Antinociceptive Potential of Ximenia americana L. Bark Extract and Caffeic Acid: Insights into Pain Modulation Pathways
Abstract
1. Introduction
2. Results
2.1. HEXA Failed in Inducing Central Nervous System Effects in Mice
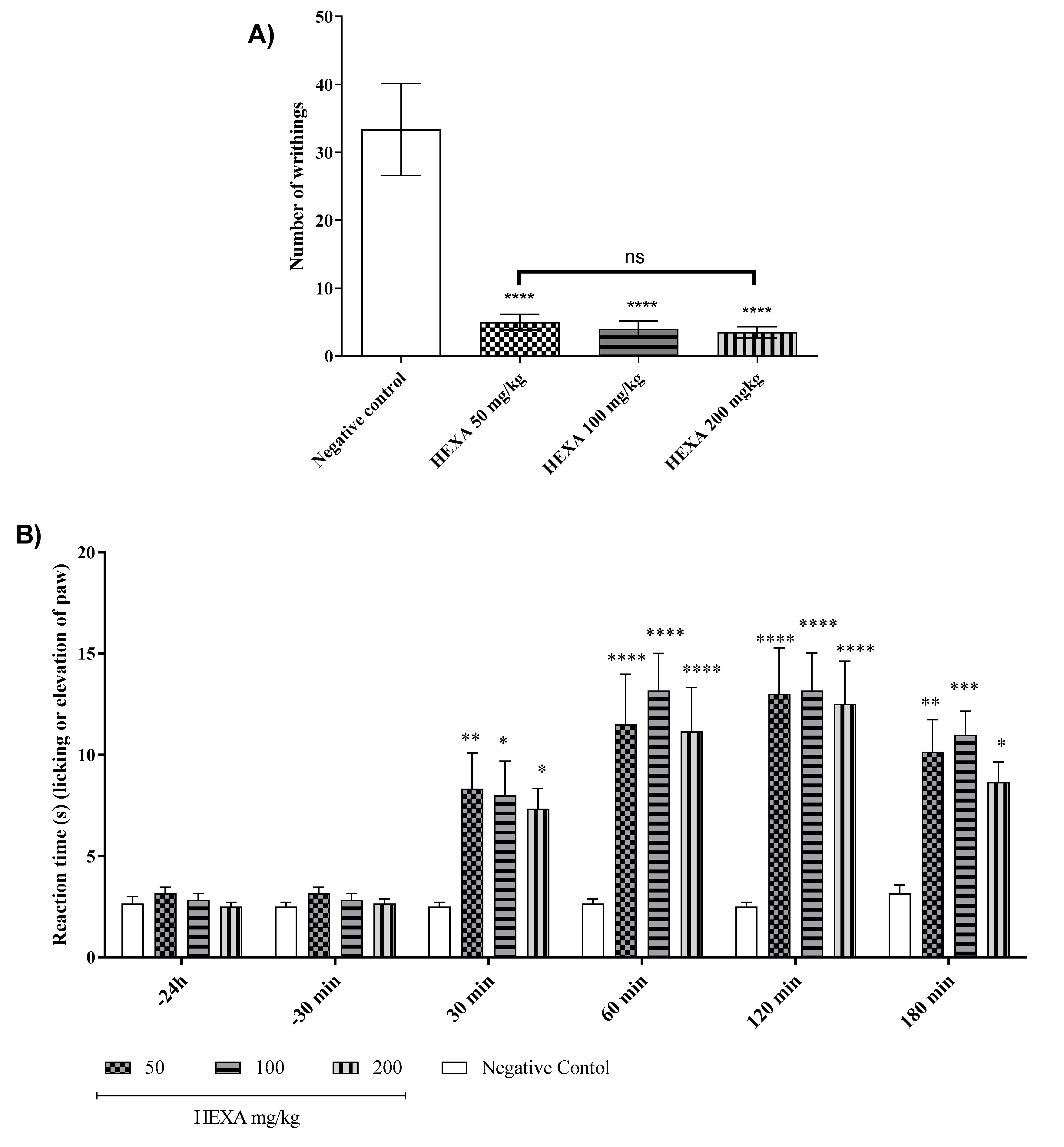
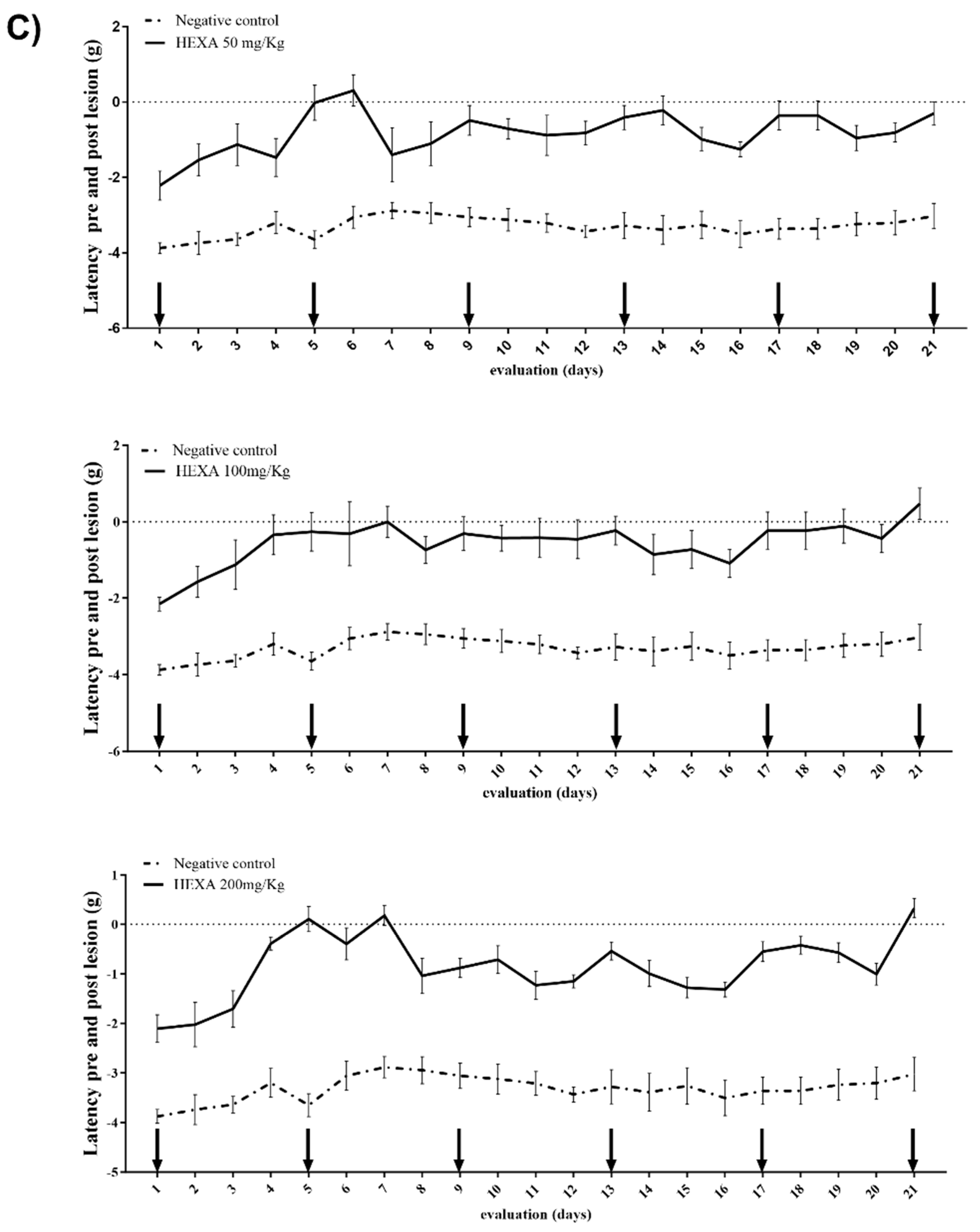
2.2. HEXA Has Antinociceptive Activity In Vivo: Central and Peripheral Effects
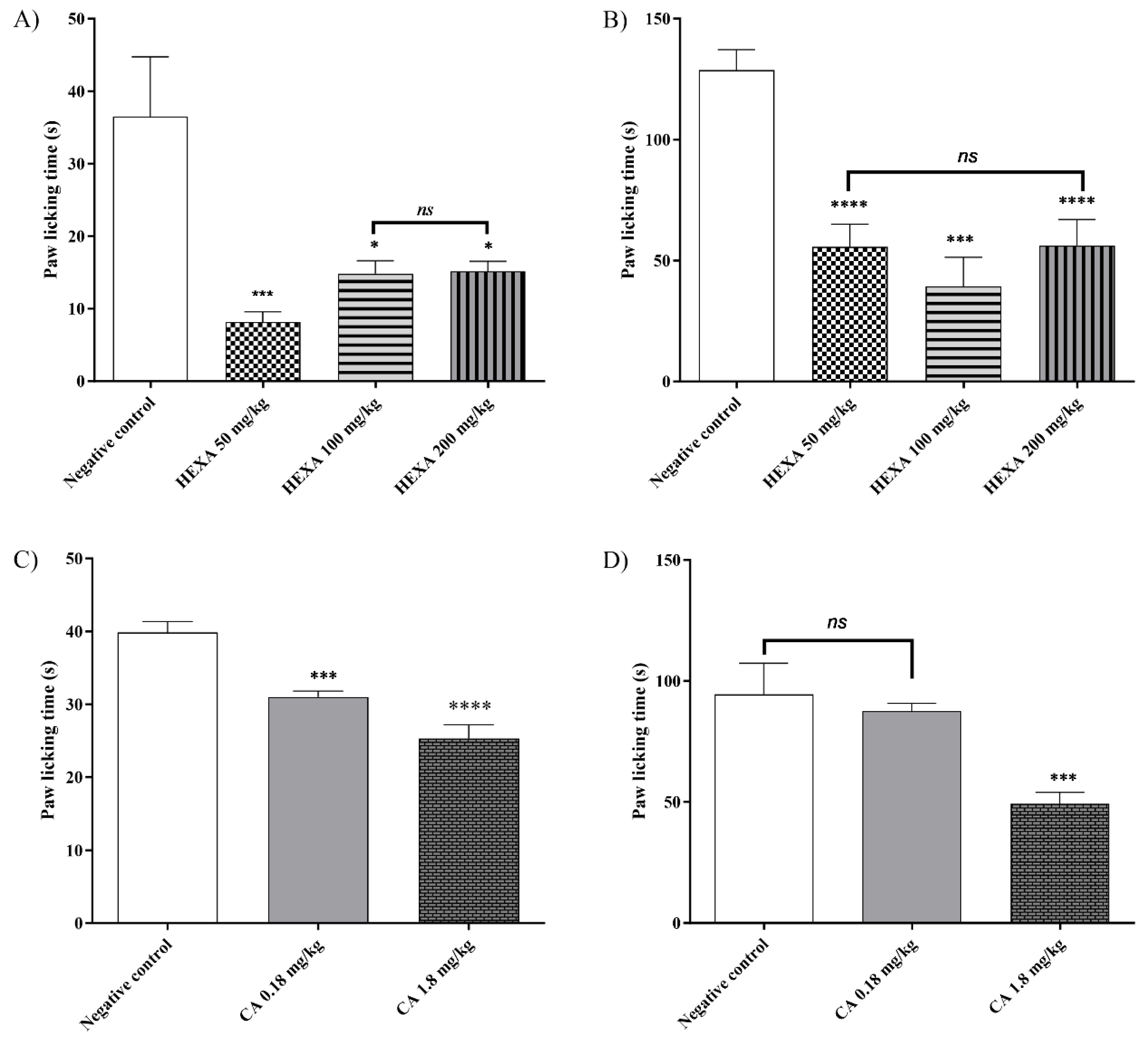
2.3. Formalin-Induced Nociception Test of HEXA and CA
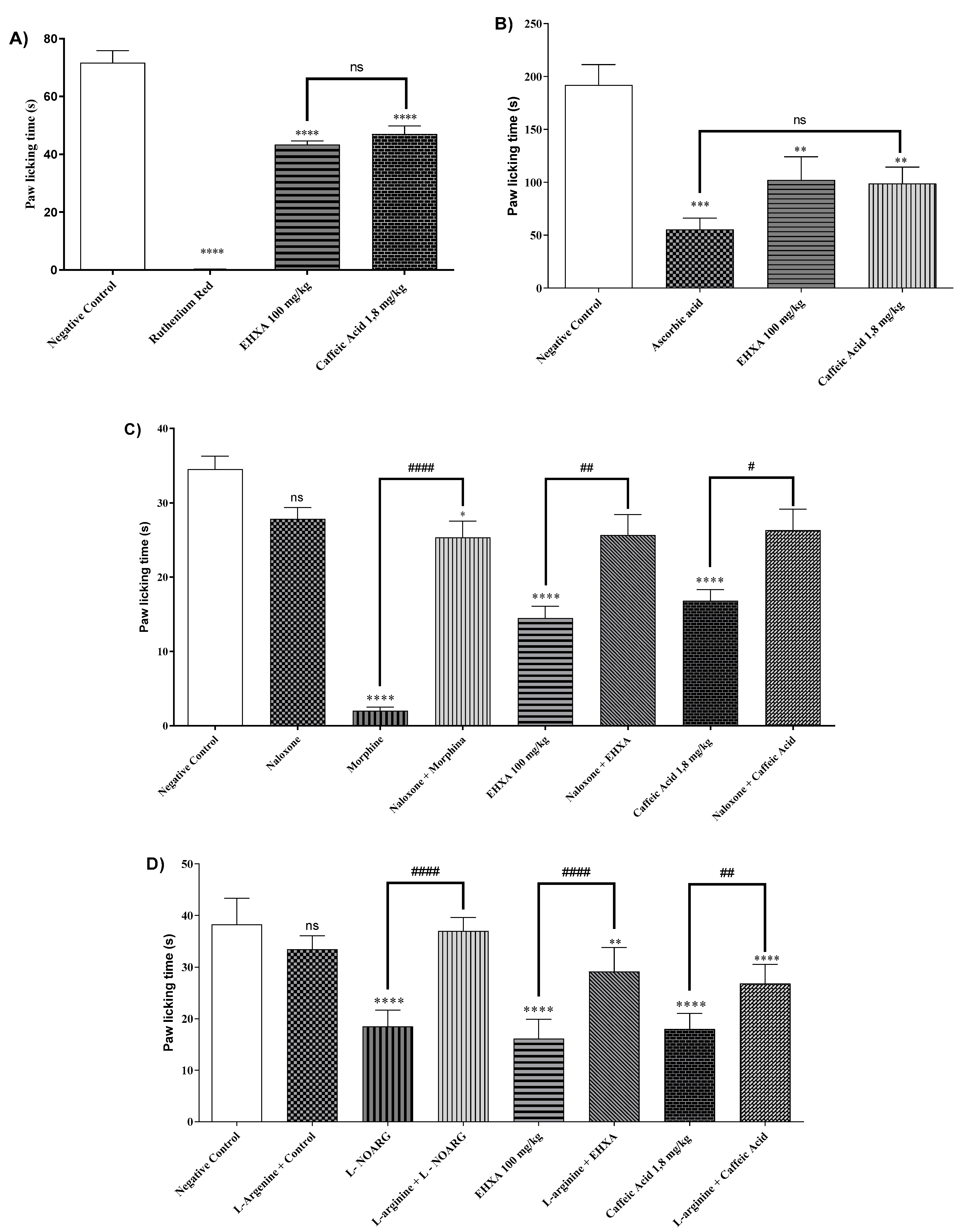
2.4. Analysis of Signaling Pathways Underlying the Analgesic Effect of HEXA and CA
2.4.1. Vanilloid System
2.4.2. Glutamatergic Pathway
2.4.3. Opioid Pathway
2.4.4. L-Arginine/Nitric Oxide/cGMP Pathway
2.4.5. Cyclic Guanosine Monophosphate (cGMP) Pathway
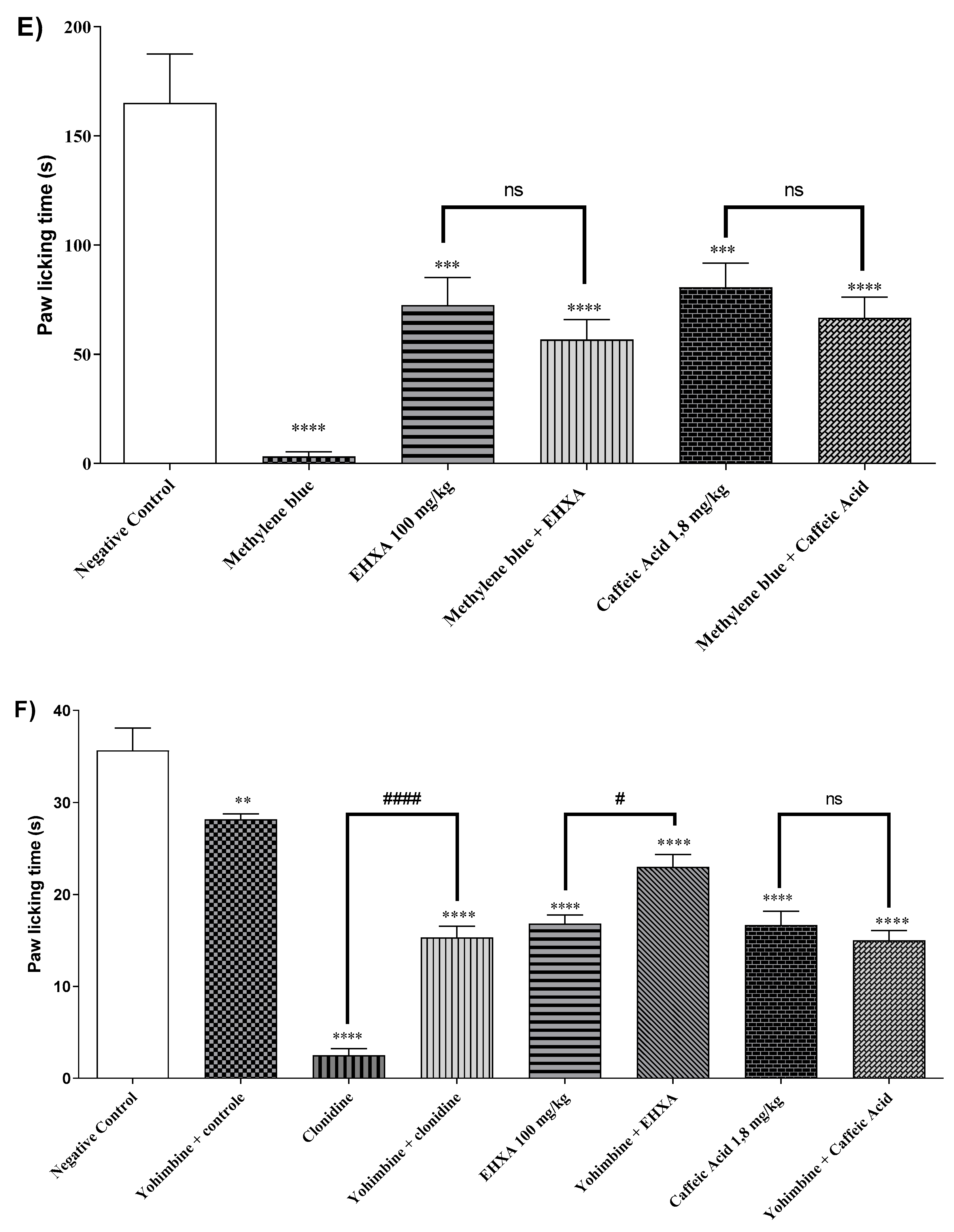
2.4.6. Involvement of the α2-Adrenergic Receptor
2.4.7. K+ATP Channel-Dependent Signaling
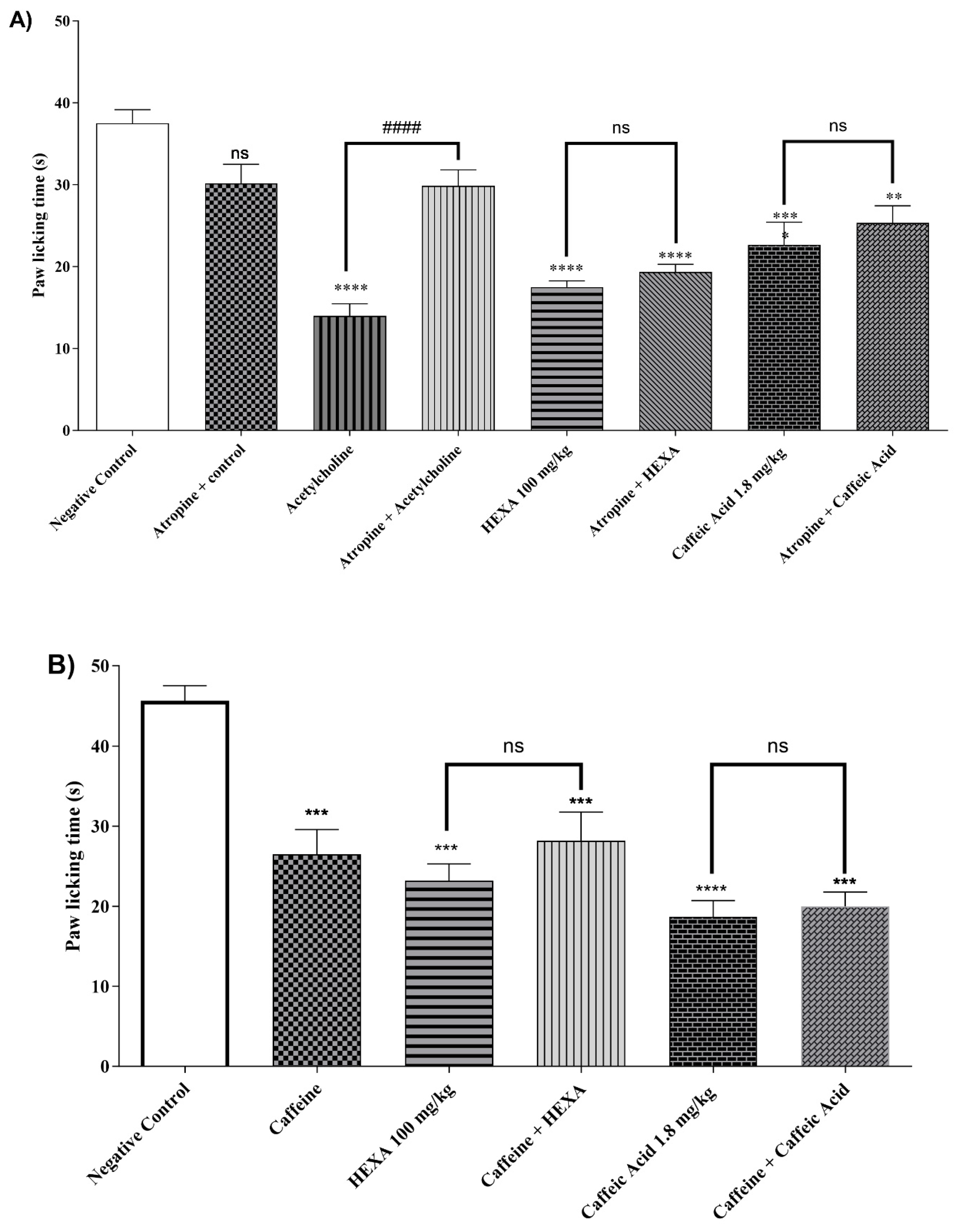
2.4.8. Cholinergic Pathway
2.4.9. Adenosinergic Pathway
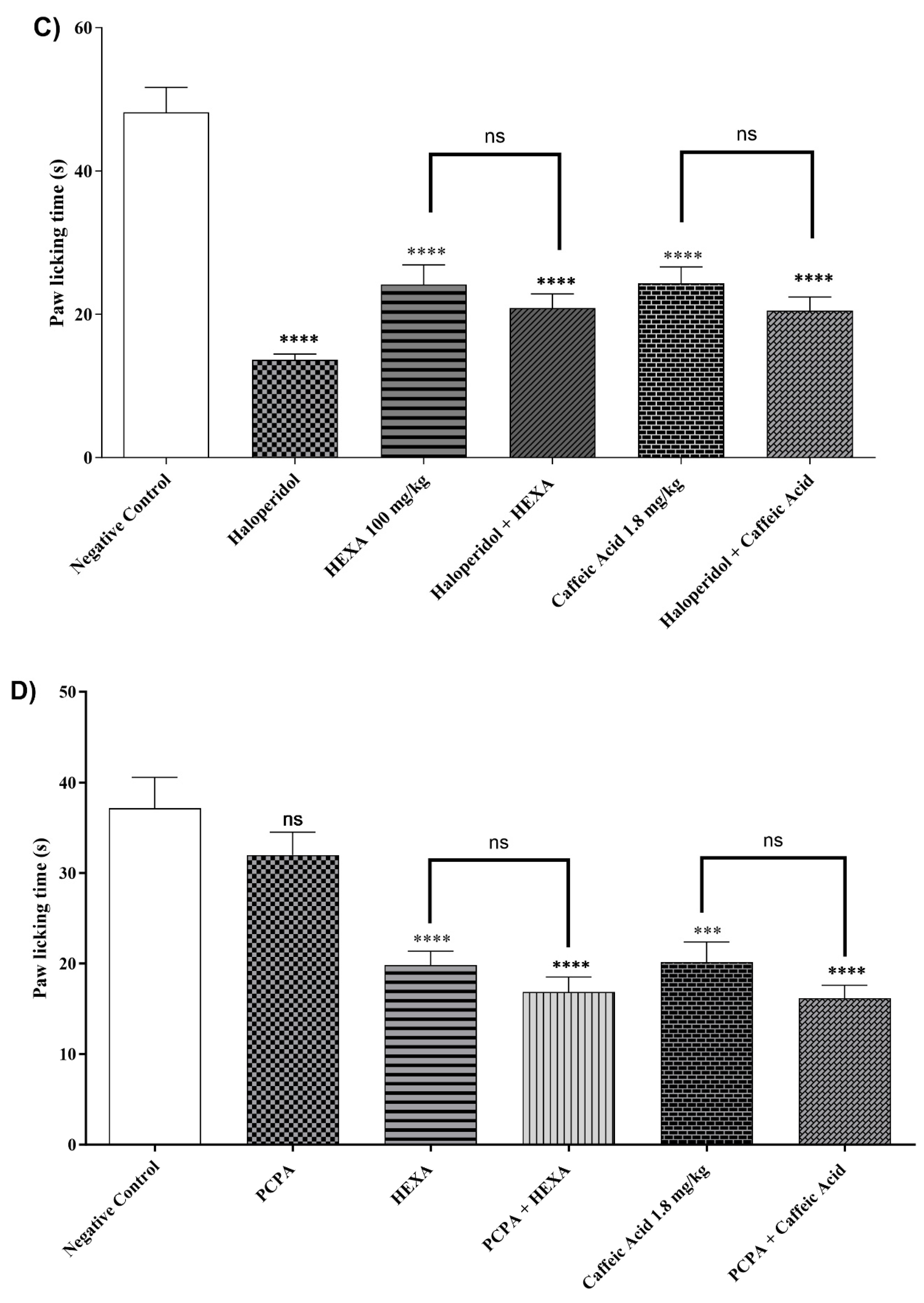
2.4.10. Dopaminergic System
2.4.11. Involvement of the Serotonergic System
3. Discussion
4. Materials and Methods
4.1. Drugs, Reagents, and Doses
4.2. Chemical Profile by Electrospray Ionization–Mass Spectrometry (ESI-MS) Analysis
Compound Annotation
4.3. Animals
4.4. Ethical Information
4.5. Evaluation of the Effects of HEXA on the Central Nervous System
4.5.1. Rotarod Performance Test
4.5.2. Open-Field Test
4.6. Screening for the Antinociceptive Effect
4.6.1. Acetic Acid-Induced Abdominal Contortions
4.6.2. The Formalin Test
4.7. Evaluation of Central and Peripheral Antinociceptive Responses
4.7.1. Hot Plate Test
4.7.2. Mechanical Pressure Hypernociception (Von Frey Test)
4.8. Investigation of the Signaling Pathways Associated with the Analgesic Effect of HEXA and AC
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baral, P.; Udit, S.; Chiu, I.M. Pain and Immunity: Implications for Host Defence. Nat. Rev. Immunol. 2019, 19, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Huh, Y.; Ji, R.R. Roles of Inflammation, Neurogenic Inflammation, and Neuroinflammation in Pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.; Bruera, E. Balancing Opioid Analgesia with the Risk of Nonmedical Opioid Use in Patients with Cancer. Nat. Rev. Clin. Oncol. 2019, 16, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic Pain: An Update on Burden, Best Practices, and New Advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Day, R.O.; Graham, G.G. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). BMJ 2013, 346, f3195. [Google Scholar] [CrossRef]
- Lima, C.P.; Silva, H.R.D.O.; Pogian, V.B.; Santos, V.G. Pharmaceutical Evaluation of the Risks of the Use of Non-Steroid Anti-Inflammatories. Unisanta Health Sci. 2020, 4, 1–20. [Google Scholar]
- Silva, L.A.; Gerica, O.; Al, H. Review on Non-Steroid Antiinflammatory: Acetylsalicylic Acid. Rev. Iniciaç. Cient. Ext. 2018, 1, 169–174. [Google Scholar]
- Mohammad, F.; Hasan, E.; Wedad, A.; Mohamed, E.G. Natural Antioxidant Flavonoids in Formalin-Induced Mice Paw Inflammation; Inhibition of Mitochondrial Sorbitol Dehydrogenase Activity. J. Biochem. Mol. Toxicol. 2017, 31, e21896. [Google Scholar] [CrossRef]
- Mussin, J.; Giusiano, G. Ethno–Phytopharmacology: Product Validation Process Based on Traditional Knowledge of Medicinal Plants. In Agricultural, Forestry and Bioindustry Biotechnology and Biodiscovery; Springer: Berlin/Heidelberg, Germany, 2020; pp. 331–353. [Google Scholar]
- Ribeiro, D.A.; de Oliveira, L.G.S.; de Macêdo, D.G.; de Menezes, I.R.A.; da Costa, J.G.M.; da Silva, M.A.P.; Lacerda, S.R.; de Almeida Souza, M.M. Promising Medicinal Plants for Bioprospection in a Cerrado Area of Chapada Do Araripe, Northeastern Brazil. J. Ethnopharmacol. 2014, 155, 1522–1533. [Google Scholar] [CrossRef]
- Sreekeesoon, D.P.; Mahomoodally, M.F. Ethnopharmacological Analysis of Medicinal Plants and Animals Used in the Treatment and Management of Pain in Mauritius. J. Ethnopharmacol. 2014, 157, 181–200. [Google Scholar] [CrossRef]
- Da Silva-Leite, K.E.S.; Assreuy, A.M.S.; Mendonça, L.F.; Damasceno, L.E.A.; De Queiroz, M.G.R.; Mourão, P.A.S.; Pires, A.F.; Pereira, M.G. Polysaccharide Rich Fractions from Barks of Ximenia americana Inhibit Peripheral Inflammatory Nociception in Mice Antinociceptive Effect of Ximenia americana Polysaccharide Rich Fractions. Rev. Bras. Farmacogn. 2017, 27, 339–345. [Google Scholar] [CrossRef]
- Jahromi, B.; Pirvulescu, I.; Candido, K.D.; Knezevic, N.N. Herbal Medicine for Pain Management: Efficacy and Drug Interactions. Pharmaceutics 2021, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.G.; De Souza, P.A.; De Morais, P.L.D.; Dos Santos, E.C.; Moura, R.D.; Menezes, J.B. Caracterização Do Fruto de Ameixa Silvestre (Ximenia americana L.). Rev. Bras. Frutic. 2008, 30, 311–314. [Google Scholar] [CrossRef]
- Shettar, A.K.; Sateesh, M.K.; Kaliwal, B.B.; Vedamurthy, A.B. In Vitro Antidiabetic Activities and GC-MS Phytochemical Analysis of Ximenia americana Extracts. S. Afr. J. Bot. 2017, 111, 202–211. [Google Scholar] [CrossRef]
- Almeida, M.L.B.; Freitas, W.E.D.S.; De Morais, P.L.D.; Sarmento, J.D.A.; Alves, R.E. Bioactive Compounds and Antioxidant Potential Fruit of Ximenia americana L. Food Chem. 2016, 192, 1078–1082. [Google Scholar] [CrossRef]
- Aragão, T.P.; dos Prazeres, L.D.K.T.; Brito, S.A.; Neto, P.J.R.; Rolim, L.A.; da Silva Almeida, J.R.G.; Caldas, G.F.R.; Wanderley, A.G. alves Contribution of Secondary Metabolites to the Gastroprotective Effect of Aqueous Extract of Ximenia americana L. (Olacaceae) Stem Bark in Rats. Molecules 2018, 23, 112. [Google Scholar] [CrossRef]
- de Menezes, I.R.A.; da Costa, R.H.S.; Augusti Boligon, A.; Rolón, M.; Coronel, C.; Vega, C.; Melo Coutinho, H.D.; da Costa, M.S.; Tintino, S.R.; Silva Pereira, R.L.; et al. Ximenia americana L. Enhances the Antibiotic Activity and Inhibit the Development of Kinetoplastid Parasites. Comp. Immunol. Microbiol. Infect. Dis. 2019, 64, 40–46. [Google Scholar] [CrossRef]
- Da Palma, A.F.M.; Marques, L.K.M.; Carneiro, R.D.S.; Carvalho, G.F.S.; Ferreira, D.C.L.; Sant’Ana, A.E.G.; Maia Filho, A.L.M.; Marques, R.B.; Alves, W.D.S.; Uchôa, V.T.; et al. Evaluation of Hydroalcoholic Extracts of Stem and Leaves of Ximenia americana L. In the Healing of Excisional Acute Wounds in Mice. Rev. Virtual Quim. 2020, 12, 37–50. [Google Scholar] [CrossRef]
- da Silva, B.A.F.; da Costa, R.H.S.; Fernandes, C.N.; Leite, L.H.I.; Ribeiro-Filho, J.; Garcia, T.R.; Coutinho, H.D.M.; Wanderley, A.G.; de Menezes, I.R.A. HPLC Profile and Antiedematogenic Activity of Ximenia americana L. (Olacaceae) in Mice Models of Skin Inflammation. Food Chem. Toxicol. 2018, 119, 199–205. [Google Scholar] [CrossRef]
- Soro, T.Y.; Zahoui, O.S.; Nene-bi, A.S. Analgesic Activity of the Fractions of the Aqueous Extract of Ximenia americana (Linné) (Olacaceae). Int. J. Pharmacol. Toxicol. 2016, 4, 1. [Google Scholar] [CrossRef][Green Version]
- Kefelegn, G.A.; Desta, B. Ximenia americana: Economic importance, medicinal value, and current status in Ethiopia. Sci. World J. 2021, 1, 8880021. [Google Scholar] [CrossRef] [PubMed]
- da Costa, R.H.S.; Martins, A.O.B.P.B.; de Oliveira, M.R.C.; Alcântara, I.S.; Ferreira, F.F.; dos Santos, F.F.C.; de Araújo Delmondes, G.; da Cunha, F.A.B.; Coutinho, H.D.M.; de Menezes, I.R.A. Acaricide Activity of the Ximenia americana L. (Olacaceae) Stem Bark Hydroethanolic Extract against Rhipicephalus (Boophilus) Microplus. Biologia 2022, 77, 1667–1674. [Google Scholar] [CrossRef]
- Cizmarova, B.; Hubkova, B.; Bolerazska, B.; Marekova, M.; Birkova, A. Caffeic Acid: A Brief Overview of Its Presence, Metabolism, and Bioactivity. Bioact. Compd. Health Dis. 2020, 3, 74–81. [Google Scholar] [CrossRef]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef]
- Nasr Bouzaiene, N.; Kilani Jaziri, S.; Kovacic, H.; Chekir-Ghedira, L.; Ghedira, K.; Luis, J. The Effects of Caffeic, Coumaric and Ferulic Acids on Proliferation, Superoxide Production, Adhesion and Migration of Human Tumor Cells in Vitro. Eur. J. Pharmacol. 2015, 766, 99–105. [Google Scholar] [CrossRef]
- Zielińska, D.; Zieliński, H.; Laparra-Llopis, J.M.; Szawara-Nowak, D.; Honke, J.; Giménez-Bastida, J.A. Caffeic Acid Modulates Processes Associated with Intestinal Inflammation. Nutrients 2021, 13, 554. [Google Scholar] [CrossRef]
- Park, S.H.; Sim, Y.B.; Kim, S.M.; Lee, J.K.; Jung, J.S.; Suh, H.W. The Effect of Caffeic Acid on the Antinociception and Mechanisms in Mouse. J. Appl. Biol. Chem. 2011, 54, 177–182. [Google Scholar] [CrossRef]
- Crevar-Sakač, M.; Vujić, Z.; Kotur-Stevuljević, J.; Ivanišević, J.; Jelić-Ivanović, Z.; Milenković, M.; Markelić, M.; Vujčić, Z. Uticaj Atorvastatina i Tinkture Lista Artičoke Na Oksidativni Stres Kod Pacova Sa Hiperholesterolemijom. Vojnosanit. Pregl. 2016, 73, 178–187. [Google Scholar] [CrossRef]
- Wu, Z.-J.; Ma, X.-L.; Fang, D.-M.; Qi, H.-Y.; Ren, W.-J.; Zhang, G.-L. Analysis of Caffeic Acid Derivatives from Osmanthus Yunnanensis Using Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry. Eur. J. Mass Spectrom. 2009, 15, 415–429. [Google Scholar] [CrossRef]
- Pessoa, R.T.; Alcântara, I.S.; da Silva, L.Y.S.; da Costa, R.H.S.; Silva, T.M.; de Morais Oliveira-Tintino, C.D.; Ramos, A.G.B.; de Oliveira, M.R.C.; Martins, A.O.B.P.B.; de Lacerda, B.C.G.V.; et al. Ximenia americana L.: Chemical Characterization and Gastroprotective Effect. Analytica 2023, 4, 141–158. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, S.; Wang, L.; Zhang, N.; Shi, Y.; Zhou, H.; Liu, X.; Shao, L.; Liu, X.; Chen, J.; et al. Reproductive and Developmental Toxicity Study of Caffeic Acid in Mice. Food Chem. Toxicol. 2019, 123, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Brandão, D.O.; Fernandes, F.H.A.; Júnior, F.J.L.R.; Silva, P.C.D.; Santana, C.P.; De Medeiros, F.D.; Véras, G.; Medeiros, A.C.D. Validation of UPLC Method for Determination of Gallic Acid from Ximenia americana L. Planta Med. 2014, 80, P2O60. [Google Scholar] [CrossRef]
- de Araújo, M.R.S.; da Costa Assunção, J.C.; Dantas, I.N.F.; Costa-Lotufo, L.V.; Monte, F.J.Q. Chemical Constituents of Ximenia americana. Nat. Prod. Commun. 2008, 3, 1934578X0800300605. [Google Scholar] [CrossRef]
- Le, N.H.T.; Malterud, K.E.; Diallo, D.; Paulsen, B.S.; Nergård, C.S.; Wangensteen, H. Bioactive Polyphenols in Ximenia americana and the Traditional Use among Malian Healers. J. Ethnopharmacol. 2012, 139, 858–862. [Google Scholar] [CrossRef]
- Queiroz Monte, F.J.; de Lemos, T.L.G.; de Arajo, M.R.S.; Sousa Gomes, E. de Ximenia americana: Chemistry, Pharmacology and Biological Properties, a Review. Phytochem.—A Glob. Perspect. Their Role Nutr. Health 2012, 1, 429–450. [Google Scholar] [CrossRef][Green Version]
- Siddaiah, M.; Jayavcera, K.N.; Mallikarjuna, R.P.; Ravindra, R.K.; Yasodha, K.Y.; Narender, R.G. Phytochemical Screening and Analgesic Activity of Methanolic Extract of Ximenia americana. J. Pharm. Chem. 2009, 3, 23–25. [Google Scholar]
- Hemamalini, K.; Srikanth, A.; Sunny, G.; Praneethkumar, H. Phytochemical Screening and Analgesic Activity of Methanolic Extract of Ximenia americana. J. Curr. Pharma Res. 2011, 1, 153. [Google Scholar]
- Coutinho, M.A.S.; Muzitano, M.F.; Costa, S.S. Flavonoids: Potential Therapeutic Agents for the Inflammatory Process. Rev. Virtual Quím. 2009, 1, 241–256. [Google Scholar] [CrossRef]
- Dias, T.L.M.F.; Melo, G.M.A.; Da Silva, Y.K.C.; Queiroz, A.C.; Goulart, H.F.; Alexandre-Moreira, M.S.; Santana, A.E.G.; Uchôa, V.T. Antinociceptive and Anti-Inflammatory Activities of the Ethanolic Extract, of Fractions and of Epicatechin Isolated from the Stem Bark of Ximenia americana L. (Oleacaceae). Rev. Virtual Quim. 2018, 10, 86–101. [Google Scholar] [CrossRef]
- Kiessoun, K.; Ouattara, N.; Arsène, M.; Souza, A.; Sytar, O.; Brestic, M.; Mamoudou, D.H. Anti-Nociceptive and Anti-Inflammatory Properties of Polyphenol-Rich Fractions of Roots from Ximenia americana L., (Olacaceae), in Experimental Mice. Int. J. Pharm. Pharm. Res. 2018, 12, 281–297. [Google Scholar]
- Matejczyk, M.; Świsłocka, R.; Golonko, A.; Lewandowski, W.; Hawrylik, E. Cytotoxic, Genotoxic and Antimicrobial Activity of Caffeic and Rosmarinic Acids and Their Lithium, Sodium and Potassium Salts as Potential Anticancer Compounds. Adv. Med. Sci. 2018, 63, 14–21. [Google Scholar] [CrossRef] [PubMed]
- de Alencar Silva, A.; Pereira-de-Morais, L.; Rodrigues da Silva, R.E.; de Menezes Dantas, D.; Brito Milfont, C.G.; Gomes, M.F.; Araújo, I.M.; Kerntopf, M.R.; Alencar de Menezes, I.R.; Barbosa, R. Pharmacological Screening of the Phenolic Compound Caffeic Acid Using Rat Aorta, Uterus and Ileum Smooth Muscle. Chem. Biol. Interact. 2020, 332, 109269. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Pedulli, G.F.; Cabrini, L.; Zambonin, L.; Landi, L. Solvent and PH Effects on the Antioxidant Activity of Caffeic and Other Phenolic Acids. J. Agric. Food Chem. 2006, 54, 2932–2937. [Google Scholar] [CrossRef] [PubMed]
- Kolgazi, M.; Cilingir, S.; Yilmaz, O.; Gemici, M.; Yazar, H.; Ozer, S.; Acikel-Elmas, M.; Arbak, S.; Suyen, G.G. Caffeic Acid Attenuates Gastric Mucosal Damage Induced by Ethanol in Rats via Nitric Oxide Modulation. Chem. Biol. Interact. 2021, 334, 109351. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.J.; De Almeida, R.N.; Falcão, A.C.G.M.; de Fátima Agra, M.; De Sousa, M.d.F.V.; Barbosa-Filho, J.M. Avaliação Da Atividade Anticonvulsivante de Plantas Do Nordeste Brasileiro. Acta Farm. Bonaer. 2002, 21, 179–184. [Google Scholar]
- da Silva-Leite, K.E.S.; Girão, D.K.F.B.; de Freitas Pires, A.; Assreuy, A.M.S.; de Moraes, P.A.F.; Cunha, A.P.; Ricardo, N.M.P.S.; Criddle, D.N.; de Souza, M.H.L.P.; Pereira, M.G.; et al. Ximenia americana Heteropolysaccharides Ameliorate Inflammation and Visceral Hypernociception in Murine Caerulein-Induced Acute Pancreatitis: Involvement of CB2 Receptors. Biomed. Pharmacother. 2018, 106, 1317–1324. [Google Scholar] [CrossRef]
- Zhen, J.; Guo, Y.; Villani, T.; Carr, S.; Brendler, T.; Mumbengegwi, D.R.; Kong, A.N.T.; Simon, J.E.; Wu, Q. Phytochemical Analysis and Anti-Inflammatory Activity of the Extracts of the African Medicinal Plant Ximenia Caffra. J. Anal. Methods Chem. 2015, 2015, 948262. [Google Scholar] [CrossRef]
- Ferreira, J.; Campos, M.M.; Pesquero, J.B.; Araújo, R.C.; Bader, M.; Calixto, J.B. Evidence for the Participation of Kinins in Freund’s Adjuvant-Induced Inflammatory and Nociceptive Responses in Kinin B1 and B2 Receptor Knockout Mice. Neuropharmacology 2001, 41, 1006–1012. [Google Scholar] [CrossRef]
- Billiau, A.; Matthys, P. Modes of Action of Freund’s Adjuvants in Experimental Models of Autoimmune Diseases. J. Leukoc. Biol. 2001, 70, 849–860. [Google Scholar] [CrossRef]
- Dutra, R.C.; Campos, M.M.; Santos, A.R.S.; Calixto, J.B. Medicinal Plants in Brazil: Pharmacological Studies, Drug Discovery, Challenges and Perspectives. Pharmacol. Res. 2016, 112, 4–29. [Google Scholar] [CrossRef] [PubMed]
- Sandkühler, J. Models and Mechanisms of Hyperalgesia and Allodynia. Physiol. Rev. 2009, 89, 707–758. [Google Scholar] [CrossRef] [PubMed]
- Gamaro, G.D.; Suyenaga, E.; Borsoi, M.; Lermen, J.; Pereira, P.; Ardenghi, P. Effect of Rosmarinic and Caffeic Acids on Inflammatory and Nociception Process in Rats. ISRN Pharmacol. 2011, 2011, 451682. [Google Scholar] [CrossRef] [PubMed]
- Leal, S.S.; Uchôa, V.T.; Figuerêdo-Silva, J.; Soares, R.B.; Mota, D.M.; De Alencar, R.C.; Filho, A.L.M.M.; Sant’Ana, A.E.G.; Beltrame Junior, M. Eficácia Da Fonoforese Com Ximenia americana L. Na Inflamação de Tendão de Ratos. Rev. Bras. Med. Esporte 2016, 22, 355–360. [Google Scholar] [CrossRef]
- Dhawan, B.N.; Cesselin, F.; Raghubir, R.; Reisine, T.; Bradley, P.B.; Portoghese, P.S.; Hamon, M. International Union of Pharmacology. XII. Classification of Opioid Receptors. Pharmacol. Rev. 1996, 48, 567–592. [Google Scholar]
- Stein, C.; Hassan, A.H.S.; Lehrberger, K.; Stein, C.; Giefing, J.; Yassouridis, A. Local Analgesic Effect of Endogenous Opioid Peptides. Lancet 1993, 342, 321–324. [Google Scholar] [CrossRef]
- Jin, W.Y.; Liu, Z.; Liu, D.; Yu, L.C. Antinociceptive Effects of Galanin in the Central Nucleus of Amygdala of Rats, an Involvement of Opioid Receptors. Brain Res. 2010, 1320, 16–21. [Google Scholar] [CrossRef]
- Shih, C.C.; Hwang, H.R.; Chang, C.I.; Su, H.M.; Chen, P.C.; Kuo, H.M.; Li, P.J.; Wang, H.M.D.; Tsui, K.H.; Lin, Y.C.; et al. Anti-Inflammatory and Antinociceptive Effects of Ethyl Acetate Fraction of an Edible Red Macroalgae Sarcodia Ceylanica. Int. J. Mol. Sci. 2017, 18, 2437. [Google Scholar] [CrossRef]
- Iacopucci, A.P.; Mello, R.O.; Barbosa-Silva, R.; Melo-Thomas, L. L-NOARG-Induced Catalepsy Can Be Influenced by Glutamatergic Neurotransmission Mediated by NMDA Receptors in the Inferior Colliculus. Behav. Brain Res. 2012, 234, 149–154. [Google Scholar] [CrossRef]
- Kim, S.R.; Jung, Y.R.; Kim, D.H.; An, H.J.; Kim, M.K.; Kim, N.D.; Chung, H.Y. Caffeic Acid Regulates LPS-Induced NF-ΚB Activation through NIK/IKK and c-Src/ERK Signaling Pathways in Endothelial Cells. Arch. Pharm. Res. 2014, 37, 539–547. [Google Scholar] [CrossRef]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The Cloned Capsaicin Receptor Integrates Multiple Pain-Producing Stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Devesa, I.; Planells-Cases, R.; Fernández-Ballester, G.; González-Ros, J.M.; Ferrer-Montiel, A.; Fernández-Carvajal, A. Role of the Transient Receptor Potential Vanilloid 1 in Inflammation and Sepsis. J. Inflamm. Res. 2011, 4, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Gamse, R.; Holzer, P.; Lembeck, F. Indirect Evidence for Presynaptic Location of Opiate Receptors on Chemosensitive Primary Sensory Neurones. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1979, 308, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.L.; Lima-Júnior, R.C.P.; Melo, C.M.; David, J.P.; David, J.M.; Campos, A.R.; Santos, F.A.; Rao, V.S.N. Oleanolic Acid, a Pentacyclic Triterpene Attenuates Capsaicin-Induced Nociception in Mice: Possible Mechanisms. Pharmacol. Res. 2006, 54, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Beirith, A.; Santos, A.R.S.; Calixto, J.B. Mechanisms Underlying the Nociception and Paw Oedema Caused by Injection of Glutamate into the Mouse Paw. Brain Res. 2002, 924, 219–228. [Google Scholar] [CrossRef]
- Vorobeychik, Y.; Willoughby, C.D.; Mao, J. NMDA Receptor Antagonists in the Treatment of Pain. In Comprehensive Treatment of Chronic Pain by Medical, Interventional, and Integrative Approaches; Springer: New York, NY, USA, 2013; pp. 61–67. [Google Scholar]
- Soares, A.C.; Leite, R.; Tatsuo, M.A.K.F.; Duarte, I.D.G. Activation of ATP-Sensitive K+ Channels: Mechanism of Peripheral Antinociceptive Action of the Nitric Oxide Donor, Sodium Nitroprusside. Eur. J. Pharmacol. 2000, 400, 67–71. [Google Scholar] [CrossRef]
- Garthwaite, J. Glutamate, Nitric Oxide and Cell-Cell Signalling in the Nervous System. Trends Neurosci. 1991, 14, 60–67. [Google Scholar] [CrossRef]
- Kimura, M.; Saito, S.; Obata, H. Dexmedetomidine Decreases Hyperalgesia in Neuropathic Pain by Increasing Acetylcholine in the Spinal Cord. Neurosci. Lett. 2012, 529, 70–74. [Google Scholar] [CrossRef]
- Obata, H. Analgesic Mechanisms of Antidepressants for Neuropathic Pain. Int. J. Mol. Sci. 2017, 18, 2483. [Google Scholar] [CrossRef]
- Chan, A.K.M.; Cheung, C.W.; Chong, Y.K. Alpha-2 Agonists in Acute Pain Management. Expert Opin. Pharmacother. 2010, 11, 2849–2868. [Google Scholar] [CrossRef]
- Lopes, L.D.S.; Marques, R.B.; Fernandes, H.B.; Pereira, S.D.S.; Ayres, M.C.C.; Chaves, M.H.; Almeida, F.R.C. Mechanisms of the Antinociceptive Action of (-) Epicatechin Obtained from the Hydroalcoholic Fraction of Combretum Leprosum Mart & Eic in Rodents. J. Biomed. Sci. 2012, 19, 2–7. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with GNPS. Nat. Biotechnol. 2016, 34, 828. [Google Scholar] [CrossRef] [PubMed]
- Dunham, N.W.; Miya, T.S. A Note on a Simple Apparatus for Detecting Neurological Deficit in Rats and Mice. J. Am. Pharm. Assoc. 1957, 46, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Lapa, A.J.J.; Souccar, C.; Lima-Landman, M.T.R.; Castro, M.S.D.A.; Lima, T.C.M. Pharmacological Activity Evaluation Methods of Medicinal Plants; Sociedade Brasileira de Plantas Medicinais: São Paulo, Brasil, 2008. [Google Scholar]
- Gaichu, D.M.; Mawia, A.M.; Gitonga, G.M.; Ngugi, M.P.; Mburu, D.N. Phytochemical screening and antipyretic activities of dichloromethane-methanolic leaf and stem bark extracts of Ximenia americana in rat models. J. Herbmed Pharmacol. 2017, 6, 3–107. [Google Scholar]
- Koster, R.; Anderson, M.; De Beer, E.J. Acetic Acid for Analgesic Screening. Fed. Proc. 1959, 18, 412–417. [Google Scholar]
- Tjølsen, A.; Berge, O.-G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The Formalin Test: An Evaluation of the Method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- Bannon, A.; Malmberg, A. Models of Nociception: Hot-Plate, Tail-Flick, and Formalin Tests in Rodents. Curr. Protoc. Neurosci. 2007, 41, 8–9. [Google Scholar] [CrossRef]
- Martinov, T.; Mack, M.; Sykes, A.; Chatterjea, D. Measuring Changes in Tactile Sensitivity in the Hind Paw of Mice Using an Electronic von Frey Apparatus. J. Vis. Exp. 2013, 82, e51212. [Google Scholar] [CrossRef]
- Tena, B.; Escobar, B.; Arguis, M.J.; Cantero, C.; Rios, J.; Gomar, C. Reproducibility of Electronic von Frey and von Frey Monofilaments Testing. Clin. J. Pain 2012, 28, 318–323. [Google Scholar] [CrossRef]
- Stevenson, G.W.; Bilsky, E.J.; Negus, S.S. Targeting Pain-Suppressed Behaviors in Preclinical Assays of Pain and Analgesia: Effects of Morphine on Acetic Acid-Suppressed Feeding in C57BL/6J Mice. J. Pain 2006, 7, 408–416. [Google Scholar] [CrossRef]
- Miller, E.C.; Swanson, A.B.; Phillips, D.H.; Fletcher, T.L.; Liem, A.; Miller, J.A. Structure-Activity Studies of the Carcinogenicities in the Mouse and Rat of Some Naturally Occurring and Synthetic Alkenylbenzene Derivatives Related to Safrole and Estragole. Cancer Res. 1983, 43, 1124–1134. [Google Scholar] [PubMed]
- Schechtmann, G.; Song, Z.; Ultenius, C.; Meyerson, B.A.; Linderoth, B. Cholinergic Mechanisms Involved in the Pain Relieving Effect of Spinal Cord Stimulation in a Model of Neuropathy. Pain 2008, 139, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.K.; Sessle, B.J.; Cairns, B.E.; Hu, J.W. Neural Mechanisms of Temporomandibular Joint and Masticatory Muscle Pain: A Possible Role for Peripheral Glutamate Receptor Mechanisms. Pain Res. Manag. 2005, 10, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhuo, M. Silent Glutamatergic Synapses and Nociception in Mammalian Spinal Cord. Nature 1998, 393, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.D.; Bell, J.A.; London, E.D. Regulation of the NMDA Receptor by Redox Phenomena: Inhibitory Role of Ascorbate. Brain Res. 1990, 537, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Zeraati, F.; Araghchian, M.; Farjoo, M.H. Ascorbic Acid Interaction With Analgesic Effect of Morphine and Tramadol in Mice. Anesthesiol. Pain Med. 2014, 4, e19529. [Google Scholar] [CrossRef]
- Santos, A.R.S.; Gadotti, V.M.; Oliveira, G.L.; Tibola, D.; Paszcuk, A.F.; Neto, A.; Spindola, H.M.; Souza, M.M.; Rodrigues, A.L.S.; Calixto, J.B. Mechanisms Involved in the Antinociception Caused by Agmatine in Mice. Neuropharmacology 2005, 48, 1021–1034. [Google Scholar] [CrossRef]
- Ocaña, M.; Cendán, C.M.; Cobos, E.J.; Entrena, J.M.; Baeyens, J.M. Potassium Channels and Pain: Present Realities and Future Opportunities. Eur. J. Pharmacol. 2004, 500, 203–219. [Google Scholar] [CrossRef]
- Ferré, S.; Diamond, I.; Goldberg, S.R.; Yao, L.; Hourani, S.M.O.; Huang, Z.L.; Urade, Y.; Kitchen, I. Adenosine A2A Receptors in Ventral Striatum, Hypothalamus and Nociceptive Circuitry. Prog. Neurobiol. 2007, 83, 332–347. [Google Scholar] [CrossRef]
- Finan, P.H.; Smith, M.T. The Comorbidity of Insomnia, Chronic Pain, and Depression: Dopamine as a Putative Mechanism. Sleep Med. Rev. 2013, 17, 173–183. [Google Scholar] [CrossRef]
- Wood, P.B. Mesolimbic Dopaminergic Mechanisms and Pain Control. Pain 2006, 120, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Maleki, N.; Nayebi, A.M.; Garjani, A. Effects of Central and Peripheral Depletion of Serotonergic System on Carrageenan-Induced Paw Oedema. Int. Immunopharmacol. 2005, 5, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Rygh, L.J.; Dickenson, A.H. Bad News from the Brain: Descending 5-HT Pathways That Control Spinal Pain Processing. Trends Pharmacol. Sci. 2004, 25, 613–617. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pessoa, R.T.; Santos da Silva, L.Y.; Alcântara, I.S.; Silva, T.M.; Silva, E.d.S.; da Costa, R.H.S.; da Silva, A.B.; Ribeiro-Filho, J.; Pereira Bezerra Martins, A.O.B.; Coutinho, H.D.M.; et al. Antinociceptive Potential of Ximenia americana L. Bark Extract and Caffeic Acid: Insights into Pain Modulation Pathways. Pharmaceuticals 2024, 17, 1671. https://doi.org/10.3390/ph17121671
Pessoa RT, Santos da Silva LY, Alcântara IS, Silva TM, Silva EdS, da Costa RHS, da Silva AB, Ribeiro-Filho J, Pereira Bezerra Martins AOB, Coutinho HDM, et al. Antinociceptive Potential of Ximenia americana L. Bark Extract and Caffeic Acid: Insights into Pain Modulation Pathways. Pharmaceuticals. 2024; 17(12):1671. https://doi.org/10.3390/ph17121671
Chicago/Turabian StylePessoa, Renata Torres, Lucas Yure Santos da Silva, Isabel Sousa Alcântara, Tarcísio Mendes Silva, Eduardo dos Santos Silva, Roger Henrique Sousa da Costa, Aparecida Barros da Silva, Jaime Ribeiro-Filho, Anita Oliveira Brito Pereira Bezerra Martins, Henrique Douglas Melo Coutinho, and et al. 2024. "Antinociceptive Potential of Ximenia americana L. Bark Extract and Caffeic Acid: Insights into Pain Modulation Pathways" Pharmaceuticals 17, no. 12: 1671. https://doi.org/10.3390/ph17121671
APA StylePessoa, R. T., Santos da Silva, L. Y., Alcântara, I. S., Silva, T. M., Silva, E. d. S., da Costa, R. H. S., da Silva, A. B., Ribeiro-Filho, J., Pereira Bezerra Martins, A. O. B., Coutinho, H. D. M., Sousa, J. C. P., Chaves, A. R., Marreto, R. N., & de Menezes, I. R. A. (2024). Antinociceptive Potential of Ximenia americana L. Bark Extract and Caffeic Acid: Insights into Pain Modulation Pathways. Pharmaceuticals, 17(12), 1671. https://doi.org/10.3390/ph17121671










