PCSK9 Inhibitors: Focus on Evolocumab and Its Impact on Atherosclerosis Progression
Abstract
1. Introduction
2. The Evolution and Significance of PCSK9
3. PCSK9 Inhibitors
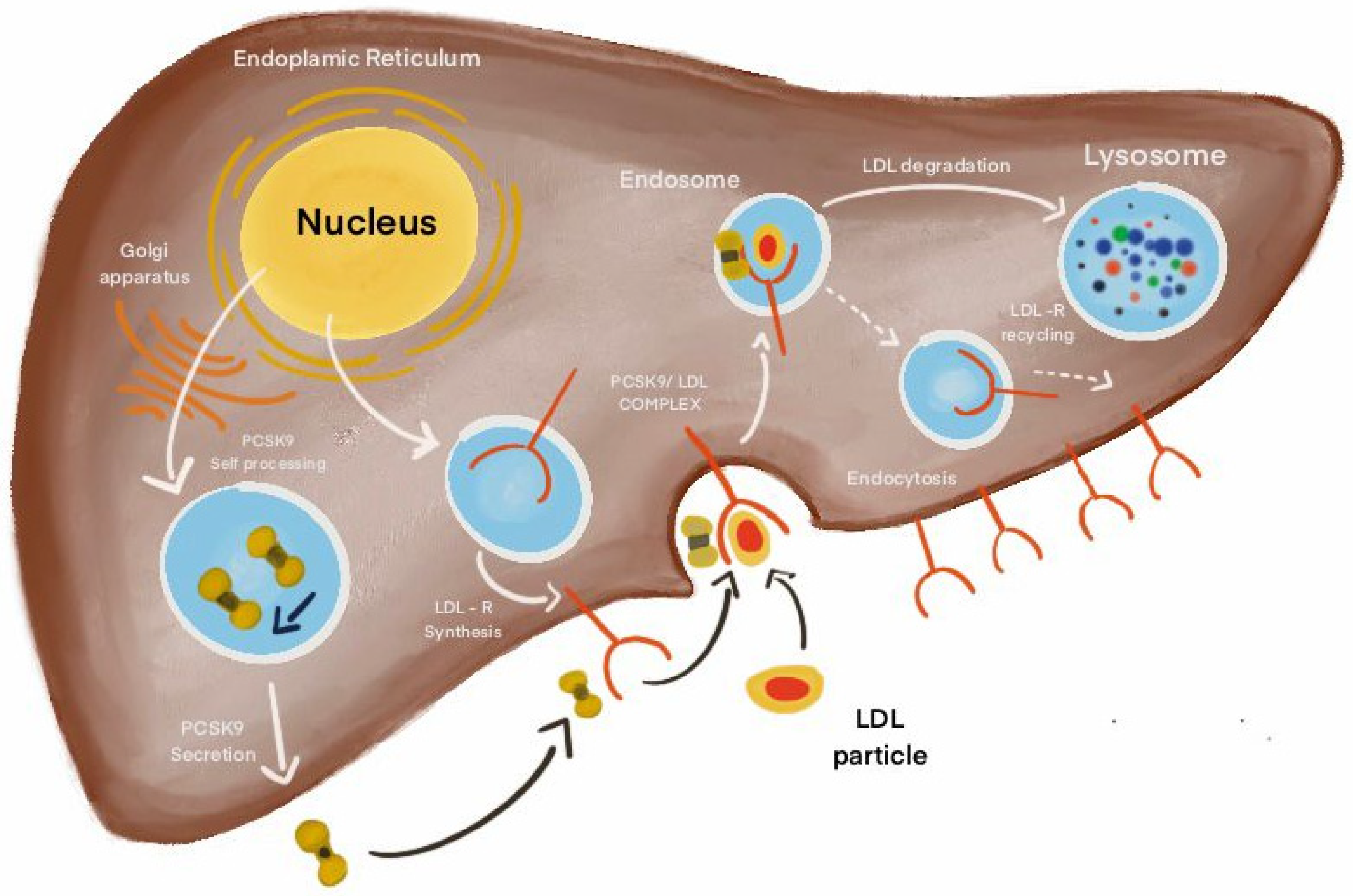
4. The Molecular Pathways of PCSK9
5. Monoclonal Antibodies Against PCSK9
6. Evolocumab: A Key Therapy for Reducing Atherosclerosis Risk
6.1. Findings from Clinical Trials Using PCSK9 Inhibitors
6.2. Clinical Studies Evaluating Patient Health Outcomes
7. Long-Term Efficacy and Safety of Evolocumab: Insights from Clinical Studies
8. PCSK9 Inhibitor Access Challenges: Understanding the Landscape and Its Cost-Effectiveness
9. Comparison of Evolocumab vs. siRNA
10. Limitations of Using PCSK9 Inhibitors
11. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Deaths from Cardiovascular Disease Surged 60% Globally over the Last 30 Years: Report (2023) World Heart Federation. Available online: https://world-heart-federation.org/news/deaths-from-cardiovascular-disease-surged-60-globally-over-the-last-30-years-report/ (accessed on 12 May 2024).
- Khan, A.; Roy, P.; Ley, K. Breaking tolerance: The autoimmune aspect of atherosclerosis. Nat. Rev. Immunol. 2024, 12, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Pang, C.; Xu, M.; Zhao, Q.; Hu, Z.; Jiang, X.; Guo, M. The role of immune system in atherosclerosis: Molecular mechanisms, controversies, and future possibilities. Hum. Immunol. 2024, 18, 110765. [Google Scholar] [CrossRef] [PubMed]
- 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Available online: https://www.ahajournals.org/doi/10.1161/CIR.0000000000001209 (accessed on 12 May 2024).
- Raedler, L.A. Praluent (Alirocumab): First PCSK9 inhibitor approved by the FDA for hypercholesterolemia. Am. Health Drug Benefits 2016, 9, 123. [Google Scholar] [PubMed]
- Baum, S.J.; Sijbrands, E.J.; Mata, P.; Watts, G.F. The doctor’s dilemma: Challenges in the diagnosis and care of homozygous familial hypercholesterolemia. J. Clin. Lipidol. 2014, 8, 542–549. [Google Scholar] [CrossRef]
- Mach, F.; Visseren, F.L.; Cater, N.B.; Salhi, N.; Soronen, J.; Ray, K.K.; Delgado, V.; Jukema, J.W.; Laufs, U.; Zamorano, J.L.; et al. Addressing residual risk beyond statin therapy: New targets in the management of dyslipidaemias–A report from the European Society of Cardiology Cardiovascular Round Table. J. Clin. Lipidol. 2024, 6, S1933-2874(24)00209-5. [Google Scholar] [CrossRef]
- Wu, M.Y.; Li, C.J.; Hou, M.F.; Chu, P.Y. New insights into the role of inflammation in the pathogenesis of atherosclerosis. Int. J. Mol. Sci. 2017, 18, 2034. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.K.; Lecis, D. Inflammation in atherosclerotic cardiovascular disease. F1000Research 2019, 8, F1000 Faculty Rev-1402. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Valanti, E.K.; Dalakoura-Karagkouni, K.; Siasos, G.; Kardassis, D.; Eliopoulos, A.G.; Sanoudou, D. Advances in biological therapies for dyslipidemias and atherosclerosis. Metabolism 2021, 116, 154461. [Google Scholar] [CrossRef]
- Gisterå, A.; Hansson, G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Ho, Y.K.; Basu, S.K.; Brown, M.S. Binding site on macrophages that mediates uptake and degradation of acetylated low-density lipoprotein, producing massive cholesterol deposition. Proc. Natl. Acad. Sci. USA 1979, 76, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Febbraio, M.; Silverstein, R.L. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J. Clin. Investig. 2009, 119, 136–145. [Google Scholar] [CrossRef]
- Robbins, C.S.; Hilgendorf, I.; Weber, G.F.; Theurl, I.; Iwamoto, Y.; Figueiredo, J.L.; Gorbatov, R.; Sukhova, G.K.; Gerhardt, L.M.; Smyth, D.; et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 2013, 19, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Roh, J.W.; Lee, O.H.; Heo, S.J.; Im, E.; Cho, D.K.; Kim, B.K. Efficacy of single-dose Evolocumab injection in early-phase acute myocardial infarction: A retrospective single-center study. Korean J. Intern. Med. 2024, 39, 793. [Google Scholar] [CrossRef] [PubMed]
- Harshfield, E.L.; Pennells, L.; Schwartz, J.E.; Willeit, P.; Kaptoge, S.; Bell, S.; Shaffer, J.A.; Bolton, T.; Spackman, S.; Wassertheil-Smoller, S.; et al. Association between depressive symptoms and incident cardiovascular diseases. JAMA 2020, 324, 2396–2405. [Google Scholar] [CrossRef]
- DeVon, H.A.; Burke, L.A.; Vuckovic, K.M.; Haugland, T.; Eckhardt, A.L.; Patmon, F.; Rosenfeld, A.G. Symptoms suggestive of acute coronary syndrome: When is sex important? J. Cardiovasc. Nurs. 2017, 32, 383–392. [Google Scholar] [CrossRef]
- Jurgens, C.Y.; Lee, C.S.; Aycock, D.M.; Masterson Creber, R.; Denfeld, Q.E.; DeVon, H.A.; Evers, L.R.; Jung, M.; Pucciarelli, G.; Streur, M.M.; et al. State of the science: The relevance of symptoms in cardiovascular disease and research: A scientific statement from the American Heart Association. Circulation 2022, 146, e173–e184. [Google Scholar] [CrossRef]
- Lechner, K.; von Schacky, C.; McKenzie, A.L.; Worm, N.; Nixdorff, U.; Lechner, B.; Kränkel, N.; Halle, M.; Krauss, R.M.; Scherr, J. Lifestyle factors and high-risk atherosclerosis: Pathways and mechanisms beyond traditional risk factors. Eur. J. Prev. Cardiol. 2020, 27, 394–406. [Google Scholar] [CrossRef]
- Al-Shaar, L.; Satija, A.; Wang, D.D.; Rimm, E.B.; Smith-Warner, S.A.; Stampfer, M.J.; Hu, F.B.; Willett, W.C. Red meat intake and risk of coronary heart disease among US men: Prospective cohort study. Bmj 2020, 371, m4141. [Google Scholar] [CrossRef]
- Zhang, B.; Xiong, K.; Cai, J.; Ma, A. Fish consumption and coronary heart disease: A meta-analysis. Nutrients 2020, 12, 2278. [Google Scholar] [CrossRef]
- Fernandez, M.L. Effects of eggs on plasma lipoproteins in healthy populations. Food Funct. 2010, 1, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Tinsley, G.; Bianco, A.; Moro, T. The influence of meal frequency and timing on health in humans: The role of fasting. Nutrients 2019, 11, 719. [Google Scholar] [CrossRef] [PubMed]
- Dhandevi, P.E.; Jeewon, R. Fruit and vegetable intake: Benefits and progress of nutrition education interventions-narrative review article. Iran. J. Public Health 2015, 44, 1309. [Google Scholar]
- 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Available online: https://emedicine.medscape.com/article/153647-guidelines?form=fpf (accessed on 21 July 2024).
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Bittner, V. The new 2019 AHA/ACC guideline on the primary prevention of cardiovascular disease. Circulation 2020, 142, 2402–2404. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J. 2018 American Heart Association/American College of Cardiology/multisociety guideline on the management of blood cholesterol–secondary prevention. JAMA Cardiol. 2019, 4, 589–591. [Google Scholar] [CrossRef]
- Oren, O.; Small, A.M.; Libby, P. Clonal hematopoiesis and atherosclerosis. J. Clin. Investig. 2024, 134, e180066. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Björkegren, J.L.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Libby, P. Inflammation and the pathogenesis of atherosclerosis. Vasc. Pharmacol. 2024, 154, 107255. [Google Scholar] [CrossRef]
- Attiq, A.; Afzal, S.; Ahmad, W.; Kandeel, M. Hegemony of inflammation in atherosclerosis and coronary artery disease. Eur. J. Pharmacol. 2024, 966, 176338. [Google Scholar] [CrossRef] [PubMed]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927. [Google Scholar] [PubMed]
- Tang, M.; Yang, S.; Zou, J.; Li, M.; Sun, Y.; Wang, M.; Li, W.; He, J.; Chen, Y.; Tang, Z. Global trends and research hotspots of PCSK9 and cardiovascular disease: A bibliometric and visual analysis. Front. Cardiovasc. Med. 2024, 11, 1336264. [Google Scholar] [CrossRef] [PubMed]
- Cao Zhang, A.M.; Ziogos, E.; Harb, T.; Gerstenblith, G.; Leucker, T.M. Emerging clinical role of proprotein convertase subtilisin/kexin type 9 inhibition–Part two: Current and emerging concepts in the clinical use of PCSK9 inhibition. Eur. J. Clin. Investig. 2024, 54, e14272. [Google Scholar] [CrossRef]
- Marco-Benedí, V.; Sánchez-Hernández, R.M.; Díaz, J.L.; Jarauta, E.; Suárez-Tembra, M.; Pintó, X.; Morillas, C.; Plana, N.; Pedro-Botet, J.; Civeira, F. PCSK9 inhibitors on the management of primary and secondary cardiovascular prevention. Lipids Health Dis. 2024, 23, 290. [Google Scholar] [CrossRef]
- Farahani, M.M.; Nasiri, A.; Salari, M.; Shamsedini, A. The therapeutic effect of PCSK9 inhibitors on dyslipidemia: One-year follow up. Eur. J. Transl. Myol. 2024, 34, 12937. [Google Scholar]
- Maligłówka, M.; Kosowski, M.; Hachuła, M.; Cyrnek, M.; Bułdak, Ł.; Basiak, M.; Bołdys, A.; Machnik, G.; Bułdak, R.J.; Okopień, B. Insight into the Evolving Role of PCSK9. Metabolites 2022, 12, 256. [Google Scholar] [CrossRef]
- Hobbs, H.H.; Cohen, J.C.; Horton, J.D. PCSK9: From nature’s loss to patient’s gain. Circulation 2024, 149, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.J.; Cannon, C.P. PCSK9 (Proprotein convertase subtilisin/kexin type 9) inhibitors: Past, present, and the future. Eur. Heart J. 2015, 36, 2415–2424. [Google Scholar] [CrossRef]
- Mullard, A. Nine paths to PCSK9 inhibition. Nat. Rev. Drug Discov. 2017, 16, 299–302. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Hegele, R.A.; Fazio, S.; Cannon, C.P. The evolving future of PCSK9 inhibitors. J. Am. Coll. Cardiol. 2018, 72, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Safarova, M.; Bimal, T.; Soffer, D.E.; Hirsh, B.; Shapiro, M.D.; Mintz, G.; Cha, A.; Gianos, E. Advances in targeting LDL cholesterol: PCSK9 inhibitors and beyond. Am. J. Prev. Cardiol. 2024, 19, 100701. [Google Scholar] [CrossRef]
- Mhaimeed, O.; Burney, Z.A.; Schott, S.L.; Kohli, P.; Marvel, F.A.; Martin, S.S. The importance of LDL-C lowering in atherosclerotic cardiovascular disease prevention: Lower for longer is better. Am. J. Prev. Cardiol. 2024, 18, 100649. [Google Scholar] [CrossRef] [PubMed]
- Seidah, N.G.; Benjannet, S.; Wickham, L.; Marcinkiewicz, J.; Jasmin, S.B.; Stifani, S.; Basak, A.; Prat, A.; Chrétien, M. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): Liver regeneration and neuronal differentiation. Proc. Natl. Acad. Sci. USA 2003, 100, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Yurtseven, E.; Ural, D.; Baysal, K.; Tokgözoğlu, L. An update on the role of PCSK9 in atherosclerosis. J. Atheroscler. Thromb. 2020, 27, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Seidah, N.G.; Awan, Z.; Chrétien, M.; Mbikay, M. PCSK9: A key modulator of cardiovascular health. Circ. Res. 2014, 114, 1022–1036. [Google Scholar] [CrossRef]
- Marschner, K.; Kollmann, K.; Schweizer, M.; Braulke, T.; Pohl, S. A key enzyme in the biogenesis of lysosomes is a protease that regulates cholesterol metabolism. Science 2011, 333, 87–90. [Google Scholar] [CrossRef]
- Leander, K.; Mälarstig, A.; Van’t Hooft, F.; Hyde, C.; Hellénius, M.; Troutt, J.; Konrad, R.; Öhrvik, J.; Hamsten, A.; de Faire, U. Circulating Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Predicts Future Risk of Cardiovascular Events Independently of Established Risk Factors. Circulation 2016, 133, 1230–1239. [Google Scholar] [CrossRef]
- Stein, E.A.; Mellis, S.; Yancopoulos, G.D.; Stahl, N.; Logan, D.; Smith, W.B.; Lisbon, E.; Gutierrez, M.; Webb, C.; Wu, R.; et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N. Engl. J. Med. 2012, 366, 1108–1118. [Google Scholar] [CrossRef]
- Tang, Z.; Peng, J.; Ren, Z.; Yang, J.; Li, T.; Li, T.; Wang, Z.; Wei, D.; Liu, L.; Zheng, X.; et al. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-κB pathway. Atherosclerosis 2017, 262, 113–122. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, S.; Wang, X.; Deng, X.; Fan, Y.; Sun, C.; Wang, Y.; Mehta, J. Hemodynamic shear stress via ROS modulates PCSK9 expression in human vascular endothelial and smooth muscle cells and along the mouse aorta. Antioxid. Redox Signal 2015, 22, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Grune, J.; Meyborg, H.; Bezhaeva, T.; Kappert, K.; Hillmeister, P.; Kintscher, U.; Pieske, B.; Stawowy, P. PCSK9 regulates the chemokine receptor CCR2 on monocytes. Biochem. Biophys. Res. Commun. 2017, 485, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Deng, X.; Zhang, P.; Wang, X.; Fan, Y.; Zhou, S.; Mu, S.; Mehta, J.L.; Ding, Z. Blood flow patterns regulate PCSK9 secretion via MyD88-mediated pro-inflammatory cytokines. Cardiovasc. Res. 2020, 116, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Lu, C.; Ryan, R.O. A two-step binding model of PCSK9 interaction with the low-density lipoprotein receptor. J. Biol. Chem. 2011, 286, 5464–5470. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Hu, L.; Zhang, J.; Yang, W.; Liu, X.; Jia, D.; Yao, Z.; Chang, L.; Pan, G.; Zhong, H.; et al. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Enhances Platelet Activation, Thrombosis, and Myocardial Infarct Expansion by Binding to Platelet CD36. Circulation 2021, 143, 45–61. [Google Scholar] [CrossRef]
- Page, M.M.; Watts, G.F. PCSK 9 in context: A contemporary review of an important biological target for the prevention and treatment of atherosclerotic cardiovascular disease. Diabetes Obes. Metab. 2018, 20, 270–282. [Google Scholar] [CrossRef]
- Mahboobnia, K.; Pirro, M.; Marini, E.; Grignani, F.; Bezsonov, E.E.; Jamialahmadi, T.; Sahebkar, A. PCSK9 and cancer: Rethinking the link. Biomed. Pharmacother. 2021, 140, 111758. [Google Scholar] [CrossRef]
- Benjannet, S.; Rhainds, D.; Essalmani, R.; Mayne, J.; Wickham, L.; Jin, W.; Asselin, M.C.; Hamelin, J.; Varret, M.; Allard, D.; et al. NARC-1/PCSK9 and its natural mutants: Zymogen cleavage and effects on the low-density lipoprotein (LDL) receptor and LDL cholesterol. J. Biol. Chem. 2004, 279, 48865–48875. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Palmer-Smith, H.; Basak, A. Regulatory effects of peptides from the pro and catalytic domains of proprotein convertase subtilisin/kexin 9 (PCSK9) on low-density lipoprotein receptor (LDL-R). Curr. Med. Chem. 2010, 17, 2168–2182. [Google Scholar] [CrossRef] [PubMed]
- Catapano, A.L.; Pirillo, A.; Norata, G.D. New Pharmacological Approaches to Target PCSK9. Curr. Atheroscler. Rep. 2020, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Dicembrini, I.; Giannini, S.; Ragghianti, B.; Mannucci, E.; Monami, M. Effects of PCSK9 inhibitors on LDL cholesterol, cardiovascular morbidity, and all-cause mortality: A systematic review and meta-analysis of randomized controlled trials. J. Endocrinol. Investig. 2019, 42, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.; Berge, K.E.; Berg, T.; Leren, T.P. Affinity and Kinetics of Proprotein Convertase Subtilisin/Kexin Type 9 Binding to Low-Density Lipoprotein Receptors on HepG2 Cells. FEBS J. 2011, 278, 2938–2950. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-D.; Lee, S.E.; Yang, J.; Lee, H.-C.; Shin, D.; Lee, H.; Lee, J.; Jin, S.; Kim, S.; Lee, S.J.; et al. Cyclase-Associated Protein 1 Is a Binding Partner of Proprotein Convertase Subtilisin/Kexin Type-9 and Is Required for the Degradation of Low-Density Lipoprotein Receptors by Proprotein Convertase Subtilisin/Kexin Type-9. Eur. Heart J. 2020, 41, 239–252. [Google Scholar] [CrossRef]
- Cammisotto, V.; Baratta, F.; Castellani, V.; Bartimoccia, S.; Nocella, C.; D’Erasmo, L.; Cocomello, N.; Barale, C.; Scicali, R.; Di Pino, A.; et al. Proprotein Convertase Subtilisin Kexin Type 9 Inhibitors Reduce Platelet Activation Modulating ox-LDL Pathways. Int. J. Mol. Sci. 2021, 22, 7193. [Google Scholar] [CrossRef]
- Gurbel, P.; Jeong, Y.; Navarese, E.; Tantry, U. Platelet-Mediated Thrombosis: From Bench to Bedside. Circ. Res. 2016, 118, 1380–1391. [Google Scholar] [CrossRef]
- Ito, M.K.; Santos, R.D. PCSK9 inhibition with monoclonal antibodies: Modern management of hypercholesterolemia. J. Clin. Pharmacol. 2017, 57, 7–32. [Google Scholar] [CrossRef]
- Ghasempour, G.; Zamani-Garmsiri, F.; Shaikhnia, F.; Soleimani, A.A.; Hosseini Fard, S.R.; Leila, J.; Teimuri, S.; Parvaz, N.; Mohammadi, P.; Najafi, M. Efficacy and Safety of Alirocumab and Evolocumab as Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitors in Familial Hypercholesterolemia: A Systematic Review and Meta-Analysis. Curr. Med. Chem. 2024, 31, 223–241. [Google Scholar] [CrossRef]
- Ray, K.K.; Bruckert, E.; Peronne-Filardi, P.; Ebenbichler, C.; Vogt, A.; Bridges, I.; Sibartie, M.; Dhalwani, N. Long-term persistence with Evolocumab treatment and sustained reductions in LDL-cholesterol levels over 30 months: Final results from the European observational HEYMANS study. Atherosclerosis 2023, 366, 14–21. [Google Scholar] [CrossRef]
- McClintick, D.J.; O’Donoghue, M.L.; De Ferrari, G.M.; Ferreira, J.; Ran, X.; Im, K.; López, J.A.; Elliott-Davey, M.; Wang, B.; Monsalvo, M.L.; et al. Long-term efficacy of Evolocumab in patients with or without multivessel coronary disease. J. Am. Coll. Cardiol. 2024, 83, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Ziogos, E.; Harb, T.; Valenta, I.; Vavuranakis, M.A.; Williams, M.S.; Blaha, M.J.; Jones, S.R.; Schindler, T.H.; Gerstenblith, G.; Leucker, T.M. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibition with Evolocumab decreases myocardial inflammation in individuals with acute coronary syndrome (ACS). Eur. Heart J. 2023, 44 (Suppl. S2), ehad655.1380. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Tate, A.; Mar, P.; Grushko, O.; Chen, Q.; Goonewardena, S.N. Inhibition of PCSK9 with Evolocumab modulates lipoproteins and monocyte activation in high-risk ASCVD subjects. Atherosclerosis 2024, 392, 117529. [Google Scholar] [CrossRef]
- Bohula, E.A.; Marston, N.A.; Ruzza, A.; Murphy, S.A.; De Ferrari, G.M.; Diaz, R.; Leiter, L.A.; Elliott-Davey, M.; Wang, H.; Bhatia, A.K.; et al. Rationale and design of the effect of Evolocumab in patients at high cardiovascular risk without prior myocardial infarction or stroke (VESALIUS-CV) trial. Am. Heart J. 2024, 269, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Deedwania, P.; Murphy, S.A.; Scheen, A.; Badariene, J.; Pineda, A.L.; Honarpour, N.; Keech, A.C.; Sever, P.S.; Pedersen, T.R.; Sabatine, M.S.; et al. Efficacy and safety of PCSK9 inhibition with Evolocumab in reducing cardiovascular events in patients with metabolic syndrome receiving statin therapy: Secondary analysis from the FOURIER randomized clinical trial. JAMA Cardiol. 2021, 6, 139–147. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Pedersen, T.R.; Saver, J.L.; Sever, P.S.; Keech, A.C.; Bohula, E.A.; Murphy, S.A.; Wasserman, S.M.; Honarpour, N.; Wang, H.; et al. Stroke prevention with the PCSK9 (proprotein convertase subtilisin-kexin type 9) inhibitor Evolocumab added to statin in high-risk patients with stable atherosclerosis. Stroke 2020, 51, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Cordero, A.; Rodríguez-Mañero, M.; Fácila, L.; Fernández-Olmo, M.R.; Gómez-Martínez, M.J.; Valle, A.; Castellano, J.M.; Toro, M.M.; Seijas-Amigo, J.; Vicedo, A.; et al. Prevention of myocardial infarction and stroke with PCSK9 inhibitors treatment: A metanalysis of recent randomized clinical trials. J. Diabetes Metab. Disord. 2020, 19, 759–765. [Google Scholar] [CrossRef]
- Keech, A.C.; Oyama, K.; Sever, P.S.; Tang, M.; Murphy, S.A.; Hirayama, A.; Lu, C.; Tay, L.; Deedwania, P.C.; Siu, C.W.; et al. Efficacy and Safety of Long-Term Evolocumab Use Among Asian Subjects―A Subgroup Analysis of the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) Trial. Circ. J. 2021, 85, 2063–2070. [Google Scholar] [CrossRef]
- Choi, H.D.; Kim, J.H. An Updated Meta-Analysis for Safety Evaluation of Alirocumab and Evolocumab as PCSK9 Inhibitors. Cardiovasc. Ther. 2023, 2023, 7362551. [Google Scholar] [CrossRef]
- Everett, B.M.; Smith, R.J.; Hiatt, W.R. Reducing LDL with PCSK9 inhibitors—the clinical benefit of lipid drugs. N. Engl. J. Med. 2015, 373, 1588–1591. [Google Scholar] [CrossRef]
- Xu, L.; Yan, X.; Tang, Z.; Feng, B. Association between circulating oxidized OxLDL/LDL-C ratio and the severity of coronary atherosclerosis, along with other emerging biomarkers of cardiovascular disease in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2022, 191, 110040. [Google Scholar] [CrossRef] [PubMed]
- Nesti, L.; Mengozzi, A.; Natali, A. Statins, LDL Cholesterol control, cardiovascular disease prevention, and atherosclerosis progression: A clinical perspective. Am. J. Cardiovasc. Drugs 2020, 20, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.A.; Raal, F.J. Targeting LDL: Is lower better and is it safe? Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Paraskevaidis, I.; Farmakis, D.; Papingiotis, G.; Tsougos, E. Inflammation and Heart Failure: Searching for the Enemy—Reaching the Entelechy. J. Cardiovasc. Dev. Dis. 2023, 10, 19. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Puri, R.; Anderson, T.; Ballantyne, C.M.; Cho, L.; Kastelein, J.J.P.; Koenig, W.; Somaratne, R.; Kassahun, H.; Yang, J.; et al. Effect of Evolocumab on progression of coronary disease in statin-treated patients: The GLAGOV randomized clinical trial. JAMA 2016, 316, 2373–2384. [Google Scholar] [CrossRef]
- Clark, D.J.; Lessio, S.; O’Donoghue, M.; Tsalamandris, C.; Schainfeld, R.; Rosenfield, K. Mechanisms and predictors of carotid artery stent restenosis: A serial intravascular ultrasound study. J. Am. Coll. Cardiol. 2006, 47, 2390–2396. [Google Scholar] [CrossRef]
- White, C.M. Therapeutic potential and critical analysis of the PCSK9 monoclonal antibodies Evolocumab and Alirocumab. Ann. Pharmacother. 2015, 49, 1327–1335. [Google Scholar] [CrossRef]
- Rexhaj, E.; Bär, S.; Soria, R.; Ueki, Y.; Häner, J.D.; Otsuka, T.; Kavaliauskaite, R.; Siontis, G.C.; Stortecky, S.; Shibutani, H.; et al. Effects of Alirocumab on endothelial function and coronary atherosclerosis in myocardial infarction: A PACMAN-AMI randomized clinical trial substudy. Atherosclerosis 2024, 392, 117504. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Losdat, S.; Shibutani, H.; Ueki, Y.; Otsuka, T.; Haener, J.; Fahrni, G.; Iglesias, J.F.; Spirk, D.; Van Geuns, R.J.; et al. Interrelation between baseline plaque characteristics and changes in coronary atherosclerosis with the PCSK9-inhibitor Alirocumab: Insights from the PACMAN-AMI randomized trial. Eur. Heart J. 2022, 43 (Suppl. S2), ehac544.1206. [Google Scholar] [CrossRef]
- Luo, J.; Liao, W.; Wang, X.; Xu, R.; Li, W.; Li, W.; Liu, K.; Huang, K.; Ma, Y.; Wang, T.; et al. PCSK9 inhibitors for anti-inflammation in atherosclerosis: Protocol for a systematic review and meta-analysis of randomized controlled trials. BMJ Open 2022, 12, e062046. [Google Scholar] [CrossRef]
- Goodman, S.G.; Steg, P.G.; Poulouin, Y.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Garon, G.; Harrington, R.A.; Jukema, J.W.; Manvelian, G.; et al. Long-Term Efficacy, Safety, and Tolerability of Alirocumab in 8242 Patients Eligible for 3 to 5 Years of Placebo-Controlled Observation in the ODYSSEY OUTCOMES Trial. J. Am. Heart Assoc. 2023, 12, e029216. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, M.L.; Fazio, S.; Giugliano, R.P.; Stroes, E.S.; Kanevsky, E.; Gouni-Berthold, I.; Im, K.; Lira Pineda, A.; Wasserman, S.M.; Češka, R.; et al. Lipoprotein (a), PCSK9 inhibition, and cardiovascular risk: Insights from the FOURIER trial. Circulation 2019, 139, 1483–1492. [Google Scholar] [CrossRef]
- Edmiston, J.B.; Brooks, N.; Tavori, H.; Minnier, J.; Duell, B.; Purnell, J.Q.; Kaufman, T.; Wojcik, C.; Voros, S.; Fazio, S.; et al. Discordant response of low-density lipoprotein cholesterol and lipoprotein (a) levels to monoclonal antibodies targeting proprotein convertase subtilisin/kexin type 9. J. Clin. Lipidol. 2017, 11, 667–673. [Google Scholar] [CrossRef]
- Mahmood, T.; Minnier, J.; Ito, M.K.; Li, Q.H.; Koren, A.; Kam, I.W.; Fazio, S.; Shapiro, M.D. Discordant responses of plasma low-density lipoprotein cholesterol and lipoprotein (a) to Alirocumab: A pooled analysis from 10 ODYSSEY Phase 3 studies. Eur. J. Prev. Cardiol. 2021, 28, 816–822. [Google Scholar] [CrossRef]
- Guedeney, P.; Giustino, G.; Sorrentino, S.; Claessen, B.E.; Camaj, A.; Kalkman, D.N.; Vogel, B.; Sartori, S.; De Rosa, S.; Baber, U.; et al. Efficacy and safety of Alirocumab and Evolocumab: A systematic review and meta-analysis of randomized controlled trials. Eur. Heart J. 2022, 43, e17–e25. [Google Scholar] [CrossRef] [PubMed]
- Koren, M.J.; Sabatine, M.S.; Giugliano, R.P.; Langslet, G.; Wiviott, S.D.; Ruzza, A.; Ma, Y.; Hamer, A.W.; Wasserman, S.M.; Raal, F.J. Long-term efficacy and safety of Evolocumab in patients with hypercholesterolemia. J. Am. Coll. Cardiol. 2019, 74, 2132–2146. [Google Scholar] [CrossRef]
- Snel, M.; Descamps, O.S. Long-term safety and effectiveness of Alirocumab and Evolocumab in familial hypercholesterolemia (FH) in Belgium. Acta Cardiol. 2024, 79, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Li, C.; Tu, Y.; Li, Z.; Zhang, M. Additive effects of Ezetimibe, Evolocumab, and Alirocumab on plaque burden and lipid content as assessed by intravascular ultrasound: A PRISMA-compliant meta-analysis. Medicine 2022, 101, e31199. [Google Scholar] [CrossRef]
- MacDougall, D.E.; Baum, S.J.; Ahmed, C.D.; McGowan, M.P.; Wilemon, K.A. Trends in Patient Access to and Utilization of Prescribed PCSK9 Inhibitors in a Large US Claims Database from 2015 to 2021. Circ. Cardiovasc. Qual. Outcomes 2024, 17, e009988. [Google Scholar] [CrossRef]
- Broder, M.S.; Zambrano, J.M.; Lee, J.; Marken, R.S. Systematic bias in predictions of new drugs’ budget impact: Analysis of a sample of recent US drug launches. Curr. Med. Res. Opin. 2018, 34, 765–773. [Google Scholar] [CrossRef]
- Mercep, I.; Strikic, D.; Hrabac, P.; Pecin, I.; Reiner, Ž. PCSK9 inhibition: From effectiveness to cost-effectiveness. Front. Cardiovasc. Med. 2024, 11, 1339487. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Chen, F. Research progress on the clinical application of PCSK9 inhibitors in coronary heart disease. Chin. J. Clin. Res. 2024, 37, 826–869. [Google Scholar]
- Fonarow, G.C.; Keech, A.C.; Pedersen, T.R.; Giugliano, R.P.; Sever, P.S.; Lindgren, P.; van Hout, B.; Villa, G.; Qian, Y.; Somaratne, R.; et al. Cost-effectiveness of Evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017, 2, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Gandra, S.R.; Villa, G.; Fonarow, G.C.; Lothgren, M.; Lindgren, P.; Somaratne, R.; van Hout, B. Cost-effectiveness of LDL-C lowering with Evolocumab in patients with high cardiovascular risk in the United States. Clin. Cardiol. 2016, 39, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Blom, D.J.; Chen, J.; Yuan, Z.; Borges, J.L.; Monsalvo, M.L.; Wang, N.; Hamer, A.W.; Ge, J. Effects of Evolocumab therapy and low LDL-C levels on vitamin E and steroid hormones in Chinese and global patients with type 2 diabetes. Endocrinol. Diabetes Metab. 2020, 3, e00123. [Google Scholar] [CrossRef]
- Ebenezer, O.; Comoglio, P.; Wong, G.K.-S.; Tuszynski, J.A. Development of Novel SiRNA Therapeutics: A Review with a Focus on Inclisiran for the Treatment of Hypercholesterolemia. Int. J. Mol. Sci. 2023, 24, 4019. [Google Scholar] [CrossRef]
- Roth, E.M.; McKenney, J.M.; Hanotin, C.; Asset, G.; Stein, E.A. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N. Engl. J. Med. 2012, 367, 1891–1900. [Google Scholar] [CrossRef]
- Stroes, E.; Colquhoun, D.; Sullivan, D.; Civeira, F.; Rosenson, R.S.; Watts, G.F.; Bruckert, E.; Cho, L.; Dent, R.; Knusel, B.; et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: The GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of Evolocumab. J. Am. Coll. Cardiol. 2014, 63, 2541–2548. [Google Scholar] [CrossRef]
| Study Type | Agents | Results | Ref. |
|---|---|---|---|
| Meta-analysis | Alirocumab and Evolocumab | The treatment decreased LDL-C levels by an average of 49.59% (with a confidence interval of −55.5% to −43.67%) compared to the placebo. | Ghasempour et al. [72] |
| Real-world European Evolocumab study: insights from HEYMANS | Evolocumab | LDL-C levels decreased by 58% within 3 months of starting Evolocumab and remained consistently lower over the subsequent 30 months. | Ray et al. [73] |
| FOURIER extended open-label trial (FOURIER-EOLT) study | Evolocumab | Evolocumab treatment led to greater cardiovascular risk reductions in patients with multivessel disease (MVD), with risk reductions increasing over time to 37–38% in MVD patients and 23–28% in non-MVD patients. | McClintick et al. [74] |
| Placebo-controlled, randomized trial | Evolocumab | Achieved significant results in the reduction in cardiac inflammation compared to placebo. | Ziogos et al. [75] |
| Double-blind, placebo-controlled trial | Evolocumab | Evolocumab achieved a reduction in LDL-C levels by 68.8% (p < 0.0001) at 2 weeks and by 52.8% (p < 0.0001) at 12 weeks. | Rosenson et al. [76] |
| VESALIUS-CV trial | Evolocumab | Latest ongoing (results pending). | Bohula et al. [77] |
| FOURIER randomized clinical trial | Evolocumab | Evolocumab achieved reductions in LDL cholesterol levels across both groups. | Deedwania et al. [78] |
| FOURIER randomized, double-blind trial | Evolocumab | Evolocumab achieved a notable decrease in total strokes (1.5% versus 1.9%) and ischemic strokes (1.2% versus 1.6%) compared to the placebo. | Giugliano et al. [79] |
| Recent meta-analysis of randomized clinical trials | Evolocumab, Alirocumab, and bococizumab | Treatment with PCSK9 inhibitors achieved a 19% lower risk of heart attacks and a 25% lower risk of strokes compared to controls. | Cordero et al. [80] |
| FOURIER trial | Evolocumab | The results showed that Evolocumab effectively reduces LDL-C levels and demonstrates comparable efficacy in reducing cardiovascular events among Asian individuals. | Keech et al. [81] |
| References | Study Design | Participants | Duration | Efficacy (LDL-C Reduction) | Key Findings (Side Effects/Safety) |
|---|---|---|---|---|---|
| [94] | Meta-analysis of 39 randomized controlled trials (RCTs) | 66,478 in total, 35,896 receiving PCSK9 inhibitors | 2.3 years (avg follow-up) | - Reduced risk of MI, ischemic stroke, and coronary artery bypass graft | No significant impact on cardiovascular mortality; no increased risk of neuropsychological issues, liver enzyme elevations, muscle breakdown syndrome, or new-onset diabetes. |
| [98] | Real-world study on FH patients | 239 patients with FH | 3 years | 54% reduction in LDL-C in the first year, maintained over the study period | PCSK9 inhibitors are safe and well tolerated, with no significant adverse effects; 93% of patients used PCSK9 inhibitors with statins; half met EAS cholesterol targets. |
| [99] | 49 RCTs (updated safety evaluation) | 66,068 participants | N/A | N/A | No difference for adverse events (AEs); Alirocumab reduced diabetes-related AEs; no difference in neurocognitive and neurological AEs; Evolocumab did not show benefits in diabetes. |
| [100] | Randomized controlled trial | Evolocumab group vs. standard medical treatment | 5 years (1-year RCT, 4-year extension) | 56% reduction in LDL-C over 5 years | Similar side effect profile to standard treatment group; no harmful antibodies detected; few patients discontinued due to side effects. |
| [101] | 9 studies (RCTs) | 1836 participants | N/A | Significant LDL-C and total cholesterol reductions | Significant reductions in LDL-C and total cholesterol, as well as regression of coronary atheroma volume in PCSK9 inhibitor groups, particularly in patients on statins. |
| Country | Drug | Cost | QALY |
|---|---|---|---|
| Germany | Evolocumab | EUR 62,722 | 0.55 |
| Alirocumab | EUR 87,002 | 0.87 | |
| United Kingdom | Evolocumab | GBP 45,279 | 0.53 |
| Alirocumab | GBP 46,375 | 0.86 | |
| China | Evolocumab | CNY 18,714 | 1.25 |
| Saudi Arabia | Evolocumab + Statins | USD 60,708 | 1 |
| Evolocumab + Ezetimibe | USD 41,757 | 1 | |
| Russian Federation | PCSK9 Inhibitors + Inclisiran | RUB 3.6 million | 1 |
| Category | Inclisiran | PCSK9 Inhibitors (Evolocumab, Alirocumab) | Cholesterol Absorption Inhibitor (Ezetimibe) |
|---|---|---|---|
| Mechanism of Action | siRNA silencing PCSK9 gene, reducing PCSK9 production in the liver | Monoclonal antibodies neutralizing circulating PCSK9 proteins | Inhibits cholesterol absorption in the intestine via NPC1L1 receptor |
| Administration Route | Subcutaneous injection | Subcutaneous injection | Oral tablet |
| Dosing Frequency | Every 6 months | Biweekly or monthly | Daily |
| LDL-C Reduction | 50–55% | 60–70% | 15–20% |
| Onset of Action | Gradual, with sustained effect over months | Rapid effect | Moderate |
| Compliance | High (due to infrequent dosing) | Moderate (frequent injections required) | High (simple daily dosing) |
| Side Effects | Injection-site reactions; no significant myopathy or liver toxicity | Injection-site reactions, rare allergic reactions | Mild gastrointestinal discomfort |
| Ideal Patient Profile | Long-term LDL-C control for ASCVD and familial hypercholesterolemia | High-risk patients needing rapid LDL-C lowering | Moderate-risk patients or statin-intolerant cases |
| Cost | Typically high (but less frequent injections) | High | Relatively affordable |
| Relative Advantages | Better compliance due to infrequent dosing | More effective for rapid LDL-C reduction | Non-injectable option |
| Lower injection burden compared to PCSK9 inhibitors | Ideal for acute management of post-cardiovascular events | Affordable compared to injectable therapies | |
| Useful as an add-on therapy with statins | |||
| Clinical Indications | ASCVD (for long-term LDL-C management) | High-risk ASCVD patients | Moderate-risk patients |
| Familial hypercholesterolemia | Statin intolerance | Statin-intolerant individuals | |
| Suitable for patients with compliance challenges | Situations requiring fast LDL-C reduction | Add-on for incomplete LDL-C control with statins |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abduljabbar, M.H. PCSK9 Inhibitors: Focus on Evolocumab and Its Impact on Atherosclerosis Progression. Pharmaceuticals 2024, 17, 1581. https://doi.org/10.3390/ph17121581
Abduljabbar MH. PCSK9 Inhibitors: Focus on Evolocumab and Its Impact on Atherosclerosis Progression. Pharmaceuticals. 2024; 17(12):1581. https://doi.org/10.3390/ph17121581
Chicago/Turabian StyleAbduljabbar, Maram H. 2024. "PCSK9 Inhibitors: Focus on Evolocumab and Its Impact on Atherosclerosis Progression" Pharmaceuticals 17, no. 12: 1581. https://doi.org/10.3390/ph17121581
APA StyleAbduljabbar, M. H. (2024). PCSK9 Inhibitors: Focus on Evolocumab and Its Impact on Atherosclerosis Progression. Pharmaceuticals, 17(12), 1581. https://doi.org/10.3390/ph17121581







