Synthesis of Silver Nanoparticles and Gold Nanoparticles Used as Biosensors for the Detection of Human Serum Albumin-Diagnosed Kidney Disease
Abstract
1. Introduction
2. Results and Discussion
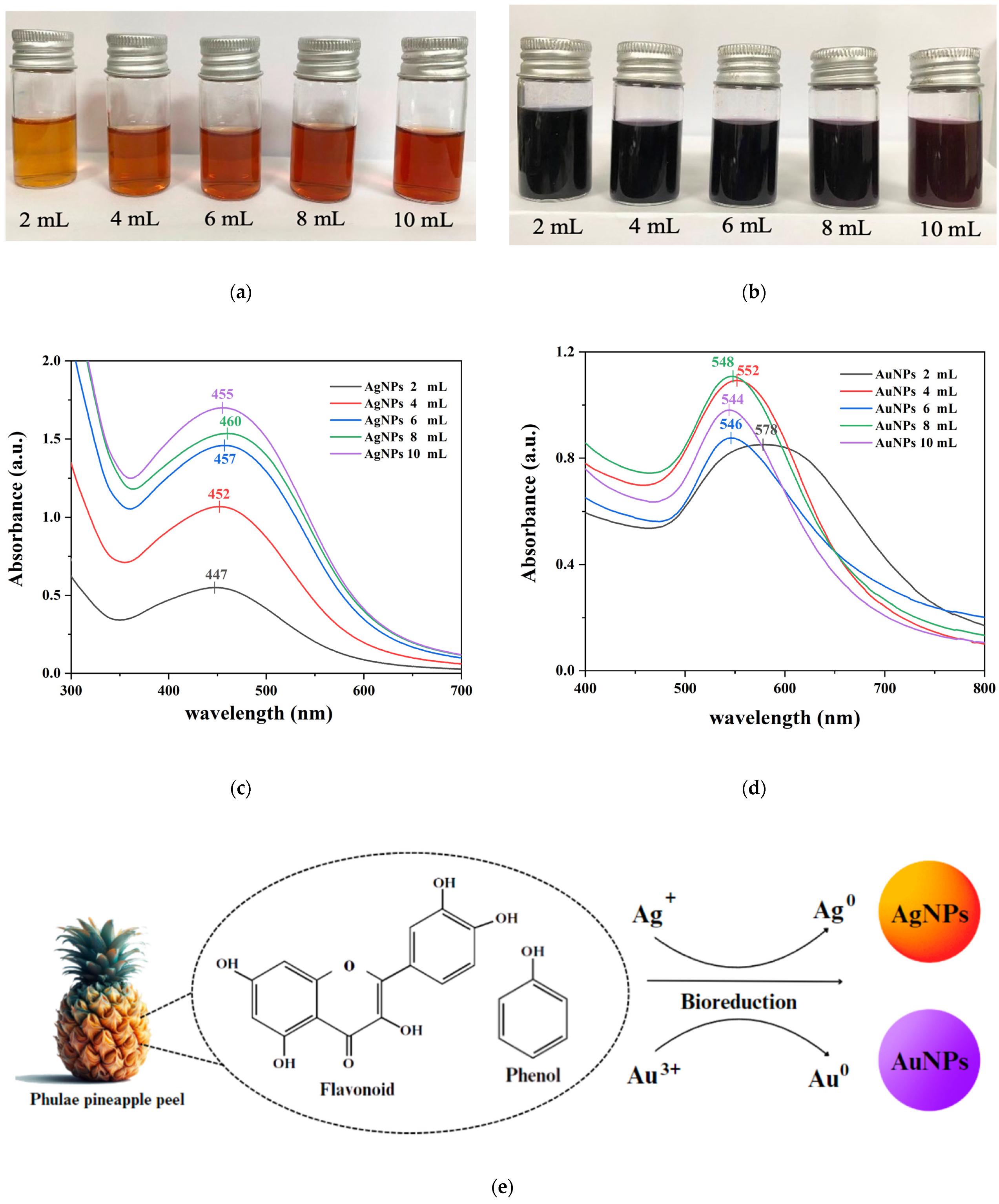
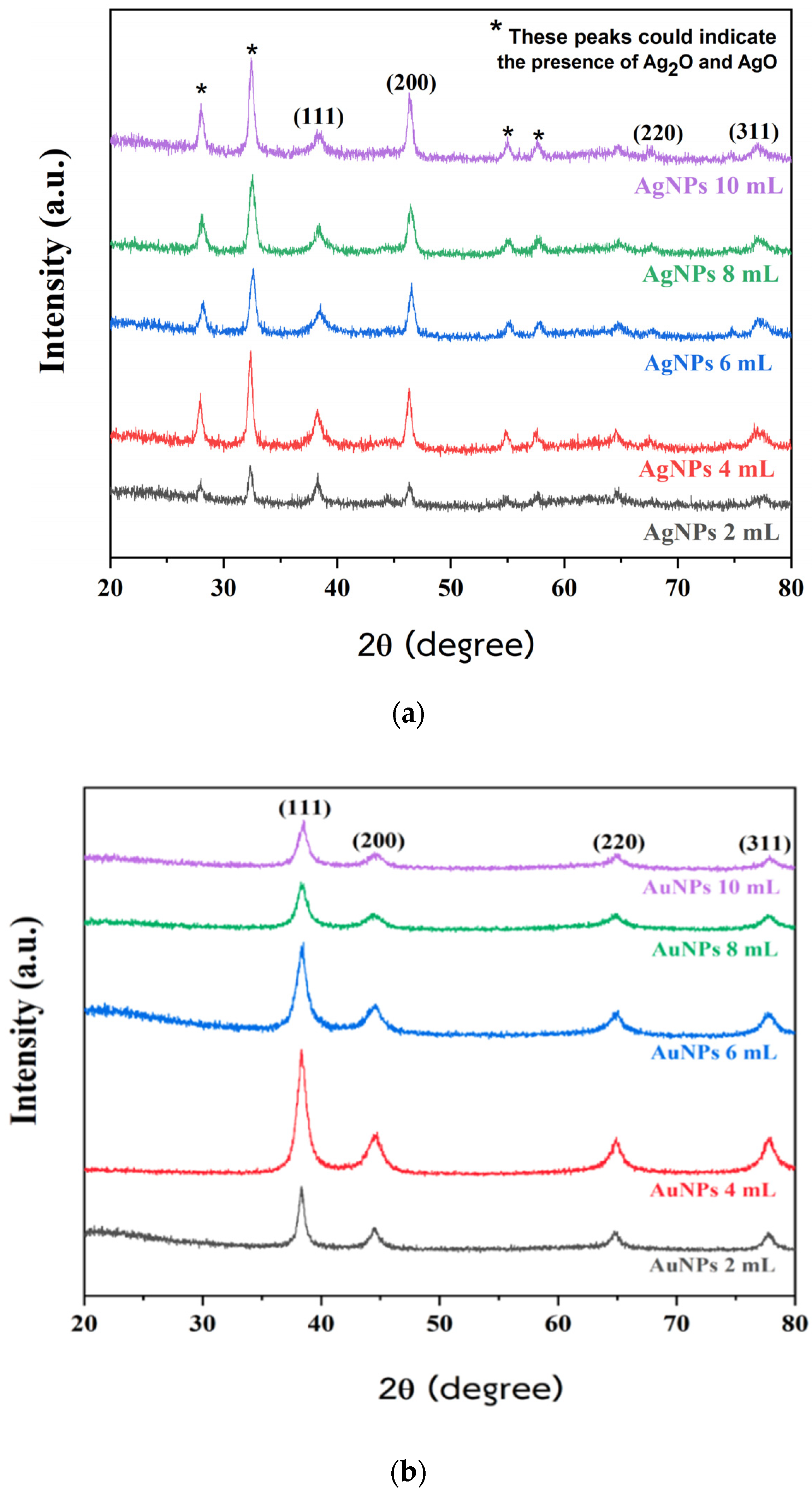
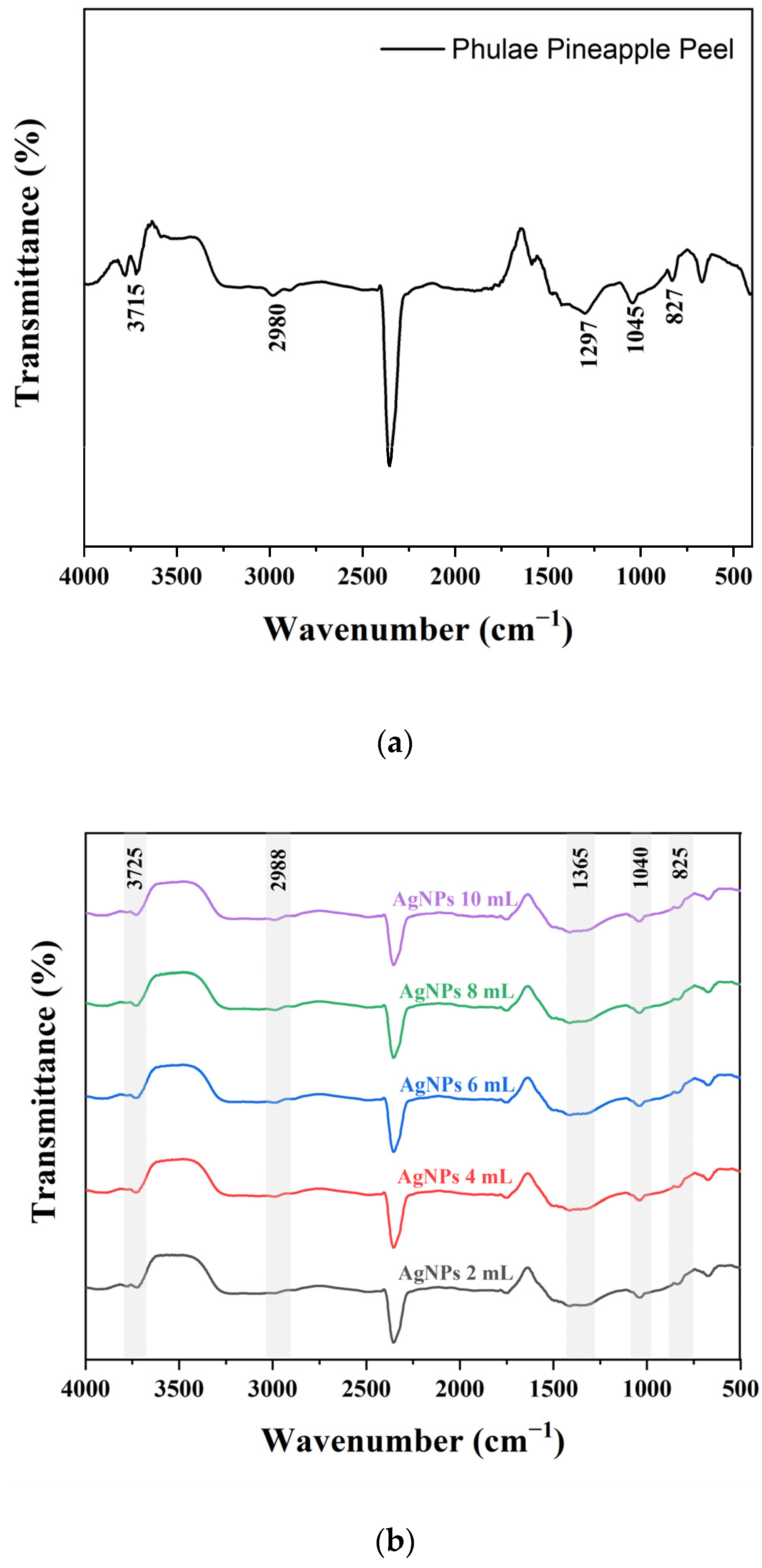
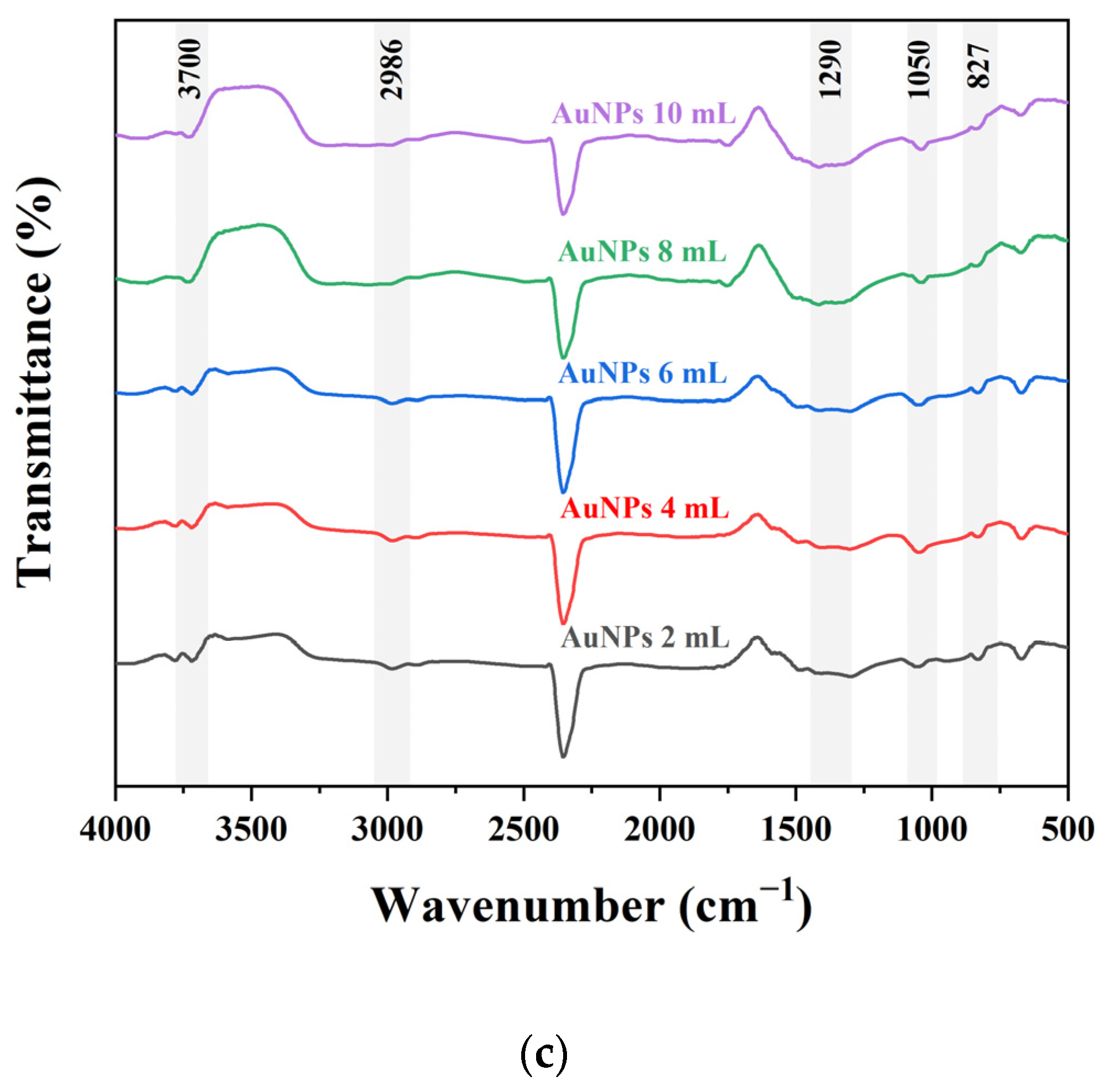
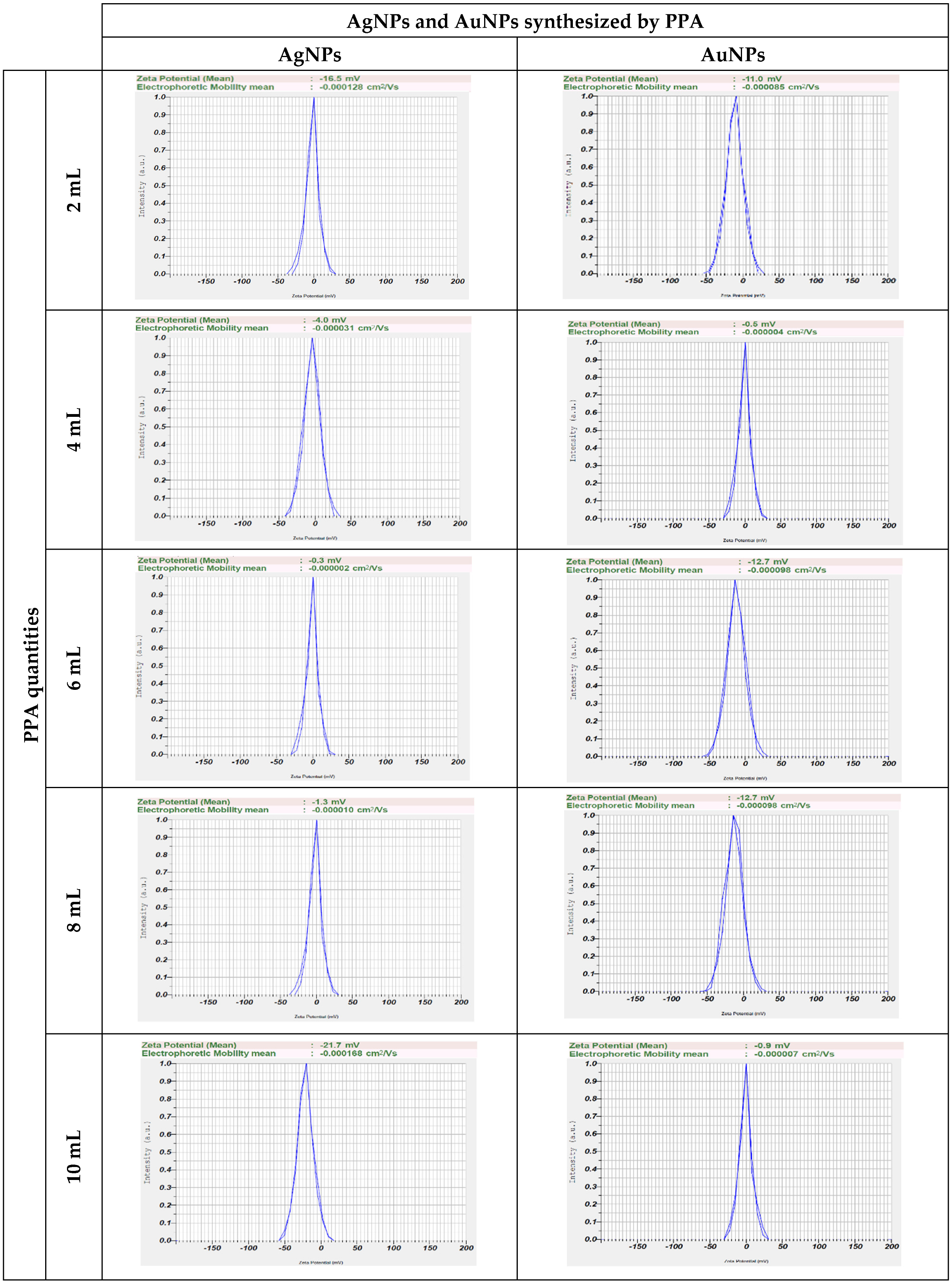
2.1. Characterization of AgNPs and AuNPs Synthesized Using PPA
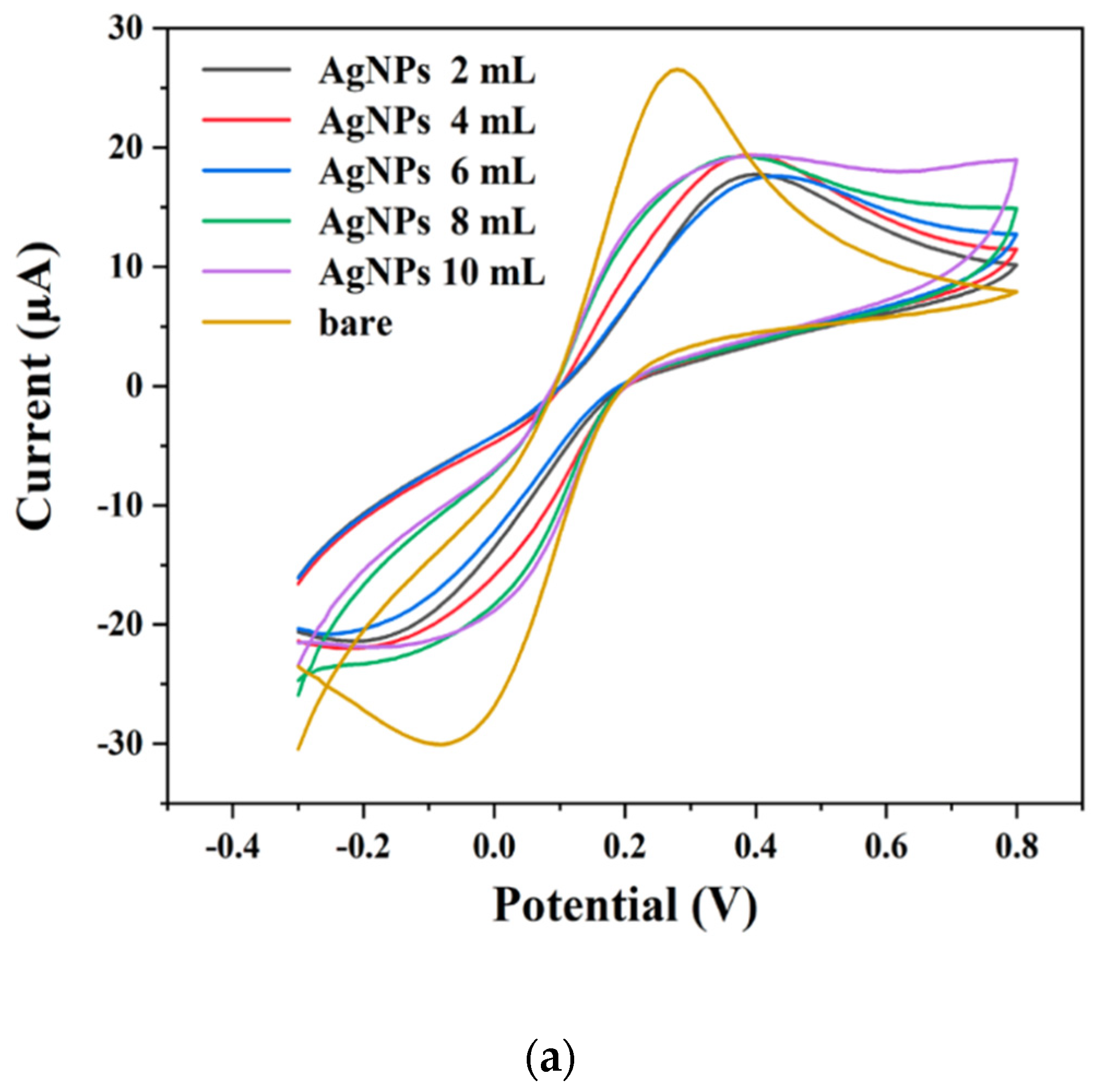
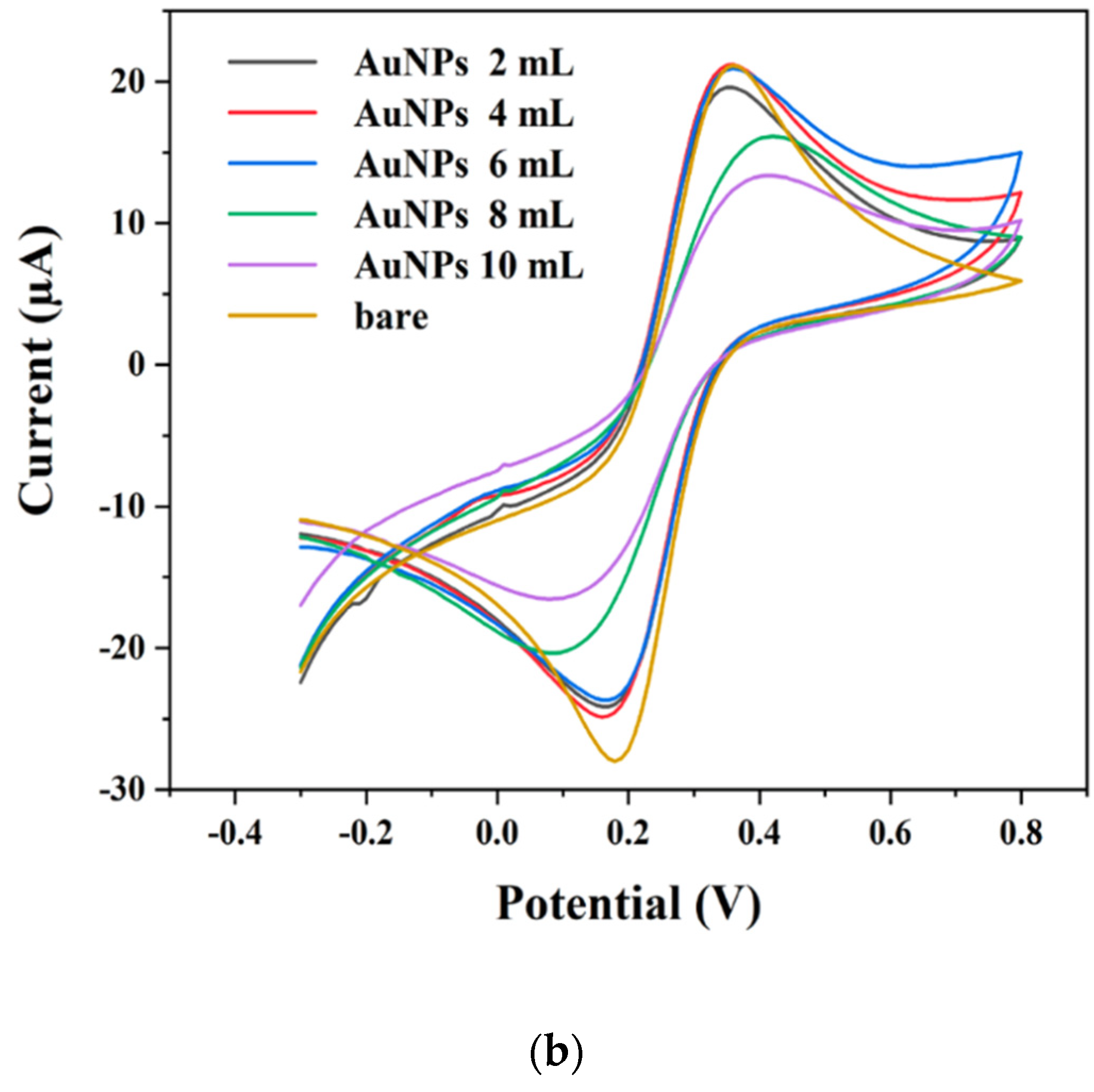
2.2. Electrochemical Properties of AgNPs and AuNPs Modified Electrodes
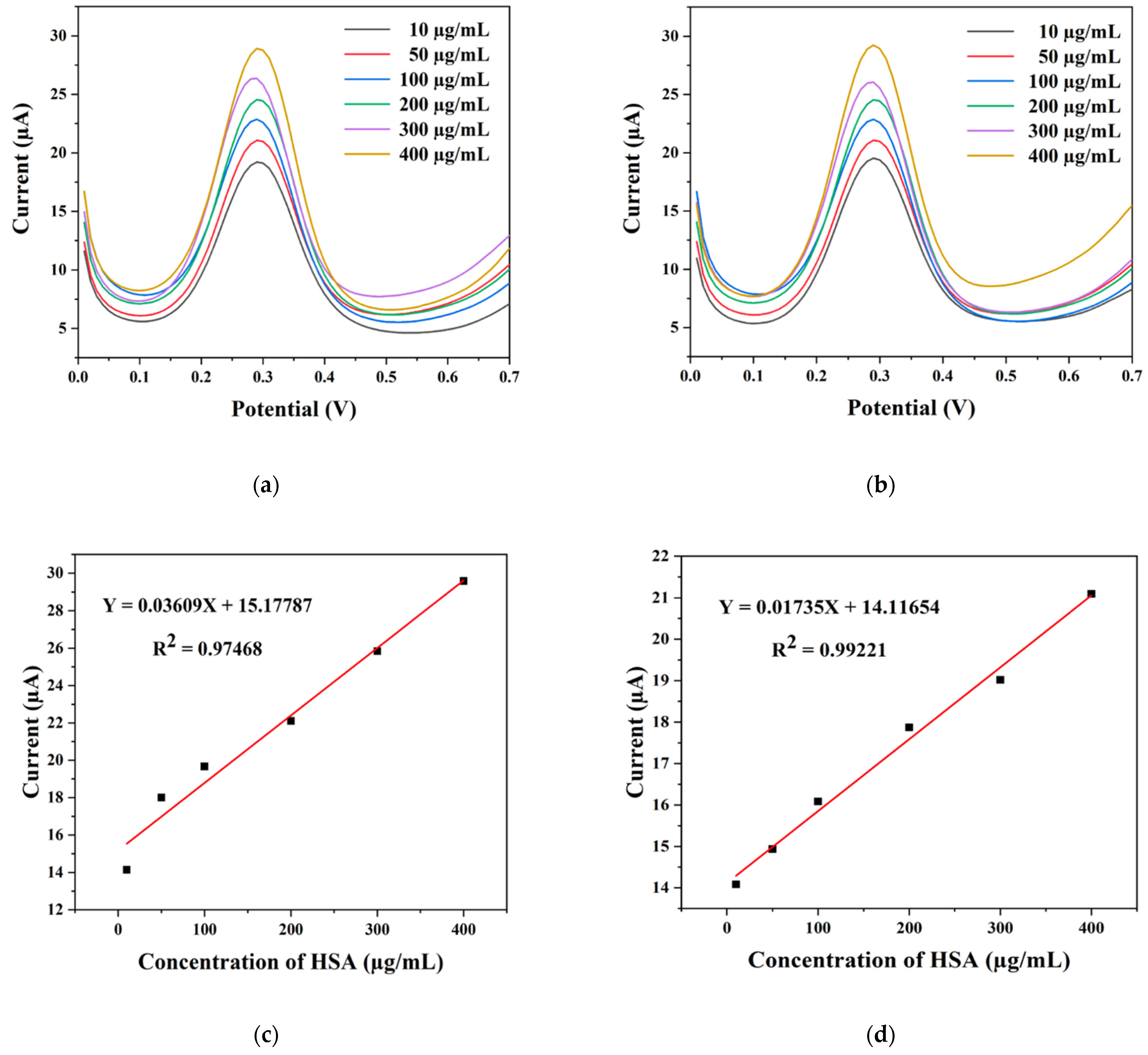
2.3. Electrochemical Detection of HSA
3. Materials and Methods
3.1. Materials
3.2. Preparation for Phulae Pineapple Peel Extract (PPA)
3.3. Synthesis of Nanoparticles
3.4. Characterization of Synthesized Nanoparticles
3.5. Measurements of Electrochemical Responses
3.6. Preparation of Electrodes Modified with Nanoparticles
3.7. Procedure for Detection of HSA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsai, J.Z.; Chen, C.J.; Settu, K.; Lin, Y.F.; Chen, C.L.; Liu, J.T. Screen-printed carbon electrode-based electrochemical immunosensor for rapid detection of microalbuminuria. Biosens. Bioelectron. 2016, 77, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Lin, C.Y. Electrochemically-functionalized CNT/ABTS nanozyme enabling sensitive and selective voltammetric detection of microalbuminuria. Anal. Chim. Acta 2022, 1197, 339517. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-C.; Ko, C.-H.; Lu, S.-Y.; Yang, C.-E.; Fu, L.-M.; Li, C.-Y. Rapid electrochemical-biosensor microchip platform for determination of microalbuminuria in CKD patients. Anal. Chim. Acta 2021, 1146, 70–76. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Ma, G.; Zhou, J.; Du, L.; Wu, H.; Zhang, X.; He, Y.; Zhou, J. TICT-based turn-on deep-red fluorescent probe for endoplasmic reticulum targeted detection of serum albumin in kidney diseases. Chem. Eng. J. 2023, 464, 142551. [Google Scholar] [CrossRef]
- Chawjiraphan, W.; Apiwat, C.; Segkhoonthod, K.; Treerattrakoon, K.; Pinpradup, P.; Sathirapongsasuti, N.; Pongprayoon, P.; Luksirikul, P.; Isarankura-Na-Ayudhya, P.; Japrung, D. Sensitive detection of albuminuria by graphene oxide-mediated fluorescence quenching aptasensor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 231, 118128. [Google Scholar] [CrossRef] [PubMed]
- Benesch, J.; Askendal, A.; Tengvall, P. Quantification of adsorbed human serum albumin at solid interfaces: A comparison between radioimmunoassay (RIA) and simple null ellipsometry. Colloids Surf. B Biointerfaces 2000, 18, 71–81. [Google Scholar] [CrossRef]
- Magliano, D.J.; Polkinghorne, K.R.; Barr, E.L.M.; Su, Q.; Chadban, S.J.; Zimmet, P.Z.; Shaw, J.E.; Atkins, R.C. HPLC-detected albuminuria predicts mortality. J. Am. Soc. Nephrol. 2007, 18, 3171–3176. [Google Scholar] [CrossRef]
- Shi, M.; Duan, X.; Zheng, X.; Lu, D.; Ge, Y.; Zhang, N.; Liu, Y.; You, J.; Xue, H.; Yin, L. Quantification of human serum albumin by combining chymotrypsin/trypsin digestion coupled with LC-MS/MS technique. Anal. Biochem. 2023, 680, 115316. [Google Scholar] [CrossRef] [PubMed]
- Brede, C.; Hop, B.; Jørgensen, K.; Skadberg, Ø. Measurement of glycated albumin in serum and plasma by LC-MS/MS. Scand. J. Clin. Lab. Investig. 2016, 76, 195–201. [Google Scholar] [CrossRef]
- Markó, L.; Molnár, G.A.; Wagner, Z.; Koszegi, T.; Matus, Z.; Mohás, M.; Kuzma, M.; Szijártó, I.A.; Wittmann, I. Analysis of microalbuminuria with immunonephelometry and high performance liquid chromatography. Evaluation of new criteria. Orvosi Hetil. 2008, 149, 59–67. [Google Scholar] [CrossRef]
- Jiang, Z.; Huang, Y.; Liang, A.; Pan, H.; Liu, Q. Resonance scattering detection of trace microalbumin using immunonanogold probe as the catalyst of Fehling reagent–glucose reaction. Biosens. Bioelectron. 2009, 24, 1674–1678. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.-Y.; Chang, C.-J.; Wang, C.-F.; Lu, C.-H.; Chen, J.-K. Controlled antibody orientation on Fe3O4 nanoparticles and CdTe quantum dots enhanced sensitivity of a sandwich-structured electrogenerated chemiluminescence immunosensor for the determination of human serum albumin. Sens. Actuators B Chem. 2021, 336, 129710. [Google Scholar] [CrossRef]
- Murtaza, G.; Rizvi, A.S.; Irfan, M.; Li, L.; Qu, F. Determination of glycated albumin in serum and saliva by capillary electrophoresis utilizing affinity of 3-acrylamido phenylboronic acid selected by virtual screening and molecular docking. J. Chromatogr. A 2021, 1636, 461793. [Google Scholar] [CrossRef] [PubMed]
- Saeed, U.; Fatima, B.; Hussain, D.; Ashiq, R.; Naeem Ashiq, M.; Najam-ul-Haq, M. CoTe nanorods based electrochemical sensor for quantitative detection of albumin from chronic kidney disease patients. J. Electroanal. Chem. 2022, 906, 115999. [Google Scholar] [CrossRef]
- Fatoni, A.; Numnuam, A.; Kanatharana, P.; Limbut, W.; Thavarungkul, P. A novel molecularly imprinted chitosan-acrylamide, graphene, ferrocene composite cryogel biosensor used to detect microalbumin. Analyst 2014, 139, 6160–6167. [Google Scholar] [CrossRef]
- Tayyab Raza Naqvi, S.; Rasheed, T.; Naeem Ashiq, M.; Najam ul Haq, M.; Majeed, S.; Fatima, B.; Nawaz, R.; Hussain, D.; Shafi, S. Fabrication of iron modified screen printed carbon electrode for sensing of amino acids. Polyhedron 2020, 180, 114426. [Google Scholar] [CrossRef]
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M. Screen-printed electrodes for biosensing: A review (2008–2013). Microchim. Acta 2014, 181, 865–891. [Google Scholar] [CrossRef]
- Stanković, V.; Đurđić, S.; Ognjanović, M.; Antić, B.; Kalcher, K.; Mutić, J.; Stanković, D.M. Anti-human albumin monoclonal antibody immobilized on EDC-NHS functionalized carboxylic graphene/AuNPs composite as promising electrochemical HSA immunosensor. J. Electroanal. Chem. 2020, 860, 113928. [Google Scholar] [CrossRef]
- Shaikh, M.O.; Srikanth, B.; Zhu, P.-Y.; Chuang, C.-H. Impedimetric Immunosensor Utilizing Polyaniline/Gold Nanocomposite-Modified Screen-Printed Electrodes for Early Detection of Chronic Kidney Disease. Sensors 2019, 19, 3990. [Google Scholar] [CrossRef]
- Omidfar, K.; Dehdast, A.; Zarei, H.; Sourkohi, B.K.; Larijani, B. Development of urinary albumin immunosensor based on colloidal AuNP and PVA. Biosens. Bioelectron. 2011, 26, 4177–4183. [Google Scholar] [CrossRef]
- Ghosh Dastidar, M.; Murugappan, K.; RNisbet, D.; Tricoli, A. Simultaneous electrochemical detection of glycated and human serum albumin for diabetes management. Biosens. Bioelectron. 2024, 246, 115876. [Google Scholar] [CrossRef] [PubMed]
- Choosang, J.; Thavarungkul, P.; Kanatharana, P.; Numnuam, A. AuNPs/PpPD/PEDOT:PSS-Fc modified screen-printed carbon electrode label-free immunosensor for sensitive and selective determination of human serum albumin. Microchem. J. 2020, 155, 104709. [Google Scholar] [CrossRef]
- Lai, S.-Y.; Liu, J.-T.; Chen, C.-J.; Tsai, J.-Z. Detecting human serum albumin using screen-printed carbon electrode by cyclic voltammetry. J. Microbiol. Immunol. Infect. 2015, 48 (Suppl. 1), S82. [Google Scholar] [CrossRef]
- Shaikh, M.O.; Zhu, P.Y.; Wang, C.C.; Du, Y.C.; Chuang, C.H. Electrochemical immunosensor utilizing electrodeposited Au nanocrystals and dielectrophoretically trapped PS/Ag/ab-HSA nanoprobes for detection of microalbuminuria at point of care. Biosens. Bioelectron. 2019, 126, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yu, Y.; Guo, M.; Lin, B.; Zhang, L. A sensitive determination of albumin in urine by molecularly imprinted electrochemical biosensor based on dual-signal strategy. Sens. Actuators B Chem. 2019, 288, 564–570. [Google Scholar] [CrossRef]
- Smith, B.R.; Gambhir, S.S. Nanomaterials for In Vivo Imaging. Chem. Rev. 2017, 117, 901–986. [Google Scholar] [CrossRef]
- Mahobiya, S.K.; Balayan, S.; Chauhan, N.; Rosario, W.; Kuchhal, N.K.; Islam, S.S.; Jain, U. Fabricating a rapid and low-cost electrochemical biosensor with imprints of glycated albumin molecules to detect diabetes using bimetallic Au-Pt nanoparticles on μSPE. Appl. Surf. Sci. Adv. 2023, 16, 100425. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomedicine 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Nande, A.; Raut, S.; Michalska-Domanska, M.; Dhoble, S.J. Green Synthesis of Nanomaterials Using Plant Extract: A Review. Curr. Pharm. Biotechnol. 2021, 22, 1794–1811. [Google Scholar]
- Alabdallah, N.M.; Hasan, M.M. Plant-based green synthesis of silver nanoparticles and its effective role in abiotic stress tolerance in crop plants. Saudi J. Biol. Sci. 2021, 28, 5631–5639. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Félix, F.; Graciano-Verdugo, A.Z.; Moreno-Vásquez, M.J.; Lagarda-Díaz, I.; Barreras-Urbina, C.G.; Armenta-Villegas, L.; Olguín-Moreno, A.; Tapia-Hernández, J.A. Trends in Sustainable Green Synthesis of Silver Nanoparticles Using Agri-Food Waste Extracts and Their Applications in Health. J. Nanomater. 2022, 2022, 8874003. [Google Scholar] [CrossRef]
- Nadaf, S.J.; Jadhav, N.R.; Naikwadi, H.S.; Savekar, P.L.; Sapkal, I.D.; Kambli, M.M.; Desai, I.A. Green synthesis of gold and silver nanoparticles: Updates on research, patents, and future prospects. OpenNano 2022, 8, 100076. [Google Scholar] [CrossRef]
- Balkrishna, A.; Rohela, A.; Kumar, A.; Mishra, S.; Arya, V.; Kala, V.; Thakur, N.; Thakur, N.; Kumari, A.; Khan, N. Elucidating the Role of Plant Extracts Mediated Gold Nanoparticles as Smart Antimicrobials: Two-Way Attack. J. Nanomater. 2023, 2023, 4085090. [Google Scholar] [CrossRef]
- Ashique, S.; Afzal, O.; Khalid, M.; Faruque Ahmad, M.; Upadhyay, A.; Kumar, S.; Garg, A.; Ramzan, M.; Hussain, A.; Altamimi, M.A.; et al. Biogenic nanoparticles from waste fruit peels: Synthesis, applications, challenges and future perspectives. Int. J. Pharm. 2023, 643, 123223. [Google Scholar]
- Thu, S.L.; Tam, H.L.M.; Tongdeesoontorn, W.; Suthiluk, P. Quality changes and volatile compounds in fresh-cut ‘phulae’ pineapple during cold storage. Curr. Appl. Sci. Technol. 2017, 17, 162–171. [Google Scholar]
- Techavuthiporn, C.; Boonyaritthongchai, P.; Supabvanich, S. Physicochemical changes of ‘Phulae’ pineapple fruit treated with short-term anoxia during ambient storage. Food Chem. 2017, 228, 388–393. [Google Scholar] [CrossRef]
- Mahardika, D.P.; Yusrita, E.; Surya, A.; Juariah, S. Phytogenic silver nanoparticle (AgNP) from Ananas comosus (L) Merr. peel extract to inhibiting the pathogen resistance. J. Phys. Conf. Ser. 2021, 1811, 012124. [Google Scholar] [CrossRef]
- Agnihotri, S.; Sillu, D.; Sharma, G.; Arya, R.K. Photocatalytic and antibacterial potential of silver nanoparticles derived from pineapple waste: Process optimization and modeling kinetics for dye removal. Appl. Nanosci. 2018, 8, 2077–2092. [Google Scholar] [CrossRef]
- Das, G.; Patra, J.K.; Debnath, T.; Ansari, A.; Shin, H.S. Investigation of antioxidant, antibacterial, antidiabetic, and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus (L.). PLoS ONE 2019, 14, e0220950. [Google Scholar] [CrossRef]
- Basavegowda, N.; Sobczak-Kupiec, A.; Malina, D.; Yathirajan, H.S.; Keerthi, V.R.; Chandrashekar, N.; Dinkar, S.; Liny, P. Plant Mediated Synthesis of Gold Nanoparticles Using Fruit Extracts of Ananas comosus (L.) (Pineapple) And Evaluation of Biological Activities. Adv. Mater. Lett. 2013, 4, 332–337. [Google Scholar] [CrossRef]
- Hamdiani, S.; Shih, Y.-F. A Green Method for Synthesis of Silver-Nanoparticles-Diatomite (AgNPs-D) Composite from Pineapple (Ananas comosus) Leaf Extract. Indones. J. Chem. 2021, 21, 740. [Google Scholar] [CrossRef]
- Huq, M.; Ashrafudoulla, M.; Rahman, M.M.; Balusamy, S.; Akter, S. Green Synthesis and Potential Antibacterial Applications of Bioactive Silver Nanoparticles: A Review. Polymers 2022, 14, 742. [Google Scholar] [CrossRef]
- Ahmad, N. Green Synthesis of Silver Nanoparticles Using Extracts of Ananas comosus. Green Sustain. Chem. 2012, 02, 141–147. [Google Scholar] [CrossRef]
- Pechyen, C.; Ponsanti, K.; Tangnorawich, B.; Ngernyuang, N. Waste fruit peel—Mediated green synthesis of biocompatible gold nanoparticles. J. Mater. Res. Technol. 2021, 14, 2982–2991. [Google Scholar] [CrossRef]
- Alikhani, N.; Hekmati, M.; Karmakar, B.; Veisi, H. Green synthesis of gold nanoparticles (Au NPs) using Rosa canina fruit extractand evaluation of its catalytic activity in the degradation of organic dye pollutants of water. Inorg. Chem. Commun. 2022, 139, 109351. [Google Scholar] [CrossRef]
- Kumari, P.; Meena, A. Green synthesis of gold nanoparticles from Lawsoniainermis and its catalytic activities following the Langmuir-Hinshelwood mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125447. [Google Scholar] [CrossRef]
- Jaffar, S.S.; Saallah, S.; Misson, M.; Siddiquee, S.; Roslan, J.; Lenggoro, W. Green Synthesis of Flower-Like Carrageenan-Silver Nanoparticles and Elucidation of Its Physicochemical and Antibacterial Properties. Molecules 2023, 28, 907. [Google Scholar] [CrossRef]
- Ning, T.; Luo, Y.; Liu, P.; Lu, A. A novel Ag nanoparticles purification method and the conductive ink based on the purified Ag nanoparticles for printed electronics. J. Nanoparticle Res. 2022, 24, 15. [Google Scholar] [CrossRef]
- Vamshi, B.S.; Sharma, V.; Ahmad, W.; Kumar, V.; Sharma, S.; Joshi, N.C.; Hussain, A.; Kohli, D.; Shabaaz Begum, J.P.; Kumar, S. Green Synthesis of Silver Nanoparticles Generated by the Photoionization Process for Anti-biofilm Application. Catal. Lett. 2023, 154, 2151–2161. [Google Scholar] [CrossRef]
- Alharbi, N.; Bhakyaraj, K.; Gopinath, K.; Govindarajan, M.; Kumaraguru, S.; Mohan, S.; Periyannan, K.; Km, S.; Khaled, J.; Benelli, G. Gum-Mediated Fabrication of Eco-Friendly Gold Nanoparticles Promoting Cell Division and Pollen Germination in Plant Cells. J. Clust. Sci. 2017, 28, 507–517. [Google Scholar] [CrossRef]
- Gautam, P.; Kumar, S.; Singh, R.; Shyanti, R.; Swaroop, S.; Ram, B.; Chauhan, A. Biologically Synthesized Gold Nanoparticles using Ocimum sanctum (Tulsi Leaf Extract) Induced Anti-Tumor Response in a T Cell Daltons Lymphoma. J. Cell Sci. Ther. 2017, 8, 6. [Google Scholar] [CrossRef]
- Rautela, A.; Rani, J.; Debnath, M. Green synthesis of silver nanoparticles from Tectona grandis seeds extract: Characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol. 2019, 10, 5. [Google Scholar] [CrossRef]
- Willian, N.; Syukri, S.; Zulhadjri, Z.; Pardi, H.; Arief, S. Marine plant mediated green synthesis of silver nanoparticles using mangrove Rhizophora stylosa: Effect of variable process and their antibacterial activity. F1000Res 2021, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Yadav, D.; Mitra, S.; Mukhopadhyay, K. Biosynthesis of Silver Nanoparticles Using Culture Supernatant of Shewanella sp. ARY1 and Their Antibacterial Activity. Int. J. Nanomed. 2020, 15, 8295–8310. [Google Scholar] [CrossRef]
- Yang, H.; Ren, Y.-y.; Wang, T.; Wang, C. Preparation and antibacterial activities of Ag/Ag+/Ag3+ nanoparticle composites made by pomegranate (Punica granatum) rind extract. Results Phys. 2016, 6, 299–304. [Google Scholar] [CrossRef]
- Poadang, S.; Yongvanich, N.; Phongtongpasuk, S. Synthesis, Characterization, and Antibacterial Properties of Silver Nanoparticles Prepared from Aqueous Peel Extract of Pineapple, Ananas comosus. Chiang Mai Univ. J. Nat. Sci. 2017, 16, 2. [Google Scholar] [CrossRef]
- Tadesse, M.; Kasaw, E.; Lübben, J. Valorization of Banana Peel Using Carbonization: Potential Use in the Sustainable Manufacturing of Flexible Supercapacitors. Micromachines 2023, 14, 330. [Google Scholar] [CrossRef]
- El-Nafaty, U.; Misau, I.; Abdulsalam, S. Biosorption and Kinetic Studies on Oil Removal from Produced Water Using Banana Peel. Civ. Environ. Res. 2013, 3, 125–136. [Google Scholar]
- Klinbumrung, A.; Panya, R.; Pung-Ngama, A.; Nasomjai, P.; Saowalakmeka, J.; Sirirak, R. Green synthesis of ZnO nanoparticles by pineapple peel extract from various alkali sources. J. Asian Ceram. Soc. 2022, 10, 755–765. [Google Scholar] [CrossRef]
- Rosales, E.; Escudero-Curiel, S.; Pazos, M.; Sanromán, M. Sustainable Removal of Cr(VI) by Lime Peel and Pineapple Core Wastes. Appl. Sci. 2019, 9, 1967. [Google Scholar] [CrossRef]
- Machmudah, S.; Shiddiqi, Q.; Karisma, A.; Widiyastuti, W.; Diono, W.; Kanda, H.; Winardi, S.; Goto, M. Subcritical Water Extraction of Xanthone from Mangosteen (Garcinia mangostana Linn) Pericarp. J. Adv. Chem. Eng. 2015, 5, 1000117. [Google Scholar] [CrossRef]
- Sengar, A.S.; Sunil, C.K.; Rawson, A.; Venkatachalapathy, N. Identification of volatile compounds, physicochemical and techno-functional properties of pineapple processing waste (PPW). J. Food Meas. Charact. 2022, 16, 1146–1158. [Google Scholar] [CrossRef]
- Raut, R.W.; Mendhulkar, V.D.; Kashid, S.B. Photosensitized synthesis of silver nanoparticles using Withania somnifera leaf powder and silver nitrate. J. Photochem. Photobiol. B Biol. 2014, 132, 45–55. [Google Scholar] [CrossRef]
- Pechyen, C.; Ponsanti, K.; Tangnorawich, B.; Ngernyuang, N. Biogenic synthesis of gold nanoparticles mediated by Spondias dulcis (Anacardiaceae) peel extract and its cytotoxic activity in human breast cancer cell. Toxicol. Rep. 2022, 9, 1092–1098. [Google Scholar] [CrossRef]
- Le Nhat Trang, N.; Thi Nguyet Nga, D.; Hoang, V.T.; Ngo, X.D.; Tuyet Nhung, P.; Le, A.T. Bio-AgNPs-based electrochemical nanosensors for the sensitive determination of 4-nitrophenol in tomato samples: The roles of natural plant extracts in physicochemical parameters and sensing performance. RSC Adv. 2022, 12, 6007–6017. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongwattana, T.; Chaiyo, R.; Ponsanti, K.; Tangnorawich, B.; Pratumpong, P.; Toommee, S.; Jenjob, R.; Yang, S.-G.; Parcharoen, Y.; Natphopsuk, S.; et al. Synthesis of Silver Nanoparticles and Gold Nanoparticles Used as Biosensors for the Detection of Human Serum Albumin-Diagnosed Kidney Disease. Pharmaceuticals 2024, 17, 1421. https://doi.org/10.3390/ph17111421
Thongwattana T, Chaiyo R, Ponsanti K, Tangnorawich B, Pratumpong P, Toommee S, Jenjob R, Yang S-G, Parcharoen Y, Natphopsuk S, et al. Synthesis of Silver Nanoparticles and Gold Nanoparticles Used as Biosensors for the Detection of Human Serum Albumin-Diagnosed Kidney Disease. Pharmaceuticals. 2024; 17(11):1421. https://doi.org/10.3390/ph17111421
Chicago/Turabian StyleThongwattana, Tiarpa, Ronnakorn Chaiyo, Khanittha Ponsanti, Benchamaporn Tangnorawich, Patcharee Pratumpong, Surachet Toommee, Ratchapol Jenjob, Su-Geun Yang, Yardnapar Parcharoen, Sitakan Natphopsuk, and et al. 2024. "Synthesis of Silver Nanoparticles and Gold Nanoparticles Used as Biosensors for the Detection of Human Serum Albumin-Diagnosed Kidney Disease" Pharmaceuticals 17, no. 11: 1421. https://doi.org/10.3390/ph17111421
APA StyleThongwattana, T., Chaiyo, R., Ponsanti, K., Tangnorawich, B., Pratumpong, P., Toommee, S., Jenjob, R., Yang, S.-G., Parcharoen, Y., Natphopsuk, S., & Pechyen, C. (2024). Synthesis of Silver Nanoparticles and Gold Nanoparticles Used as Biosensors for the Detection of Human Serum Albumin-Diagnosed Kidney Disease. Pharmaceuticals, 17(11), 1421. https://doi.org/10.3390/ph17111421






