Vesicle-Transported Multidrug Resistance as a Possible Therapeutic Target of Natural Compounds
Abstract
1. Introduction
2. Results
2.1. Multidrug-Resistant Cells Produce More EVs than Their Sensitive Cellular Counterparts
2.2. MDR Cells Are Able to Carry Out Horizontal Transfer of P-gp through the Release of EVs onto Sensitive Cells
2.3. Natural Compounds Are Able to Reverte Resistance Acquisition via Vesicles
2.4. P-gp Transfer Occurs in 3D Breast Cancer Model
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture
4.2. Extracellular Vesicle Isolation
4.3. Immunofluorescence Analysis
4.4. Western Blotting Analysis
4.5. Acridine Orange Staining
4.6. Nanoparticle Tracking Analysis (NTA)
4.7. Cell Culture with EVs
4.8. Cell Culture in Conditioned Medium
4.9. Cell Co-Culture
4.10. Evaluation of the Reversal of Acquired Resistance
4.11. Cancer Spheroid Culture and Immunofluorescence
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- To, K.K.W.; Huang, Z.; Zhang, H.; Ashby, C.R., Jr.; Fu, L. Utilizing non-coding RNA-mediated regulation of ATP binding cassette (ABC) transporters to overcome multidrug resistance to cancer chemotherapy. Drug Resist. Updates 2024, 73, 101058. [Google Scholar] [CrossRef] [PubMed]
- Assaraf, Y.G.; Brozovic, A.; Gonçalves, A.C.; Jurkovicova, D.; Linē, A.; Machuqueiro, M.; Saponara, S.; Sarmento-Ribeiro, A.B.; Xavier, C.P.R.; Vasconcelos, M.H. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist. Updates 2019, 46, 100645. [Google Scholar] [CrossRef] [PubMed]
- Nussinov, R.; Tsai, C.J.; Jang, H. Anticancer drug resistance: An update and perspective. Drug Resist. Updates 2021, 59, 100796. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fletcher, J.I.; Williams, R.T.; Henderson, M.J.; Norris, M.D.; Haber, M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist. Updates 2016, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- da Silveira Júnior, L.S.; Soares, V.L.; da Silva, A.S.J.; Gil, E.A.; Araújo, M.d.G.P.d.; Gonçalves, C.A.M.; Paiva, A.d.S.; de Oliveira, T.M.M.; Oliveira, G.H.d.M.; e Silva, D.G.K.C.; et al. P-glycoprotein and multidrug resistance-associated protein-1 expression in acute myeloid leukemia: Biological and prognosis implications. Int. J. Lab. Hematol. 2020, 42, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Mandić, D.; Nežić, L.; Amdžić, L.; Vojinović, N.; Gajanin, R.; Popović, M.; Đeri, J.; Balint, M.T.; Dumanović, J.; Milovanović, Z.; et al. Overexpression of MRP1/ABCC1, Survivin and BCRP/ABCC2 predicts the resistance of diffuse large B-cell lymphoma to R-CHOP Treatment. Cancers 2023, 15, 4106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pote, M.S.; Gacche, R.N. ATP-binding cassette efflux transporters and MDR in cancer. Drug Discov. Today 2023, 28, 103537. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.; Caja, S.; Strano Moraes, M.C.; Couto, N.; Costa-Silva, B. Exosome-based cell-cell communication in the tumor microenvironment. Front. Cell Dev. Biol. 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Namee, N.M.; O’Driscoll, L. Extracellular vesicles and anti-cancer drug resistance. Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiao, D.; Chen, L.; Xu, M.; Chen, S.; Huang, L.; Wang, F.; Chen, Z.; Cai, J.; Fu, L. Chemotherapeutic drugs stimulate the release and recycling of extracellular vesicles to assist cancer cells in developing an urgent chemoresistance. Mol. Cancer 2019, 18, 182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Osteikoetxea, X.; Benke, M.; Rodriguez, M.; Pálóczi, K.; Sódar, B.W.; Szvicsek, Z.; Szabó-Taylor, K.; Vukman, K.V.; Kittel, Á.; Wiener, Z.; et al. Detection and proteomic characterization of extracellular vesicles in human pancreatic juice. Biochem. Biophys. Res. Commun. 2018, 499, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, C.; Rani, S.; O’Brien, K.; O’Neill, A.; Prencipe, M.; Sheikh, R.; Webb, G.; McDermott, R.; Watson, W.; Crown, J.; et al. Docetaxel-resistance in prostate cancer: Evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS ONE 2012, 7, e50999. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barzegar, M.; Allahbakhshian Farsan, M.; Amiri, V.; Mohammadi, S.; Shahsavan, S.; Mirzaeian, A.; HosseinMohammadi, M. AML-derived extracellular vesicles confer de novo chemoresistance to leukemic myeloblast cells by promoting drug export genes expression and ROS inhibition. Iran. J. Pharm. Res. 2021, 20, 384–397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xavier, C.P.R.; Belisario, D.C.; Rebelo, R.; Assaraf, Y.G.; Giovannetti, E.; Kopecka, J.; Vasconcelos, M.H. The role of extracellular vesicles in the transfer of drug resistance competences to cancer cells. Drug Resist. Updates 2022, 62, 100833. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, V.V.; Svistunov, A.A.; Chubarev, V.N.; Dostdar, S.A.; Sokolov, A.V.; Brzecka, A.; Sukocheva, O.; Neganova, M.E.; Klochkov, S.G.; Somasundaram, S.G.; et al. Extracellular vesicles in cancer nanomedicine. Semin. Cancer Biol. 2021, 69, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Shin, K.J.; Chae, Y.C. Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer. Exp. Mol. Med. 2024, 56, 877–889. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levchenko, A.; Mehta, B.M.; Niu, X.; Kang, G.; Villafania, L.; Way, D.; Polycarpe, D.; Sadelain, M.; Larson, S.M. Intercellular transfer of P-glycoprotein mediates acquired multidrug resistance in tumor cells. Proc. Natl. Acad. Sci. USA 2005, 102, 1933–1938. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bebawy, M.; Combes, V.; Lee, E.; Jaiswal, R.; Gong, J.; Bonhoure, A.; Grau, G.E.R. Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells. Leukemia 2009, 23, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Bai, X.; Ni, J.; Zhang, H.; Duan, W.; Graham, P.; Li, Y. Exosomes and breast cancer drug resistance. Cell Death Dis. 2020, 11, 987. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sousa, D.; Lima, R.T.; Vasconcelos, M.H. Intercellular transfer of cancer drug resistance traits by extracellular vesicles. Trends Mol. Med. 2015, 21, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Cai, Y.; He, D.; Zou, C.; Zhang, P.; Lo, C.Y.; Xu, Z.; Chan, F.L.; Yu, S.; Chen, Y.; et al. Transient receptor potential channel TRPC5 is essential for P-glycoprotein induction in drug-resistant cancer cells. Proc. Natl. Acad. Sci. USA 2012, 109, 16282–16287, Erratum in Proc. Natl. Acad. Sci. USA 2023, 120, e2301544120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bandari, S.K.; Tripathi, K.; Rangarajan, S.; Sanderson, R.D. Therapy-induced chemoexosomes: Sinister small extracellular vesicles that support tumor survival and progression. Cancer Lett. 2020, 493, 113–119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pokharel, D.; Padula, M.P.; Lu, J.F.; Jaiswal, R.; Djordjevic, S.P.; Bebawy, M. The role of CD44 and ERM proteins in expression and functionality of P-glycoprotein in breast cancer cells. Molecules 2016, 21, 290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maacha, S.; Bhat, A.A.; Jimenez, L.; Raza, A.; Haris, M.; Uddin, S.; Grivel, J.-C. Extracellular vesicles-mediated intercellular communication: Roles in the tumor microenvironment and anti-cancer drug resistance. Mol. Cancer 2019, 18, 55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Federici, C.; Petrucci, F.; Caimi, S.; Cesolini, A.; Logozzi, M.; Borghi, M.; D’Ilio, S.; Lugini, L.; Violante, N.; Azzarito, T.; et al. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS ONE 2014, 9, e88193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roseblade, A.; Luk, F.; Ung, A.; Bebawy, M. Targeting microparticle biogenesis: A novel approach to the circumvention of cancer multidrug resistance. Curr. Cancer Drug Targets. 2015, 15, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Jorfi, S.; Ansa-Addo, E.A.; Kholia, S.; Stratton, D.; Valley, S.; Lange, S.; Inal, J. Inhibition of microvesiculation sensitizes prostate cancer cells to chemotherapy and reduces docetaxel dose required to limit tumor growth in vivo. Sci. Rep. 2015, 5, 13006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muralidharan-Chari, V.; Kohan, H.G.; Asimakopoulos, A.G.; Sudha, T.; Sell, S.; Kannan, K.; Boroujerdi, M.; Davis, P.J.; Mousa, S.A. Microvesicle removal of anticancer drugs contributes to drug resistance in human pancreatic cancer cells. Oncotarget 2016, 7, 50365–50379. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mitani, F.; Lin, J.; Sakamoto, T.; Uehara, R.; Hikita, T.; Yoshida, T.; Setiawan, A.; Arai, M.; Oneyama, C. Asteltoxin inhibits extracellular vesicle production through AMPK/mTOR-mediated activation of lysosome function. Sci. Rep. 2022, 12, 6674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.; Xu, C.; Hua, Y.; Sun, L.; Cheng, K.; Jia, Z.; Han, Y.; Dong, J.; Cui, Y.; Yang, Z. Exosomes play an important role in the process of psoralen reverse multidrug resistance of breast cancer. J. Exp. Clin. Cancer Res. 2016, 35, 186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Labbozzetta, M.; Poma, P.; Tutone, M.; McCubrey, J.A.; Sajeva, M.; Notarbartolo, M. Phytol and heptacosane are possible tools to overcome multidrug resistance in an in vitro model of acute myeloid leukemia. Pharmaceuticals 2022, 15, 356. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Rodrigues, V.; Sousa, M.E.; Vasconcelos, M.H. Curcumin as a Modulator of P-Glycoprotein in Cancer: Challenges and Perspectives. Pharmaceuticals 2016, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhao, P.; Li, Y.; Yang, D.; Hu, P.; Li, L.; Cheng, Y.; Yao, H. Reversal of P-glycoprotein-mediated multidrug resistance by novel curcumin analogues in paclitaxel-resistant human breast cancer cells. Biochem. Cell Biol. 2020, 98, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Poma, P.; Notarbartolo, M.; Labbozzetta, M.; Maurici, A.; Carina, V.; Alaimo, A.; Rizzi, M.; Simoni, D.; D’Alessandro, N. The antitumor activities of curcumin and of its isoxazole analogue are not affected by multiple gene expression changes in an MDR model of the MCF-7 breast cancer cell line: Analysis of the possible molecular basis. Int. J. Mol. Med. 2007, 20, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Notarbartolo, M.; Cervello, M.; Dusonchet, L.; Cusimano, A.; D’Alessandro, N. Resistance to diverse apoptotic triggers in multidrug resistant HL60 cells and its possible relationship to the expression of P-glycoprotein, Fas and of the novel anti-apoptosis factors IAP (inhibitory of apoptosis proteins). Cancer Lett. 2002, 180, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Luk, F.; Jaiswal, R.; George, A.M.; Grau, G.E.; Bebawy, M. Microparticle drug sequestration provides a parallel pathway in the acquisition of cancer drug resistance. Eur. J. Pharmacol. 2013, 721, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Bucci-Muñoz, M.; Gola, A.M.; Rigalli, J.P.; Ceballos, M.P.; Ruiz, M.L. Extracellular vesicles and cancer multidrug resistance: Undesirable intercellular messengers? Life 2023, 13, 1633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Souza-Schorey, C.; Schorey, J.S. Regulation and mechanisms of extracellular vesicle biogenesis and secretion. Essays Biochem. 2018, 62, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Sousa, D.; Lima, R.T.; Lopes-Rodrigues, V.; Gonzalez, E.; Royo, F.; Xavier, C.P.R.; Falcón-Pérez, J.M.; Vasconcelos, M.H. Different ability of multidrug-resistant and -sensitive counterpart cells to release and capture extracellular vesicles. Cells 2021, 10, 2886. [Google Scholar] [CrossRef]
- Lopes-Rodrigues, V.; Di Luca, A.; Sousa, D.; Seca, H.; Meleady, P.; Henry, M.; Lima, R.T.; O’Connor, R.; Vasconcelos, M.H. Multidrug resistant tumour cells shed more microvesicle-like EVs and less exosomes than their drug-sensitive counterpart cells. Biochim. Biophys. Acta 2016, 1860, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell 2020, 182, 1044–1061.e18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Labbozzetta, M.; Poma, P.; Notarbartolo, M. Natural inhibitors of P-glycoprotein in acute myeloid leukemia. Int. J. Mol. Sci. 2023, 24, 4140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taverna, S.; Rigogliuso, S.; Salamone, M.; Vittorelli, M.L. Intracellular trafficking of endogenous fibroblast growth factor-2. FEBS J. 2008, 275, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.; O’Driscoll, L. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. J. Extracell. Vesicles 2019, 9, 1703244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, S.; Jutzy, J.M.; Aspe, J.R.; McGregor, D.W.; Neidigh, J.W.; Wall, N.R. Survivin is released from cancer cells via exosomes. Apoptosis 2011, 16, 1–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

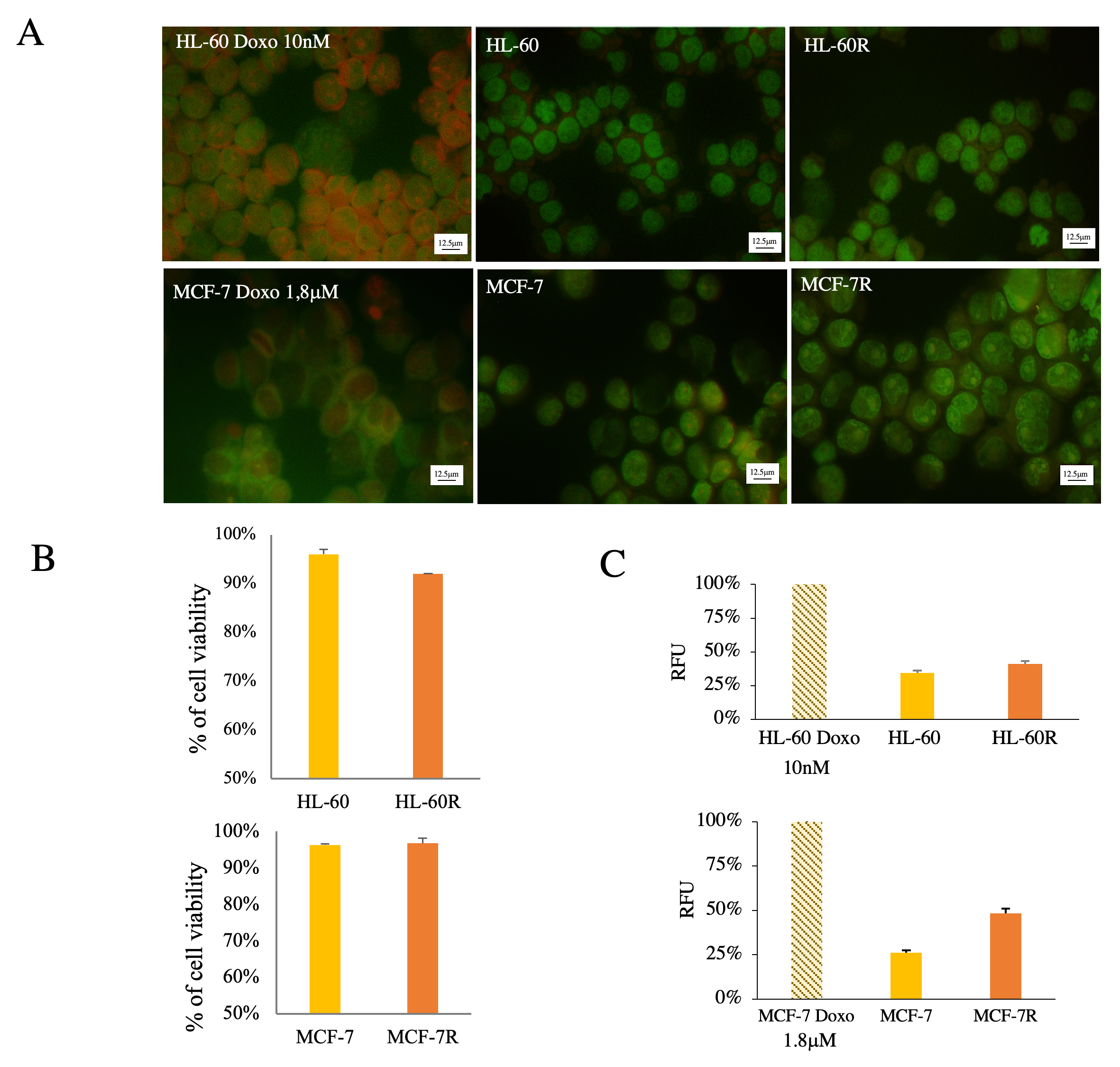


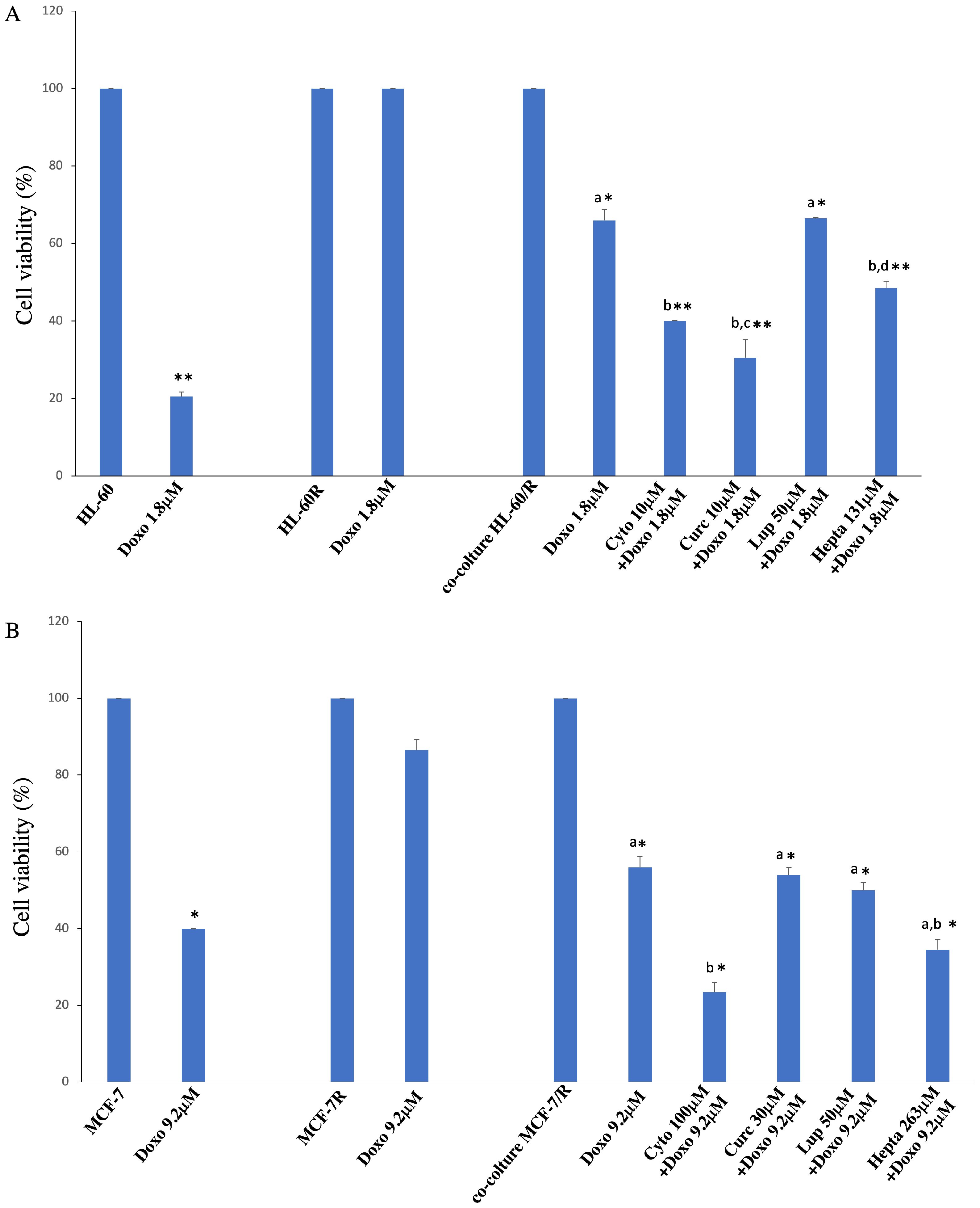
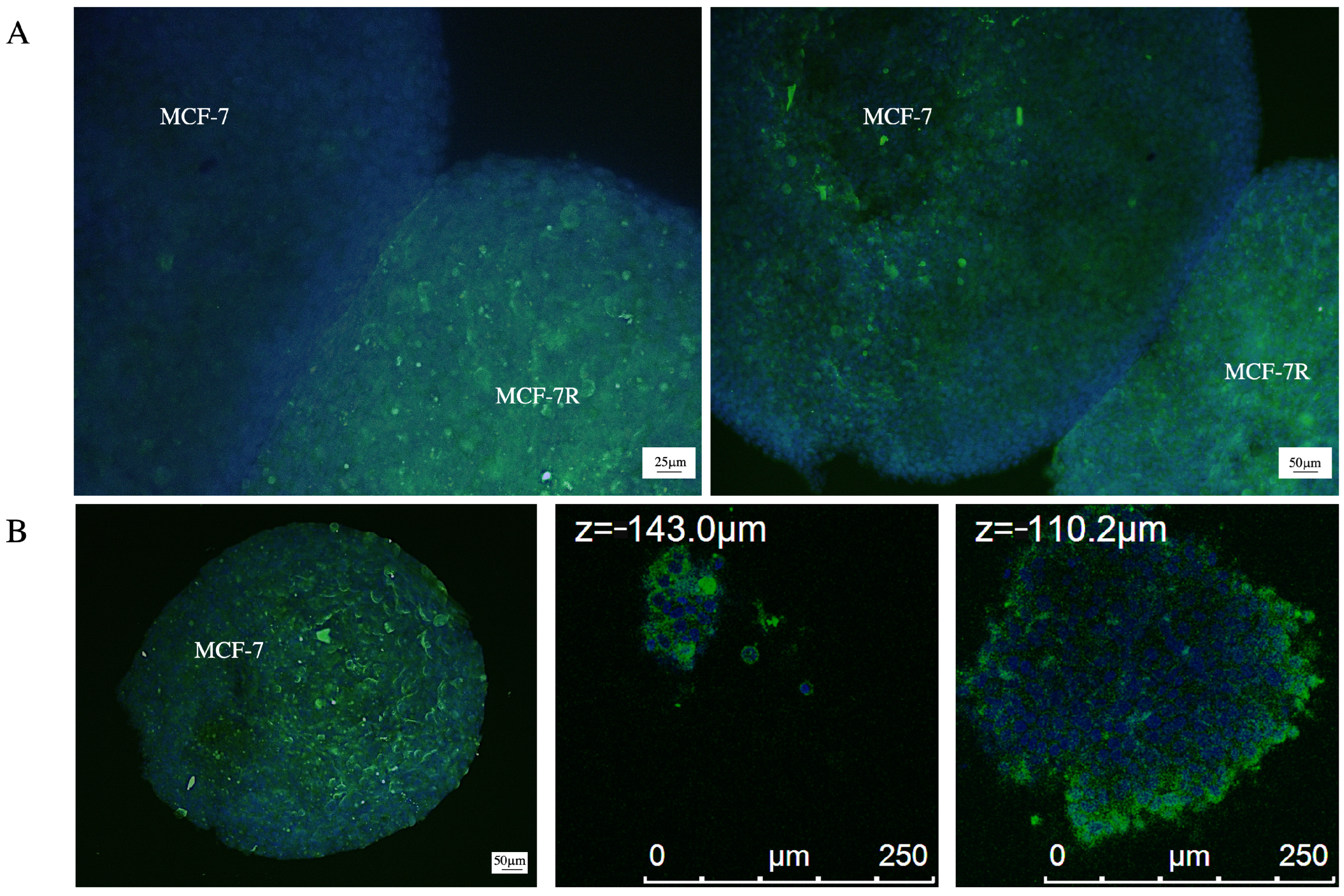
| HL-60R (IC50 ± SE) | MCF-7R (IC50 ± SE) | |
|---|---|---|
| Cytochalasin D | 17.0 ± 5.3 µM | >500.0 µM |
| Curcumin | 13.0 ± 0.7 µM | 30.0 ± 0.3 µM |
| Lupeol | >100.0 µM | >100.0 µM |
| Heptacosane | >263.0 µM | >263.0 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigogliuso, S.; Cusimano, A.; Condorelli, L.; Labbozzetta, M.; Schiera, G.; Poma, P.; Notarbartolo, M. Vesicle-Transported Multidrug Resistance as a Possible Therapeutic Target of Natural Compounds. Pharmaceuticals 2024, 17, 1358. https://doi.org/10.3390/ph17101358
Rigogliuso S, Cusimano A, Condorelli L, Labbozzetta M, Schiera G, Poma P, Notarbartolo M. Vesicle-Transported Multidrug Resistance as a Possible Therapeutic Target of Natural Compounds. Pharmaceuticals. 2024; 17(10):1358. https://doi.org/10.3390/ph17101358
Chicago/Turabian StyleRigogliuso, Salvatrice, Alessandra Cusimano, Lucia Condorelli, Manuela Labbozzetta, Gabriella Schiera, Paola Poma, and Monica Notarbartolo. 2024. "Vesicle-Transported Multidrug Resistance as a Possible Therapeutic Target of Natural Compounds" Pharmaceuticals 17, no. 10: 1358. https://doi.org/10.3390/ph17101358
APA StyleRigogliuso, S., Cusimano, A., Condorelli, L., Labbozzetta, M., Schiera, G., Poma, P., & Notarbartolo, M. (2024). Vesicle-Transported Multidrug Resistance as a Possible Therapeutic Target of Natural Compounds. Pharmaceuticals, 17(10), 1358. https://doi.org/10.3390/ph17101358








