The Bi-(AID-1-T) G-Quadruplex Has a Janus Effect on Primary and Recurrent Gliomas: Anti-Proliferation and Pro-Migration
Abstract
1. Introduction
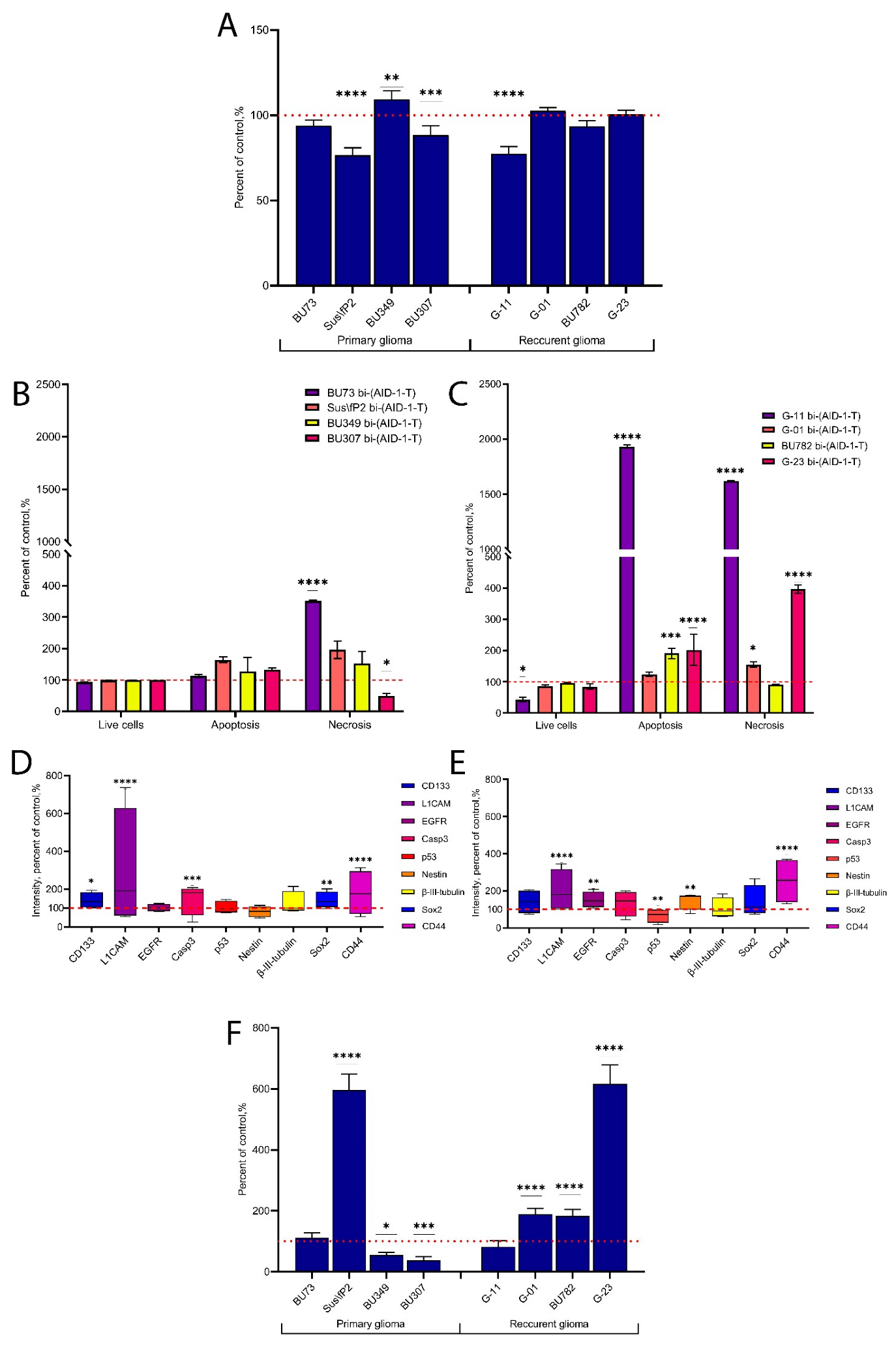
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Aptamer Treatment
4.3. MTS Assay
4.4. Flow Cytometry
4.5. Immunocytochemistry (ICC)
4.6. Assessment of Cell Penetration and Distribution
4.7. Assessment of Cell Migratory Activity
4.8. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Feldheim, J.; Kessler, A.F.; Feldheim, J.J.; Schulz, E.; Wend, D.; Lazaridis, L.; Kleinschnitz, C.; Glas, M.; Ernestus, R.-I.; Brandner, S.; et al. Effects of Long-Term Temozolomide Treatment on Glioblastoma and Astrocytoma WHO Grade 4 Stem-like Cells. Int. J. Mol. Sci. 2022, 23, 5238. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, Y.; Nonoguchi, N.; Okuzaki, D.; Wada, Y.; Motooka, D.; Hirota, Y.; Toho, T.; Yoshikawa, N.; Furuse, M.; Kawabata, S.; et al. Chronic Pathophysiological Changes in the Normal Brain Parenchyma Caused by Radiotherapy Accelerate Glioma Progression. Sci. Rep. 2021, 11, 22110. [Google Scholar] [CrossRef] [PubMed]
- Osuka, S.; Van Meir, E.G. Overcoming Therapeutic Resistance in Glioblastoma: The Way Forward. J. Clin. Investig. 2017, 127, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Dinevska, M.; Gazibegovic, N.; Morokoff, A.P.; Kaye, A.H.; Drummond, K.J.; Mantamadiotis, T.; Stylli, S.S. Inhibition of Radiation and Temozolomide-Induced Glioblastoma Invadopodia Activity Using Ion Channel Drugs. Cancers 2020, 12, 2888. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xiang, J. Aptamers, the Nucleic Acid Antibodies, in Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 2793. [Google Scholar] [CrossRef]
- Monaco, I.; Camorani, S.; Colecchia, D.; Locatelli, E.; Calandro, P.; Oudin, A.; Niclou, S.; Arra, C.; Chiariello, M.; Cerchia, L.; et al. Aptamer Functionalization of Nanosystems for Glioblastoma Targeting through the Blood–Brain Barrier. J. Med. Chem. 2017, 60, 4510–4516. [Google Scholar] [CrossRef]
- Roxo, C.; Kotkowiak, W.; Pasternak, A. G-Quadruplex-Forming Aptamers—Characteristics, Applications, and Perspectives. Molecules 2019, 24, 3781. [Google Scholar] [CrossRef]
- Carvalho, J.; Mergny, J.-L.; Salgado, G.F.; Queiroz, J.A.; Cruz, C. G-Quadruplex, Friend or Foe: The Role of the G-Quartet in Anticancer Strategies. Trends Mol. Med. 2020, 26, 848–861. [Google Scholar] [CrossRef]
- Legatova, V.; Samoylenkova, N.; Arutyunyan, A.; Tashlitsky, V.; Zavyalova, E.; Usachev, D.; Pavlova, G.; Kopylov, A. Covalent Bi-Modular Parallel and Antiparallel G-Quadruplex DNA Nanocostructs Reduce Viability of Patient Glioma Primary Cell Cultures. Int. J. Mol. Sci. 2021, 22, 3372. [Google Scholar] [CrossRef] [PubMed]
- Antipova, O.; Samoylenkova, N.; Savchenko, E.; Zavyalova, E.; Revishchin, A.; Pavlova, G.; Kopylov, A. Bimodular Antiparallel G-Quadruplex Nanoconstruct with Antiproliferative Activity. Molecules 2019, 24, 3625. [Google Scholar] [CrossRef] [PubMed]
- Kopylov, A.M.; Samoylenkova, N.; Bizayeva, A.; Arutyunyan, A.; Tashlitsky, V.; Golbin, D.; Usachev, D.; Pavlova, G. P13.19 Bi-Modular G-Quadruplex DNA-Crypto-Aptamers Diminish Viability of Glioma Primary Cell Cultures of Patients. Neuro-Oncology 2021, 23 (Suppl. 2), ii36–ii37. [Google Scholar] [CrossRef]
- Caragher, S.; Chalmers, A.J.; Gomez-Roman, N. Glioblastoma’s Next Top Model: Novel Culture Systems for Brain Cancer Radiotherapy Research. Cancers 2019, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Ishida, J.; Kurozumi, K.; Ichikawa, T.; Otani, Y.; Oka, T.; Tomita, Y.; Hattori, Y.; Uneda, A.; Matsumoto, Y.; et al. δ-Catenin Promotes Bevacizumab-Induced Glioma Invasion. Mol. Cancer Ther. 2019, 18, 812–822. [Google Scholar] [CrossRef]
- Merrick, M.; Mimlitz, M.J.; Weeder, C.; Akhter, H.; Bray, A.; Walther, A.; Nwakama, C.; Bamesberger, J.; Djam, H.; Abid, K.; et al. In Vitro Radiotherapy and Chemotherapy Alter Migration of Brain Cancer Cells before Cell Death. Biochem. Biophys. Rep. 2021, 27, 101071. [Google Scholar] [CrossRef]
- Kochanowski, P.; Catapano, J.; Pudełek, M.; Wróbel, T.; Madeja, Z.; Ryszawy, D.; Czyż, J. Temozolomide Induces the Acquisition of Invasive Phenotype by O6-Methylguanine-DNA Methyltransferase (MGMT)+ Glioblastoma Cells in a Snail-1/Cx43-Dependent Manner. Int. J. Mol. Sci. 2021, 22, 4150. [Google Scholar] [CrossRef]
- Arevalo, O.D.; Soto, C.; Rabiei, P.; Kamali, A.; Ballester, L.Y.; Esquenazi, Y.; Zhu, J.-J.; Riascos, R.F. Assessment of Glioblastoma Response in the Era of Bevacizumab: Longstanding and Emergent Challenges in the Imaging Evaluation of Pseudoresponse. Front. Neurol. 2019, 10, 460. [Google Scholar] [CrossRef]
- Shankar, A.; Kumar, S.; Iskander, A.; Varma, N.R.; Janic, B.; Decarvalho, A.; Mikkelsen, T.; Frank, J.A.; Ali, M.M.; Knight, R.A.; et al. Subcurative Radiation Significantly Increases Cell Proliferation, Invasion, and Migration of Primary Glioblastoma Multiforme in Vivo. Chin. J. Cancer 2014, 33, 148–158. [Google Scholar] [CrossRef]
- Falk, A.T.; Moncharmont, C.; Guilbert, M.; Guy, J.-B.; Alphonse, G.; Trone, J.-C.; Rivoirard, R.; Gilormini, M.; Toillon, R.-A.; Rodriguez-Lafrasse, C.; et al. Radiation-Induces Increased Tumor Cell Aggressiveness of Tumors of the Glioblastomas? Bull. Du Cancer 2014, 101, 876–880. [Google Scholar] [CrossRef]
- Campos, B.; Olsen, L.R.; Urup, T.; Poulsen, H.S. A Comprehensive Profile of Recurrent Glioblastoma. Oncogene 2016, 35, 5819–5825. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, H.; Yan, W.; Yang, P.; Bao, Z.; Zhang, C.; Jiang, T.; You, Y. Genetic and Clinical Characteristics of Primary and Secondary Glioblastoma Is Associated with Differential Molecular Subtype Distribution. Oncotarget 2015, 6, 7318–7324. [Google Scholar] [CrossRef] [PubMed]
- Kubelt, C.; Hattermann, K.; Sebens, S.; Mehdorn, H.M.; Held-Feindt, J. Epithelial-to-Mesenchymal Transition in Paired Human Primary and Recurrent Glioblastomas. Int. J. Oncol. 2015, 46, 2515–2525. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-A.; Chang, C.-Y.; Hsueh, K.-W.; Su, H.-L.; Chiou, T.-W.; Lin, S.-Z.; Harn, H.-J. Migration/Invasion of Malignant Gliomas and Implications for Therapeutic Treatment. Int. J. Mol. Sci. 2018, 19, 1115. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhu, F.; Dai, L.; Liu, X.; Shao, L.; Hao, L.; Cang, S.; Cheng, J. Caspase-3 in Glioma Indicates an Unfavorable Prognosis by Involving Surrounding Angiogenesis and Tumor Cell Repopulation. J. Neuro-Oncol. 2023, 163, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, W.; Chen, B.; Jiang, T.; Wang, Z. Prognostic and Predictive Value of P53 in Low MGMT Expressing Glioblastoma Treated with Surgery, Radiation and Adjuvant Temozolomide Chemotherapy. Neurol. Res. 2010, 32, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, X.; Hao, S.; Su, Z.; Zhang, P.; Li, Y.; Song, G.; Yu, L.; Wang, J.; Ji, N.; et al. Analysis of Treatment Tolerance and Factors Associated with Overall Survival in Elderly Patients with Glioblastoma. World Neurosurg. 2016, 95, 77–84. [Google Scholar] [CrossRef]
- Matsuda, Y.; Ishiwata, T.; Yoshimura, H.; Hagio, M.; Arai, T. Inhibition of Nestin Suppresses Stem Cell Phenotype of Glioblastomas through the Alteration of Post-Translational Modification of Heat Shock Protein HSPA8/HSC71. Cancer Lett. 2015, 357, 602–611. [Google Scholar] [CrossRef]
- Mooney, K.L.; Choy, W.; Sidhu, S.; Pelargos, P.; Bui, T.T.; Voth, B.; Barnette, N.; Yang, I. The Role of CD44 in Glioblastoma Multiforme. J. Clin. Neurosci. 2016, 34, 1–5. [Google Scholar] [CrossRef]
- Mohanan, V.; Temburni, M.K.; Kappes, J.C.; Galileo, D.S. L1CAM Stimulates Glioma Cell Motility and Proliferation through the Fibroblast Growth Factor Receptor. Clin. Exp. Metastasis 2013, 30, 507–520. [Google Scholar] [CrossRef]
- Giordano, M.; Cavallaro, U. Different Shades of L1CAM in the Pathophysiology of Cancer Stem Cells. J. Clin. Med. 2020, 9, 1502. [Google Scholar] [CrossRef] [PubMed]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Cesarini, V.; Scopa, C.; Silvestris, D.A.; Scafidi, A.; Petrera, V.; Del Baldo, G.; Gallo, A. Aptamer-Based In Vivo Therapeutic Targeting of Glioblastoma. Molecules 2020, 25, 4267. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, S.; Brancato, V.; Affinito, A.; Salvatore, M.; Cavaliere, C.; Condorelli, G. The Role of RNA and DNA Aptamers in Glioblastoma Diagnosis and Therapy: A Systematic Review of the Literature. Cancers 2020, 12, 2173. [Google Scholar] [CrossRef]
- Wang, J.-M.; Huang, F.-C.; Kuo, M.H.-J.; Wang, Z.-F.; Tseng, T.-Y.; Chang, L.-C.; Yen, S.-J.; Chang, T.-C.; Lin, J.-J. Inhibition of Cancer Cell Migration and Invasion through Suppressing the Wnt1-Mediating Signal Pathway by G-Quadruplex Structure Stabilizers. J. Biol. Chem. 2014, 289, 14612–14623. [Google Scholar] [CrossRef]
- Chang, L.-C.; Chen, T.-C.; Chen, S.-J.; Chen, C.-L.; Lee, C.-C.; Wu, S.-H.; Yen, Y.; Huang, H.-S.; Lin, J.-J. Identification of a New Class of WNT1 Inhibitor: Cancer Cells Migration, G-Quadruplex Stabilization and Target Validation. Oncotarget 2016, 7, 67986–68001. [Google Scholar] [CrossRef]
- Zheng, X.-H.; Nie, X.; Liu, H.-Y.; Fang, Y.-M.; Zhao, Y.; Xia, L.-X. TMPyP4 Promotes Cancer Cell Migration at Low Doses, but Induces Cell Death at High Doses. Sci. Rep. 2016, 6, 26592. [Google Scholar] [CrossRef]
- Esposito, V.; Russo, A.; Amato, T.; Vellecco, V.; Bucci, M.; Mayol, L.; Russo, G.; Virgilio, A.; Galeone, A. The ‘Janus Face’ of the Thrombin Binding Aptamer: Investigating the Anticoagulant and Antiproliferative Properties through Straightforward Chemical Modifications. Bioorganic Chem. 2018, 76, 202–209. [Google Scholar] [CrossRef]


| Name | Grade | IDH1 Status | Tumor Location | |
|---|---|---|---|---|
| Primary glioma | BU73 | III | wild type | Right temporal lobe |
| Sus\fP2 | IV | wild type | Left temporal lobe | |
| BU349 | IV | wild type | Left frontal lobe | |
| BU307 | IV | wild type | Left temporal lobe | |
| Recurrent glioma | G-11 | III-IV | wild type | Right frontal–temporal–insular region |
| G-01 | IV | wild type | Left frontal lobe | |
| BU782 | IV | wild type | Parieto-occipital region | |
| G-23 | IV | wild type | Left frontal parasagittal region |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlova, S.; Fab, L.; Savchenko, E.; Ryabova, A.; Ryzhova, M.; Revishchin, A.; Pronin, I.; Usachev, D.; Kopylov, A.; Pavlova, G. The Bi-(AID-1-T) G-Quadruplex Has a Janus Effect on Primary and Recurrent Gliomas: Anti-Proliferation and Pro-Migration. Pharmaceuticals 2024, 17, 74. https://doi.org/10.3390/ph17010074
Pavlova S, Fab L, Savchenko E, Ryabova A, Ryzhova M, Revishchin A, Pronin I, Usachev D, Kopylov A, Pavlova G. The Bi-(AID-1-T) G-Quadruplex Has a Janus Effect on Primary and Recurrent Gliomas: Anti-Proliferation and Pro-Migration. Pharmaceuticals. 2024; 17(1):74. https://doi.org/10.3390/ph17010074
Chicago/Turabian StylePavlova, Svetlana, Lika Fab, Ekaterina Savchenko, Anastasia Ryabova, Marina Ryzhova, Alexander Revishchin, Igor Pronin, Dmitry Usachev, Alexey Kopylov, and Galina Pavlova. 2024. "The Bi-(AID-1-T) G-Quadruplex Has a Janus Effect on Primary and Recurrent Gliomas: Anti-Proliferation and Pro-Migration" Pharmaceuticals 17, no. 1: 74. https://doi.org/10.3390/ph17010074
APA StylePavlova, S., Fab, L., Savchenko, E., Ryabova, A., Ryzhova, M., Revishchin, A., Pronin, I., Usachev, D., Kopylov, A., & Pavlova, G. (2024). The Bi-(AID-1-T) G-Quadruplex Has a Janus Effect on Primary and Recurrent Gliomas: Anti-Proliferation and Pro-Migration. Pharmaceuticals, 17(1), 74. https://doi.org/10.3390/ph17010074







