Abstract
Moringa oleifera L., commonly known as Kelor in Indonesia and miracle tree in English, has a rich history of utilization for medicinal, nutritional, and water treatment purposes dating back to ancient times. The plant is renowned for its abundance of vitamins, minerals, and various chemical constituents, making it a valuable resource. Among its notable pharmacological properties are its effectiveness as an anti-diabetic, anti-diarrheal, anti-helmintic, anti-leishmanial, anti-fungal, anti-bacterial, anti-allergic, anti-cancer, anti-inflammatory, and anti-oxidant agent. In this comprehensive review, we delve into the extensive pharmacological applications and phytochemical constituents of M. oleifera and its application in dental health.
1. Introduction
Moringa oleifera L. comes from the Moringaeceae family and is commonly known as Kelor in Indonesia, Sahajan in India, and Horseradish tree or Drumstick tree in English. It is also described as a miracle tree due to its nutritional value, diverse functions, and medicinal properties. M. oleifera can grow up to 12 m in tropical and subtropical environments. Although it is native to South Asia, the cultivation itself has already spread to the Middle East, Africa, Asia, and other areas. Traditionally, M. oleifera has been used in medicine, skincare, breastmilk production, and even food. Almost all parts of M. oleifera can be useful. Nowadays, M. oleifera is also used for water purification, animal feed, as a bio-stimulant, bio-pesticide, and biomass as biodiesel production in industrial and agricultural processes [1,2,3,4,5,6]. Table 1 describes the traditional usage of M. oleifera.

Table 1.
Traditional usage of M. oleifera.
As a food and stimulant, M. oleifera is known to have an abundance of nutritional value and is comparatively easy to cultivate because of its rapid growth and good adaptability to climate change. Thus, in poor countries, M. oleifera is used as a source of proteins, calories, minerals, and vitamins. It has been reported that dry leaves of M. oleifera contain more calories, protein, carbohydrate, fiber, vitamin B, calcium, magnesium, phosphorus, potassium, copper, and iron than fresh leaves. Meanwhile, fresh leaves contain more vitamin C and E. Between the leaf, seed, and pod of M. oleifera, proteins, vitamin E, and magnesium have been found to be more abundant in the seed [7,8,9].
Due to many its traditional usages, research has been conducted to prove its ability as medicine. It is reported to have pharmacological properties such as anti-diabetic, anti-diarrheal, anti-helmintic, anti-leishmanial, anti-fungi, anti-bacterial, anti-allergic, anti-cancer, anti-inflammatory, and anti-oxidant. Hastuty and Nitia reported the efficiency of M. oleifera leaf extract to raise hemoglobin levels in young girls. They showed a value of 10.83 g/dL before treatment, while after treatment with M. oleifera, the hemoglobin levels increased to 12.72 g/dL [10]. Yuliastuti and Kurnia also reported the effect of M. oleifera on hemoglobin levels in anemic pregnant women. The result showed a significant difference, where before treatment, the respondents showed hemoglobin levels about 10.2 g/dL, and after treatment these increased to 10.8 g/dL (p = 0.003 < 0.05) [11].
Dental health has been a concern for researchers to this day. The various infections that can occur in the teeth cause a decline in health. Dental infections occur due to the growth of various kinds of microbes in the dental and oral area. Among these are odontogenic infections and periradicular periodontitis that occur in the root canal system caused by anaerobic bacteria such as Porphyromonas gingivalis, Enteroccus faecalis, and Candida albicans [12,13,14]. E. faecalis is reported to infect root canals up to 30–80% [15]. In addition, based on polymerase chain reaction (PCR) analysis, the bacteria Tannerella forysthia, Treponema denticola, Dialister pneumosintes, and Prevotella tannerae were also reported to infect root canals with high prevalence [16]. These infections occur due to biofilm formation by microbes on the tooth area [17]. The use of antibiotics is a very useful treatment due to their effectiveness, low cost, and compatibility. However, resistance to antibiotic agents by microbes has been identified. Gram-negative bacteria have been reported to be resistant to beta-lactam antibiotics due to an enzyme that can open the ring in the beta-lactam structure, thus inactivating the action of the drug [18].
Therefore, natural product drug discovery research is of particular interest to researchers as a natural antibacterial agent. The main parameters in determining antibacterial activity are the inhibition zone value, minimum inhibition concentration (MIC) as a reference for the minimum concentration that can inhibit bacterial growth, and minimum bactericidal concentration (MBC) as a reference for the minimum concentration needed to kill a microorganism [19]. Based on the bioactivity and phytochemicals contained in M. oleifera, this plant has become one of the natural sources for dental disease treatment. M. oleifera seed extract was reported to provide MIC and antibiofilm values against P. gingivalis of 12.5 mg/mL and 6.25 mg/mL, respectively [20]. M. oleifera nanosuspension can inhibit Aggregatibacteri actinomycetemcomitans, P. gingivalis, Prevotella intermedia, Fusobacterium nucleatum with an MIC and MBC of 25% and 12.5%, respectively [21]. Based on this description, this review will explain the bioactivity contained in M. oleifera and describe the role of the plant for dental health and the chemical components contained therein.
2. Phytochemical Constituent
The pharmacological effects of M. oleifera are influenced by its phytochemical components. Previous studies have reported that there are several groups of compounds that are unique to each part of M. oleifera. The flowers are known to contain flavonoids, alkaloids, sucrose, and amino acids such as kaempferitrin, isoquercitrin, and rhamnetin. Furthermore, the stem contains alkaloid compounds such as moringinine and moringin, octacosanoic acid, β-sitosterol, and 4-hydroxymellein. The seed contains high contents of 4-(α-l-rhamnosyloxy) phenylacetonitrile, benzylglucosinolate, 4-(α-l-rhamnosyloxy) benzylisothiocyanate, O-ethyl-4-(α-l-rhamnosyloxy) benzyl, and 4-(α-l-rhamnopyranosyloxy)-benzylglucosinolate carbamate, while the fruit contains cytokines. In addition, the whole pods were specific for O-[2′-hydroxy-3′-(2′′-heptenyloxy)]-propyl undecanoate, methyl-p-hydroxybenzoate, thiocarbamates, isothiocyanate, nitrile, and O-ethyl-4-[(α-l-rhamnosyloxy)-benzyl] carbamate [22]. The seeds of M. oleifera contain total flavonoids 144.07 mg/kg, total polyphenols 145.16 mg/100 g, and proanthocyanidines 140.49 mg/kg. In addition, the oil of M. oleifera contains 18.24 mg rutin equivalent/g (total flavonoids), 37.94 mg ascorbic acid equivalent/g (total antioxidant capacity), and 40.17 mg GA equivalent/g (total phenols). Based on this description, the following are some of the structures of phytochemical components contained in M. oleifera [23].
2.1. Phenolic
Niazirin (1) was obtained through an ethanol and butanol extraction of the seeds and leaves of M. oleifera. It was reported to inhibit α-glucosidase inhibitor with an IC50 value of 382.2 µM. [24,25,26,27,28]. Caffeoylquinic acid (2), 4-O-caffeoylquinic acid (3), 4-O-(3′-O-α-D-glucopyranosyl)-caffeoylquinic acid (4), 4-O-(4′-O-α-D-glucopyranosyl)-caffeoylquinic acid (5), 4-O-β-D-glucopyranoside benzoic acid (6), 5-O-caffeoylquinic acid (7), benzaldehyde 4-O-α-L-rhamnopyranoside (8), chlorogenic acid (9), methyl caffeoylquinate (10), methyl 4-caffeoylquinate (11), and 3,4-dihydroxybenzoic acid (12) could be obtained through an ethyl acetate and butanol extraction of M. oleifera leaves. The structures of the compounds are shown in Figure 1 [24,25,26,27,28].
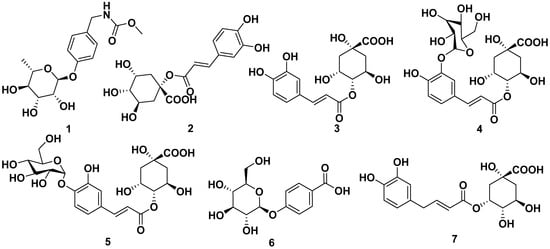
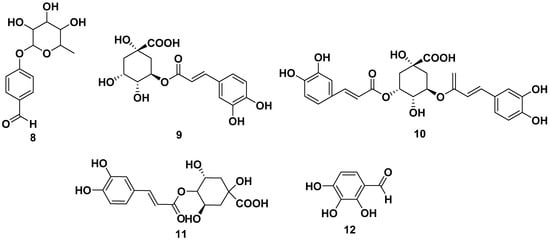
Figure 1.
Phenolic compounds in ethanol and butanol extracts of seeds and leaves of M. oleifera.
Other phenolic compounds that could be isolated from various parts of M. oleifera were caffeic acid (13), gallic acid (14), p-coumaric (15), and vanillin (16). Cryptochlorogenic acid (17) also could be obtained from M. oleifera, and it was also reported to have anticancer activity against MCF-7 with an IC50 value of 20.8 M. The structures are shown in Figure 2 [28,29,30].
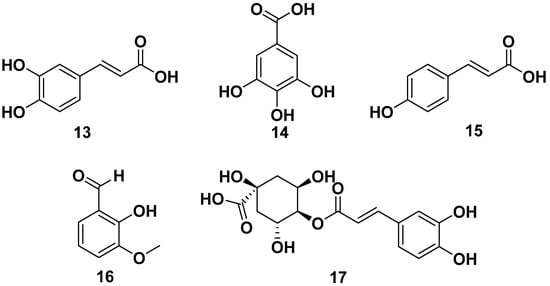
Figure 2.
Phenolic compounds of other parts of M. oleifera.
2.2. Glucosinolate
Glucosinolate compounds, as shown in Figure 3, could be isolated from the ethanol extracts of seeds of M. oleifera. These include the following compounds: 4-(3′-O-acetyl-α-L-rhamnosyloxy) benzyl isothiocyanate (18), 4-(α-L-rhamnopyranosyloxy)-benzylglucosinolate (19), 4-(α-L-rhamnosyl) benzyl ethyl ester (20), moringaside C (21), moringaside D (22), moringaside E (23), moringaside F (24), moringaside G (25), moringin (26), niazimicin (27), and glucomoringin (28). Furthermore, compound 23 exhibited α-glucosidase inhibitory activity with an IC50 value of 382.8 µM, meanwhile compound 26 had a reported an anti-adipogenic effect and an anticancer activity against HeLa cells with an IC50 value of 9.2 µg/mL. Meanwhile, compound 28 exhibited anti-allergic properties with IC50 values towards β-hexosaminidase and histamine releases of 10.43 and 27.22 µM, respectively. It is also reported to has antiviral properties against H1N1 with an IC50 value of 0.98 µg/mL [13,15,26,30,31,32,33,34,35,36,37].
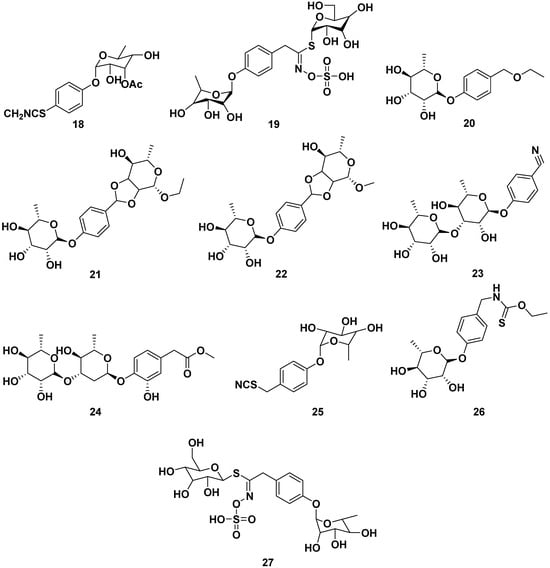
Figure 3.
Glucosinolate compounds of seed ethanol extract of M. oleifera.
Other glucosinolates found in M. oleifera were 4-(4′-O-acetyl-α-L-rhamnosyloxy) benzyl isothiocyanate (29), benzyl glucosinolate (30), 4-[(2′-O-acetyl-α-L-rhamnosyloxy) benzyl] isothiocyanate (31), 4-[(3′-O-acetyl-α-L-rhamnosyloxy) benzyl] isothiocyanate (32), niazinin (33), and niazinin B (34). Furthermore, compound 31 and 32 exhibited NO inhibitory activity with IC50 values of 1.67 and 2.66 µM, respectively, meanwhile compound 33 exhibited antileishmanial properties with an IC50 value of 5.25 mM. The structures are shown in Figure 4 [24,38,39,40,41].
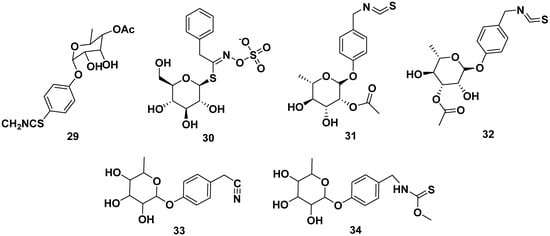
Figure 4.
Glucosinolate compounds of other parts of M. oleifera.
2.3. Flavonoid
The leaves, barks, and seeds of M. oleifera contained various flavonoid compounds, as shown in Figure 5. The flavonoids reported were astragalin (35), isoquercitrin (36), kaempferol (37), kaempferol 3-O-glucoside (38), kaempferol acetyl glycoside (39), kaempferol-3-O-(6″-malonyl-glucoside) (40), quercetin (41), quercetin 3-O-β-D-glucopyranoside (42), quercetin-3-acetyl-glucoside (43), quercetin-3-O-(6″-malonyl-glucoside) (44), quercetin-3-O-β-D-(6″-O-3-hydroxy-3-methylglutaryl)-glucoside (45), rutin (46), vitexin (47), and 3,5,6-trihydroxy-2-(2,3,4,5,6-pentahydroxyphenyl)-4Hchromen-4-one (48). Those compounds could be obtained through extraction with methanol, ethanol, butanol, and ethyl acetate. Compounds 35, 36, and 46 were reported to inhibit CYP3A4 and CYP2D6, with IC50 values of 69.5 and 90 µM for compound 35, whereas compounds 36 and 46 were reported to be CYP3AP inhibitors with IC50 values of 65.5 and 60 µM, respectively.
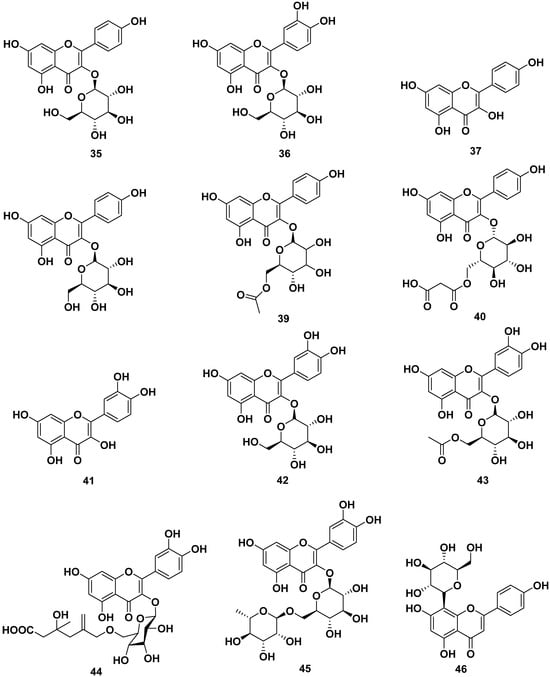
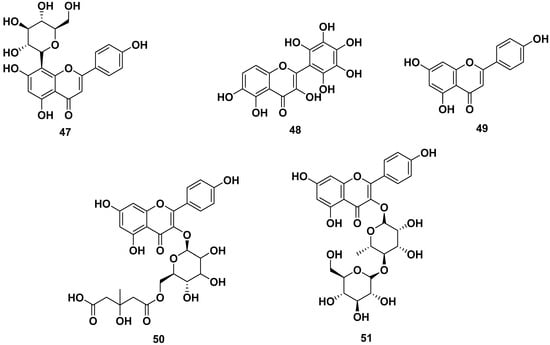
Figure 5.
Flavonoid compounds of leaves, barks, and seeds of M. oleifera.
Compounds 37, 41, and 48 were reported to have anti-allergic properties by inhibiting β-hexosaminidase and histamine release, with IC50 values of 29.39 and 46.94 µM, respectively, for compound 37. Compound 41 exhibited IC50 values of 19.07 and 7.77 µM, respectively. Compound 48 showed IC50 values of 17.70 and 44.87 µM, respectively. Furthermore, compound 46 also inhibited α-glucosidase and pancreatic lipase with IC50 values of 40 and 35 µg/mL, respectively. Compound 47 also exhibited antiviral properties against virus H1N1 with an IC50 value of 3.42 µg/mL [13,24,25,26,27,33,39,42,43,44,45,46].
Apigenin (49), kaempferol-3-O-[methyl-(S)-3-hydroxy-3-methylglutaroyl(1→6)]-β-d-glucopyranoside (50), and multiflorin-B (51) also could be obtained from M. oleifera ethanol extracts [42,47,48].
2.4. Fatty Acid
Figure 6 shows the structure of fatty acid compounds from the ethanol, methanol, and ethyl acetate extracts of leaves, seeds, and flowers of M. oleifera. The compounds are glycerol-1-(9-octadecanoate) (52), heneicosanoic acid (53), monoacetyl glycerol (54), monacosan-15-one (55), octacosanol (56), oleic acid (57), 3,4-methyleneazelaic acid (58), and triolein acid (59). Compound 57 exhibited anti-allergic properties by inhibiting β-hexosaminidase and histamine release, with IC50 values of 53.76 and 56.05 µM, respectively [40,42,49,50,51,52,53].
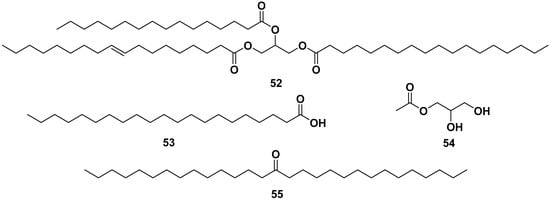
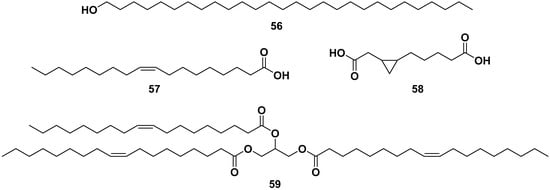
Figure 6.
Fatty acid compounds of leaves, seeds, and flowers of ethanol, methanol, and ethyl acetate extracts of M. oleifera.
2.5. Ester
Ethyl geranyl acetate (60), ethyl-(E)-undec-6-enoate (61), methyl heptanoate (62), methyl-4-(α-L-rhamnopyranosyloxy) benzyl carbamate (63), O-ethyl-4-(α-l-rhamnosyloxy)benzyl carbamate (64), and 2-formyl-5-methyl-1H-pyrrol-1-ylbutanoic acid (65) were ester groups isolated from leaves, flowers, and seeds of ethanol, methanol, and n-hexane M. oleifera extracts. The structures are shown in Figure 7. Compound 61 exhibited anti-allergic properties by inhibiting β-hexosaminidase and histamine release with IC50 values of 82.68 and 82.07 µM, respectively [42,49,50,53,54,55].
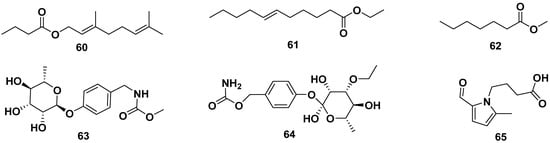
Figure 7.
Ester compounds of leaves, flowers, and seeds of ethanol, methanol, and n-hexane extracts of M. oleifera.
2.6. Alkaloid
The roots, seeds, and leaves of M. oleifera contain alkaloids, as shown in Figure 8. Some of them could be obtained from butanol extraction. The alkaloids contained in M. oleifera include the following: marumoside A (66), marumoside B (67), aurantiamide acetate (68), hostine D (69), methyl 4-(α-L-rhamnopyranosyloxy) benzylcarbamate (70), pyrrolemorine A (71), pyrrolemorine B (72), pyrrolemorine C (73), pyrrolemorine D (74), pyrrolemorine E (75), pyrrolemorine F (76), pyrrolemorine G (77), pyrrolemarumine (78), tangutorid E (79), and tangutorid F (80). Compounds 71 and 75 demonstrated notable neuroprotective effects. At a concentration of 0.1 μM, they effectively mitigated PC12 cell damage caused by oxygen glucose deprivation and concurrently reduced the expression of NF-kB [42,47,56,57].
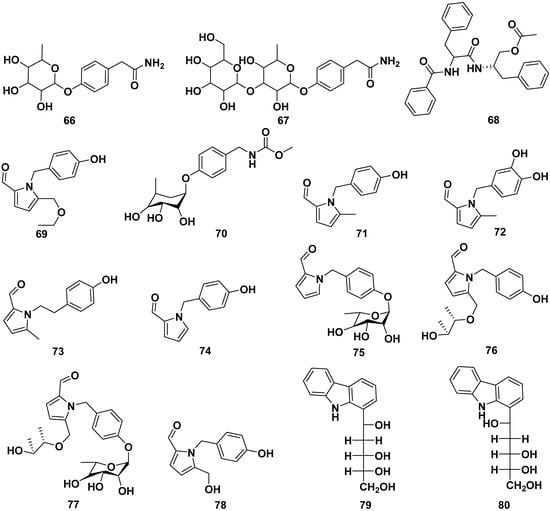
Figure 8.
Alkaloid compounds of roots, seeds, and leaves of M. oleifera.
2.7. Sterol
All parts of M. oleifera contain sterol compounds, as shown in Figure 9. The compounds could be isolated from methanol, ethanol, ethyl acetate, and acetone extracts. The following are sterols isolated from various parts of M. oleifera: β-sitosterone (81), stigmasterol (82), β-sitosterol-3-O-glucoside (83), β-sitosteryl oleate (84), and 24-methylene-9,19-cyclolanostan-3-ol (85). Compounds 82 and 83 exhibited anti-allergic properties by inhibiting β-hexosaminidase and histamine release with IC50 values of 75.92 and 38.27 µM, respectively, for compound 82; meanwhile, compound 83 only inhibited β-hexosaminidase release with an IC50 value of 24.93 µM. Moreover, compound 82 exhibited anti-inflammatory characteristics by inhibiting caspase 1 and NF-kB. It also exhibited anti-adipogenic properties by reducing the S and G2/M phases, inhibiting ROS, and enhancing glucose uptake [13,31,37,58,59,60,61].
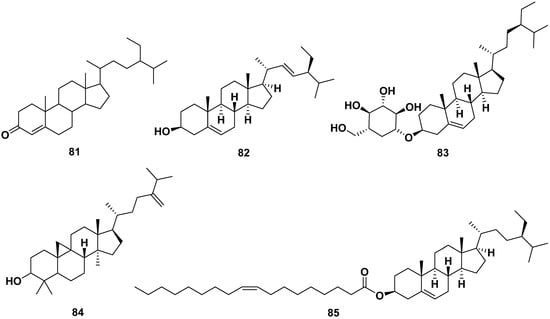
Figure 9.
Sterol compounds of M. oleifera.
2.8. Terpene
(S) Linalyl-β-D-glucoside (86), (S) linalyl-β-primeveroside (87), lupeol (88), tuberonic acid (89), γ-diosphenol (90), and 2,2,4,4-tetramethyl-6-(1-oxobutyl)-1,3,5-cyclohexanetrione (91) were terpenes isolated from M. oleifera. Compound 88 exhibited anti-adipogenic properties by reducing the S and G2/M phases, inhibiting ROS, and enhancing glucose uptake. The structure of the compounds is shown in Figure 10 [52,54,62].
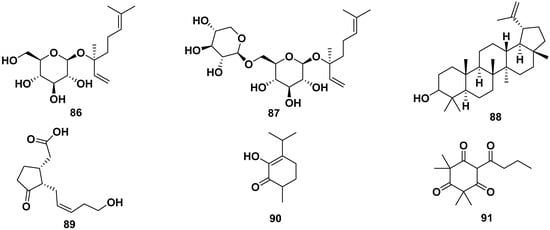
Figure 10.
Terpene compounds of M. oleifera.
2.9. Other Compounds
The leaf extract of M. oleifera contained 4-hydroxyphenylacetonitrile (92), lutein (93), adenosine (94), uridine (95), 3-pyridinecarboxamide (96), 5-hydroxymethyl-2-furaldehyde (97), 5-hydroxymethyl-2-furancarboxylic acid (98), bis-isothiocyanatomethyl benzene (99), and pyropheophorbide-a (100). Compound 92 exhibited activity to induce the secretion of insulin. Other compounds isolated from M. oleifera were L-tryptophan (101), benzyl β-D-glucopyranoside (102), benzyl-β-primeveroside (103), (+)-pinoresinol-4-O-β-D-glucopyranoside (104), isolariciresinol-3a-O-β-D-glucopyranoside (105), lariciresinol-9-O-β-D-glucopyranoside (106), fluoropyrazine (107), (10-hydroxy-1,3-dimethylchrysen-3-yl)-5-hydroxypentan-1-one (108), hexademethylated 3β,11β-dihydroxyfriedelane (109), 6,7-dipropanone-5-hydroxyphenyl-3-methylphenanthrene-1-carboxylic acid (110), (2R)-2-phenylmethoxybutane-1,4-diol (111), (2S)-2-phenylmethoxybutane-1,4-diol (112), 2-hexenyl-β-D-glucopyranoside (113), omoringone (114), 1,3-dibenzyl urea (115), 1-hydroxy-3-phenylpropan-2-yl benzoate (116), 1-octadecene (117), 2,3,4-trihydroxybenzaldehyde (118), 3,4-dihydroxy benzonitrile (119), 3,7,11,15-tetramethyl-2-hexadecen-1-ol (120), 3-hydroxy-β-ionone (121), N-benzyl S-ethyl thioformate (122), benzyl benzylcarbamate (123), methyl-4-hydroxybenzoate (124), and pyrrolezanthine (125). The structures of these compounds are shown in Figure 11.
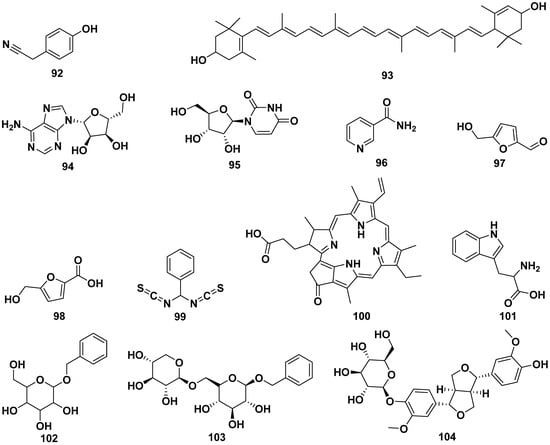
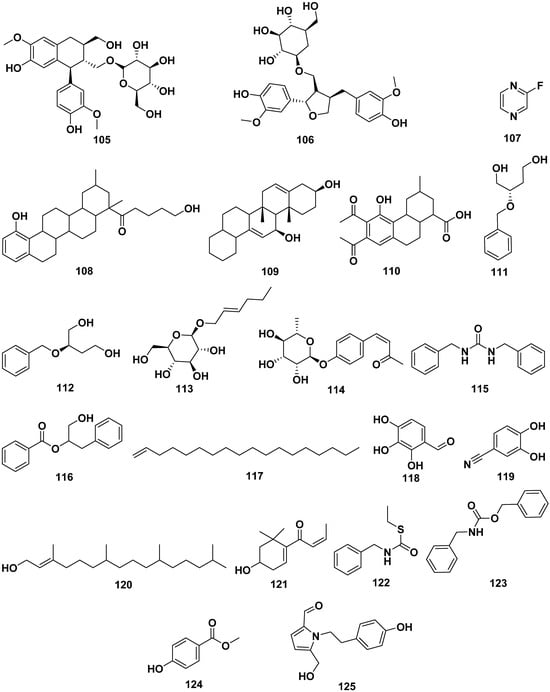
Figure 11.
The structures of compounds of leaf extract of M. oleifera.
Compound 104 displayed a potent inhibition of CYP3A4, with an IC50 value of 41.5 µg/mL, while compound 105 exhibited CYP3A4 inhibition with an IC50 value of 100 µg/mL. Compound 107 also demonstrated CYP3A4 inhibitory activity, with an IC50 value of 72.5 µg/mL. In addition to their enzyme inhibitory effects, compounds 109 and 110 showcased strong antioxidant properties, as indicated by DPPH IC50 values of 0.475 and 0.671 mg/mL, respectively. Furthermore, compound 122 exhibited significant antibacterial activity, with MIC values of 32 µg/mL against pathogens such as S. dysenteriae, S. boydii, and S. aureus [13,25,30,37,41,43,51,58,63,64,65,66,67,68,69].
3. Pharmacological Properties
3.1. Anti-Hemorrhage
Anti-hemorrhage properties in medicine are used to prevent excessive bleeding due to injury or surgery. Excessive bleeding could lead to death. Adeyemi et al. investigated the anti-hemorrhage properties of M. oleifera extracts. The experiment was studied by utilizing the venom of Echis ocellatus. The result reported that the ethanol extract of M. oleifera showed the highest effictiveness in neutralizing hemorrhage, with a dose of 800 mg/kg for 2 mL of 0.22 mg/kg venom. They also investigated the incubation factor and demonstrated its enhanced potency when pre-incubated with venom from E. ocellatus [43].
3.2. Anti-Allergic
Allergy is a condition where the immune system in the body mistakenly identifies substances as harmful and triggers reactions that affect various parts of the body. The development of anti-allergy medicine has been evolving to improve treatment and understand allergy mechanisms. Rani et al. evaluated the effectiveness of the anti-allergic properties of the leaves, pods, and seeds of M. oleifera. The extracts were macerated with ethanol at 80% and yielded nine compounds from the isolation. The study showed that the extracts of M. oleifera could inhibit the early and late phases of allergic reactions. In particular, the leaf extracts could better suppress the release of β-hexosaminidase (IC50 7.17 µg/mL), IL-4 (IC50 2.32 µg/mL), and TNF-α (IC50 1.2 µg/mL) compared to ketotifen fumarate as a positive control. M. oleifera seed extract could inhibit histamine release better compared to other extracts and the positive control, with an IC50 value of 5.97 µg/mL. Further study showed that compared to other isolated compounds, glucomoringin (28) had a better inhibition against beta-hexosaminidase in the early phase and TNF-α release in the late phase (IC50 10.43 µg/mL); meanwhile, quercetin (41) had a better inhibition against histamine release (IC50 7.77 µg/mL). In the last allergic phase, β-sitosterol-3-O-glucoside (83) showed a better inhibition against IL-4 release compared to other isolated compounds, with an IC50 value of 7.33 µg/mL [50].
3.3. Antimicrobial
Antimicrobial properties of compounds always gain interest due to their crucial role in preventing infectious diseases. In an era where microorganisms keep evolving and show resistance to antimicrobial agents, the development of natural antimicrobial agents has become urgent. Various parts of M. oleifera extracts with antibacterial activity were studied against E. coli and S. aureus using the agar well diffusion method. The study showed that the 80% methanol extract of leaves, pulp, and seed had the best inhibition against E. coli. The 70 and 80% methanol extract of flowers showed the same value against S. aureus, and the aqueous pulp extract showed a better result against S. aureus compared to other extracts [70]. Abadallah and Ali studied the comparison of aqueous and ethanol extracts of M. oleifera against several bacteria. The MIC value and zone inhibition of the ethanol extract showed better results compared to the aqueous extract. Shigella spp. was the most susceptible to both extracts [71]. The extracts of M. oleifera showed potential for antibacterial and anti-fungal properties, as shown in Table 2.

Table 2.
Antimicrobial activity of M. oleifera against (A) Gram negative bacteria, (B) Gram positive bacteria, and (C) fungi.
The antiviral activities of M. oleifera extracts against several viruses are shown in Table 3.

Table 3.
Antiviral activity of M. oleifera against several viruses.
3.4. Anthelminthic
Parasitic worm infections can infect various parts of the body, which could lead to health issues for the host. This could lead to the economic loss of livestock. To prevent these losses, the development of anthelmintic medicine has gained interest due to parasitic worm that affect not only affect livestock but also human health. Utilizing earthworms 3–5 cm in length and 0.1–0.2 cm in width, Nilani et al. evaluated the anthelminthic properties of the seed oil M. oleifera. The seed oil was divided into two concentrations, 25 and 50 mg/mL, they exhibit anthelminthic properties, with paralysis times of 21 and 16 min respectively while the death time 30 and 24 min, respectively. The study also reported that oleic acid (57) at a concentration of 25 mg/mL contained in seed oil showed a paralysis time 23 min and a death time 33 min [36].
3.5. Antihypertensive
Hypertension is a cardiovascular disease that causes sustained blood pressure levels. It could lead to health complications, including heart disease, kidney, and stroke. Randriamboajonvy et al. utilizing spontaneous hypertensive rats (SHR) as experiment model to demonstrate the effect of M. oleifera seed oil. The result showed a significant reduction in nocturnal heart rate without a change in diurnal heart rate after ten days of treatment. The use of seed oil in SHR increased the capacity of the left ventricle during diastole, which was substantially lower in SHR rats as in comparison with WKY (control) rats. Ejection fraction, a measure of systolic ventricular function, was substantially reduced in both SHR groups (control and seed oil-treated) in comparison with to WKY rats. This suggests that seed oil treatment did not have a positive effect on systolic ventricular function in SHR. The increased isovolumic relaxation time, indicative of diastolic function impairment in SHR, was completely reversed by seed oil treatment. M. oleifera seed oil treatment also led to a reduction in cardiomyocyte size in SHR seed oil-treated hearts compared to those in SHR control hearts. Furthermore, the study explored the potential involvement of peroxisome proliferator-activated receptor (PPAR) signaling pathways in seed oil’s protective effect against cardiac fibrosis in SHR. The expression of PPARα and PPARδ in cardiac tissue was assessed, revealing increased staining in the left ventricle of SHR seed oil-treated rats compared to SHR controls. These findings collectively suggest a beneficial impact of M. oleifera seed oil on cardiac structure and function in SHR, accompanied by an upregulation of PPAR-α and δ signaling pathways [37].
Acuram et al. studied the antihypertensive properties of methanol and ethyl acetate extracts related to inhibition of angiotensin converting enzyme (ACE), furthermore, blood pressure was also investigated. The hypertension was induced in mice with Nω-nitro-L-arginine methyl ester (L-name). The result demonstrated that compared to methanol extract, ethyl acetate showed more significant inhibition of ACE and lowering blood pressure on the last day [85].
3.6. Antileishmanial
Infection from the genus Leishmanial affecting health issues in tropical and subtropical regions such as Asia, Africa, America, and Mediterranean. This infection is transmitted from sandflies to humans. Kaur et al. studied the anti-leishmanial properties of M. oleifera extract against promastigotes of Leishmania donavani. The roots were extracted with 70% ethanol and the leaves were extracted with methanol. The roots ethanolic extract and the leaves’ methanolic extract exhibited moderate inhibitory activity, with IC50 values of 83.0 and 47.5 μg/mL, respectively. Upon fractionation, the methanolic extract of leaves showed enhanced antileishmanial activity, particularly in its ethyl acetate fraction, which displayed increased potency with an IC50 value of 27.5 mg/mL. Niazin was isolated from ethyl acetate fraction gave antileishmanial properties with IC50 value of 5.25 mM [41].
3.7. Wound Healing
Tofiq et al. macerated the leaves of M. oleifera with 70% ethanol. The experiment was conducted in 7 groups and observed wound healing properties. The result demonstrated that ointment with 10% concentration of extract formulation showed a better effect, representing less scarring, brighter skin, and more regenerated hair follicles compared to gentamicin ointment. Ramadhany et al. made M. oleifera leaves extract by soxhlet with ethanol. The extract was made into 4 and 15% gel and investigated their wound healing properties on gingival wounds. The study analyzes the neutrophils, fibroblasts, angiogenesis, and epithelial thickness for seven days. It resulted in 15% M. oleifera gel extract having a better effect in reducing neutrophils, increasing the number fibroblasts and angiogenesis. However, 4% M. oleifera gel extract was better at increasing epithelial thickness [86].
3.8. Antioxidant
Antioxidant properties are used to decrease oxidative stress, which could lead to damaging tissues. Antioxidants function as electron donors to free radicals and neutralize them. Thus, made antioxidant properties become one of most researched topics. Antioxidant activities of M. oleifera by several bio-assay methods are shown in Table 4.

Table 4.
Antioxidant activity of M. oleifera by several bio-assay methods.
3.9. Anti-diarrheal
Misra et al. evaluated the anti-diarrheal potential of M. oleifera. Leaves of M. oleifera were extracted with petroleum ether and then subjected to ethanol for seven days. The animal models were divided into six groups, as control and as doses of extract, and castor oil was used to induce diarrhea. The study showed that the ethanol extract successfully acted as an anti-diarrheal within 52 min, with an extract dose 150 mg/kg, and showed total stools of 0.130 mg [90].
3.10. Hepatoprotective
Pari and Kumar conducted an evaluation of the hepatoprotective properties of an ethanol extract obtained from Moringa oleifera leaves in rats with liver damage caused by anti-tubercular medications such as isoniazid (INH), rifampicin (RMP), and pyrazinamide (PZA). This extract had considerable protective effects when administered orally, as indicated by its impact on numerous parameters. This included the serum levels of glutamic pyruvic transaminase (alanine aminotransferase), glutamic oxaloacetic transaminase (aspartate aminotransferase), bilirubin, and alkaline phosphatase, as well as the levels of lipids and lipid peroxidation in the liver [91].
Khalid et al. assessed the potential protective effects of M. oleifera leaf powder and a 70% ethanol extract of M. oleifera leaves in alleviating liver and kidney dysfunction induced by polycystic ovary syndrome (PCOS) in female albino mice. PCOS was induced by administering intramuscular injections of testosterone enanthate at a dose of 1.0 mg/100 g body weight for a duration of thirty-five days. The study conducted assessments of renal function (RFT), liver function (LFT), and the oxidative stress biomarker malondialdehyde (MDA) in the serum at intervals of 0, 7, and 14 days. The mice that received treatments of M. oleifera exhibited significant reductions in the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin, urea, and creatinine compared to the PCOS-induced controls. Conversely, there was a notable increase in the levels of total protein, albumin, globulin, and the albumin/globulin (A/G) ratio. Furthermore, oxidative stress levels exhibited significant reductions in response to treatments, exposure duration, and their combined effect. The findings of this study suggest that both Moringa oleifera leaf powder and extract have the potential to reduce oxidative stress and improve renal and hepatic activity in female albino mice with PCOS-induced dysfunction [58].
3.11. Anti-Inflammatory
Inflammation is a recognized physiological reaction that serves to safeguard the body against infections and promote the healing of tissue injuries. However, persistent or chronic inflammation can potentially support the growth of various disorders and diseases associated with inflammation. Sulaiman et al. assessed the anti-inflammatory potential of an M. oleifera aqueous extract. The assay was conducted by carrageenan-induced paw edema. The extract was made at different doses and showed inhibitory effects in a dose-dependent manner. At dose 100 mg/kg, after five hours, the inhibition showed a value of 50%. The suggested anti-inflammatory mechanism was the extract which contained flavonoids with an inhibitory action against NF-k [64].
Fard et al. studied the anti-inflammatory potential of an M. oleifera extract on macrophages stimulated with lipopolysaccharide (LPS) in RAW264.7 macrophages. The anti-inflammatory properties of ethanol extracts derived from M. oleifera’s bioactive leaves were assessed by examining their ability to inhibit nitric oxide (NO) production using the Griess reaction and to modulate the expression of pro-inflammatory mediators in macrophages. The ethanol leaf extract exhibited a significant inhibition of various inflammatory markers, including NO production, as well as prostaglandin E2, TNF-α, IL-6, and IL-1β secretion. Concurrently, the bioactive extract dose-dependently stimulated the production of IL-10. Furthermore, the ethanol extract effectively suppressed the protein expression of inflammatory markers such as inducible NO synthase, cyclooxygenase-2, and NF-kB p65, also in a dose-dependent manner [65].
Previously, Xu et al. investigated the antioxidant properties of M. oleifera, and also investigated its anti-inflammatory properties. The study was conducted by determining the NO production of RAW264.7 macrophages. The study revealed that the leaf extract gave a lower NO production, at 100 µg/mL, while the seed extract presented higher NO production. It concluded that the leaf extract had better anti-inflammatory properties compared to the seed extract [46].
An ethyl acetate fraction of M. oleifera was assessed to have anti-inflammatory potential against LPS-induced macrophages. The study showed that macrophages which were treated with the ethyl acetate fraction exhibited a reduction in the production of pro-inflammatory mediators. This reduction was observed between the mRNA and protein levels. The study findings indicated that the fraction downregulated the mRNA expression of various inflammatory markers, including IL-1, IL-6, TNF-α, PTGS2, NF-kB P50, and RelA. Furthermore, the fraction effectively inhibited the expression of inflammatory mediators such as IL-6, TNF-α, and cyclooxygenase-2. Notably, the fraction effects included the inhibition of IkB-α phosphorylation and the ability to decrease the expression of nuclear factor NF-kB p65, thereby impeding its nuclear translocation [52].
3.12. Anti-Diabetic
One of the traditional usages of M. oleifera was as a diabetic medicine. Gupta et al., and Al-Malki and El Rabey studied the proper effects of M. oleifera as an anti-diabetic medicine. Pod and seed powders of M. oleifera were used in various doses. The animal model used was a streptozotocin-induced diabetic male rat. The parameters used were the determination of IL-6, immunoglobulin A, immunoglobulin G, fasting blood sugar, glycosylated hemoglobin, albumin, potassium, sodium, creatinine, and uric acid. Both studies showed that M. oleifera extracts could restore the abnormalities to a slightly normal level; however, a higher dose was suspected to be more effective. The water consumption of all subject groups also returned to normal after treatment [59,60].
Previously, Hamed et al. examined the antioxidant properties of M. oleifera leaf extracts. Furthermore, they also investigated the anti-diabetic activity of purified flavonoids from crude extracts by determining their α-glucosidase inhibitory effects. The results showed that purified flavonoids inhibited α-glucosidase by 54.41% at 100 µg/mL and 99.01% at 800 µg/mL in an uncompetitive manner [48]. Chen et al. reported that the ethanol leaf extracts of M. oleifera could be used as anti-diabetic medicine. They investigated the activity by determining the α-glucosidase value. The study showed an IC50 value of 123 µg/mL [61].
3.13. Anticancer
Jung evaluated the anticancer properties of M. oleifera leaf extracts against A549 human lung cancer cells. The evaluation was conducted by MTT assay, and the changes and apoptotic effects were observed. The observation revealed that M. oleifera extract treatment resulted in a dose-dependent downregulation of caspase-3 and an upregulation of cleaved caspase-3, indicating an induction of apoptosis. There was a significant dose-dependent downregulation of Akt, p-IkB, NF-kB, p-Erk, β-catenin, and cyclin D1, all of which play roles in cell survival and proliferation. Treatment with soluble M. oleifera extracts for 48 h resulted in a significantly reduced release of reactive oxygen species (ROS) in comparison to the untreated control group. This implies a decrease in oxidative stress. In summary, the study concluded that MOL treatment induced apoptosis, inhibited the growth of tumor cells, and reduced the levels of internal reactive oxygen species (ROS) in human lung cancer cells. These outcomes emphasize the potential of M. oleifera leaf extracts as a promising candidate for further research and development in the context of lung cancer therapy [66]. The anticancer activities of M. oleifera against several cancer cell types are shown in Table 5.

Table 5.
Anticancer activity of M. oleifera against several cancer cell types.
Based on the data attained by Do et al., as shown in Table 3, this study demonstrated that M. oleifera extract induced apoptosis in A375 cells, as evidenced by chromatin condensation and the externalization of phosphatidylserine (PS), which are characteristic features of apoptotic cell death. The apoptosis process was initiated by the activation of caspase-9 and caspase-3/7, the cleavage of PARP, and the translocation of apoptosis-inducing factor into the nucleus. These findings provide insights into the apoptotic mechanisms triggered by M. oleifera extract in A375 cells [69]. Meanwhile, Pappas et al. assessed the gene expression of M. oleifera aqueous wild and cultivated leaf extracts against human pancreas cancer cells AsPC-1, MCF-7, and HCT-116 colon cancer cells. The aqueous extract exerted its effects by down-regulating p53 expression in all tested cell lines and by down-regulating c-myc in AsPC-1 cells. Additionally, specific marker genes associated with each cell line, such as BRCA-1 in MCF-7, mta-1 in AsPC-1, and Ki-67 in HTC116 cells, were down-regulated. Furthermore, the survivin (BIRC5) gene, an inhibitor of apoptosis, was down-regulated in all three cell lines, suggesting a shared target mechanism of Moringa constituents across these cell lines [92].
Kumar et al. discovered that an extract of M. oleifera leaves demonstrated effective inhibition of Dalton lymphoma (DL) cell proliferation. This inhibition was characterized by alterations in mitochondrial membrane potential (ΔΨm) and noticeable changes in overall cell morphology. Notably, DL cells treated with the extract experienced cell cycle arrest at the G2/M phase and a significant upregulation in the expression of p53 and p21. Furthermore, the treatment resulted in increased levels of pro-apoptotic markers, including Bax, Cytochrome-c (Cyt-c), and caspase-3, while reducing the expression levels of the anti-apoptotic Bcl-2 protein. These changes strongly suggest the induction of apoptosis in DL cells. Mechanistically, the anticancer efficacy of M. oleifera extract was attributed to the inactivation of the MEK/ERK-mediated pathway in DL cells. Additionally, it is noteworthy that the inhibition of DL growth by MOML was accompanied by apoptosis induction and improvements in hematological parameters in DL-induced mice [99].
4. Utilization of M. oleifera in Dental Health
Research on several bacteria that are pathogenic to dental health has been carried out, such as Streptococcus mutans, Enterococcus faecalis, Staphylacoccus aureus, and others. Table 6 shows some data on the antibacterial activity of M. oleifera against oral pathogenic bacteria.

Table 6.
Antibacterial activity of M. oleifera to some oral pathogenic bacteria.
Jwa evaluated the aqueous and ethanol extracts of M. oleifera against S. mutans, which were found in the cariogenic biofilm of dental caries. Both extracts reduced the bacteria’s growth at concentrations of 25 and 6.25 µg/mL, respectively. At the same concentration, heated ethanol extract exhibited inhibitory activity better than non-heated extracts. This study showed that the ethanol extract was more effective than the aqueous extract against S. mutans [109]. Soraya et al. investigated the anti-bacterial properties of M. oleifera gel in inhibiting the growth of S. mutans, which were involved in the pathogenesis of dental caries. After 48 h, it was observed that the 12.5% concentration exhibited the highest effectiveness in reducing S. mutans growth. Within 24 h, the 6.25 and 3.125% concentrations displayed remarkable capabilities in suppressing S. mutans growth. Notably, the 6.25% concentration showed superior efficacy in reducing the formation of S. mutans biofilms. The application of M. oleifera gel extract created conditions in which S. mutans, a commensal bacterium, struggled to form a biofilm, with inhibition levels surpassing 70%. This was evidenced by the absence of substantial biofilm development. It is worth mentioning that at all tested concentrations, M. oleifera exhibited a toxic effect on S. mutans cells. The ethanol extract gel of M. oleifera demonstrated the ability to curtail both the growth and biofilm formation of S. mutans on tooth surfaces while concurrently exerting toxicity on S. mutans cells, potentially due to the presence of anti-bacterial compounds [110]. The anti-bacterial properties of herbal toothpaste, formulated with M. oleifera root essential oil, were assessed against bacteria commonly associated with tooth plaque, namely S. mutans and S. aureus. The Muller Hinton agar well diffusion method was used for this evaluation The findings of this study demonstrated that S. mutans exhibited susceptibility to the herbal toothpaste, as indicated by a significant inhibition zone measuring 31 mm [111].
Alharbi et al. examined the antimicrobial efficacy of M. oleifera leaf extract, octenidine dihydrochloride (OCT), sodium hypochlorite (NaOCl), and their combinations as an intracanal irrigant against E. faecalis. Decoronation and root canal preparation were performed on single-rooted mandibular premolars. After autoclaving, each root specimen was inoculated with E. faecalis and incubated at 37 °C for 48 h. Subsequently, based on the irrigation solution used, the specimens were split into six groups: 2.5% NaOCl (Group 1), 0.1% OCT (Group 2), M. oleifera leaf extract (Group 3), a combination of M. oleifera extract and 1.25% NaOCl (Group 4), a combination of M. oleifera extract and OCT (Group 5), and normal saline (Group 6). Both M. oleifera extract and 0.1% OCT demonstrated antibacterial effects against E. faecalis comparable to 2.5% NaOCl and could be considered as potential root canal irrigants. Furthermore, combination groups exhibited superior anti-microbial activity compared to individual irrigants [112].
Rochyani investigated the inhibitory potential of M. oleifera leaf extract on the biofilm formation of E. faecalis bacteria. The experiment was divided into several groups, including a negative control group (CMC solvent 0.1%), a positive control group (ChKM), and four test groups extracts of 20, 40, 60, and 80%, respectively. The results indicate that the M. oleifera leaf extract demonstrates a significant inhibitory effect on the biofilm formation of E. faecalis bacteria. Notably, the inhibitory effect observed in the 80% extract was found to be substantially greater than that achieved by the positive control group using ChKM [113].
Shanmugapriya et al. investigated the antimicrobial effects of M. oleifera extracts on pooled plaque collected from orthodontic patients. To achieve this, M. oleifera extracts were prepared through maceration. Subgingival plaque samples were collected, and the microorganisms present were cultured under anaerobic conditions. The microorganisms were subsequently subjected to treatment with the extracts, and their MIC and MBC were determined. Additionally, the cytotoxic effects of the extracts were assessed using a brine shrimp assay. The results of the study revealed that the 5% aqueous extract of M. oleifera exhibited a dose-dependent antimicrobial activity against oral anaerobic organisms. Notably, this anti-microbial effect became more pronounced with longer exposure times of the treated samples. Furthermore, in the cytotoxicity assay, the aqueous extract showed superior performance in lower concentrations in comparison to the ethanol extract. This was evident from the higher number of live nauplii observed in the aqueous extract group, indicating its lower cytotoxicity [114].
Sopandani et al. evaluated the antibacterial efficacy of M. oleifera extract at various concentrations (25, 50, 75, and 100%) when used as an irrigation solution against E. faecalis within the root canal ex vivo. Quantitative polymerase chain reaction (qPCR) was employed to assess the population of E. faecalis within the root canal following treatment with M. oleifera extract. The results indicated that M. oleifera extract solutions at concentrations of 75 and 100% exhibited comparable effectiveness to a 5.25% sodium hypochlorite (NaOCl) solution as a positive control [114].
Rieuwpassa et al. assessed the effectiveness of M. oleifera leaf extract in modulating the anti-inflammatory cytokine IL-1 of the bacteria Porphyromonas gingivalis, a key contributor to chronic periodontitis. The study revealed a significant variation in IL-1 levels across different observation days. Administration of the extract led to a reduction in pro-inflammatory cytokine IL-1 levels, as evident from the observations on days D0, D1, D3, D5, and D7 in the experimental Wistar rats induced with Porphyromonas gingivalis bacteria [115].
Kumar et al. evaluated the antibacterial effectiveness of a 5% M. oleifera mouthwash enhanced with silver nanoparticles against oral aerobic microorganisms. The mouthwash was prepared by utilizing a 5% M. oleifera aqueous extract for the synthesis of silver nanoparticles. The characterization of the mouthwash was performed through scanning electron microscopy analysis and energy dispersive X-ray analysis. To assess its anti-bacterial properties, the mouthwash was tested against S. mutans, S. aureus, E. faecalis, and C. albicans using the agar well diffusion assay. The results indicated that the 5% Moringa oleifera-silver nanoparticle mouthwash exhibited a pronounced effect on S. aureus and a comparable impact on S. mutans [114].
5. Conclusions
M. oleifera has a variety of pharmacological effects, such as anti-hemorrhage, anti-allergic, antimicrobial, anthelmintic, antihypertensive, antileukemia, antioxidant, anti-diabetic, hepatoprotection, anti-inflammatory, and anticancer effects. This plant is effective against dental infections. Antimicrobial research against microbes that cause dental infections has been carried out on the leaves of M. oleifera both in aqueous and ethanol extracts. This plant is reported to be active in vitro to inhibit several oral bacteria such as E. faecalis, S. mutans, P. gingivalis, S. aureus, and C. albicans, and has been tested ex vivo. The chemical components contained in M. oleifera are phenolics, glucosinolates, flavonoids, fatty acids, esters, alkaloids, sterols, terpenes, and several other compounds. Based on the pharmacological effects and chemical components contained in M. oleifera, this plant has the potential to be further developed to produce an antibacterial agent product, especially for dental health. The search for bioactive compounds in M. oleifera that have a major role as antibacterials in oral pathogens can be carried out through isolation. Furthermore, both extracts and isolated compounds from M. oleifera can be further traced for their activity through in vitro, in vivo, and clinical trials.
Author Contributions
Conceptualization, M.F.A.; methodology, M.F.A.; software; S.A.P.; validation, T.A. and S.A.P.; formal analysis, M.F.A. and T.A.; investigation, T.A.; resources, M.F.A. and S.A.P.; data curation, S.A.P. and D.K.; writing—original draft preparation, M.F.A. and T.A.; writing—review and editing, M.F.A., D.K. and S.A.P.; visualization, S.A.P. and M.F.A.; supervision, M.F.A. and D.K.; project administration, S.A.P. and T.A.; funding acquisition, M.F.A. and T.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author is grateful to Trisakti University for all research facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Granella, S.J.; Bechlin, T.R.; Christ, D.; Coelho, S.R.M.; de Oliveira Paz, C.H. An approach to recent applications of Moringa oleifera in the agricultural and biofuel industries. S. Afr. J. Bot. 2021, 137, 110–116. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: An overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef]
- Matic, I.; Guidi, A.; Kenzo, M.; Mattei, M.; Galgani, A. Investigation of medicinal plants traditionally used as dietary supplements: A review on Moringa oleifera. J. Public Health Afr. 2018, 9, 191–199. [Google Scholar] [CrossRef]
- Mishra, G.; Singh, P.; Verma, R.; Kumar, S.; Srivastav, S.; Jha, K.; Khosa, R. Traditional uses, phytochemistry and pharmacological properties of Moringa oleifera plant: An overview. Der Pharm. Lett. 2011, 3, 141–164. [Google Scholar]
- Padayachee, B.; Baijnath, H. An updated comprehensive review of the medicinal, phytochemical and pharmacological properties of Moringa oleifera. S. Afr. J. Bot. 2020, 129, 304–316. [Google Scholar] [CrossRef]
- Valdivié-Navarro, M.; Martínez-Aguilar, Y.; Mesa-Fleitas, O.; Botello-León, A.; Hurtado, C.B.; Velázquez-Martí, B. Review of Moringa oleifera as forage meal (leaves plus stems) intended for the feeding of non-ruminant animals. Anim. Feed. Sci. Technol. 2020, 260, 114338. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Sales, J.A.; Pereira, V.S.; Castelo, D.d.S.C.M.; de Aguiar Cordeiro, R.; de Souza Sampaio, C.M.; Paiva, M.d.A.N.; Dos Santos, J.B.F.; Sidrim, J.J.C.; Rocha, M.F.G. Research advances on the multiple uses of Moringa oleifera: A sustainable alternative for socially neglected population. Asian Pac. J. Trop. Med. 2017, 10, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef]
- Trigo, C.; Castello, M.L.; Ortola, M.D.; Garcia-Mares, F.J.; Desamparados Soriano, M. Moringa oleifera: An unknown crop in developed countries with great potential for industry and adapted to climate change. Foods 2020, 10, 31. [Google Scholar] [CrossRef]
- Hastuty, Y.D. Ekstrak daun kelor dan efeknya pada kadar hemoglobin remaja putri. J. Kesehat. Poltekkes Plb. 2022, 17, 121–127. [Google Scholar] [CrossRef]
- Yuliastuti, S.; Kurnia, H. Pengaruh pemberian serbuk halus daun kelor (Moringa oleifera) terhadap kadar hb ibu hamil trimester iii dengan anemia di wilayah kerja puskesmas mangunreja kab. Tasikmalaya the influence of Moringa oleifera fine powder. Media Inf. 2021, 17, 122–127. [Google Scholar] [CrossRef]
- Ferraz, C.C.R.; Henry, M.A.; Hargreaves, K.M.; Diogenes, A. Lipopolysaccharide from Porphyromonas gingivalis sensitizes capsaicin-sensitive nociceptors. J. Endod. 2011, 37, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jiang, X.; Lin, D.; Chen, Y.; Tong, Z. The starvation resistance and biofilm formation of Enterococcus faecalis in coexistence with Candida albicans, Streptococcus gordonii, Actinomyces viscosus, or Lactobacillus acidophilus. J. Endod. 2016, 42, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Miranda, T.T.; Vianna, C.R.; Rodrigues, L.; Rosa, C.A.; Corrêa Jr, A. Differential proteinase patterns among Candida albicans strains isolated from root canal and lingual dorsum: Possible roles in periapical disease. J. Endod. 2015, 41, 841–845. [Google Scholar] [CrossRef]
- Salem, A.S.; Tompkins, G.R.; Cathro, P.R. Alkaline tolerance and biofilm formation of Root Canal isolates of Enterococcus faecalis: An in Vitro Study. J. Endod. 2022, 48, 542–547.e544. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.P.; Montagner, F.; Jacinto, R.C.; Zaia, A.A.; Ferraz, C.C.R.; Souza-Filho, F.J. Polymerase chain reaction of Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia in primary endodontic infections. J. Endod. 2007, 33, 1049–1052. [Google Scholar] [CrossRef]
- Senges, C.; Wrbas, K.-T.; Altenburger, M.; Follo, M.; Spitzmüller, B.; Wittmer, A.; Hellwig, E.; Al-Ahmad, A. Bacterial and Candida albicans adhesion on different root canal filling materials and sealers. J. Endod. 2011, 37, 1247–1252. [Google Scholar] [CrossRef]
- Montagner, F.; Jacinto, R.C.; Signoretti, F.G.C.; de Mattos, V.S.; Grecca, F.S.; de Almeida Gomes, B.P.F. Beta-lactamic resistance profiles in Porphyromonas, Prevotella, and Parvimonas species isolated from acute endodontic infections. J. Endod. 2014, 40, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Putri, S.A.; Nur Shadrina, A.A.; Julaeha, E.; Kurnia, D. Potential Nevadensin from Ocimum basilicum as Antibacterial Agent against Streptococcus mutans: In Vitro and In Silico Studies. Comb. Chem. High Throughput Screen. 2023, 26, 1746–1754. [Google Scholar] [CrossRef]
- Madhloom, F. Antimicrobial Effect of Moringa oleifera L. and Red Pomegranate against Clinically Isolated Porphyromonas gingivalis: In vitro Study. Arch. Razi Inst. 2022, 77, 1405. [Google Scholar] [CrossRef]
- Nugraha, A.P.; Triwardhani, A.; Sitalaksmi, R.M.; Ramadhani, N.F.; Luthfi, M.; Ulfa, N.M. Phytochemical, antioxidant, and antibacterial activity of Moringa oleifera nanosuspension against peri-implantitis bacteria: An in vitro study. J. Oral Biol. Craniofacial Res. 2023, 13, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Tiwari, P.; Sahu, P.K.; Kumar, S. A review of the phytochemical and pharmacological characteristics of Moringa oleifera. J. Pharm. Bioallied Sci. 2018, 10, 181. [Google Scholar]
- Özcan, M. Moringa spp: Composition and bioactive properties. S. Afr. J. Bot. 2020, 129, 25–31. [Google Scholar] [CrossRef]
- Cheenpracha, S.; Park, E.-J.; Yoshida, W.Y.; Barit, C.; Wall, M.; Pezzuto, J.M.; Chang, L.C. Potential anti-inflammatory phenolic glycosides from the medicinal plant Moringa oleifera fruits. Bioorg. Med. Chem. 2010, 18, 6598–6602. [Google Scholar] [CrossRef]
- Lar, P.; Ojile, E.; Dashe, E.; Oluoma, J. Antibacterial activity on Moringa oleifera seed extracts on some gram negative bacterial isolates. Afr. J. Nat. Sci. 2011, 14, 57–62. [Google Scholar]
- Zaffer, M.; Ganie, S.A.; Gulia, S.S.; Yadav, S.S.; Singh, R.; Ganguly, S. Antifungal efficacy of Moringa oleifera Lam. AJPCT 2015, 3, 28–33. [Google Scholar]
- El-Meidany, W.M.; Abdel-Gawad, F.K.; Mahmoud, S.H.; Ali, M.A. In vitro antiviral effect of cinnamon oil, Moringa oleifera extract, Manuka honey, and Nigella sativa oil against SARS-CoV-2 compared to remdesivir. Bull. Natl. Res. Cent. 2023, 47, 156. [Google Scholar] [CrossRef]
- Allam, O.G.; Kutkat, O.; Gaballah, M.; El-Halawany, A.M.; Mostafa, A.; Shouman, S.; Ali, M.A.; El Farouk, O. Virucidal effect of Moringa oleifera against SARS-CoV-2 and Influenza A/H1N1. Afr. J. Biol. Sci. 2023, 19, 69–78. [Google Scholar] [CrossRef]
- Xiong, Y.; Rajoka, M.S.R.; Mehwish, H.M.; Zhang, M.; Liang, N.; Li, C.; He, Z. Virucidal activity of Moringa A from Moringa oleifera seeds against Influenza A Viruses by regulating TFEB. Int. Immunopharmacol. 2021, 95, 107561. [Google Scholar] [CrossRef] [PubMed]
- Nasr-Eldin, M.A.; Abdelhamid, A.; Baraka, D. Antibiofilm and antiviral potential of leaf extracts from Moringa oleifera and rosemary (Rosmarinus officinalis Lam.). Egypt. J. Microbiol. 2017, 52, 129–139. [Google Scholar] [CrossRef]
- Mahbub, K.R.; Hoq, M.M.; Ahmed, M.M.; Sarker, A. In vitro antibacterial activity of Crescentia cujete and Moringa oleifera. Bangladesh Res. Publ. J. 2011, 5, 337–343. [Google Scholar]
- Effendi, D.N.; Yuliawati, K.M.; Patricia, V.M. Uji Aktivitas Antibakteri Ekstrak Daun Kelor (Moringa oleifera L.) Terhadap Bakteri Staphylococcus epidermidis. Proc. Bdg. Conf. Ser. Pharm. 2023, 3, 528–533. [Google Scholar]
- Ashraf, M.; Alam, S.S.; Fatima, M.; Altaf, I.; Khan, F.; Afzal, A. Comparative anti-influenza potential of Moringa oleifera leaves and amantadine invitro. Pak. Postgrad. Med. J. 2017, 28, 127–131. [Google Scholar] [CrossRef]
- Goswami, D.; Mukherjee, P.K.; Kar, A.; Ojha, D.; Roy, S.; Chattopadhyay, D. Screening of Ethnomedicinal Plants of Diverse Culture for Antiviral Potentials; NISCAIR-CSIR: New Delhi, India, 2016. [Google Scholar]
- Kurokawa, M.; Wadhwani, A.; Kai, H.; Hidaka, M.; Yoshida, H.; Sugita, C.; Watanabe, W.; Matsuno, K.; Hagiwara, A. Activation of cellular immunity in herpes simplex virus type 1-infected mice by the oral administration of aqueous extract of Moringa oleifera Lam. leaves. Phytother. Res. 2016, 30, 797–804. [Google Scholar] [CrossRef]
- Nilani, P.; Pinaka, M.K.; Duraisamy, B.; Dhamodaran, P.; Jeyaprakash, M. Anthelmintic activity of Moringa oleifera seed oil-validation of traditional use. J. Adv. Sci. Res. 2012, 3, 65–66. [Google Scholar]
- Randriamboavonjy, J.I.; Loirand, G.; Vaillant, N.; Lauzier, B.; Derbré, S.; Michalet, S.; Pacaud, P.; Tesse, A. Cardiac protective effects of Moringa oleifera seeds in spontaneous hypertensive rats. Am. J. Hypertens. 2016, 29, 873–881. [Google Scholar] [CrossRef]
- Bennett, R.N.; Mellon, F.A.; Foidl, N.; Pratt, J.H.; Dupont, M.S.; Perkins, L.; Kroon, P.A. Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L.(horseradish tree) and Moringa stenopetala L. J. Agric. Food Chem. 2003, 51, 3546–3553. [Google Scholar] [CrossRef]
- Huang, L.; Yuan, C.; Wang, Y. Bioactivity-guided identification of anti-adipogenic isothiocyanates in the moringa (Moringa oleifera) seed and investigation of the structure-activity relationship. Molecules 2020, 25, 2504. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Ng, V.A.S.; Shen, C.-C. Chemical constituents of Moringa oleifera Lam. seeds. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 495–498. [Google Scholar]
- Kaur, A.; Kaur, P.K.; Singh, S.; Singh, I.P. Antileishmanial compounds from Moringa oleifera Lam. Z. Für Naturforschung C 2014, 69, 110–116. [Google Scholar] [CrossRef]
- Jiang, M.-Y.; Lu, H.; Pu, X.-Y.; Li, Y.-H.; Tian, K.; Xiong, Y.; Wang, W.; Huang, X.-Z. Laxative Metabolites from the Leaves of Moringa oleifera. J. Agric. Food Chem. 2020, 68, 7850–7860. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, S.; Larayetan, R.; Onoja, A.; Ajayi, A.; Yahaya, A.; Ogunmola, O.O.; Adeyi, A.; Chijioke, O. Anti-hemorrhagic activity of ethanol extract of Moringa oleifera leaf on envenomed albino rats. Sci. Afr. 2021, 12, e00742. [Google Scholar] [CrossRef]
- Atawodi, S.E.; Atawodi, J.C.; Idakwo, G.A.; Pfundstein, B.; Haubner, R.; Wurtele, G.; Bartsch, H.; Owen, R.W. Evaluation of the polyphenol content and antioxidant properties of methanol extracts of the leaves, stem, and root barks of Moringa oleifera Lam. J. Med. Food 2010, 13, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Deng, J.; Li, H.; He, Y.; Lan, T.; Wu, D.; Gong, H.; Zhang, Y.; Chen, Z. Optimization of phenolic compound extraction from Chinese Moringa oleifera leaves and antioxidant activities. J. Food Qual. 2019, 2019, 5346279. [Google Scholar] [CrossRef]
- Xu, Y.-B.; Chen, G.-L.; Guo, M.-Q. Antioxidant and anti-inflammatory activities of the crude extracts of Moringa oleifera from Kenya and their correlations with flavonoids. Antioxidants 2019, 8, 296. [Google Scholar] [CrossRef]
- Sahakitpichan, P.; Mahidol, C.; Disadee, W.; Ruchirawat, S.; Kanchanapoom, T. Unusual glycosides of pyrrole alkaloid and 4′-hydroxyphenylethanamide from leaves of Moringa oleifera. Phytochemistry 2011, 72, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Karthivashan, G.; Tangestani Fard, M.; Arulselvan, P.; Abas, F.; Fakurazi, S. Identification of bioactive candidate compounds responsible for oxidative challenge from hydro-ethanolic extract of Moringa oleifera leaves. J. Food Sci. 2013, 78, C1368–C1375. [Google Scholar] [CrossRef]
- Guevara, A.P.; Vargas, C.; Sakurai, H.; Fujiwara, Y.; Hashimoto, K.; Maoka, T.; Kozuka, M.; Ito, Y.; Tokuda, H.; Nishino, H. An antitumor promoter from Moringa oleifera Lam. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 1999, 440, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Abd Rani, N.Z.; Kumolosasi, E.; Jasamai, M.; Jamal, J.A.; Lam, K.W.; Husain, K. In vitro anti-allergic activity of Moringa oleifera Lam. extracts and their isolated compounds. BMC Complement. Altern. Med. 2019, 19, 361. [Google Scholar] [CrossRef]
- Igbo, U.E.; Igoli, J.O.; Onyiriuka, S.O.; Ogukwe, C.E.; Ayuk, A.A.; Gray, A.I. Isolation and characterization of Pyropheophorbide-a from Moringa oleifera Lam. Trop. J. Nat. Prod. Res. 2019, 3, 314–318. [Google Scholar] [CrossRef]
- Luetragoon, T.; Pankla Sranujit, R.; Noysang, C.; Thongsri, Y.; Potup, P.; Suphrom, N.; Nuengchamnong, N.; Usuwanthim, K. Bioactive compounds in Moringa oleifera Lam. leaves inhibit the pro-inflammatory mediators in lipopolysaccharide-induced human monocyte-derived macrophages. Molecules 2020, 25, 191. [Google Scholar] [CrossRef]
- Punia, J.; Singh, R. Antioxidant potential and nutritional content of stem, bark and pod of Drumstick tree (Moringa oleifera Lam.) from semi-arid region of Haryana. J. Indian Chem. Soc. 2017, 94, 103–110. [Google Scholar]
- Fantoukh, O.I.; Albadry, M.A.; Parveen, A.; Hawwal, M.F.; Majrashi, T.; Ali, Z.; Khan, S.I.; Chittiboyina, A.G.; Khan, I.A. Isolation, synthesis, and drug interaction potential of secondary metabolites derived from the leaves of miracle tree (Moringa oleifera) against CYP3A4 and CYP2D6 isozymes. Phytomedicine 2019, 60, 153010. [Google Scholar] [CrossRef]
- Oluduro, O.; Aderiye, B.; Connolly, J.; Akintayo, E.; Famurewa, O. Characterization and antimicrobial activity of 4-([beta]-d-glucopyranosyl-1 [arrow right] 4-[alpha]-l-rhamnopyranosyloxy)-benzyl thiocarboxamide; a novel bioactive compound from Moringa oleifera seed extract. Folia Microbiol. 2010, 55, 422. [Google Scholar] [CrossRef]
- Li, F.-H.; Wang, H.-Q.; Su, X.-M.; Li, C.-K.; Li, B.-M.; Chen, R.-Y.; Kang, J. Constituents isolated from n-butanol extract of leaves of Moringa oleifera. Zhongguo Zhong Yao Za Zhi/Zhongguo Zhongyao Zazhi/China J. Chin. Mater. Medica 2018, 43, 114–118. [Google Scholar]
- Sashidhara, K.V.; Singh, S.P.; Kant, R.; Maulik, P.R.; Sarkar, J.; Kanojiya, S.; Kumar, K.R. Cytotoxic cycloartane triterpene and rare isomeric bisclerodane diterpenes from the leaves of Polyalthia longifolia var. pendula. Bioorg. Med. Chem. Lett. 2010, 20, 5767–5771. [Google Scholar] [CrossRef]
- Khalid, S.; Arshad, M.; Raza, K.; Mahmood, S.; Siddique, F.; Aziz, N.; Khan, S.; Khalid, W.; AL-Farga, A.; Aqlan, F. Assessment of hepatoprotective, nephroprotective efficacy, and antioxidative potential of Moringa oleifera leaf powder and ethanolic extract against PCOS-induced female albino mice (Mus Musculus). Food Sci. Nutr. 2023, 11, 7206–7217. [Google Scholar] [CrossRef]
- Al-Malki, A.L.; El Rabey, H.A. The antidiabetic effect of low doses of Moringa oleifera Lam. seeds on streptozotocin induced diabetes and diabetic nephropathy in male rats. BioMed Res. Int. 2015, 2015, 381040. [Google Scholar] [CrossRef]
- Gupta, R.; Mathur, M.; Bajaj, V.K.; Katariya, P.; Yadav, S.; Kamal, R.; Gupta, R.S. Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. J. Diabetes 2012, 4, 164–171. [Google Scholar] [CrossRef]
- Chen, G.-L.; Xu, Y.-B.; Wu, J.-L.; Li, N.; Guo, M.-Q. Hypoglycemic and hypolipidemic effects of Moringa oleifera leaves and their functional chemical constituents. Food Chem. 2020, 333, 127478. [Google Scholar] [CrossRef]
- Vasanth, K.; Minakshi, G.C.; Velu, K.; Priya, T.; Kumar, R.M.; Kaliappan, I.; Dubey, G.P. Anti-adipogenic β-sitosterol and lupeol from Moringa oleifera suppress adipocyte differentiation through regulation of cell cycle progression. J. Food Biochem. 2022, 46, e14170. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.H.; Atiyah, M.M. Anti-fungal activities of aqueous and alcoholic leaf extracts of Moringa oleifera Lam. on Candida albicans isolated from diabetic foot infections. In Proceedings of the AIP Conference Proceedings; AIP Publishing: New York, NY, USA, 2023. [Google Scholar]
- Sulaiman, M.R.; Zakaria, Z.; Bujarimin, A.; Somchit, M.; Israf, D.; Moin, S. Evaluation of Moringa oleifera aqueous extract for antinociceptive and anti-inflammatory activities in animal models. Pharm. Biol. 2008, 46, 838–845. [Google Scholar] [CrossRef]
- Ariyani, F.; Amin, I.; Fardiaz, D. Ekstrak Air Daun Sirih (Piper betle Linn) sebagai Antioksidan Alami pada Pengolahan Ikan Patin (Pangasius hypophthalmus) Asin Kering. J. Pascapanen Dan Bioteknol. Kelaut. Dan Perikan. 2015, 10, 45–59. [Google Scholar] [CrossRef]
- Jung, I.L. Soluble extract from Moringa oleifera leaves with a new anticancer activity. PLoS ONE 2014, 9, e95492. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, E.A.; Sharaf-Eldin, M.A.; Wadaan, M. In vitro evaluation of cytotoxic activities of essential oil from Moringa oleifera seeds on HeLa, HepG2, MCF-7, CACO-2 and L929 cell lines. Asian Pac. J. Cancer Prev 2015, 16, 4671–4675. [Google Scholar] [CrossRef]
- Nair, S.; Varalakshmi, K. Anticancer, cytotoxic potential of Moringa oleifera extracts on HeLa cell line. J. Nat. Pharm. 2011, 2, 138–142. [Google Scholar]
- Do, B.H.; Nguyen, T.P.T.; Ho, N.Q.C.; Le, T.L.; Hoang, N.S.; Doan, C.C. Mitochondria-mediated Caspase-dependent and Caspase-independent apoptosis induced by aqueous extract from Moringa oleifera leaves in human melanoma cells. Mol. Biol. Rep. 2020, 47, 3675–3689. [Google Scholar] [CrossRef] [PubMed]
- Dodiya, B.; Amin, B.; Kamlaben, S.; Patel, P. Antibacterial activity and phytochemical screening of different parts of Moringa oleifera against selected gram positive and gram negative bacteria. J. Pharm. Chem. Biol. Sci. 2015, 3, 421–425. [Google Scholar]
- Abadallah, M.; Ali, M. Antibacterial activity of Moringa oleifera leaf extracts against bacteria isolated from patients attending general Sani Abacha specialist hospital damaturu. J. Allied Pharm. Sci. 2019, 1, 61–66. [Google Scholar]
- Brilhante, R.S.N.; Sales, J.A.; de Souza Sampaio, C.M.; Barbosa, F.G.; Paiva, M.d.A.N.; de Melo Guedes, G.M.; de Alencar, L.P.; de Ponte, Y.B.; Bandeira, T.d.J.P.G.; Moreira, J.L.B. Vibrio spp. from Macrobrachium amazonicum prawn farming are inhibited by Moringa oleifera extracts. Asian Pac. J. Trop. Med. 2015, 8, 919–922. [Google Scholar] [CrossRef]
- Morgan, C.; Opio, C.; Migabo, S. Chemical composition of Moringa (Moringa oleifera) root powder solution and effects of Moringa root powder on E. coli growth in contaminated water. S. Afr. J. Bot. 2020, 129, 243–248. [Google Scholar] [CrossRef]
- Zahran, E.M.; Mohamad, S.A.; Yahia, R.; Badawi, A.M.; Sayed, A.M.; Abdelmohsen, U.R. Anti-otomycotic potential of nanoparticles of Moringa oleifera leaf extract: An integrated in vitro, in silico and phase 0 clinical study. Food Funct. 2022, 13, 11083–11096. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, J.R.O.; Silva, G.C.; Costa, R.A.; Vieira, G.H.F.; Fonteles Filho, A.A.; dos Fernandes Vieira, R.H.S. In vitro antibacterial effect of aqueous and ethanolic Moringa leaf extracts. Asian Pac. J. Trop. Med. 2011, 4, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, M.A.; Hossain, M.S.; Chowdhury, M.E.H.; Haque, M. In vitro antimicrobial activity of methanolic extract of Moringa olieifera lam. fruits. J. Pharmacogn. Phytochem. 2012, 1, 94–98. [Google Scholar]
- Moyo, B.; Masika, P.J.; Muchenje, V. Antimicrobial activities of Moringa oleifera Lam leaf extracts. Afr. J. Biotechnol. 2012, 11, 2797–2802. [Google Scholar] [CrossRef]
- Kumar, V.; Pandey, N.; Mohan, N.; Singh, R.P. Antibacterial & antioxidant activity of different extract of Moringa oleifera Leaves–an in vitro study. Int. J. Pharm. Sci. Rev. Res. 2012, 12, 89–94. [Google Scholar]
- Fouad, E.A.; Elnaga, A.S.A.; Kandil, M.M. Antibacterial efficacy of Moringa oleifera leaf extract against pyogenic bacteria isolated from a dromedary camel (Camelus dromedarius) abscess. Vet. World 2019, 12, 802. [Google Scholar] [CrossRef]
- Syeda, A.M.; Riazunnisa, K. Data on GC-MS analysis, in vitro anti-oxidant and anti-microbial activity of the Catharanthus roseus and Moringa oleifera leaf extracts. Data Brief 2020, 29, 105258. [Google Scholar] [CrossRef] [PubMed]
- Aboud, A.S.; Jazar, Z.H.; Mansoor, R.F.; Zboon, H.A. Effect of ethanol and aqueous extract of Moringa oleifera on bacteria isolated from wound infection. Int. J. Sci. Res. Arch. 2023, 9, 941–949. [Google Scholar] [CrossRef]
- Cahyani, D.E.; Rusdi, B.; Mulqie, L. Antibacterial activity and klt-bioautography analysys of ethanol extract of kelor leaves (Moringa oleifera L.) against Staphylococcus aureus dan Escherichia coli Bacteries. Proc. Bdg. Conf.Ser. Pharm. 2023, 3, 168–176. [Google Scholar]
- Doughari, J.; Pukuma, M.; De, N. Antibacterial effects of Balanites aegyptiaca L. Drel. and Moringa oleifera Lam. on Salmonella typhi. Afr. J. Biotechnol. 2007, 6, 2212–2215. [Google Scholar] [CrossRef]
- Xiong, Y.; Riaz Rajoka, M.S.; Zhang, M.; He, Z. Isolation and identification of two new compounds from the seeds of Moringa oleifera and their antiviral and anti-inflammatory activities. Nat. Prod. Res. 2022, 36, 974–983. [Google Scholar] [CrossRef]
- Lovely, K.A.; Hernandez, C.L.C. Anti-hypertensive effect of Moringa oleifera Lam. Cogent Biol. 2019, 5, 1596526. [Google Scholar]
- Ramadhany, E.P.; Ambarawati, I.G.A.D.; Musyaffa, M.R. Effect of 4% and 15% moringa leaf extract gel on gingival wound healing in rats. Maj. Kedokt. Gigi Indones. 2022, 8, 192–199. [Google Scholar] [CrossRef]
- Oldoni, T.L.C.; Merlin, N.; Bicas, T.C.; Prasniewski, A.; Carpes, S.T.; Ascari, J.; de Alencar, S.M.; Massarioli, A.P.; Bagatini, M.D.; Morales, R. Antihyperglycemic activity of crude extract and isolation of phenolic compounds with antioxidant activity from Moringa oleifera Lam. leaves grown in Southern Brazil. Food Res. Int. 2021, 141, 110082. [Google Scholar] [CrossRef]
- Segwatibe, M.K.; Cosa, S.; Bassey, K. Antioxidant and Antimicrobial Evaluations of Moringa oleifera Lam Leaves Extract and Isolated Compounds. Molecules 2023, 28, 899. [Google Scholar] [CrossRef]
- Hamed, Y.S.; Abdin, M.; Rayan, A.M.; Akhtar, H.M.S.; Zeng, X. Synergistic inhibition of isolated flavonoids from Moringa oleifera leaf on α-glucosidase activity. Lwt 2021, 141, 111081. [Google Scholar] [CrossRef]
- Misra, A.; Srivastava, S.; Srivastava, M. Evaluation of anti diarrheal potential of Moringa oleifera (Lam.) leaves. J. Pharmacogn. Phytochem. 2014, 2, 43–46. [Google Scholar]
- Pari, L.; Kumar, N.A. Hepatoprotective activity of Moringa oleifera on antitubercular drug-induced liver damage in rats. J. Med. Food 2002, 5, 171–177. [Google Scholar] [CrossRef]
- Pappas, I.S.; Siomou, S.; Bozinou, E.; Lalas, S.I. Moringa oleifera leaves crude aqueous extract down-regulates of BRCA1, mta-1 and oncogenes c-myc and p53 in AsPC-1, MCF-7 and HTC-116 cells. Food Biosci. 2021, 43, 101221. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Mohamed, S.R.; Dkhil, M.A.; Thagfan, F.A.; Abdel-Gaber, R.; Soliman, D. The effect of Moringa oleifera leaf extracts against urethane-induced lung cancer in rat model. Environ. Sci. Pollut. Res. 2023, 30, 37280–37294. [Google Scholar] [CrossRef] [PubMed]
- Panchaware, P.S.; Shekokar, S.S.; Pachpor, A.G. Study of cytotoxic effects of CO2 extract of shigru (Moringa oleifera lam.) Root, in MCF-7 cell line of breast cancer. World J. Biol. Pharm. Health Sci. 2023, 15, 128–137. [Google Scholar] [CrossRef]
- Krishnamurthy, P.T.; Vardarajalu, A.; Wadhwani, A.; Patel, V. Identification and characterization of a potent anticancer fraction from the leaf extracts of Moringa oleifera L. Indian J. Exp. Biol. 2015, 53, 98–103. [Google Scholar]
- Tragulpakseerojn, J.; Yamaguchi, N.; Pamonsinlapatham, P.; Wetwitayaklung, P.; Yoneyama, T.; Ishikawa, N.; Ishibashi, M.; Apirakaramwong, A. Anti-proliferative effect of Moringa oleifera Lam (Moringaceae) leaf extract on human colon cancer HCT116 cell line. Trop. J. Pharm. Res. 2017, 16, 371–378. [Google Scholar] [CrossRef]
- Mohd Fisall, U.F.; Ismail, N.Z.; Adebayo, I.A.; Arsad, H. Dichloromethane fraction of Moringa oleifera leaf methanolic extract selectively inhibits breast cancer cells (MCF7) by induction of apoptosis via upregulation of Bax, p53 and caspase 8 expressions. Mol. Biol. Rep. 2021, 48, 4465–4475. [Google Scholar] [CrossRef] [PubMed]
- Wisitpongpun, P.; Suphrom, N.; Potup, P.; Nuengchamnong, N.; Calder, P.C.; Usuwanthim, K. In vitro bioassay-guided identification of anticancer properties from Moringa oleifera Lam. leaf against the MDA-MB-231 cell line. Pharmaceuticals 2020, 13, 464. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, P.K.; Shukla, A.; Singh, R.K.; Patel, A.K.; Yadav, L.; Kumar, S.; Kumar, N.; Acharya, A. Moringa oleifera L. leaf extract induces cell cycle arrest and mitochondrial apoptosis in Dalton’s Lymphoma: An in vitro and in vivo study. J. Ethnopharmacol. 2023, 302, 115849. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Sheikh, M.M.I.; Sharmin, S.A.; Islam, M.S.; Rahman, M.A.; Rahman, M.M.; Alam, M. Antibacterial activity of leaf juice and extracts of Moringa oleifera Lam. against some human pathogenic bacteria. CMU J. Nat. Sci. 2009, 8, 219. [Google Scholar]
- Angestia, W.; Ningrum, V.; Lee, T.L.; Lee, S.-C.; Bakar, A. Antibacterial activities of moringa olifiera freeze dried extract on staphylococcus aureus. J. Dentomaxillofacial Sci. 2020, 5, 154–157. [Google Scholar] [CrossRef]
- Arévalo-Híjar, L.; Aguilar-Luis, M.A.; Caballero-García, S.; Gonzáles-Soto, N.; Valle-Mendoza, D. Antibacterial and cytotoxic effects of Moringa oleifera (Moringa) and Azadirachta indica (Neem) methanolic extracts against strains of Enterococcus faecalis. Int. J. Dent. 2018, 2018, 1071676. [Google Scholar] [CrossRef]
- Elgamily, H.; Moussa, A.; Elboraey, A.; Hoda, E.-S.; Al-Moghazy, M.; Abdalla, A. Microbiological assessment of Moringa oleifera extracts and its incorporation in novel dental remedies against some oral pathogens. Open Access Maced. J. Med. Sci. 2016, 4, 585. [Google Scholar] [CrossRef]
- Zaffer, M.; Ahmad, S.; Sharma, R.; Mahajan, S.; Gupta, A.; Agnihotri, R.K. Antibacterial activity of bark extracts of Moringa oleifera Lam. against some selected bacteria. Pak. J. Pharm. Sci. 2014, 27, 1857–1862. [Google Scholar] [PubMed]
- Amanze, E.K.; Nwankpa, U.D.; Udekwu, C.E.; Ogbonna, H.N.; Nwokafor, C.V.; Udensi, C.G. Antibacterial activity of Moringa oleifera root bark extract against some pathogenic organisms. Asian J. Immunol. 2020, 4, 21–27. [Google Scholar]
- Ichsan, M.; Soraya, C.; Mubarak, Z.; Nur, S.; Gani, B.A. The Potency of Moringa oleifera on the Biofilm Formation, Adhesion, and Growth of Streptococcus Mutants Based on Incubation Times. J. Int. Dent. Med. Res. 2023, 16, 943–949. [Google Scholar]
- Marrufo, T.; Encarnação, S.; Silva, O.M.D.; Duarte, A.; Neto, F.F.; Barbosa, F.M.; Agostinho, A.B. Chemical characterization and determination of antioxidant and antimicrobial activities of the leaves of Moringa oleifera. Int. Netw. Environ. Manag. Confl. 2013, 2, 1–15. [Google Scholar]
- Gulzar, R.A.; Ajitha, H.S. Comparative evaluation of antimicrobial efficacy of Moringa oleifera extract and calcium hydroxide against E. faecalis. Int. J. Dent. Oral. Sci. 2021, 8, 2605–2609. [Google Scholar] [CrossRef]
- Jwa, S.-K. Efficacy of Moringa oleifera leaf extracts against cariogenic biofilm. Prev. Nutr. Food Sci. 2019, 24, 308. [Google Scholar] [CrossRef] [PubMed]
- Soraya, C.; Syafriza, D.; Gani, B.A. Antibacterial effect of Moringa oleifera gel to prevent the growth, biofilm formation, and cytotoxicity of Streptococcus mutans. J. Int. Dent. Med. Res. 2022, 15, 1053–1061. [Google Scholar]
- Amalunweze, A.; Ezumezu, C. Production of herbal toothpaste using Moringa root essential oil extract. Int. J. Adv. Biochem. Res. 2022, 6, 49–51. [Google Scholar]
- Alharbi, A.M.; Alharbi, T.M.; Alqahtani, M.S.; Elfasakhany, F.M.; Afifi, I.K.; Rajeh, M.T.; Fattouh, M.; Kenawi, L.M.M. A Comparative Evaluation of Antibacterial Efficacy of Moringa oleifera Leaf Extract, Octenidine Dihydrochloride, and Sodium Hypochlorite as Intracanal Irrigants against Enterococcus faecalis: An In Vitro Study. Int. J. Dent. 2023, 2023, 7690497. [Google Scholar] [CrossRef]
- Rochyani, L. The inhibition of leaf extract Moringaoleifera on the formation biofilm bacteria Enterococcus faecalis. DENTA 2020, 14, 44–50. [Google Scholar] [CrossRef]
- Kumar, G.K.; Ramamurthy, S.; Ulaganathan, A.; Varghese, S.; Praveen, A.A.; Saranya, V. Moringa oleifera Mouthwash Reinforced with Silver Nanoparticles–Preparation, Characterization and its Efficacy Against Oral Aerobic Microorganisms–In Vitro Study. Biomed. Pharmacol. J. 2022, 15, 2051–2059. [Google Scholar] [CrossRef]
- Rieuwpassa, I.E.; Ramadany, S.; Achmad, H.; Sitanaya, R.; Lesmana, H.; Djais, A.I.; Sesioria, A.; Inayah, N.H.; Mutmainnah, N. The Effectiveness of Moringa Leaf Extract (Moringa oleifera) Against Porphyromonas gingivalis Bacteria in Periodontitis Cases Through IL-1 Cytokine Analysis. J. Int. Dent. Med. Res. 2022, 15, 611–617. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).