors for Treating Parkinson’s Disease. ACS Med. Chem. Lett. 2022, 13, 1008–1009. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, R.W.; Sabnis, A.R. Novel C-Linked Isoquinoline Amides as LRRK2 Inhibitors for Treating Parkinson’s Disease. ACS Med. Chem. Lett. 2023, 14, 698–699. [Google Scholar] [CrossRef]
- Keylor, M.H.; Gulati, A.; Kattar, S.D.; Johnson, R.E.; Chau, R.W.; Margrey, K.A.; Ardolino, M.J.; Zarate, C.; Poremba, K.E.; Simov, V.; et al. Structure-Guided Discovery of Aminoquinazolines as Brain-Penetrant and Selective LRRK2 Inhibitors. J. Med. Chem. 2022, 65, 838–856. [Google Scholar] [CrossRef]
- Garofalo, A.W.; Bright, J.; De Lombaert, S.; Toda, A.M.A.; Zobel, K.; Andreotti, D.; Beato, C.; Bernardi, S.; Budassi, F.; Caberlotto, L.; et al. Selective Inhibitors of G2019S-LRRK2 Kinase Activity. J. Med. Chem. 2020, 63, 14821–14839. [Google Scholar] [CrossRef] [PubMed]
- Leśniak, R.K.; Nichols, R.J.; Schonemann, M.; Zhao, J.; Gajera, C.R.; Lam, G.; Nguyen, K.C.; Langston, J.W.; Smith, M.; Montine, T.J. Discovery of 1H-Pyrazole Biaryl Sulfonamides as Novel G2019S-LRRK2 Kinase Inhibitors. ACS Med. Chem. Lett. 2022, 13, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Rosse, G. Imidazoquinolines as Novel Inhibitors of LRRK2 Kinase Activity. ACS Med. Chem. Lett. 2019, 10, 148–149. [Google Scholar] [CrossRef]
- Zaldivar-Diez, J.; Li, L.; Garcia, A.M.; Zhao, W.-N.; Medina-Menendez, C.; Haggarty, S.J.; Gil, C.; Morales, A.V.; Martinez, A. Benzothiazole-Based LRRK2 Inhibitors as Wnt Enhancers and Promoters of Oligodendrocytic Fate. J. Med. Chem. 2020, 63, 2638–2655. [Google Scholar] [CrossRef]
- Williamson, D.S.; Smith, G.P.; Mikkelsen, G.K.; Jensen, T.; Acheson-Dossang, P.; Badolo, L.; Bedford, S.T.; Chell, V.; Chen, I.J.; Dokurno, P.; et al. Design and Synthesis of Pyrrolo [2,3-d]pyrimidine-Derived Leucine-Rich Repeat Kinase 2 (LRRK2) Inhibitors Using a Checkpoint Kinase 1 (CHK1)-Derived Crystallographic Surrogate. J. Med. Chem. 2021, 64, 10312–10332. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kalogeropulou, A.F.; Domingos, S.; Makukhin, N.; Nirujogi, R.S.; Singh, F.; Shpiro, N.; Saalfrank, A.; Sammler, E.; Ganley, I.G.; et al. Discovery of XL01126: A Potent, Fast, Cooperative, Selective, Orally Bioavailable, and Blood–Brain Barrier Penetrant PROTAC Degrader of Leucine-Rich Repeat Kinase 2. J. Am. Chem. Soc. 2022, 144, 16930–16952. [Google Scholar] [CrossRef]
- Schwarzschild, M.A.; Ascherio, A.; Casaceli, C.; Curhan, G.C.; Fitzgerald, R.; Kamp, C.; Lungu, C.; Macklin, E.A.; Marek, K.; Mozaffarian, D.; et al. Effect of Urate-Elevating Inosine on Early Parkinson Disease Progression: The SURE-PD3 Randomized Clinical Trial. JAMA 2021, 326, 926–939. [Google Scholar] [CrossRef]
- Choong, C.J.; Mochizuki, H. Involvement of Mitochondria in Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 17027. [Google Scholar] [CrossRef] [PubMed]
- Payne, T.; Sassani, M.; Buckley, E.; Moll, S.; Anton, A.; Appleby, M.; Maru, S.; Taylor, R.; McNeill, A.; Hoggard, N.; et al. Ursodeoxycholic acid as a novel disease-modifying treatment for Parkinson’s disease: Protocol for a two-centre, randomised, double-blind, placebo-controlled trial, The ‘UP’ study. BMJ Open 2020, 10, e038911. [Google Scholar] [CrossRef] [PubMed]
- Day, J.O.; Mullin, S. The Genetics of Parkinson’s Disease and Implications for Clinical Practice. Genes 2021, 12, 1006. [Google Scholar] [CrossRef] [PubMed]
- Kopytova, A.E.; Rychkov, G.N.; Nikolaev, M.A.; Baydakova, G.V.; Cheblokov, A.A.; Senkevich, K.A.; Bogdanova, D.A.; Bolshakova, O.I.; Miliukhina, I.V.; Bezrukikh, V.A.; et al. Ambroxol increases glucocerebrosidase (GCase) activity and restores GCase translocation in primary patient-derived macrophages in Gaucher disease and Parkinsonism. Park. Relat. Disord. 2021, 84, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.E.; Al-Jasmi, F. Exploring the efficacy and safety of Ambroxol in Gaucher disease: An overview of clinical studies. Front. Pharmacol. 2024, 15, 1335058. [Google Scholar] [CrossRef] [PubMed]
- Mullin, S.; Smith, L.; Lee, K.; D’Souza, G.; Woodgate, P.; Elflein, J.; Hällqvist, J.; Toffoli, M.; Streeter, A.; Hosking, J.; et al. Ambroxol for the Treatment of Patients With Parkinson Disease With and Without Glucocerebrosidase Gene Mutations: A Nonrandomized, Noncontrolled Trial. JAMA Neurol. 2020, 77, 427–434. [Google Scholar] [CrossRef] [PubMed]








| Drug Classification | Drug Name | Ingredient | Year | ||
|---|---|---|---|---|---|
| 1 | Levadopa and derivatices | Duopa enteral suspension | Levodopa (A) and Carbidopa (D) | 2015 | |
| Rytary | 2015 | ||||
| 2 | Non-ergoline dopamine agonist | Mirapex | Pramipexole (G) | 1997 | |
| Apokyn | Apomorphine hydrochloride (B) | 2004 | |||
| Kynmobi | 2020 | ||||
| Neupro | Rotigotine (H) | 2012 | |||
| 3 | Catechol-O-methyltransferase (COMT) inhibitor | Comtan | Entacapone (C) | 1999 | |
| Ongentys | Opicapone (K) | 2020 | |||
| 4 | Monoamine oxidase type B (MAO-B) inhibitor | Xadago | Safinamide (L) | 2017 | |
| 5 | Ohters | Cholinesterase inhibitor | Exelon Patch | Rivastigmine tartrate (E) | 2007 |
| Dopamine agonist, NMDA receptor antagonist | Gocovri | Amantadine (F) | 2017 | ||
| Adenosine A2A receptor antagonist | Nourianz | Istradefylline (I) | 2019 | ||
| Atypical antipsychotic | Nuplazid | Pimavanserin (J) | 2016 | ||
| 1-Pyrazolyl-5,6-Disubstituted Indazole | LRRK2 IC50 (μM) | N-Heteroaryl Indazole | LRRK2 IC50 (nM) | ||
| 3a | 0.09751 | 4a | 0.90 | ||
| 3b | 0.1043 | 4b | 0.80 | ||
| 3c | <0.0804 | 4c | <0.625 | ||
| 3d | 0.1570 | 4d | 0.71 | ||
| 3e | 0.2592 | 4e | <0.625 | ||
| 3f | 0.1540 | 4f | <0.625 | ||
| N-Heteroaryl Quinazolin-2-amine | LRRK2 pIC50* | Macrocyclic | LRRK2 ADP-Glo IC50 (nM) | ||
| 5a | >9.20 | 6a | <10 | ||
| 5b | >9.20 | 6b | <10 | ||
| 5c | >9.20 | 6c | <10 | ||
| 5d | >9.20 | 6d | <10 | ||
| 5e | >9.20 | 6e | <10 | ||
| 5f | >9.20 | 6f | <10 | ||
| 2-Aminoquinazoline | LRRK2 pIC50 (nM)* | N-Linked Isoquinoline | LRRK2 pIC50 (nM)* | ||
| 7a | 10.12 | 8a | 10.09 | ||
| 7b | 9.772 | 8b | 10.09 | ||
| 7c | 9.759 | 8c | 10.08 | ||
| 7d | 10.19 | 8d | 10.09 | ||
| 7e | 9.727 | 8e | 10.00 | ||
| 7f | 9.346 | 8f | 10.09 | ||
| C-Linked Isoquinoline Amide | LRRK2 pIC50 (nM)* | *pIC50 = −log10(IC50) | |||
| 9a | 10.09 | ||||
| 9b | 10.09 | ||||
| 9c | 10.09 | ||||
| 9d | 10.09 | ||||
| 9e | 10.09 | ||||
| 9f | 10.09 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Elkamhawy, A.; Rakhalskaya, P.; Lu, Q.; Nada, H.; Quan, G.; Lee, K. Small Molecules in Parkinson’s Disease Therapy: From Dopamine Pathways to New Emerging Targets. Pharmaceuticals 2024, 17, 1688. https://doi.org/10.3390/ph17121688
Lee H, Elkamhawy A, Rakhalskaya P, Lu Q, Nada H, Quan G, Lee K. Small Molecules in Parkinson’s Disease Therapy: From Dopamine Pathways to New Emerging Targets. Pharmaceuticals. 2024; 17(12):1688. https://doi.org/10.3390/ph17121688
Chicago/Turabian StyleLee, Hwayoung, Ahmed Elkamhawy, Polina Rakhalskaya, Qili Lu, Hossam Nada, Guofeng Quan, and Kyeong Lee. 2024. "Small Molecules in Parkinson’s Disease Therapy: From Dopamine Pathways to New Emerging Targets" Pharmaceuticals 17, no. 12: 1688. https://doi.org/10.3390/ph17121688
APA StyleLee, H., Elkamhawy, A., Rakhalskaya, P., Lu, Q., Nada, H., Quan, G., & Lee, K. (2024). Small Molecules in Parkinson’s Disease Therapy: From Dopamine Pathways to New Emerging Targets. Pharmaceuticals, 17(12), 1688. https://doi.org/10.3390/ph17121688







