Radiosynthesis and Preclinical Evaluation of [99mTc]Tc-Tigecycline Radiopharmaceutical to Diagnose Bacterial Infections
Abstract
1. Introduction
2. Results
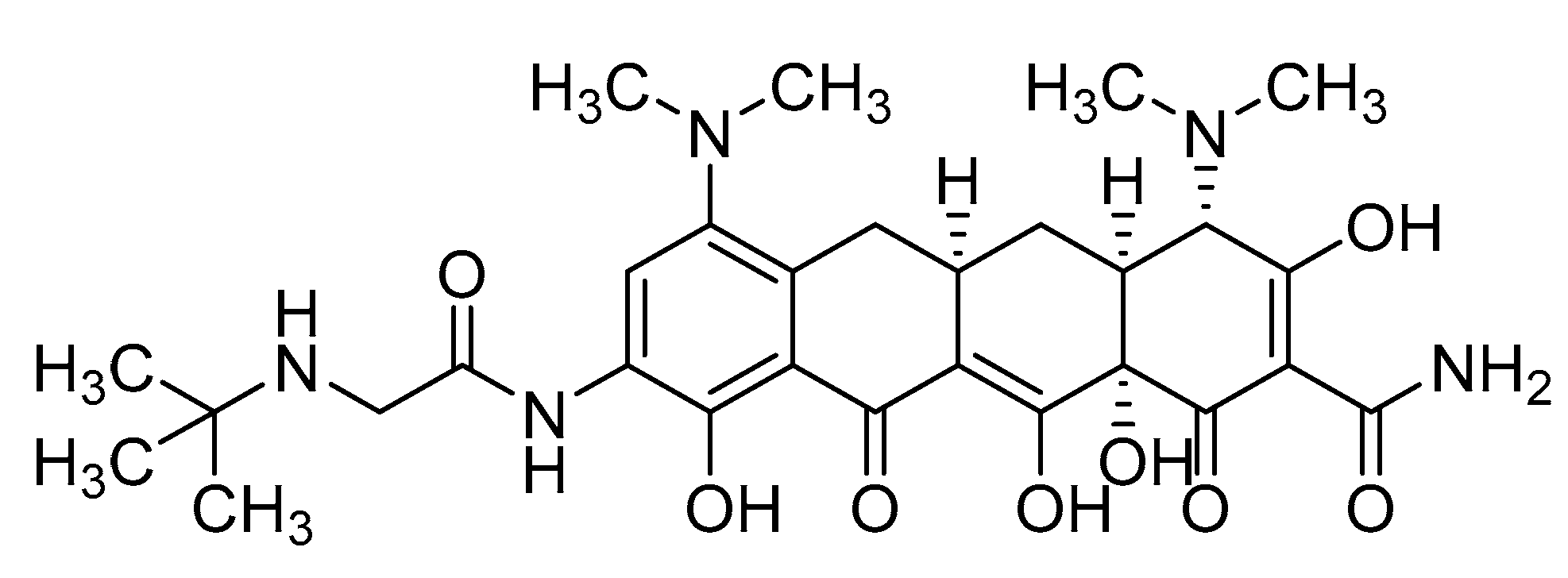
2.1. Physical Characteristics
2.2. Effect of Quality Control Parameters on Labeling Yield
2.2.1. Effect of pH
2.2.2. Effect of Ligand
2.2.3. Effect of Reducing Agent
2.2.4. Effect of Reaction Time
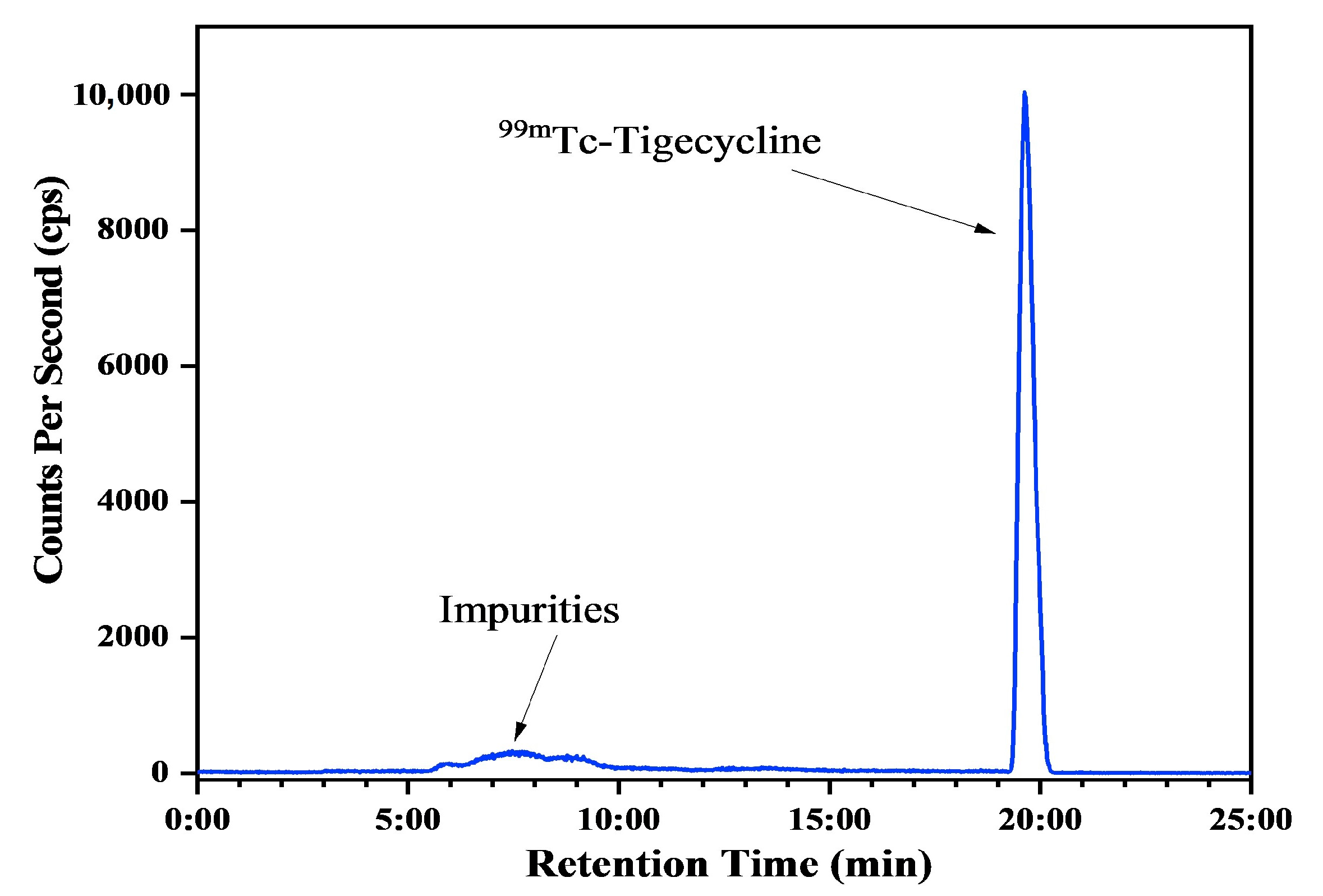
2.3. Radiolabeling Studies
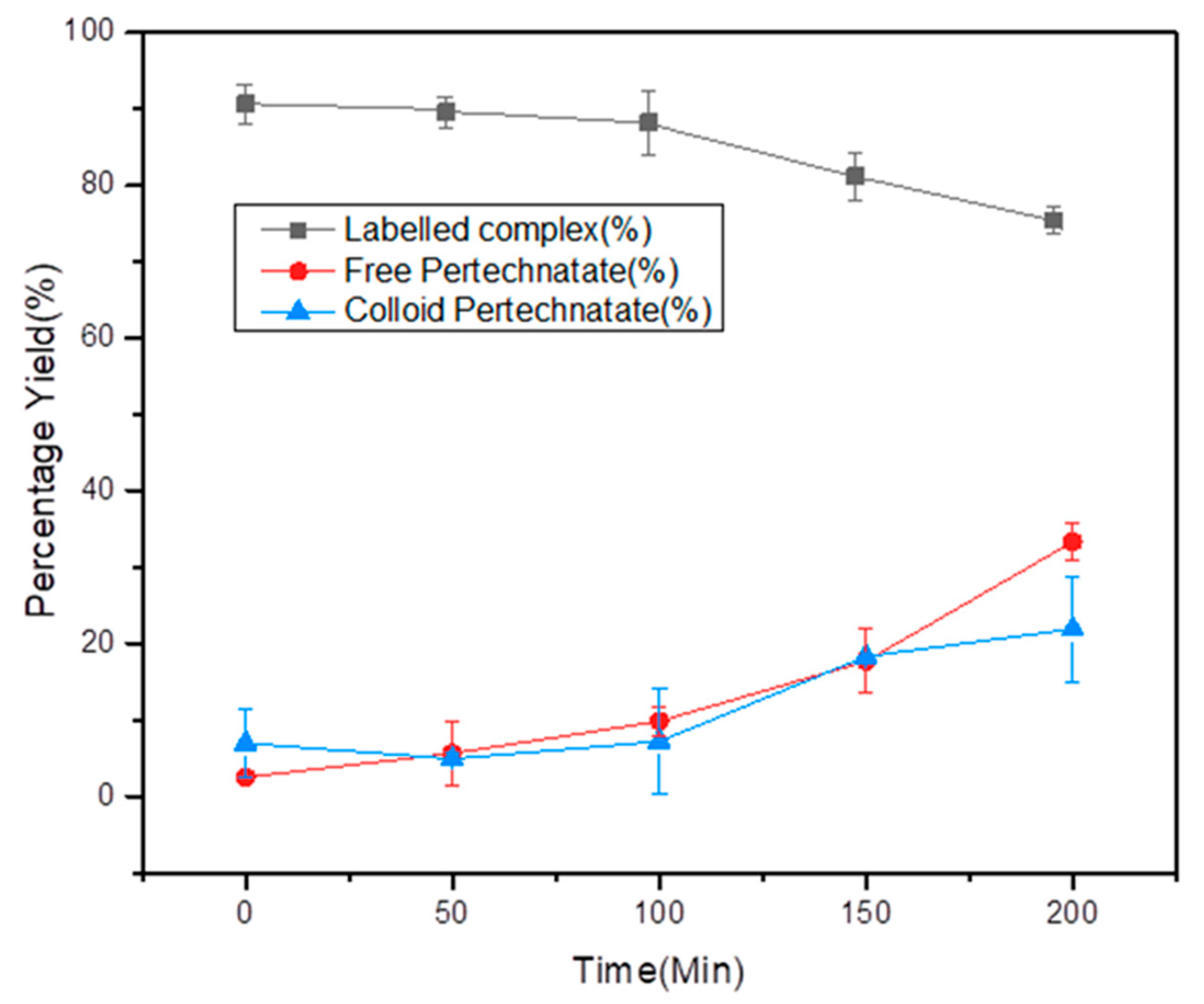
2.4. In Vitro Stability of the Complex in Saline
2.5. In Vitro Bacterial Binding of [99mTc]Tc-Tigecycline and Tigecycline
2.6. Partition Coefficient Factor Study
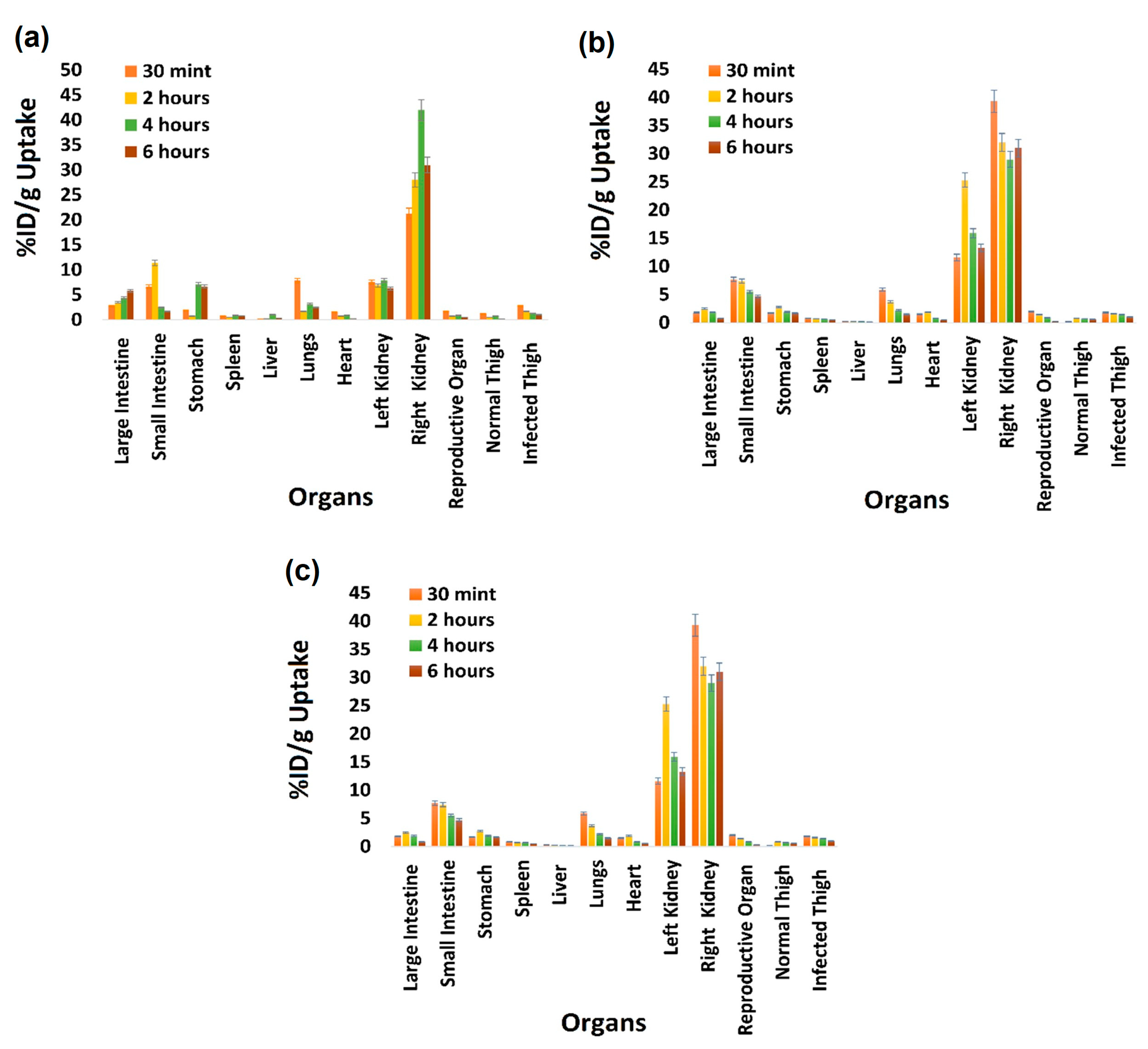
2.7. Biodistribution Study
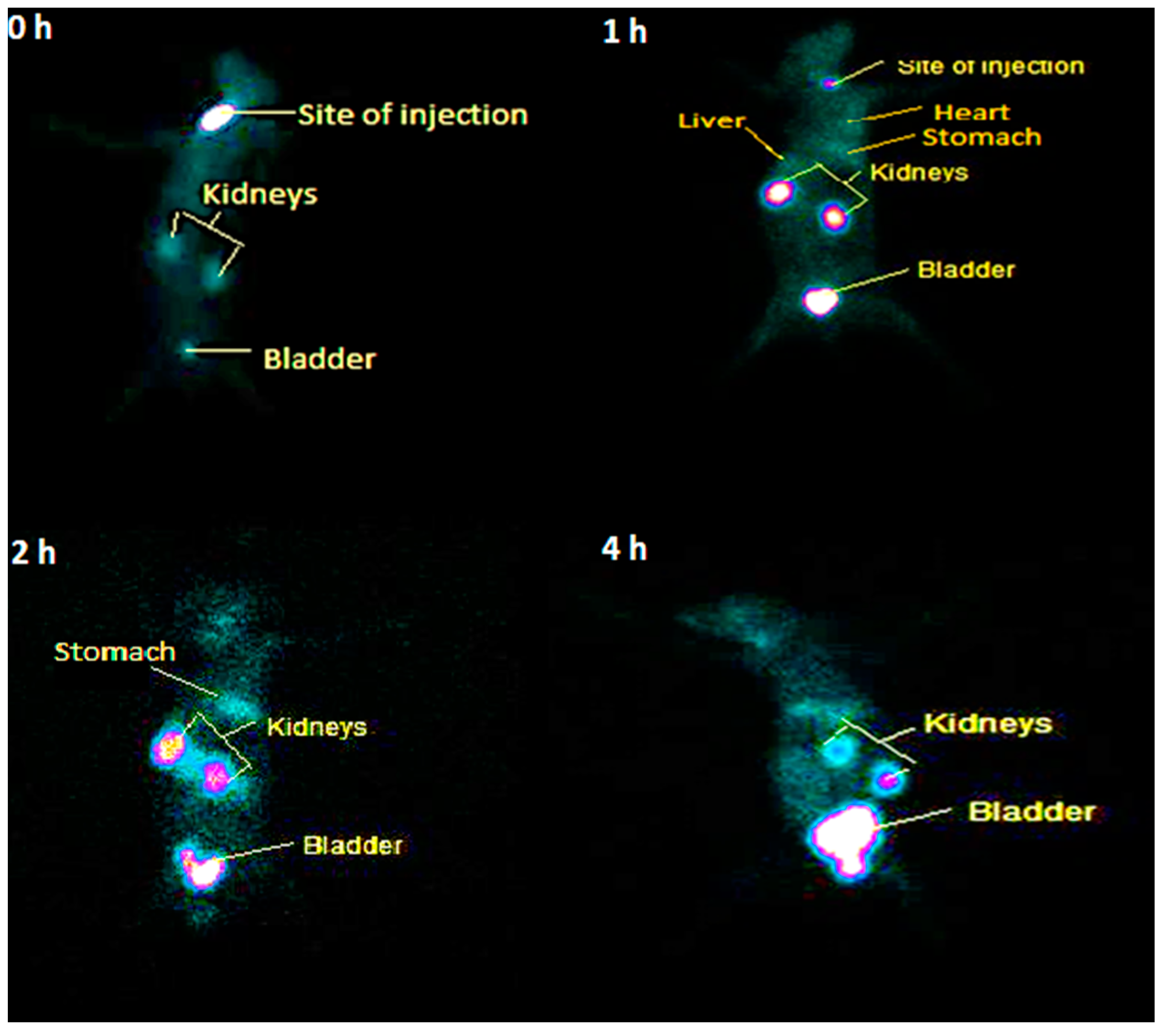
2.8. Scintigraphy Study of [99mTc]Tc-Tigecycline
3. Discussion
4. Materials and Methods
4.1. Chemicals and Radioisotope
4.2. Utensil and Equipment
4.3. Bacterial Strains and Animals
4.4. ITLC-SG Analysis of the Radiochemical Mixture
4.5. Ray-HPLC Analysis for RCP Analysis
4.6. Radiosynthesis of [99mTc]Tc-Tigecycline
Effect of Reaction Parameters
4.7. In Vitro Stability of [99mTc]Tc-Tigecycline
4.8. In Vitro Bacterial Binding Study
4.9. Partition Coefficient (Log P) Measurements
4.10. Biodistribution [99mTc]Tc-Tigecycline in Rats
4.11. [99mTc]Tc-Tigecycline Scintigraphy Study
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Momčilović, S.; Cantacessi, C.; Arsić-Arsenijević, V.; Otranto, D.; Tasić-Otašević, S. Rapid diagnosis of parasitic diseases: Current scenario and future needs. Clin. Microbiol. Infect. 2019, 25, 290–309. [Google Scholar] [CrossRef]
- Khan, N.U.H.; Naqvi, S.A.R.; Roohi, S.; Sherazi, T.A.; Khan, Z.A.; Zahoor, A.F. Technetium-99m radiolabeling and biological study of epirubicin for in vivo imaging of multi-drug-resistant Staphylococcus aureus infections via single photon emission computed tomography. Chem. Biol. Drug Des. 2019, 93, 154–162. [Google Scholar] [CrossRef]
- Cao, Y.; Sundgren, P.C.; Tsien, C.I.; Chenevert, T.T.; Junck, L. Physiologic and metabolic magnetic resonance imaging in gliomas. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 1228–1235. [Google Scholar]
- Carlson, S. A Glance At The History Of Nuclear Medicine. Acta Oncol. 1995, 34, 1095–1102. [Google Scholar] [CrossRef]
- Rizvi, S.U.F.; Siddiqui, H.L.; Parvez, M.; Ahmad, M.; Siddiqui, W.A.; Yasinzai, M.M. Antimicrobial and Antileishmanial Studies of Novel (2E)-3-(2-Chloro-6-methyl/methoxyquinolin-3-yl)-1-(Aryl)prop-2-en-1-ones. Chem. Pharm. Bull. 2010, 58, 301–306. [Google Scholar] [CrossRef]
- Ahmad, M.; Aslam, S.; Bukhari, M.H.; Montero, C.; Detorio, M.; Parvez, M.; Schinazi, R.F. Synthesis of novel pyrazolobenzothiazine 5,5-dioxide derivatives as potent anti-HIV-1 agents. Med. Chem. Res. 2014, 23, 1309–1319. [Google Scholar] [CrossRef]
- Mohsin, N.U.A.; Aslam, S.; Ahmad, M.; Irfan, M.; Al-Hussain, S.A.; Zaki, M.E.A. Cyclooxygenase-2 (COX-2) as a Target of Anticancer Agents: A Review of Novel Synthesized Scaffolds Having Anticancer and COX-2 Inhibitory Potentialities. Pharmaceuticals 2022, 15, 1471. [Google Scholar] [CrossRef]
- Zaib, S.; Farooq Rizvi, S.U.; Aslam, S.; Ahmad, M.; Al-Rashida, M.; Iqbal, J. Monoamine Oxidase Inhibition and Molecular Modeling Studies of Piperidyl-thienyl and 2-Pyrazoline Derivatives of Chalcones. Med. Chem. 2015, 11, 497–505. [Google Scholar]
- Bukhari, M.H.; Siddiqui, H.L.; Ahmad, M.; Hussain, T.; Moloney, M.G. Synthesis and anti-bacterial activities of some novel pyrazolobenzothiazine-based chalcones and their pyrimidine derivatives. Med. Chem. Res. 2012, 21, 2885–2895. [Google Scholar] [CrossRef]
- Douds, H.N.; Berens, S.V.; Long, R.F.; Caplan, G.E. 67Ga-citrate scanning in gastrointestinal malignancies. Clin. Nucl. Med. 1978, 3, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.H.; Fischman, A.J.; Needleman, M.; Wilkinson, R.; Callahan, R.J.; Khaw, B.A.; Hansen, W.P.; Kramer, P.B.; Strauss, H.W. Radiolabeled, nonspecific, polyclonal human immunoglobulin in the detection of focal inflammation by scintigraphy: Comparison with gallium-67 citrate and technetium-99m-labeled albumin. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1989, 30, 385–389. [Google Scholar]
- Roca, M.; de Vries, E.F.; Jamar, F.; Israel, O.; Signore, A. Guidelines for the labelling of leucocytes with (111)In-oxine. Inflammation/Infection Taskgroup of the European Association of Nuclear Medicine. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Karayel, E.; Ocak, M.; Birteksöz Tan, A.S. Leukocyte Labeling with Tc-99m-HMPAO: The Role of Leucocyte Numbers and Medication on the Labeling Efficacy and Image Quality. Mol. Imaging Radionucl. Ther. 2023, 32, 28–34. [Google Scholar] [CrossRef] [PubMed]
- van der Laken, C.J.; Boerman, O.C.; Oyen, W.J.; van de Ven, M.T.; Edwards, D.S.; Barrett, J.A.; van der Meer, J.W.; Corstens, F.H. Technetium-99m-labeled chemotactic peptides in acute infection and sterile inflammation. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1997, 38, 1310–1315. [Google Scholar]
- Almuhaideb, A.; Papathanasiou, N.; Bomanji, J. 18F-FDG PET/CT imaging in oncology. Ann. Saudi Med. 2011, 31, 3–13. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Iqbal, J.; Khan, M.A.; Irfanullah, J.; Jehangir, M.; Khan, B.; ul-Haq, I.; Muhammad, G.; Nadeem, M.A.; Afzal, M.S.; et al. 99mTc-labeled antimicrobial peptide ubiquicidin (29-41) accumulates less in Escherichia coli infection than in Staphlococcus aureus infection. J. Nucl. Med. 2004, 45, 849–856. [Google Scholar]
- Naqvi, S.A.R.; Drlica, K. Fluoroquinolones as imaging agents for bacterial infection. Dalton Trans. 2017, 46, 14452–14460. [Google Scholar] [CrossRef]
- Mota, F.; Ordonez, A.A.; Firth, G.; Ruiz-Bedoya, C.A.; Ma, M.T.; Jain, S.K. Radiotracer Development for Bacterial Imaging. J. Med. Chem. 2020, 63, 1964–1977. [Google Scholar] [CrossRef]
- Sonmezoglu, K.; Sonmezoglu, M.; Halac, M.; Akgün, I.; Türkmen, C.; Onsel, C.; Kanmaz, B.; Solanki, K.; Britton, K.E.; Uslu, I. Usefulness of 99mTc-ciprofloxacin (infecton) scan in diagnosis of chronic orthopedic infections: Comparative study with 99mTc-HMPAO leukocyte scintigraphy. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2001, 42, 567–574. [Google Scholar]
- Sarda, L.; Crémieux, A.C.; Lebellec, Y.; Meulemans, A.; Lebtahi, R.; Hayem, G.; Génin, R.; Delahaye, N.; Huten, D.; Le Guludec, D. Inability of 99mTc-ciprofloxacin scintigraphy to discriminate between septic and sterile osteoarticular diseases. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2003, 44, 920–926. [Google Scholar]
- Naqvi, S.A.R.; Roohi, S.; Iqbal, A.; Sherazi, T.A.; Zahoor, A.F.; Imran, M. Ciprofloxacin: From infection therapy to molecular imaging. Mol. Biol. Rep. 2018, 45, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.A.R.; Jabbar, T.; Alharbi, M.A.; Noureen, A.; Alharbi, N.K.; Sherazi, T.A.; Shahzadi, A.; Ahmed, A.E.; Afzal, M.S.; Imran, M.B. Radiosynthesis, quality control, biodistribution, and infection-imaging study of a new (99m)Tc-labeled ertapenem radiopharmaceutical. Front. Chem. 2022, 10, 1020387. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, R.; Naqvi, S.A.R.; Gillani, S.J.H.; Zahoor, A.F.; Jielani, A.; Saeed, N. (99m) Tc-tazobactam, a novel infection imaging agent: Radiosynthesis, quality control, biodistribution, and infection imaging studies. J. Label. Compd. Radiopharm. 2017, 60, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.A.R.; Roohi, S.; Sabir, H.; Shahzad, S.A.; Aziz, A.; Rasheed, R. Susceptibility of (99m)Tc-Ciprofloxacin for Common Infection Causing Bacterial Strains Isolated from Clinical Samples: An In Vitro and In Vivo Study. Appl. Biochem. Biotechnol. 2019, 188, 424–435. [Google Scholar] [CrossRef]
- Naqvi, S.; Ishfaq, M.; Khan, Z.; Nagra, S.; Bukhari, I.; Hussain, A.; Mahmood, N.; Shahzad, S.; Haq, A. 99mTc labeled levofloxacin as an infection imaging agent: A novel method for labeling levofloxacin using cysteine·HCl as co-ligand and in vivo study. Turk. J. Chem. 2012, 36, 267–277. [Google Scholar]
- Oliva, M.E.; Rekha, A.; Yellin, A.; Pasternak, J.; Campos, M.; Rose, G.M.; Babinchak, T.; Ellis-Grosse, E.J.; Loh, E. A multicenter trial of the efficacy and safety of tigecycline versus imipenem/cilastatin in patients with complicated intra-abdominal infections [Study ID Numbers: 3074A1-301-WW; ClinicalTrials.gov Identifier: NCT00081744]. BMC Infect. Dis. 2005, 5, 88. [Google Scholar] [CrossRef]
- Yaghoubi, S.; Zekiy, A.O.; Krutova, M.; Gholami, M.; Kouhsari, E.; Sholeh, M.; Ghafouri, Z.; Maleki, F. Tigecycline antibacterial activity, clinical effectiveness, and mechanisms and epidemiology of resistance: Narrative review. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2022, 41, 1003–1022. [Google Scholar] [CrossRef]
- Schedlbauer, A.; Kaminishi, T.; Ochoa-Lizarralde, B.; Dhimole, N.; Zhou, S.; López-Alonso, J.P.; Connell, S.R.; Fucini, P. Structural characterization of an alternative mode of tigecycline binding to the bacterial ribosome. Antimicrob. Agents Chemother. 2015, 59, 2849–2854. [Google Scholar] [CrossRef]
- Crișan, G.; Moldovean-Cioroianu, N.S.; Timaru, D.-G.; Andrieș, G.; Căinap, C.; Chiș, V. Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. Int. J. Mol. Sci. 2022, 23, 5023. [Google Scholar] [CrossRef]
- Jødal, L.; Afzelius, P.; Alstrup, A.K.O.; Jensen, S.B. Radiotracers for Bone Marrow Infection Imaging. Molecules 2021, 26, 3159. [Google Scholar] [CrossRef]
- Boschi, A.; Uccelli, L.; Marvelli, L.; Cittanti, C.; Giganti, M.; Martini, P. Technetium-99m Radiopharmaceuticals for Ideal Myocardial Perfusion Imaging: Lost and Found Opportunities. Molecules 2022, 27, 1188. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Ryu, J.-S.; Shin, J.W.; Yoon, E.J.; Ha, H.-J.; Cheon, J.H.; Lee, H.K. Synthesis of 99mTc-ciprofloxacin by different methods and its biodistribution. Appl. Radiat. Isot. 2002, 57, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Spies, H.; Pietzsch, H.J. Stannous Chloride in the Preparation of 99mTc Pharmaceuticals. In Technetium-99m Pharmaceuticals: Preparation and Quality Control in Nuclear Medicine; Zolle, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 59–66. [Google Scholar]
- Erfani, M.; Rekabgardan, M.; Mortazavi, P.; Shafiei, M. Radiocomplexation and evaluation of the 99mTc-Gemifloxacin in artificially Escherichia coli infected mice. J. Radioanal. Nucl. Chem. 2016, 308, 825–833. [Google Scholar] [CrossRef]
- Sohaib, M.; Khurshid, Z.; Roohi, S. Labelling of ceftriaxone with (99m) Tc and its bio-evaluation as an infection imaging agent. J. Label. Compd. Radiopharm. 2014, 57, 652–657. [Google Scholar] [CrossRef]
- Hina, S.; Rajoka, M.I.; Roohi, S.; Haque, A.; Qasim, M. Preparation, Biodistribution, and Scintigraphic Evaluation of 99mTc-Clindamycin: An Infection Imaging Agent. Appl. Biochem. Biotechnol. 2014, 174, 1420–1433. [Google Scholar] [CrossRef] [PubMed]
- Siaens, R.H.; Rennen, H.J.; Boerman, O.C.; Dierckx, R.; Slegers, G. Synthesis and comparison of 99mTc-enrofloxacin and 99mTc-ciprofloxacin. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2004, 45, 2088–2094. [Google Scholar]
- Ïlem-Özdemir, D.; Asikoglu, M.; Ozkilic, H.; Yilmaz, F.; Hosgor-Limoncu, M.; Ayhan, S. (99m) Tc-Doxycycline hyclate: A new radiolabeled antibiotic for bacterial infection imaging. J. Label. Compd. Radiopharm. 2014, 57, 36–41. [Google Scholar] [CrossRef]
- Guillen, J. FELASA guidelines and recommendations. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 311–321. [Google Scholar]
- Naqvi, S.A.R.; Shah, S.M.A.; Kanwal, L.; Saeed, M.; Atta Ul, H.; Nisar, J.; Nisar, Z.; Akram, M. Antimicrobial and Antihypercholesterolemic Activities of Pulicaria gnaphalodes. Dose-Response Publ. Int. Hormesis Soc. 2020, 18, 1559325820904858. [Google Scholar] [CrossRef]
- Naqvi, S.A.R.; Hassan, A.J.; Janjua, M.; Abbas, N.; Zahoor, A.F.; Hassan, S.U.; Hussain, A. Radiolabeling and preclinical animal model evaluation of DTPA coupled (99m)Tc-labelled flutamide complex ([(99m)Tc]DTPA-FLUT) as a potential radiotracer for cancer imaging. Acta Radiol. 2024, 65, 940–949. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, S.M.; Jabbar, T.; Imran, M.B.; Noureen, A.; Sherazi, T.A.; Afzal, M.S.; Rab Nawaz, H.Z.; Ramadan, M.F.; Alkahtani, A.M.; Alsuwat, M.A.; et al. Radiosynthesis and Preclinical Evaluation of [99mTc]Tc-Tigecycline Radiopharmaceutical to Diagnose Bacterial Infections. Pharmaceuticals 2024, 17, 1283. https://doi.org/10.3390/ph17101283
Saleem SM, Jabbar T, Imran MB, Noureen A, Sherazi TA, Afzal MS, Rab Nawaz HZ, Ramadan MF, Alkahtani AM, Alsuwat MA, et al. Radiosynthesis and Preclinical Evaluation of [99mTc]Tc-Tigecycline Radiopharmaceutical to Diagnose Bacterial Infections. Pharmaceuticals. 2024; 17(10):1283. https://doi.org/10.3390/ph17101283
Chicago/Turabian StyleSaleem, Syeda Marab, Tania Jabbar, Muhammad Babar Imran, Asma Noureen, Tauqir A. Sherazi, Muhammad Shahzad Afzal, Hafiza Zahra Rab Nawaz, Mohamed Fawzy Ramadan, Abdullah M. Alkahtani, Meshari A. Alsuwat, and et al. 2024. "Radiosynthesis and Preclinical Evaluation of [99mTc]Tc-Tigecycline Radiopharmaceutical to Diagnose Bacterial Infections" Pharmaceuticals 17, no. 10: 1283. https://doi.org/10.3390/ph17101283
APA StyleSaleem, S. M., Jabbar, T., Imran, M. B., Noureen, A., Sherazi, T. A., Afzal, M. S., Rab Nawaz, H. Z., Ramadan, M. F., Alkahtani, A. M., Alsuwat, M. A., Almubarak, H. A., Momenah, M. A., & Naqvi, S. A. R. (2024). Radiosynthesis and Preclinical Evaluation of [99mTc]Tc-Tigecycline Radiopharmaceutical to Diagnose Bacterial Infections. Pharmaceuticals, 17(10), 1283. https://doi.org/10.3390/ph17101283







