Pyrimidines: A New Versatile Molecule in the Drug Development Field, Scope, and Future Aspects
Abstract
1. Introduction
2. Pharmacological Properties
2.1. As Anticancer Agents
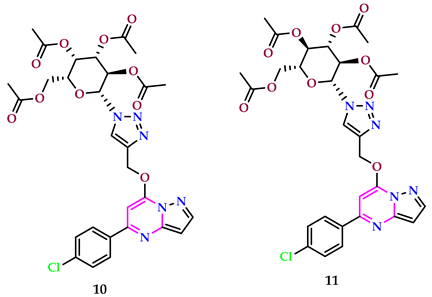
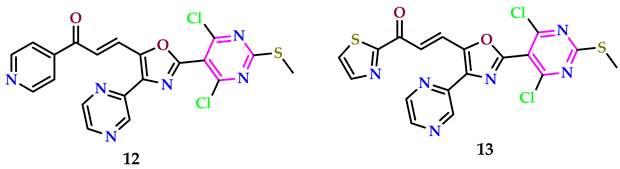


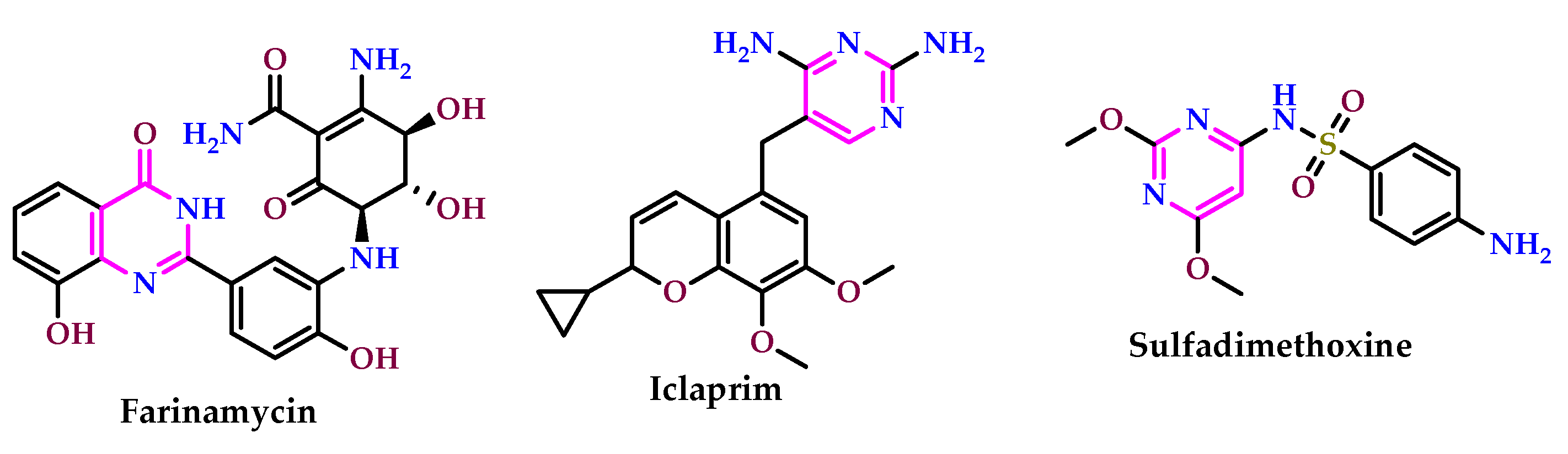
2.2. As Antimicrobial Agents
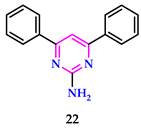

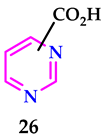

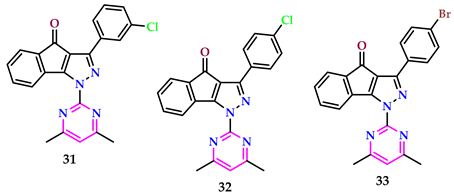



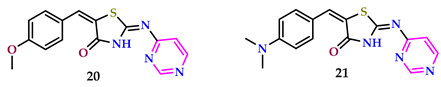
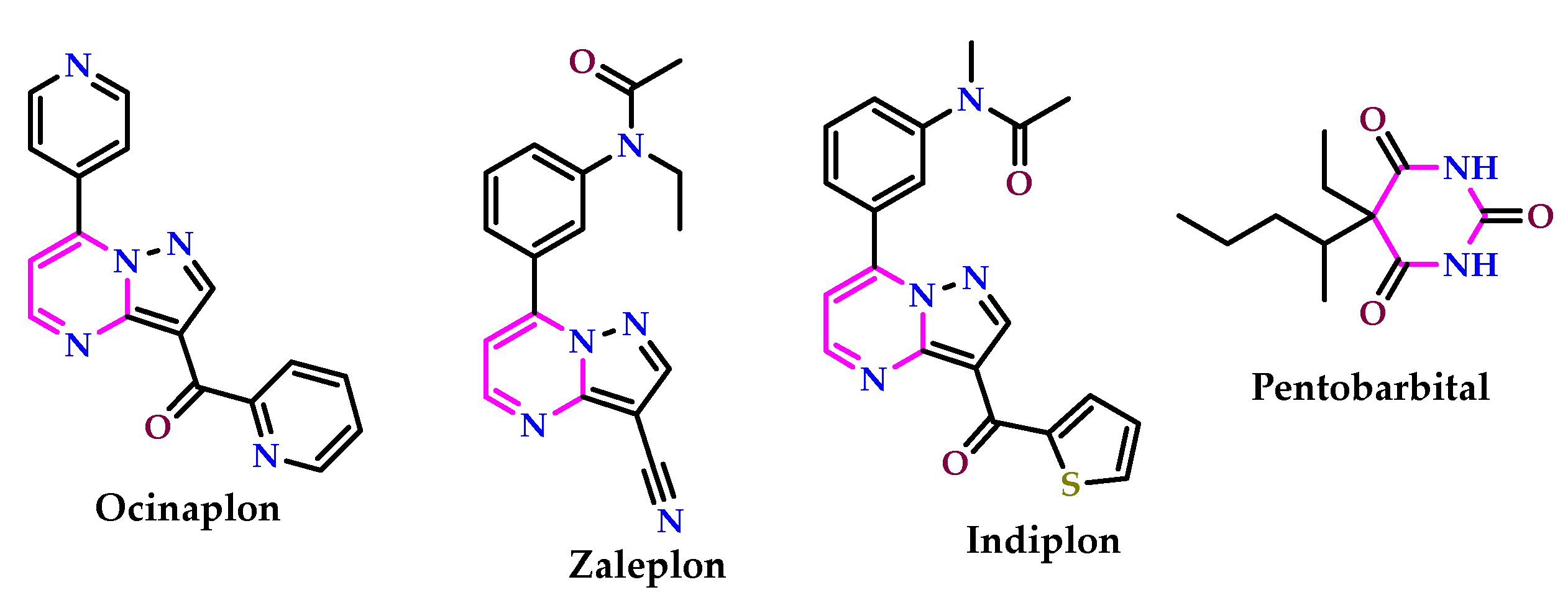
2.3. As Anti-Alzheimer’s Agents
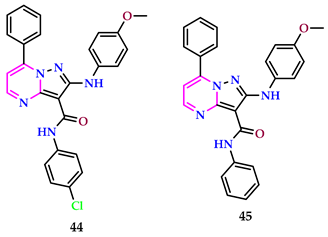
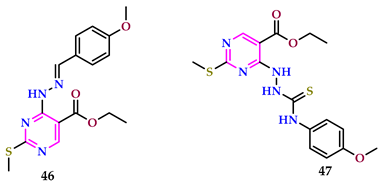
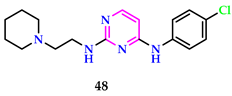
2.4. Herbicidal Properties
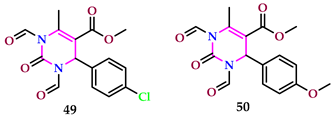
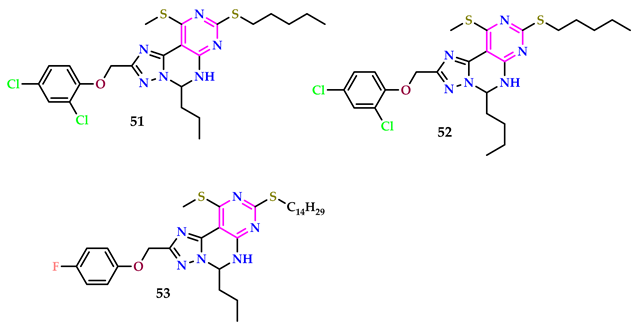
2.5. As Antioxidant
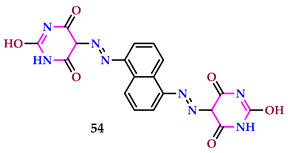
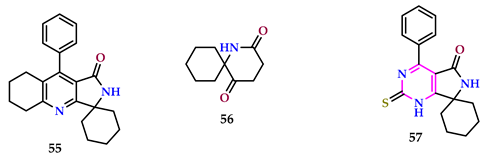
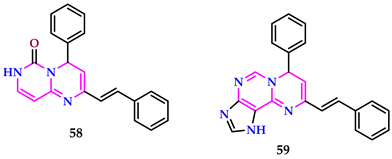
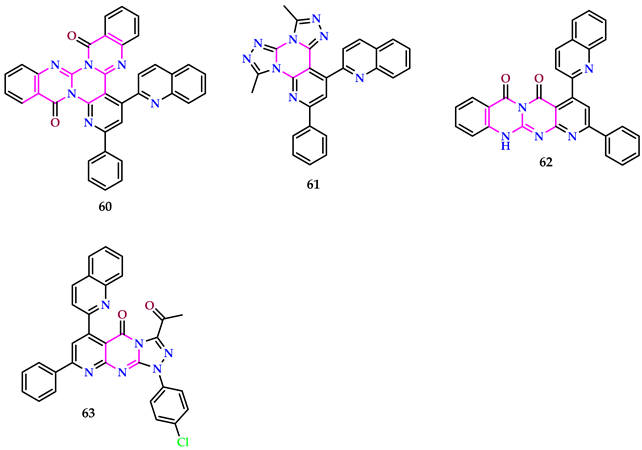
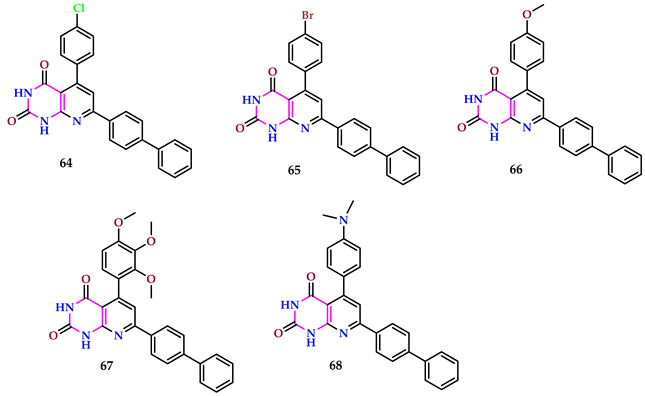
2.6. Use of Pyrimidine Core as Insecticide
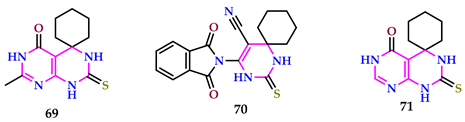
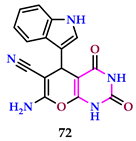
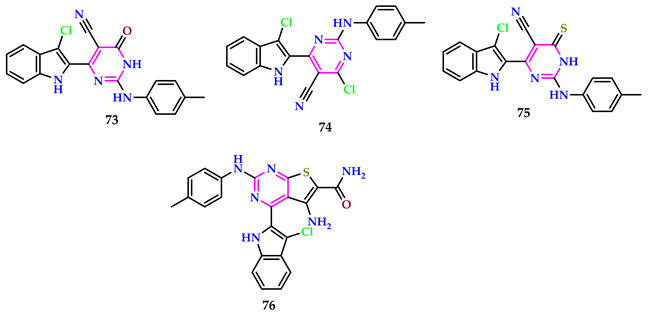
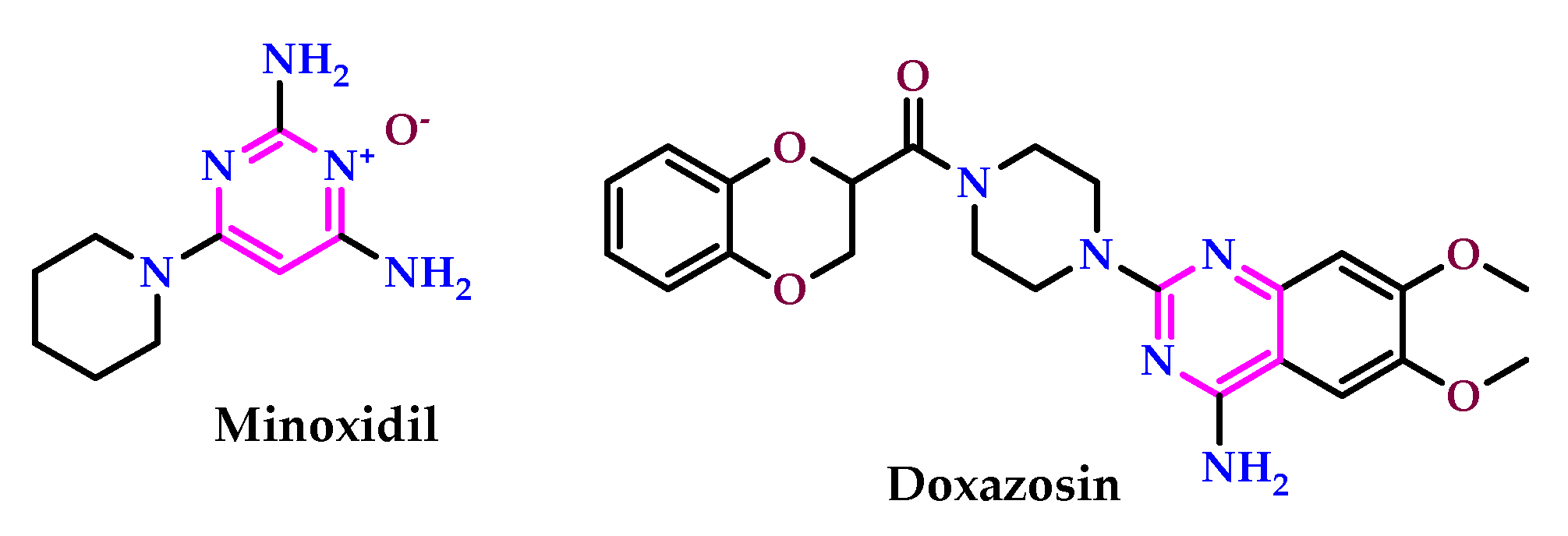
2.7. Use of Pyrimidine Core as Anti-Inflammatory
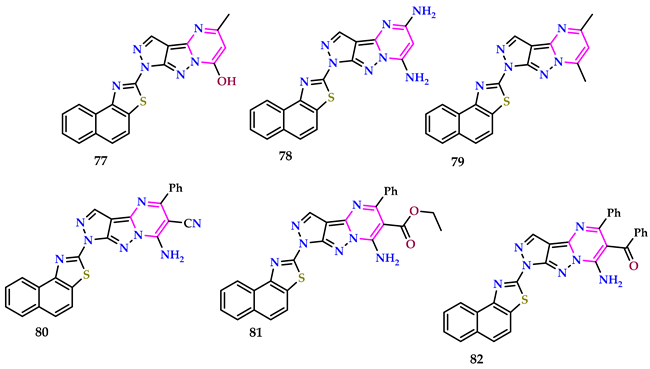
2.8. Use of Pyrimidine Core as Anti-Diabetic
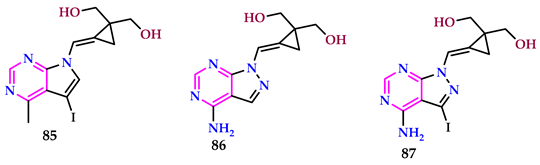
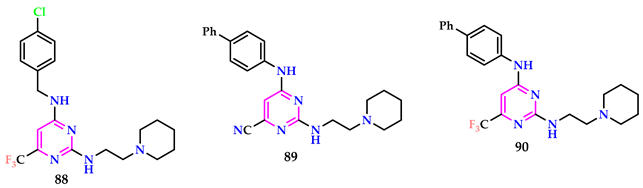
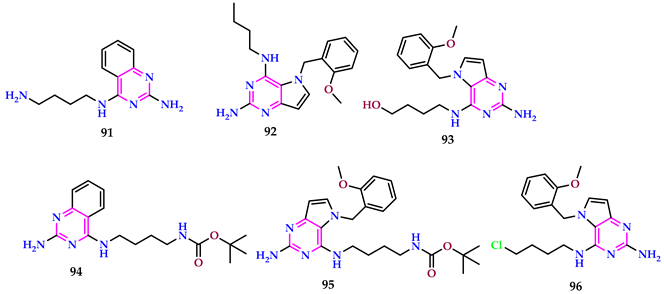
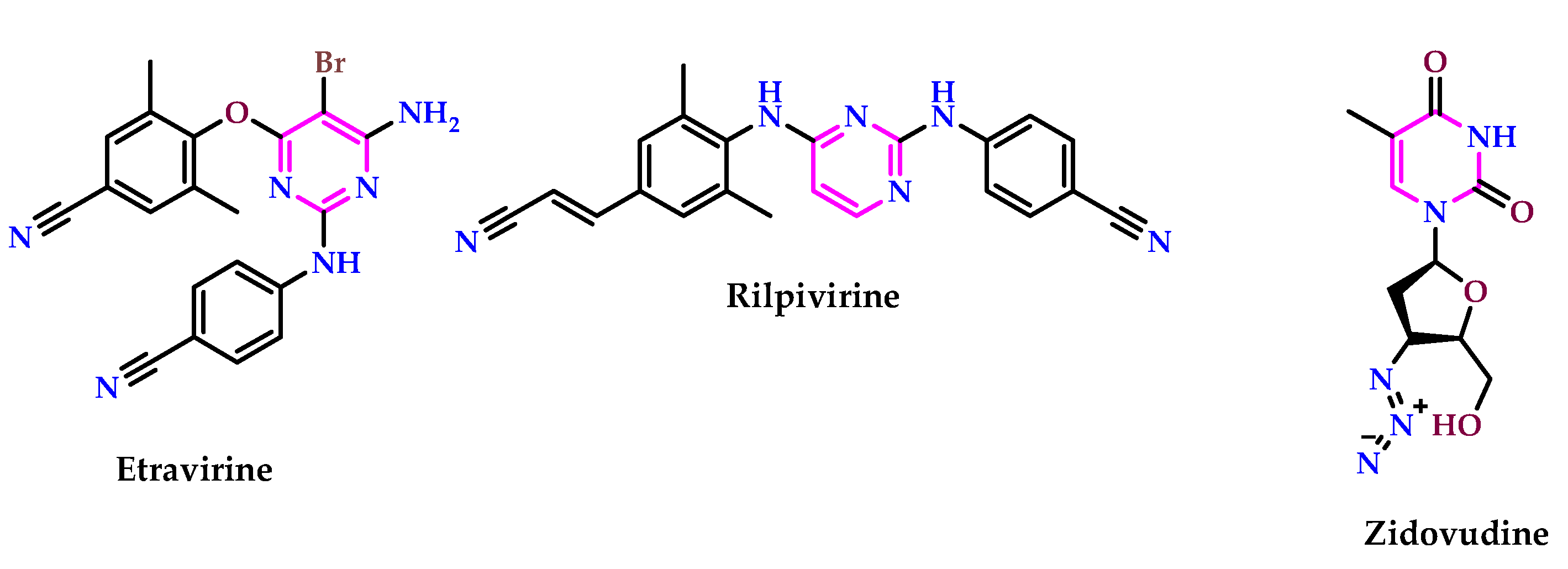
2.9. Use of Pyrimidine Core as Antiviral
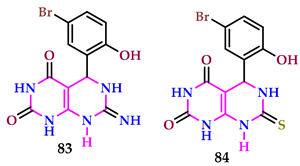
2.10. Use of Pyrimidine Core as Antitubercular
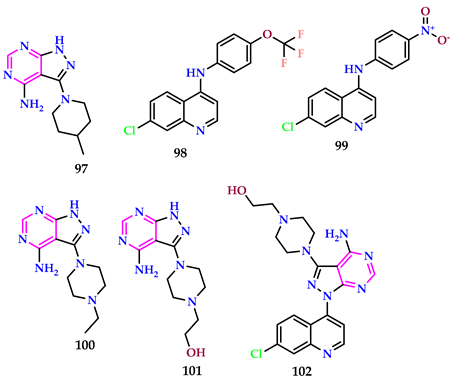
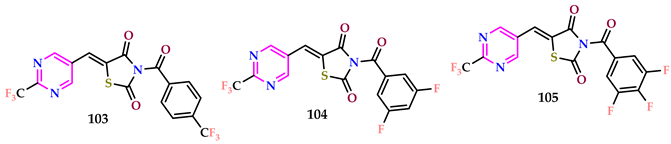
2.11. DNA Binding Studies

2.12. Auxin-like and Cytokinin-like Effect
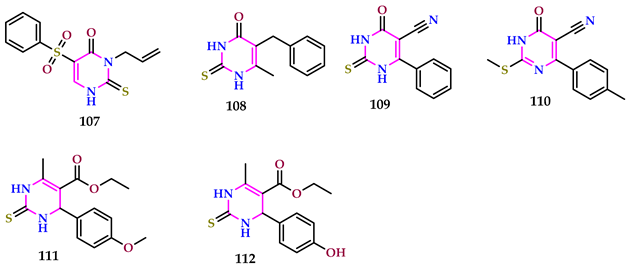
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nammalwar, B.; Bunce, R.A. Recent Advances in Pyrimidine-Based Drugs. Pharmaceuticals 2024, 17, 104. [Google Scholar] [CrossRef] [PubMed]
- Padmashali, B.; Chidananda, B.N.; Govindappa, B.; Basavaraj, S.M.; Chandrashekharappa, S.; Venugopala, K.N. Synthesis and characterization of novel 1, 6-dihydropyrimidine derivatives for their pharmacological properties. J. Appl. Pharm. Sci. 2019, 9, 117–124. [Google Scholar]
- Venugopala, K.N.; Deb, P.K.; Kamat, V.; Santosh, R.; Poojary, B.; Kugaji, M.S.; Kumbar, V.M.; Morsy, M.A.; Aldhubiab, B.; Attimarad, M. 5-(substitutedphenyl)-7-imino-7,8-dihydropyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione Analogues as Anti-inflammatory Agents. U.S. Patent No. 11,884,677, 30 January 2024. [Google Scholar]
- Venugopala, K.N.; Govender, R.; Khedr, M.A.; Venugopala, R.; Aldhubiab, B.E.; Harsha, S.; Odhav, B. Design, synthesis, and computational studies on dihydropyrimidine scaffolds as potential lipoxygenase inhibitors and cancer chemopreventive agents. Drug Des. Dev. Ther. 2015, 9, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Pan, N.; Wu, R.; Yan, C.; Zhou, M.; Fei, Q.; Li, P.; Wu, W. Design, synthesis, antifungal activity, and molecular docking of novel trifluoromethyl pyrimidine derivatives containing 1,3,4-oxadiazole and thioether moieties as potential succinate dehydrogenase inhibitors. J. Heterocycl. Chem. 2023, 60, 1768–1777. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.; das Chagas Almeida, A.; da Trindade Granato, J.; de Oliveira Lemos, A.S.; Kumar, K.; Patil, M.T.; da Silva, A.D.; Rode, A.B.; Coimbra, E.S. Imidazo [1,2-a] pyrimidine as a New Antileishmanial Pharmacophore against Leishmania amazonensis Promastigotes and Amastigotes. ACS Omega 2023, 8, 40613–40621. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Deb, P.K.; Kamat, V.; Santosh, R.; Poojary, B.; Kugaji, M.S.; Kumbar, V.M.; Morsy, M.A.; Aldhubiab, B.; Attimarad, M. 5-(3-substituted phenyl)-pyrimido[4,5-d]pyrimidine-2,4,7(1H,3H,8H)-trione Derivatives as Anticancer Agents. U.S. Patent No. 11,932,649, 19 March 2024. [Google Scholar]
- Dahabiyeh, L.A.; Hudaib, F.; Hourani, W.; Darwish, W.; Abu-Irmaileh, B.; Deb, P.K.; Venugopala, K.N.; Mohanlall, V.; Chandrashekharappa, S.; Abu-Dahab, R. Mass spectrometry-based metabolomics approach and in vitro assays revealed promising role of 2,3-dihydroquinazolin-4 (1H)-one derivatives against colorectal cancer cell lines. Eur. J. Pharm. Sci. 2023, 182, 106378. [Google Scholar] [CrossRef]
- Bhole, R.; Sarode, V.; Kothapalli, L.; Gurav, S.; Chikhale, R. Design, Synthesis and Pharmacological Evaluation of Some 2-Methylsulfanyl-1,4-Dihydropyrimidines Derivatives as an Analgesic Agent. Russ. J. Bioorganic Chem. 2023, 49, 897–904. [Google Scholar] [CrossRef]
- Wu, J.; Hou, Z.; Wang, Y.; Chen, L.; Lian, C.; Meng, Q.; Zhang, C.; Li, X.; Huang, L.; Yu, H. Discovery of 7-alkyloxy-[1,2,4]triazolo[1,5-a]pyrimidine derivatives as selective positive modulators of GABAA1 and GABAA4 receptors with potent antiepileptic activity. Bioorganic Chem. 2022, 119, 105565. [Google Scholar] [CrossRef]
- Keshari, M.; Khan, R.A.; Khalilullah, H.; Yusuf, M.; Ahmed, B. Pharmacophore modeling, design, and synthesis of potent antihypertensives, oxazolo/thiazolo-[3,2-a]-pyrimidin-3(2H)-one, and 1,5-dihydroimidazo-[1,2-a]-pyrimidin-3(2H)-one derivatives: A pilot trial. Bioorganic Med. Chem. Lett. 2020, 30, 127604. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Nayak, S.K.; Gleiser, R.M.; Sanchez-Borzone, M.E.; Garcia, D.A.; Odhav, B. Synthesis, Polymorphism, and Insecticidal Activity of Methyl 4-(4-chlorophenyl)-8-iodo-2-methyl-6-oxo-1,6-dihydro-4H-pyrimido[2,1-b]quinazoline-3-Carboxylate Against Anopheles arabiensis Mosquito. Chem. Biol. Drug Des. 2016, 88, 88–96. [Google Scholar] [CrossRef]
- Bairagi, K.M.; Younis, N.S.; Emeka, P.M.; Sangtani, E.; Gonnade, R.G.; Venugopala, K.N.; Alwassil, O.I.; Khalil, H.E.; Nayak, S.K. Antidiabetic activity of dihydropyrimidine scaffolds and structural insight by single crystal X-ray studies. Med. Chem. 2020, 16, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Hekal, H.A.; Hammad, O.M.; El-Brollosy, N.R.; Salem, M.M.; Allayeh, A.K. Design, synthesis, docking, and antiviral evaluation of some novel pyrimidinone-based α-aminophosphonates as potent H1N1 and HCoV-229E inhibitors. Bioorganic Chem. 2024, 147, 107353. [Google Scholar] [CrossRef] [PubMed]
- Chitikina, S.S.; Buddiga, P.; Deb, P.K.; Mailavaram, R.P.; Venugopala, K.N.; Nair, A.B.; Al-Jaidi, B.; Kar, S. Synthesis and anthelmintic activity of some novel (E)-2-methyl/propyl-4-(2-(substitutedbenzylidene)hydrazinyl)-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidines. Med. Chem. Res. 2020, 29, 1600–1610. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Tratrat, C.; Pillay, M.; Chandrashekharappa, S.; Al-Attraqchi, O.H.A.; Aldhubiab, B.E.; Attimarad, M.; Alwassil, O.I.; Nair, A.B.; Sreeharsha, N. In silico design and synthesis of tetrahydropyrimidinones and tetrahydropyrimidinethiones as potential thymidylate kinase inhibitors exerting anti-TB activity against Mycobacterium tuberculosis. Drug Des. Dev. Ther. 2020, 14, 1027–1039. [Google Scholar] [CrossRef]
- Duraisamy, R.; Al-Shar’i, N.A.; Chandrashekharappa, S.; Deb, P.K.; Gleiser, R.M.; Tratrat, C.; Chopra, D.; Muthukurpalya Bhojegowd, M.R.; Thirumalai, D.; Morsy, M.A. Synthesis, biological evaluation, and computational investigation of ethyl 2,4,6-trisubstituted-1,4-dihydropyrimidine-5-carboxylates as potential larvicidal agents against Anopheles arabiensis. J. Biomol. Struct. Dyn. 2023, 42, 4016–4028. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Shinu, P.; Tratrat, C.; Deb, P.K.; Gleiser, R.M.; Chandrashekharappa, S.; Chopra, D.; Attimarad, M.; Nair, A.B.; Sreeharsha, N. 1,2,3-Triazolyl-tetrahydropyrimidine Conjugates as Potential Sterol Carrier Protein-2 Inhibitors: Larvicidal Activity against the Malaria Vector Anopheles arabiensis and In Silico Molecular Docking Study. Molecules 2022, 27, 2676. [Google Scholar] [CrossRef] [PubMed]
- Bairagi, K.M.; Venugopala, K.N.; Mondal, P.K.; Gleiser, R.M.; Chopra, D.; García, D.; Odhav, B.; Nayak, S.K. Larvicidal study of tetrahydropyrimidine scaffolds against Anopheles arabiensis and structural insight by single crystal X-ray studies. Chem. Biol. Drug Des. 2018, 92, 1924–1932. [Google Scholar] [CrossRef]
- Moharam, M.M.; Saleh, E.A.M.; Hassan, I.; Husain, K. Synthesis, Antifungal, and Antioxidant Evaluation of New Class of Thiazolo[3,2-a]pyrimidine and Pyrimido[5,4-d]thiazolo[3,2-a]pyrimidine Derived from α, α-Ketene Dithioacetals as Five and Six-membered Heterocycles Analogues. Russ. J. Bioorganic Chem. 2023, 49, 1119–1136. [Google Scholar] [CrossRef]
- Abdel-Raheem, S.A.; Fouad, M.R.; Gad, M.A.; El-Dean, A.M.K.; Tolba, M.S. Environmentally green synthesis and characterization of some novel bioactive pyrimidines with excellent bioefficacy and safety profile towards soil organisms. J. Environ. Chem. Eng. 2023, 11, 110839. [Google Scholar] [CrossRef]
- Haque, J.; Ansari, K.; Srivastava, V.; Quraishi, M.; Obot, I. Pyrimidine derivatives as novel acidizing corrosion inhibitors for N80 steel useful for petroleum industry: A combined experimental and theoretical approach. J. Ind. Eng. Chem. 2017, 49, 176–188. [Google Scholar] [CrossRef]
- Ghazoui, A.; Saddik, R.; Benchat, N.; Guenbour, M.; Hammouti, B.; Al-Deyab, S.; Zarrouk, A. Comparative study of pyridine and pyrimidine derivatives as corrosion inhibitors of C38 steel in molar HCl. Int. J. Electrochem. Sci. 2012, 7, 7080–7097. [Google Scholar] [CrossRef]
- Upadhyay, K.; Kumar, A. Pyrimidine based highly sensitive fluorescent receptor for Al3+ showing dual signalling mechanism. Org. Biomol. Chem. 2010, 8, 4892–4897. [Google Scholar] [CrossRef] [PubMed]
- Al-Masoudi, N.A.; Al-Salihi, N.J.; Marich, Y.A.; Markus, T. Synthesis, and fluorescence properties of coumarin and benzocoumarin derivatives conjugated pyrimidine scaffolds for biological imaging applications. J. Fluoresc. 2015, 25, 1847–1854. [Google Scholar] [CrossRef]
- West, T.P.; Chu, C.P. Utilization of pyrimidines and pyrimidine analogues by fluorescent pseudomonads. Microbios 1986, 47, 149–157. [Google Scholar]
- Verbitskiy, E.V.; Cheprakova, E.M.; Subbotina, J.O.; Schepochkin, A.V.; Slepukhin, P.A.; Rusinov, G.L.; Charushin, V.N.; Chupakhin, O.N.; Makarova, N.I.; Metelitsa, A.V. Synthesis, spectral and electrochemical properties of pyrimidine-containing dyes as photosensitizers for dye-sensitized solar cells. Dye. Pigment. 2014, 100, 201–214. [Google Scholar] [CrossRef]
- Achelle, S.; Malval, J.P.; Aloïse, S.; Barsella, A.; Spangenberg, A.; Mager, L.; Akdas-Kilig, H.; Fillaut, J.L.; Caro, B.; Robin-le Guen, F. Synthesis, Photophysics and Nonlinear Optical Properties of Stilbenoid Pyrimidine-Based Dyes Bearing Methylenepyran Donor Groups. ChemPhysChem 2013, 14, 2725–2736. [Google Scholar] [CrossRef]
- Sayed, A.Z.; Aboul-Fetouh, M.S.; Nassar, H.S. Synthesis, biological activity and dyeing performance of some novel azo disperse dyes incorporating pyrazolo[1,5-a]pyrimidines for dyeing of polyester fabrics. J. Mol. Struct. 2012, 1010, 146–151. [Google Scholar] [CrossRef]
- Park, I.S.; Komiyama, H.; Yasuda, T. Pyrimidine-based twisted donor–acceptor delayed fluorescence molecules: A new universal platform for highly efficient blue electroluminescence. Chem. Sci. 2017, 8, 953–960. [Google Scholar] [CrossRef]
- Ganesan, P.; Ranganathan, R.; Chi, Y.; Liu, X.K.; Lee, C.S.; Liu, S.H.; Lee, G.H.; Lin, T.C.; Chen, Y.T.; Chou, P.T. Functional Pyrimidine-Based Thermally Activated Delay Fluorescence Emitters: Photophysics, Mechanochromism, and Fabrication of Organic Light-Emitting Diodes. Chem.—A Eur. J. 2017, 23, 2858–2866. [Google Scholar] [CrossRef]
- Irfan, A.; Muhammad, S.; Chaudhry, A.R.; Al-Sehemi, A.G.; Jin, R. Tuning of optoelectronic and charge transport properties in star shaped anthracenothiophene-pyrimidine derivatives as multifunctional materials. Optik 2017, 149, 321–331. [Google Scholar] [CrossRef]
- Irfan, A.; Al-Sehemi, A.G.; Assiri, M.A.; Mumtaz, M.W. Exploring the electronic, optical and charge transfer properties of acene-based organic semiconductor materials. Bull. Mater. Sci. 2019, 42, 1–7. [Google Scholar] [CrossRef]
- Zarenezhad, E.; Farjam, M.; Iraji, A. Synthesis and biological activity of pyrimidines-containing hybrids: Focusing on pharmacological application. J. Mol. Struct. 2021, 1230, 129833. [Google Scholar] [CrossRef]
- Kamat, V.; Reddy, D.S.; Kumar, A. Catalytic role in Biginelli reaction: Synthesis and biological property studies of 2-oxo/thioxo-1,2,3,4-tetrahydropyrimidines. Arch. Der Pharm. 2023, 356, 2300008. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, G.; Khanna, A.; Tyagi, R.; Mishra, V.K.; Narayana, C.; Sagar, R. Copper-catalyzed synthesis of pyrazolo[1,5-a]pyrimidine based triazole-linked glycohybrids: Mechanistic insights and bio-applications. Sci. Rep. 2024, 14, 529. [Google Scholar] [CrossRef]
- Sabita, G.; Savitha, R.; Divya, K.; Shivakumar, E.; Bhaskar, K. Design, Synthesis and Biological Evaluation of Chalcone Incorporated of Pyrimidine-Pyrazine-Oxazoles as Anticancer Agents. Chem. Data Collect. 2024, 51, 101128. [Google Scholar] [CrossRef]
- Sivaiah, G.; Raghu, M.; Prasad, S.B.; Anusuya, A.; Kumar, K.Y.; Alharethy, F.; Prashanth, M.; Jeon, B.-H. Synthesis, biological evaluation and molecular docking studies of new pyrimidine derivatives as potent dual EGFR/HDAC inhibitors. J. Mol. Struct. 2024, 1309, 138223. [Google Scholar] [CrossRef]
- Vemuluri, S.P.; Somarapu, V.L.; Eppakayala, L. Design, synthesis and anticancer evaluation of various aryl amide derivatives of thiazole-benzothiazole-pyrimidines. Results Chem. 2024, 7, 101403. [Google Scholar] [CrossRef]
- Jame, R. Synthesis, photophysical properties, anticancer evaluation, and molecular docking studies of new pyrimidine linked 4-arylidene-thiazolidin-4-ones as potent anticancer agents. Luminescence 2024, 39, e4672. [Google Scholar] [CrossRef]
- Limaye, A.S.; Rananaware, P.; Ghosh, A.; Rajashekarreddy, T.; Raghavendrarao, N.; Brahmkhatri, V.; Hegde, R.V.; Dateer, R.B. Greener Approach for Synthesis of δ-MnO2 Nanoparticles: Access to Pharmaceutically Important Pyrimidines and their Antimicrobial Activity Studies. ACS Appl. Bio Mater. 2024, 7, 1790–1800. [Google Scholar] [CrossRef]
- Bryndal, I.; Stolarczyk, M.; Mikołajczyk, A.; Krupińska, M.; Pyra, A.; Mączyński, M.; Matera-Witkiewicz, A. Pyrimidine Schiff Bases: Synthesis, Structural Characterization and Recent Studies on Biological Activities. Int. J. Mol. Sci. 2024, 25, 2076. [Google Scholar] [CrossRef]
- Yao, X.; Ma, J.-W.; Yao, N.-T.; Yin, F.; Zhang, R.-F. Exploring the Synthesis, Structure and Bioactivity of Pyrimidine Carboxylic Acid-Derived Organic Antimony (V) Complexes: Cytostatic and Antimicrobial Evaluations. J. Organomet. Chem. 2024, 1012, 123128. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ghasemzadeh, M.A.; Javadi, A. Synthesis of ZIF-8/ZnFe2O4/GO-OSO3H nanocomposite as a superior and reusable heterogeneous catalyst for the preparation of pyrimidine derivatives and investigation of their antimicrobial activities. Heliyon 2024, 10, e26339. [Google Scholar] [CrossRef]
- Mor, S.; Punia, R.; Khatri, M.; Kumar, D.; Kumar, A.; Jindal, D.K.; Singh, N.; Sharma, R.; Ahmed, M.; Shukla, S. Synthesis, biological evaluations and in silico studies on pyrimidine-appended fused pyrazolones as anticancer and antimicrobial agents. J. Mol. Struct. 2024, 1296, 136759. [Google Scholar] [CrossRef]
- Almakhzoum, K.A.A.H.; Almaqtari, M.A. Synthesis and Characterization of Some Novel 6-(Heteroatom-substituted) Pyrimidine Derivatives and Study the Biological Activity. PSM Biol. Res. 2024, 9, 30–40. [Google Scholar]
- Mamand, S.O.; Abdul, D.A.; Ayoob, M.M.; Hussein, A.J.; Samad, M.K.; Hawaiz, F.E. Traditional, one-pot three-component synthesis and anti-bacterial evaluations of some new pyrimidine derivatives. Inorg. Chem. Commun. 2024, 160, 111875. [Google Scholar] [CrossRef]
- Uysal, K.; Yıldırım, M.; Karakuş, H.; Yıldırım, A. A simple, facile and greener route to thiazolo[3,2-c]pyrimidinones in semi-aqueous medium and their antibacterial properties. Synth. Commun. 2024, 54, 491–503. [Google Scholar] [CrossRef]
- Almehizia, A.A.; Aboulthana, W.M.; Naglah, A.M.; Hassan, A.S. In vitro biological studies and computational prediction-based analyses of pyrazolo[1,5-a]pyrimidine derivatives. RSC Adv. 2024, 14, 8397–8408. [Google Scholar] [CrossRef]
- Kahvecioglu, D.; Yilmaz Ozguven, S.; Sicak, Y.; Tok, F.; Öztürk, M.; Kocyigit-Kaymakcioglu, B. Synthesis and Molecular Docking Analysis of Novel Hydrazone and Thiosemicarbazide Derivatives Incorporating a Pyrimidine Ring: Exploring Neuroprotective Activity. arXiv 2024. [Google Scholar] [CrossRef]
- Pant, S.; Kumar, K.R.; Rana, P.; Anthwal, T.; Ali, S.M.; Gupta, M.; Chauhan, M.; Nain, S. Novel Substituted Pyrimidine Derivatives as Potential Anti-Alzheimer’s Agents: Synthesis, Biological, and Molecular Docking Studies. ACS Chem. Neurosci. 2024, 15, 783–797. [Google Scholar] [CrossRef]
- Nand, V. Synthesis, characterization, herbicidal activities and in silico studies of some highly functionalized pyrimidine derivatives. Pharma Innov. J. 2024, 13, 150–154. [Google Scholar]
- Luo, J.; Nie, H.; He, L.; Zhao, A.; Wang, T. New library of pyrimido[5,4-e][1,2,4]triazolo[1,5-c]pyrimidine derivatives: Synthesis, herbicidal activity, and molecular docking study. J. Mol. Struct. 2024, 1300, 137246. [Google Scholar] [CrossRef]
- Abouzayed, F.I.; Mostafa, M.S.; Hammad, A.M.; Ghazal, B.; Abouel-Enein, S.A. Synthesis, characterization, thermal, anticancer studies, and density functional theory for potentially active pyrimidine-based complexes. Appl. Organomet. Chem. 2024, 38, e7395. [Google Scholar] [CrossRef]
- Alzahrani, A.Y.; Shehab, W.S.; Amer, A.H.; Assy, M.G.; Mouneir, S.M.; Aziz, M.A.; Hamid, A.M.A. Design, synthesis, pharmacological evaluation, and in silico studies of the activity of novel spiro pyrrolo[3,4-d]pyrimidine derivatives. RSC Adv. 2024, 14, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Bouguessa, I.; Aber, M.; Khier, N.; Dehamchia, M.; Bayou, S.; Ra, Z. Water-Mediated Synthesis, Antibacterial and Antioxidant Evaluation of New Fused Pyrimido-pyrimidine and Pyrimido-purines Derived From Nucleobases. Curr. Green Chem. 2024, 11, 75–83. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Hakami, O.; Amri, N. Synthesis, anticancer activity and molecular docking of new quinolines, quinazolines and 1,2,4-triazoles with pyrido[2,3-d]pyrimidines. Heliyon 2024, 10, e26735. [Google Scholar] [CrossRef]
- Sivanandhan, M.; Parasuraman, A. In-silico Molecular Docking and ADMET predictions of Pyrido[2,3-d]pyrimidine-2,4(1H,3H)-Dione Analogues as promising Antimicrobial, Antioxidant and Anticancer agents. Polycycl. Aromat. Compd. 2024, 44, 1273–1290. [Google Scholar] [CrossRef]
- Abbass, E.M.; Ali, A.K.; El-Farargy, A.F.; Abdel-Haleem, D.R.; Shaban, S.S. Synthesis, toxicological and in silico evaluation of novel spiro pyrimidines against Culex pipiens L. referring to chitinase enzyme. Sci. Rep. 2024, 14, 1516. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Kamel, M.S.; Alzahrani, A.Y.A.; Khalaf, M.M.; Gouda, M.; Ali, M.A.E.A.A. Green chemistry approach for rapid synthesis of indol-3-yl-4H-pyran derivatives, biological assessments, and toxicological activities against Cowpea aphid (Aphis craccivora). Bull. Chem. Soc. Ethiop. 2024, 38, 1077–1090. [Google Scholar] [CrossRef]
- Sayed, M.; Sayed, A.M.; El-Rashedy, A.A.; Saddik, A.A.; Alsaggaf, A.T.; El-Dean, A.M.K.; Hassanien, R.; Ahmed, M. Anti-inflammatory Activity and Computational Biology Study of Indole/Pyrimidine Hybrids. Curr. Org. Chem. 2024, 28, 56–64. [Google Scholar] [CrossRef]
- Bafail, R.S.; Samman, W.A. Anti-parkinsonian, anti-inflammatory, anti-microbial, analgesic, anti-hyperglycemic and anticancer activities of poly-fused ring pyrimidine derivatives. Trop. J. Pharm. Res. 2024, 23, 67–75. [Google Scholar] [CrossRef]
- Kamat, V.; Barretto, D.A.; Poojary, B.; Kumar, A.; Patil, V.B.; Hamzad, S. In vitro α-amylase and α-glucosidase inhibition study of dihydropyrimidinones synthesized via one-pot Biginelli reaction in the presence of a green catalyst. Bioorganic Chem. 2024, 143, 107085. [Google Scholar] [CrossRef] [PubMed]
- Mallidi, K.; Gundla, R.; Makam, P.; Katari, N.K.; Jonnalagadda, S.B. Dual active pyrimidine-based carbocyclic nucleoside derivatives: Synthesis, and in silico and in vitro anti-diabetic and anti-microbial studies. RSC Adv. 2024, 14, 9559–9569. [Google Scholar] [CrossRef] [PubMed]
- Gallo, F.N.; Marquez, A.B.; Fidalgo, D.M.; Dana, A.; Dellarole, M.; García, C.C.; Bollini, M. Antiviral Drug Discovery: Pyrimidine Entry Inhibitors for Zika and Dengue Viruses. Eur. J. Med. Chem. 2024, 272, 116465. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Fan, W.; Yao, C.; Wang, H.; Lu, X.; Wang, Y.; Liu, P.; Ma, Y.; Zhang, Z.; Wang, J. Design, synthesis and biological evaluation of quinazoline and pyrrolo[3,2-d]pyrimidine derivatives as TLR7 agonists for antiviral agents. Org. Biomol. Chem. 2024, 22, 2764–2773. [Google Scholar] [CrossRef]
- Cele, N.; Awolade, P.; Seboletswe, P.; Khubone, L.; Olofinsan, K.; Islam, M.S.; Jordaan, A.; Warner, D.F.; Singh, P. Synthesis, Antidiabetic and Antitubercular Evaluation of Quinoline–pyrazolopyrimidine hybrids and Quinoline-4-Arylamines. ChemistryOpen 2024, 13, e202400014. [Google Scholar] [CrossRef]
- Raghu, M.; Kumar, C.P.; Kumar, K.Y.; Prashanth, M.; Alharethy, F.; Jeon, B.-H. Synthesis, biological evaluation and molecular docking study of pyrimidine linked thiazolidinedione derivatives as potential antimicrobial and antitubercular agents. Bioorganic Med. Chem. Lett. 2024, 103, 129707. [Google Scholar] [CrossRef]
- Abdelwahab, R.E.; Ragheb, M.A.; Elwahy, A.H.; Abdelhamid, I.A.; Abdelmoniem, A.M. Conjugate and regiochemical addition of aminoazoles to 2-(4-(2,2-dicyanovinyl)phenoxy)-N-arylacetamide affording fused pyrimidines linked to phenoxy-N-arylacetamide: Antibacterial activity, molecular docking, and DNA binding studies. J. Mol. Struct. 2024, 1307, 137946. [Google Scholar] [CrossRef]
- Domínguez, A.; Gargallo, R.; Cuestas-Ayllón, C.; Grazu, V.; Fàbrega, C.; Valiuska, S.; Noé, V.; Ciudad, C.J.; Calderon, E.J.; de la Fuente, J.M. Biophysical evaluation of antiparallel triplexes for biosensing and biomedical applications. Int. J. Biol. Macromol. 2024, 264, 130540. [Google Scholar] [CrossRef]
- Anatolyivna, T.V.; YaV, A.; Vasylenko, N.; Kopich, V.; Popilnichenko, S.; Pilyo, S.; Brovarets, V. Auxin-like and Cytokinin-like Effects of New Synthetic Pyrimidine Derivatives on the Growth and Photosynthesis of Wheat. J. Plant Sci. Phytopathol. 2024, 8, 15–24. [Google Scholar]




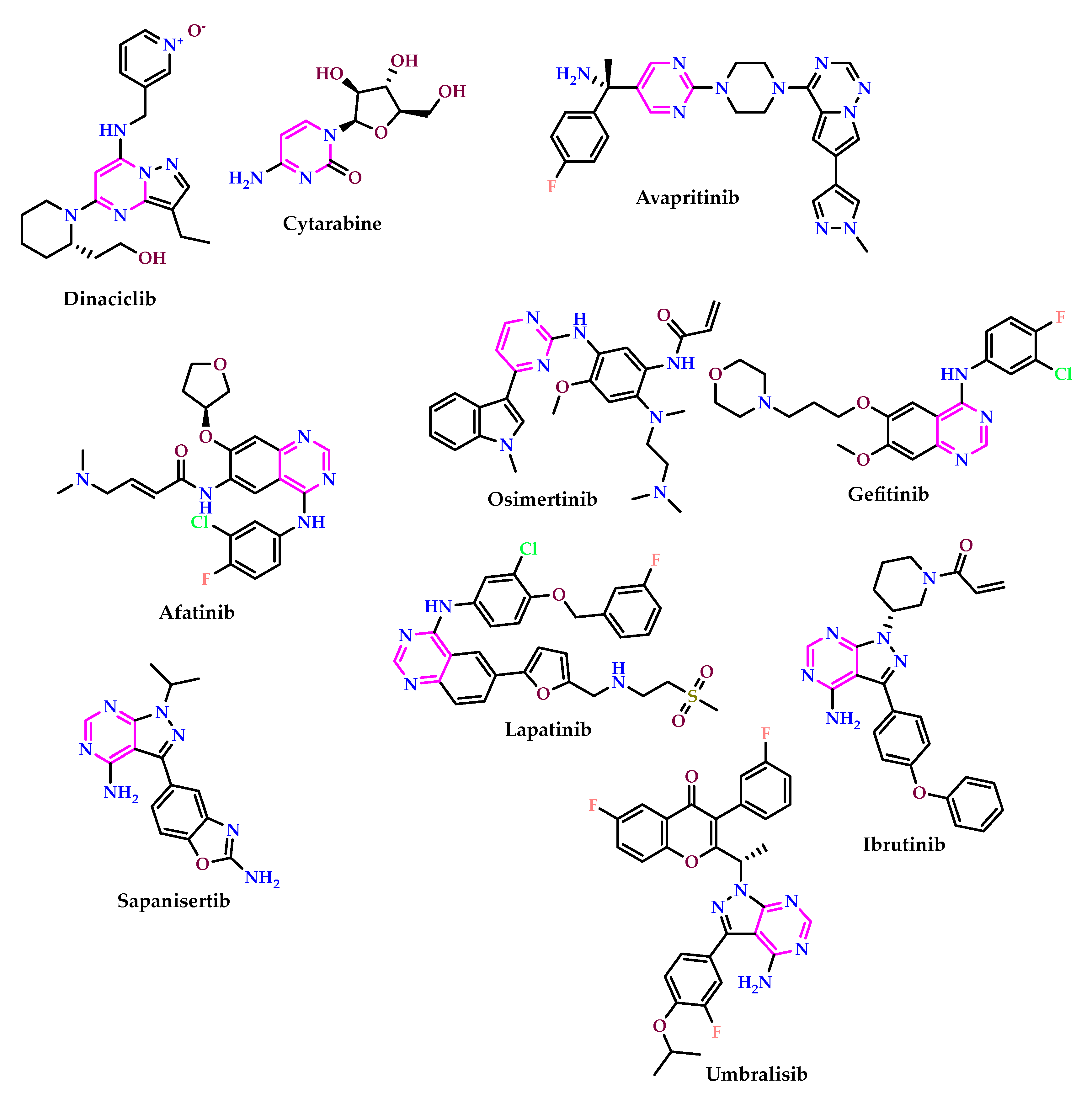
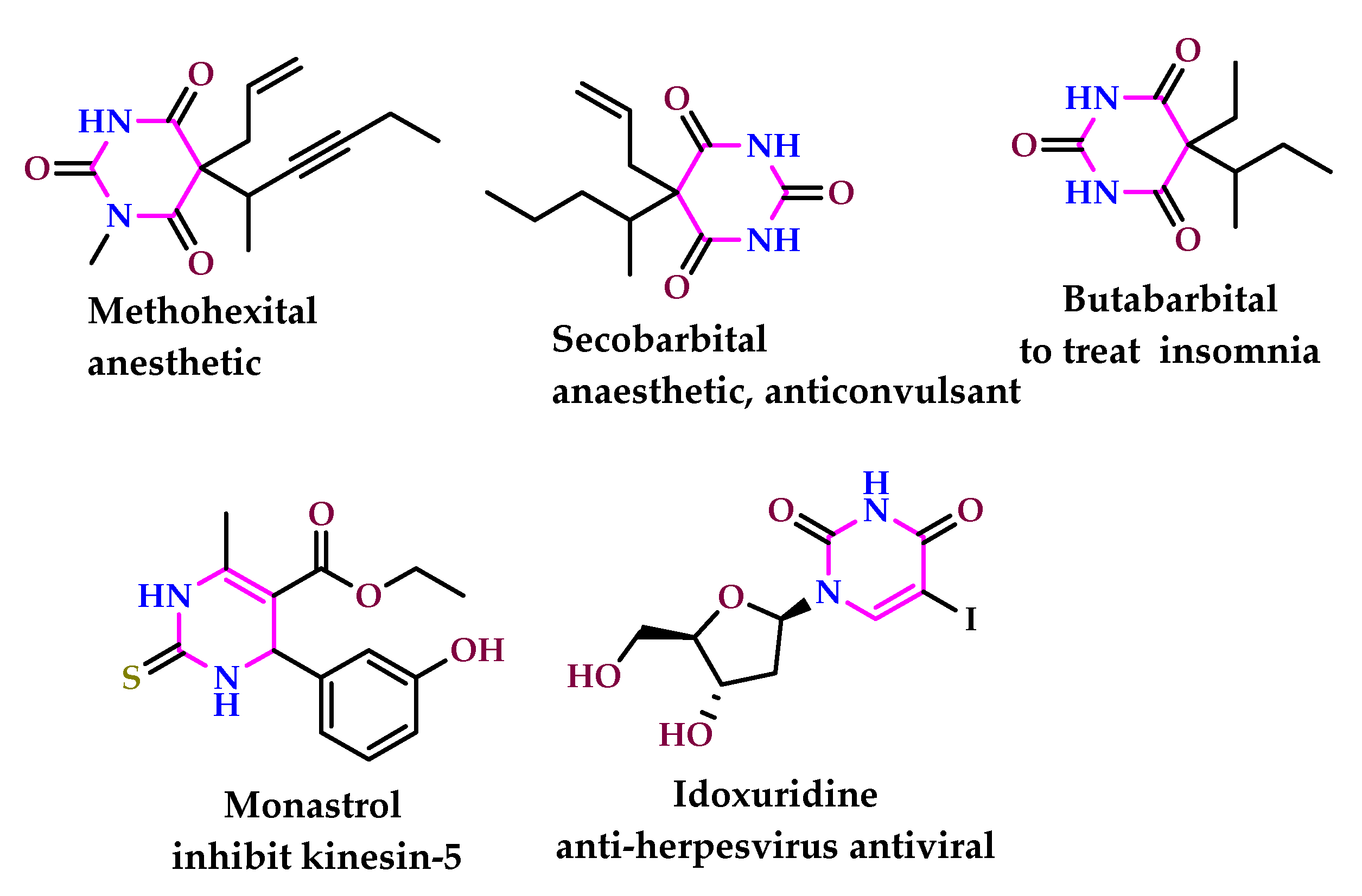

| Compound | SiHa (µM) | A549 (µM) | MCF-7 (µM) | Colo-205 (µM) |
|---|---|---|---|---|
| 12 | 0.03 ± 0.0076 | 0.01 ± 0.0054 | 0.12 ± 0.055 | 0.34 ± 0.033 |
| 13 | 0.071 ± 0.0048 | 0.09 ± 0.0018 | 0.011 ± 0.0067 | 0.16 ± 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venugopala, K.N.; Kamat, V. Pyrimidines: A New Versatile Molecule in the Drug Development Field, Scope, and Future Aspects. Pharmaceuticals 2024, 17, 1258. https://doi.org/10.3390/ph17101258
Venugopala KN, Kamat V. Pyrimidines: A New Versatile Molecule in the Drug Development Field, Scope, and Future Aspects. Pharmaceuticals. 2024; 17(10):1258. https://doi.org/10.3390/ph17101258
Chicago/Turabian StyleVenugopala, Katharigatta N., and Vinuta Kamat. 2024. "Pyrimidines: A New Versatile Molecule in the Drug Development Field, Scope, and Future Aspects" Pharmaceuticals 17, no. 10: 1258. https://doi.org/10.3390/ph17101258
APA StyleVenugopala, K. N., & Kamat, V. (2024). Pyrimidines: A New Versatile Molecule in the Drug Development Field, Scope, and Future Aspects. Pharmaceuticals, 17(10), 1258. https://doi.org/10.3390/ph17101258







