Anti-Vascular Endothelial Growth Factor Combined with Ocular Steroid Therapy for Persistent Diabetic Macular Edema: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Design and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Quality Assessment
2.4. Heterogeneity and Sensitivity Analysis
2.5. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Best-Corrected Visual Acuity
3.2.1. One Month
3.2.2. Two Months
3.2.3. Three Months
3.2.4. Six Months
3.2.5. Nine Months
3.2.6. Twelve Months
3.3. Central Retinal Thickness
3.3.1. One Month
3.3.2. Two Months
3.3.3. Three Months
3.3.4. Six Months
3.3.5. Nine Months
3.3.6. Twelve Months
3.4. Incidence of Adverse Events
3.5. Heterogeneity Analysis
3.5.1. Subgroup Analysis of Drug Types
3.5.2. Subgroup Analysis by Study Type
3.6. Quality and Risk-of-Bias Assessment
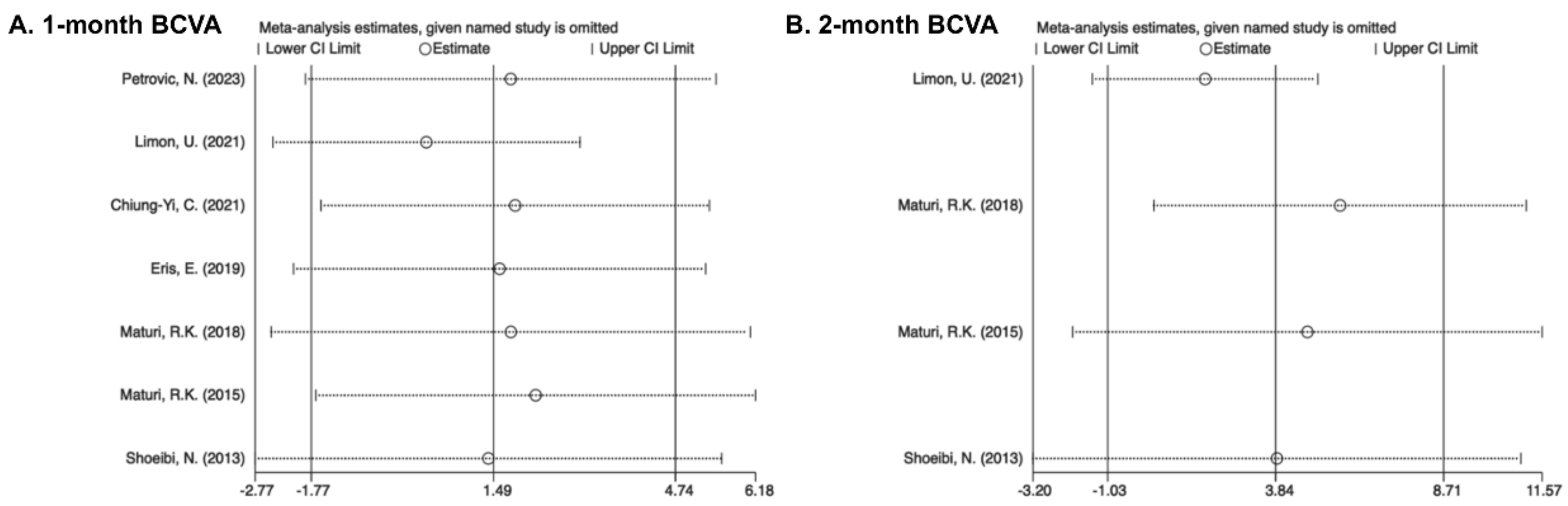
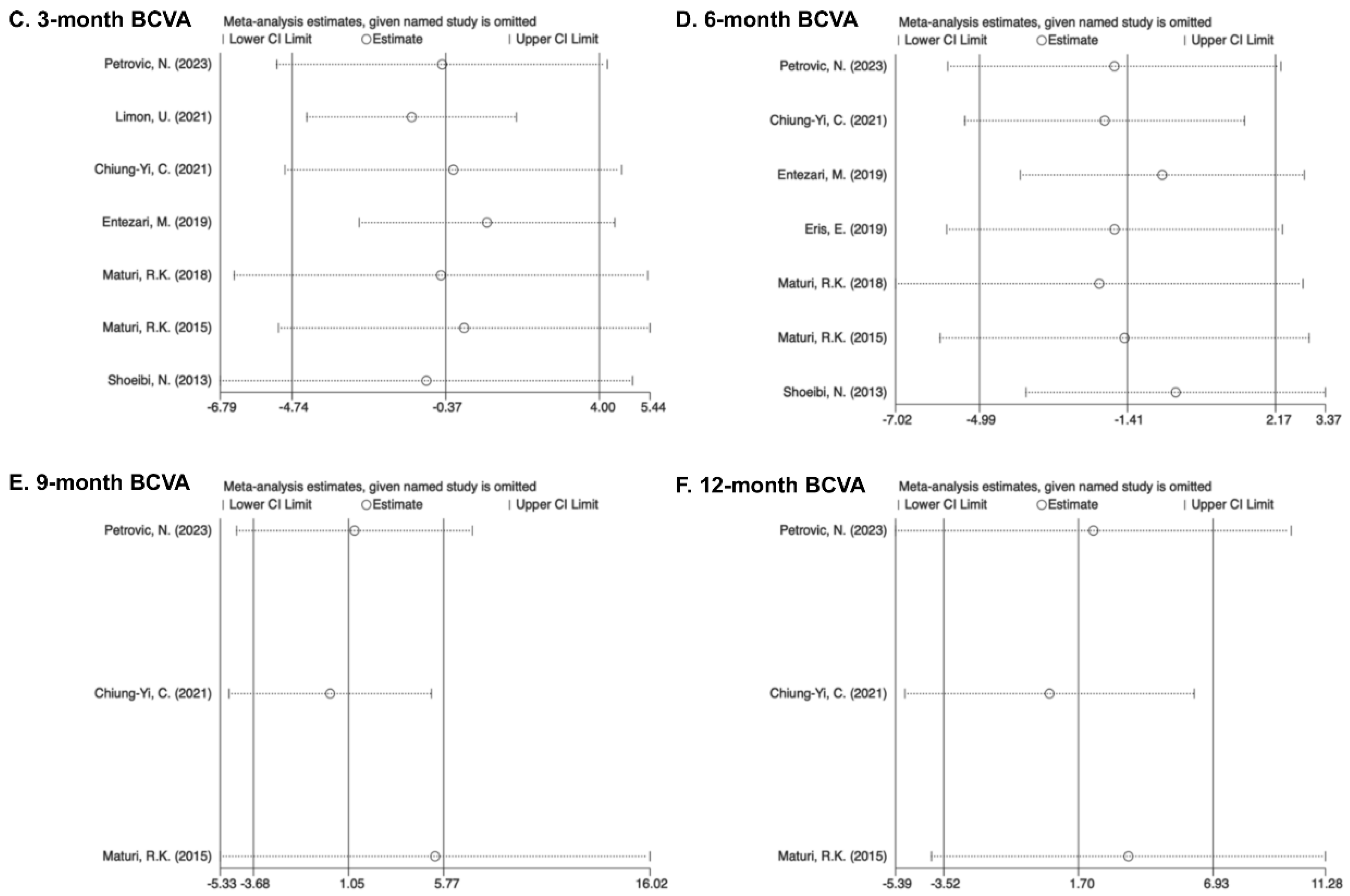
3.7. Sensitivity Analysis and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Zhang, J.; Zhang, C.; Zhang, J.; Gu, L.; Luo, D.; Qiu, Q. Diabetic Macular Edema: Current Understanding, Molecular Mechanisms and Therapeutic Implications. Cells 2022, 11, 3362. [Google Scholar] [CrossRef] [PubMed]
- Daruich, A.; Matet, A.; Moulin, A.; Kowalczuk, L.; Nicolas, M.; Sellam, A.; Rothschild, P.-R.; Omri, S.; Gélizé, E.; Jonet, L.; et al. Mechanisms of macular edema: Beyond the surface. Prog. Retin. Eye Res. 2018, 63, 20–68. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Schmitz-Valckenberg, S.; Chakravarthy, U. Age-Related Macular Degeneration: A Review. JAMA 2024, 331, 147. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Ying, G.-S.; Toth, C.A.; Daniel, E.; Grunwald, J.E.; Martin, D.F.; Maguire, M.G. Macular Morphology and Visual Acuity in Year Five of the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology 2019, 126, 252–260. [Google Scholar] [CrossRef]
- Marques, A.P.; Ramke, J.; Cairns, J.; Butt, T.; Zhang, J.H.; Jones, I.; Jovic, M.; Nandakumar, A.; Faal, H.; Taylor, H.; et al. The economics of vision impairment and its leading causes: A systematic review. eClinicalMedicine 2022, 46, 101354. [Google Scholar] [CrossRef]
- Durand, M.L. Endophthalmitis. Clin. Microbiol. Infect. 2013, 19, 227–234. [Google Scholar] [CrossRef]
- Klein, K.S.; Walsh, M.K.; Hassan, T.S.; Halperin, L.S.; Castellarin, A.A.; Roth, D.; Driscoll, S.; Prenner, J.L. Endophthalmitis After Anti-VEGF Injections. Ophthalmology 2009, 116, 1225–1225.e1. [Google Scholar] [CrossRef]
- Jampol, L.M. Pharmacologic Therapy of Aphakic and Pseudophakic Cystoid Macular Edema. Ophthalmology 1985, 92, 807–810. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Bandello, F.; Berg, K.; Chakravarthy, U.; Gerendas, B.S.; Jonas, J.; Larsen, M.; Tadayoni, R.; Loewenstein, A. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2017, 237, 185–222. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Han, Y.S.; Mir, T.A.; Kherani, S.; Hafiz, G.; Krispel, C.; Liu, T.Y.A.; Wang, J.; Scott, A.W.; Zimmer-Galler, I. Increased Frequency of Topical Steroids Provides Benefit in Patients With Recalcitrant Postsurgical Macular Edema. Am. J. Ophthalmol. 2017, 178, 163–175. [Google Scholar] [CrossRef]
- Madjedi, K.; Pereira, A.; Ballios, B.G.; Arjmand, P.; Kertes, P.J.; Brent, M.; Yan, P. Switching between anti-VEGF agents in the management of refractory diabetic macular edema: A systematic review. Surv. Ophthalmol. 2022, 67, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Fry, C.; Turner, A.; Razavi, H. Intravitreal dexamethasone versus bevacizumab in Aboriginal and Torres Strait Islander patients with diabetic macular oedema: The OASIS study (a randomised control trial). Clin. Exp. Ophthalmol. 2022, 50, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Mehta, H.; Fraser-Bell, S.; Nguyen, V.; Lim, L.L.; Gillies, M.C. Short-term vision gains at 12 weeks correlate with long-term vision gains at 2 years: Results from the BEVORDEX randomised clinical trial of bevacizumab versus dexamethasone implants for diabetic macular oedema. Br. J. Ophthalmol. 2018, 102, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Liu, Y.; Xu, H.; Gao, Y.; Qin, L.; Gou, Y.; Tao, M.; Zhang, M. Efficacy and safety of single-dose dexamethasone implantation for patients with persistent diabetic macular edema: A systematic review and meta-analysis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 405–413. [Google Scholar] [CrossRef]
- Veiga Reis, F.; Dalgalarrondo, P.; Da Silva Tavares Neto, J.E.; Wendeborn Rodrigues, M.; Scott, I.U.; Jorge, R. Combined intravitreal dexamethasone and bevacizumab injection for the treatment of persistent diabetic macular edema (DexaBe study): A phase I clinical study. Int. J. Retin. Vitr. 2023, 9, 13. [Google Scholar] [CrossRef]
- Ferris, F.L.; Kassoff, A.; Bresnick, G.H.; Bailey, I. New Visual Acuity Charts for Clinical Research. Am. J. Ophthalmol. 1982, 94, 91–96. [Google Scholar] [CrossRef]
- Khoshnood, B.; Mesbah, M.; Jeanbat, V.; Lafuma, A.; Berdeaux, G. Transforming scales of measurement of visual acuity at the group level: Visual acuity transformation. Ophthalmic Physiol. Opt. 2010, 30, 816–823. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Harbord, R.M.; Egger, M.; Sterne, J.A.C. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2006, 25, 3443–3457. [Google Scholar] [CrossRef]
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Petrovic, N.; Todorovic, D.; Sarenac Vulovic, T.; Sreckovic, S.; Zivic, F.; Risimic, D. Combined Treatment of Persistent Diabetic Macular Edema with Aflibercept and Triamcinolone Acetonide in Pseudophakic Eyes. Medicina 2023, 59, 982. [Google Scholar] [CrossRef]
- Shahid, M.H.; Rashid, F.; Tauqeer, S.; Ali, R.; Farooq, M.T.; Aleem, N. Comparison of Suprachoroidal Triamcinolone Injection with Intravitreal Bevacizumab Vs Intravitreal Bevacizumab only in Treatment of Refractory Diabetic Macular Edema. Pak. J. Med. Health Sci. 2022, 16, 301–303. [Google Scholar] [CrossRef]
- Limon, U. Early effect of simultaneous intravitreal dexamethasone and bevacizumab combination treatment in patients with persistent diabetic macular edema. J. Fr. Ophtalmol. 2021, 44, 849–854. [Google Scholar] [CrossRef]
- Chiu, C.-Y.; Huang, T.-L.; Chang, P.-Y.; Chen, F.-T.; Hsu, Y.-R.; Chen, Y.-J.; Wang, J.-K. Combined intravitreal ranibizumab and posterior subtenon triamcinolone acetonide injections for patients with diabetic macular edema refractory to intravitreal ranibizumab monotherapy. Taiwan J. Ophthalmol. 2021, 11, 251. [Google Scholar] [CrossRef]
- Eriş, E.; Perente, I.; Vural, E.; Vural, A.; Seymen, Z.; Celebi, A.R.C.; Erdogan, G.; Ozkaya, A.; Artunay, O. Evaluation of the effect of combined intravitreal ranibizumab injection and sub-tenon steroid injection in the treatment of resistant diabetic macular edema. Int. Ophthalmol. 2019, 39, 1575–1580. [Google Scholar] [CrossRef]
- Entezari, M.; Flavarjani, Z.K.; Ramezani, A.; Nikkhah, H.; Karimi, S.; Moghadam, H.F.; Daftarian, N.; Yaseri, M. Combination of intravitreal bevacizumab and erythropoietin versus intravitreal bevacizumab alone for refractory diabetic macular edema: A randomized double-blind clinical trial. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 2375–2380. [Google Scholar] [CrossRef]
- Maturi, R.K.; Glassman, A.R.; Liu, D.; Beck, R.W.; Bhavsar, A.R.; Bressler, N.M.; Jampol, L.M.; Melia, M.; Punjabi, O.S.; Salehi-Had, H.; et al. Effect of Adding Dexamethasone to Continued Ranibizumab Treatment in Patients With Persistent Diabetic Macular Edema: A DRCR Network Phase 2 Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 29. [Google Scholar] [CrossRef] [PubMed]
- Rezar-Dreindl, S.; Eibenberger, K.; Buehl, W.; Georgopoulos, M.; Weigert, G.; Krall, C.; Dunavoelgyi, R.; Schmidt-Erfurth, U.; Sacu, S. Role of Additional Dexamethasone for the Management of Persistent or Recurrent Neovascular Age-Related Macular Degeneration Under Ranibizumab Treatment. Retina 2017, 37, 962–970. [Google Scholar] [CrossRef]
- Shoeibi, N.; Ahmadieh, H.; Entezari, M.; Yaseri, M. Intravitreal Bevacizumab with or without Triamcinolone for Refractory Diabetic Macular Edema: Long-term Results of a Clinical Trial. J. Ophthalmic Vis. Res. 2013, 8, 99. [Google Scholar]
- MATURI, R.K.; Bleau, L.; Saunders, J.; Mubasher, M.; Stewart, M.W. A 12-Month, Single-Masked, Randomized Controlled Study of Eyes with Persistent Diabetic Macular Edema after Multiple Anti-Vegf Injections to Assess the Efficacy of the Dexamethasone-Delayed Delivery System as an Adjunct to Bevacizumab Compared with Continued Bevacizumab Monotherapy. Retina 2015, 35, 1604–1614. [Google Scholar] [CrossRef]
- Zehden, J.A.; Mortensen, X.M.; Reddy, A.; Zhang, A.Y. Systemic and Ocular Adverse Events with Intravitreal Anti-VEGF Therapy Used in the Treatment of Diabetic Retinopathy: A Review. Curr. Diabetes Rep. 2022, 22, 525–536. [Google Scholar] [CrossRef]
- Wells, J.A.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Bressler, N.M.; Bressler, S.B.; Brucker, A.J.; Ferris, F.L.; Hampton, G.R.; Jhaveri, C.; et al. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema. Ophthalmology 2016, 123, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.Z.; Rather, P.A.; Samarah, S.M.; Elhusseiny, A.M.; Sallam, A.B. Current and Novel Therapeutic Approaches for Treatment of Diabetic Macular Edema. Cells 2022, 11, 1950. [Google Scholar] [CrossRef]
- Cai, X.; Zhao, J.; Dang, Y. Combination Therapy with Anti-VEGF and Intravitreal Dexamethasone Implant for Macular Edema Secondary to Retinal Vein Occlusion. Curr. Eye Res. 2024, 49, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Maboud, M.; Menshawy, E.; Bahbah, E.I.; Outani, O.; Menshawy, A. Intravitreal bevacizumab versus intravitreal triamcinolone for diabetic macular edema–Systematic review, meta-analysis and meta-regression. PLoS ONE 2021, 16, e0245010. [Google Scholar] [CrossRef]
- Mehta, H.; Hennings, C.; Gillies, M.C.; Nguyen, V.; Campain, A.; Fraser-Bell, S. Anti-vascular endothelial growth factor combined with intravitreal steroids for diabetic macular oedema. Cochrane Database Syst. Rev. 2018, CD011599. [Google Scholar] [CrossRef]
- Ghasemi Falavarjani, K.; Nguyen, Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye 2013, 27, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Nanji, K.; Sarohia, G.S.; Kennedy, K.; Ceyhan, T.; McKechnie, T.; Phillips, M.; Devji, T.; Thabane, L.; Kaiser, P.; Sarraf, D.; et al. The 12- and 24-Month Effects of Intravitreal Ranibizumab, Aflibercept, and Bevacizumab on Intraocular Pressure. Ophthalmology 2022, 129, 498–508. [Google Scholar] [CrossRef] [PubMed]






| Author; Ref. | Year | Location | Design | Intervention Model | Masking | Stochastic Approach | Etiology | Gender | Modules of Intervention | Sample Size | Outcome Reported | Follow-Up Duration (Month) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Petrovic [25] | 2023 | Serbia | Non-RCT | Dual-arm | NA | Non-randomized | Persistent DME | C: male/female 6/6 M: male/female 5/7 | C: IVA 2 mg/0.05 mL plus IVTA 10 mg/0.1 mL M: IVA 1.25 mg/0.05 mL | C: 12 M: 12 | BCVA, CMT, IOP, adverse reactions | 12 |
| Shahid [26] | 2022 | Pakistan | RCT | Dual-arm | Single-masked | Randomization | Persistent DME | Total: male/female 26/14 | C: IVB 1.25 mg/0.05 mL plus CLS-TA 2 mg/0.05 mL M: IVB 1.25 mg/0.05 mL | C: 20 M: 20 | CMT, adverse reactions | 3 |
| Limon, U. [27] | 2021 | Turkey | Non-RCT | Dual-arm | NA | Non-randomized | Persistent DME | C: male/female 12/17 M:male/female 14/16 | C: IVB 1.25 mg/0.05 mL plus DEX 0.7 mg M: IVB 1.25 mg/0.05 mL | C: 35 M: 30 | BCVA, CMT, IOP, adverse reactions | 3 |
| Chiu [28] | 2021 | China | Retrospective | Dual-arm | NA | NA | Persistent DME | C: male/female 10/13 M: male/female 14/6 | C: IVR 0.5 mg plus PSTA 40 mg M: IVR 0.5 mg | C: 23 M: 20 | BCVA, CMT, adverse reactions | 12 |
| Entezari [30] | 2019 | Iran | RCT | Dual-arm | Double-blind | Randomization | Persistent DME | C: male/female 8/11 M: male/female 5/10 | C: IVB 1.25 mg/0.05 mL plus EPO 1000 μg/0.05 mL M: IVB 1.25 mg/0.05 ml | C: 24 M: 24 | BCVA, CMT, adverse reactions | 6 |
| Eris [29] | 2019 | Turkey | Retrospective | Dual-arm | Open-label | Randomization | Persistent DME | NA | C: IVR 0.5 mg plus PSTA 40 mg M: IVR 0.5 mg | C: 38 M: 34 | BCVA, CMT, IOP, adverse reactions | 6 |
| Maturi [31] | 2018 | America | RCT | Dual-arm | Double-masked | Randomization | Persistent DME | C: male/female 34/31 M: male/female 28/36 | C: IVR 0.3 mg plus DEX 0.7 mg M: IVR 0.5 mg plus sham injection | C: 63 M: 64 | BCVA, CMT, adverse reactions | 6 |
| Maturi [34] | 2015 | America | RCT | Dual-arm | Single-masked | Randomization | Persistent DME | Total: male/female 13/17 | C: IVB 1.25 mg/0.05 mL plus DEX 0.7 mg M: IVB 1.25 mg/0.05 mL | C: 21 M: 19 | BCVA, CMT, adverse reactions | 12 |
| Shoeibi [33] | 2013 | Iran | RCT | Three-arm | Tri-blind | Randomization | Persistent DME | C: male/female 7/8 M: male/female 7/9 | C: IVB 1.25 mg/0.05 mL plus IVTA 2 mg/0.05 mL M: IVB 1.25 mg/0.05 mL | C: 41 M: 37 | BCVA, CMT, adverse reactions | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Tao, Y.; Yuan, M.; Sun, X. Anti-Vascular Endothelial Growth Factor Combined with Ocular Steroid Therapy for Persistent Diabetic Macular Edema: A Systematic Review and Meta-Analysis. Pharmaceuticals 2024, 17, 1574. https://doi.org/10.3390/ph17121574
Ma Y, Tao Y, Yuan M, Sun X. Anti-Vascular Endothelial Growth Factor Combined with Ocular Steroid Therapy for Persistent Diabetic Macular Edema: A Systematic Review and Meta-Analysis. Pharmaceuticals. 2024; 17(12):1574. https://doi.org/10.3390/ph17121574
Chicago/Turabian StyleMa, Yunxi, Yunhan Tao, Mingzhu Yuan, and Xufang Sun. 2024. "Anti-Vascular Endothelial Growth Factor Combined with Ocular Steroid Therapy for Persistent Diabetic Macular Edema: A Systematic Review and Meta-Analysis" Pharmaceuticals 17, no. 12: 1574. https://doi.org/10.3390/ph17121574
APA StyleMa, Y., Tao, Y., Yuan, M., & Sun, X. (2024). Anti-Vascular Endothelial Growth Factor Combined with Ocular Steroid Therapy for Persistent Diabetic Macular Edema: A Systematic Review and Meta-Analysis. Pharmaceuticals, 17(12), 1574. https://doi.org/10.3390/ph17121574







