Investigation of Potential Drug Targets Involved in Inflammation Contributing to Alzheimer’s Disease Progression
Abstract
1. Introduction
2. Overview of Inflammation Pathways and Mechanisms
3. Major Genes and Proteins for Alzheimer’s–Inflammation Pathways and Mechanisms
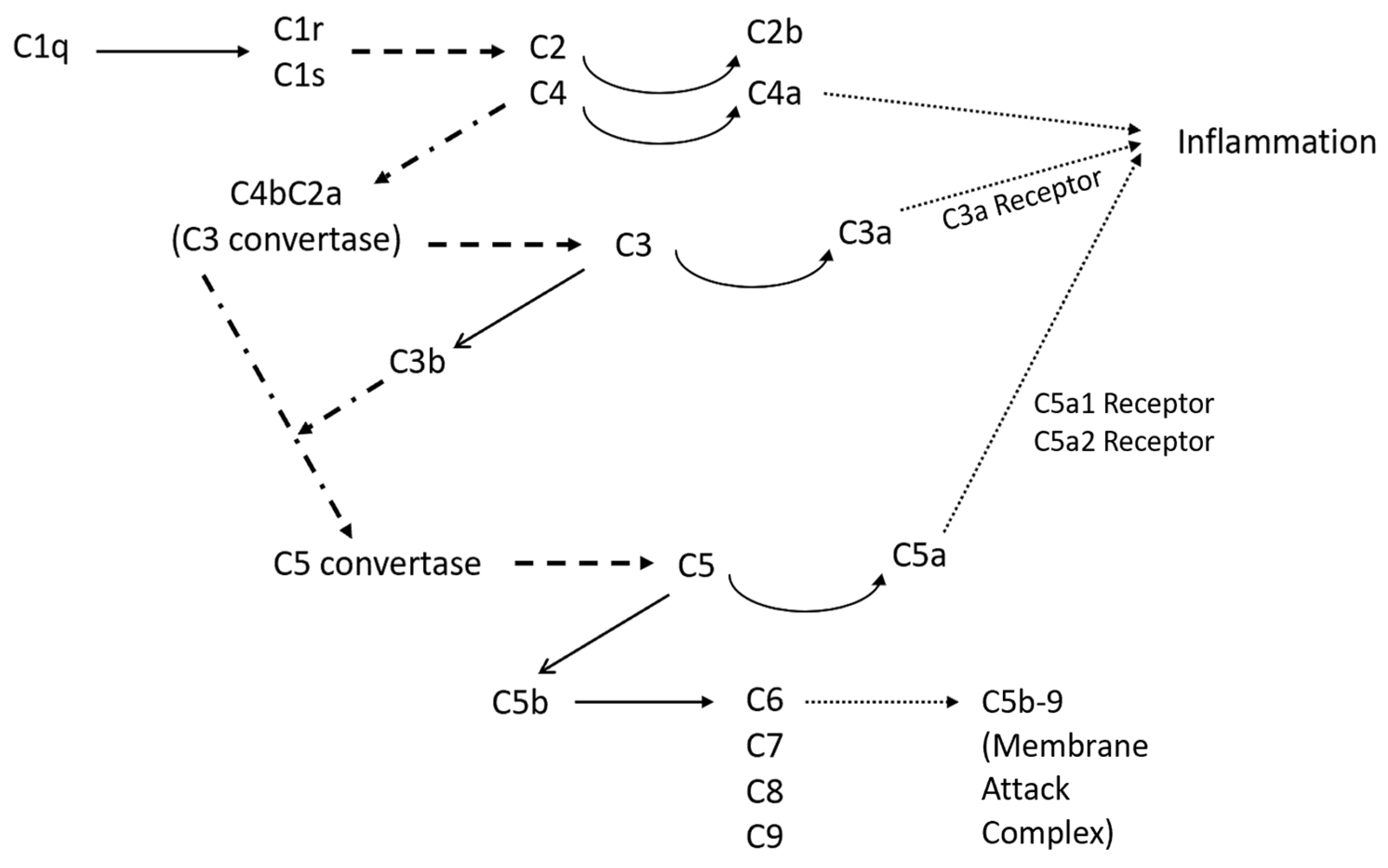
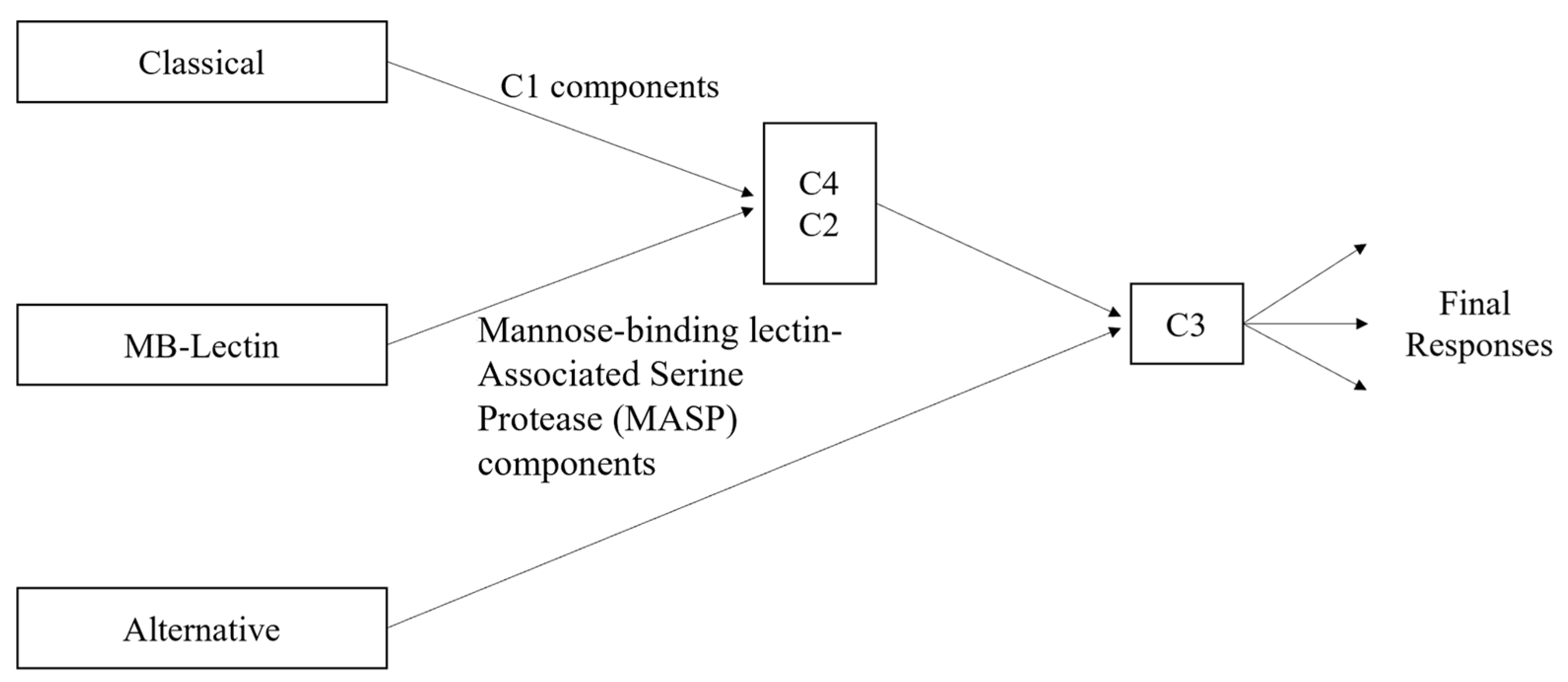
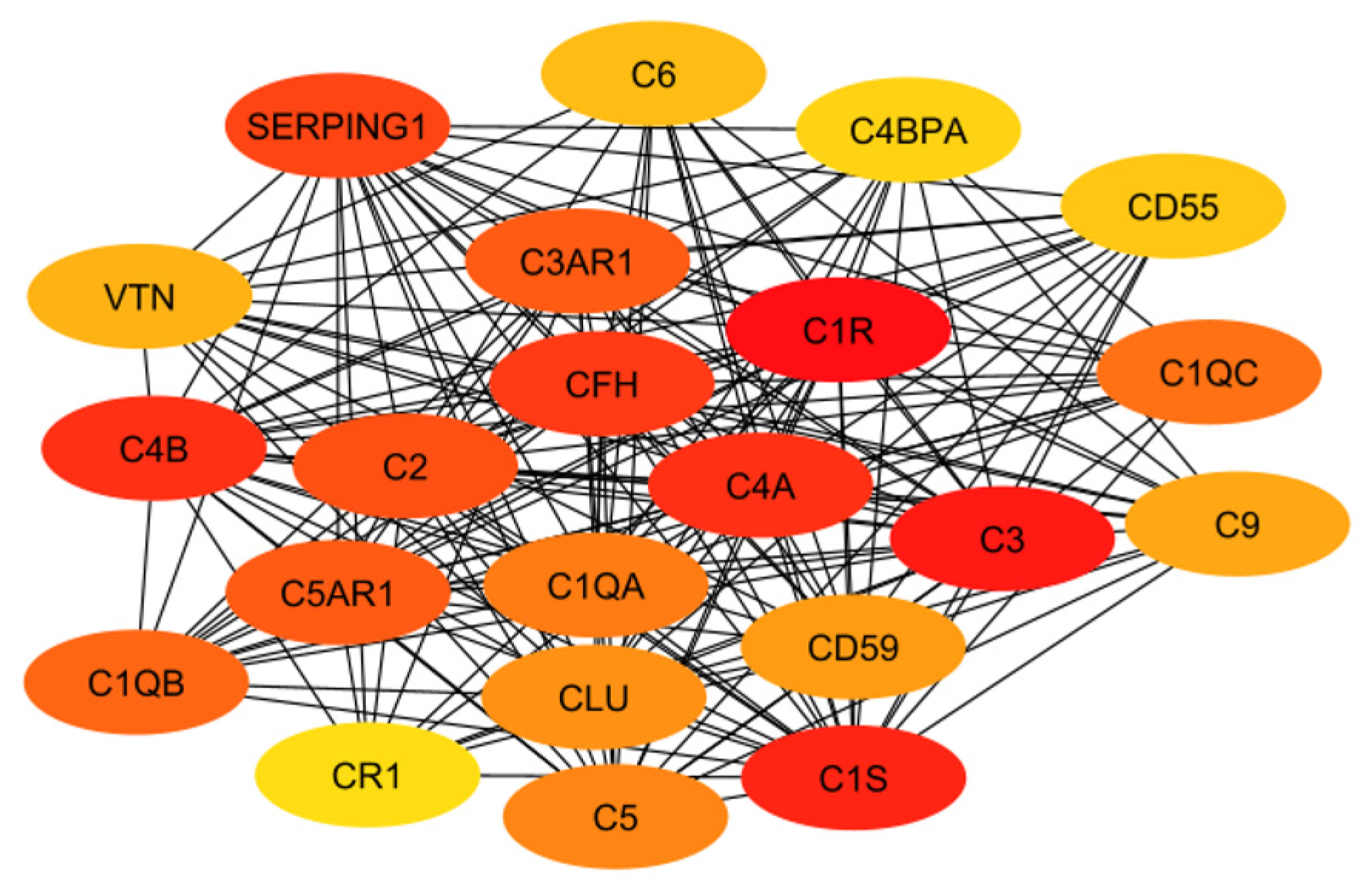
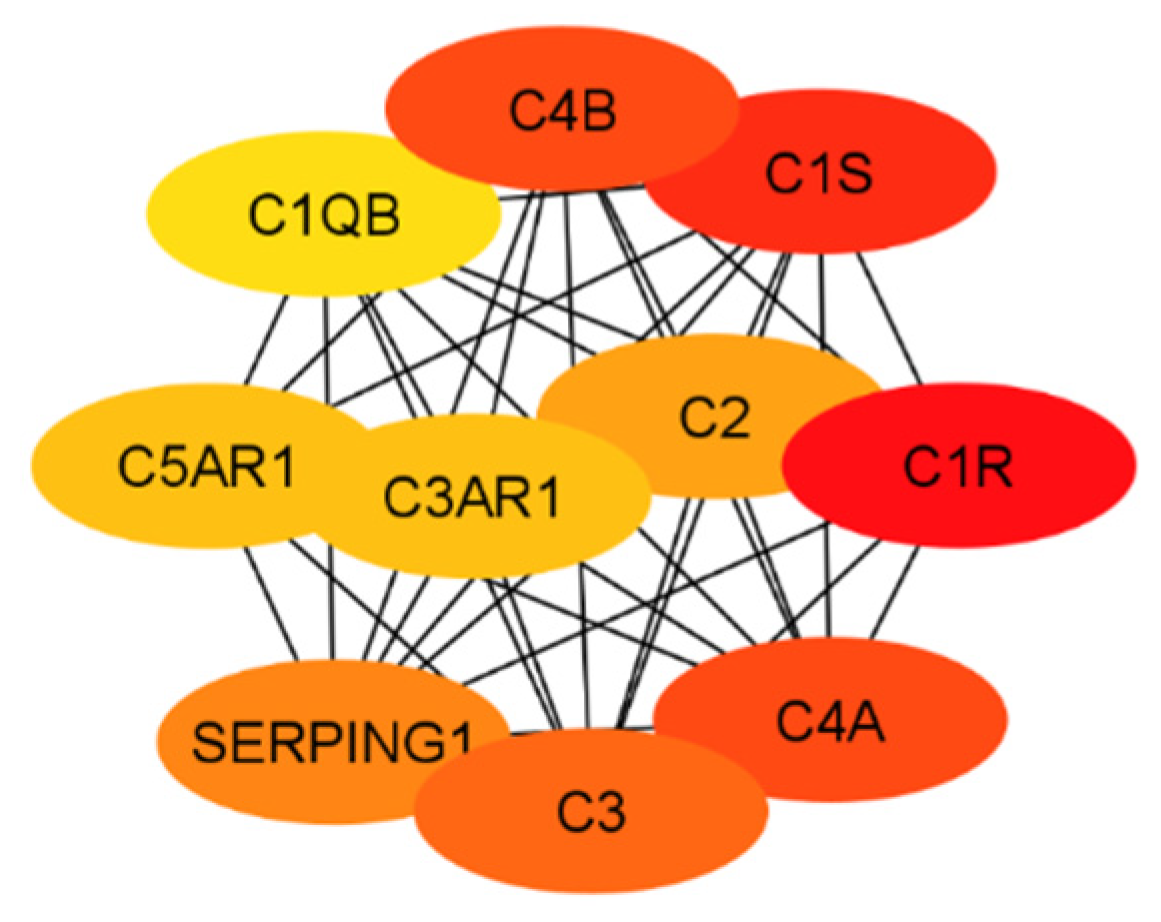
3.1. Classical Complement Pathway
3.2. GPCRs
3.3. Microglia Involvement and Key Proteins
3.4. Cytokines
3.5. Kynurenine and Inflammation Pathway Interactions
4. Discussion: Potential Drug Targets for Regulating Alzheimer’s Inflammation
Limitations and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Finder, V.H. Alzheimer’s Disease: A General Introduction and Pathomechanism. J. Alzheimer’s Dis. 2010, 22, S5–S19. [Google Scholar] [CrossRef] [PubMed]
- Tatulian, S.A. Challenges and hopes for Alzheimer’s disease. Drug Discov. Today 2022, 27, 1027–1043. [Google Scholar] [CrossRef] [PubMed]
- Green, C.; Shearer, J.; Ritchie, C.W.; Zajicek, J.P. Model-Based Economic Evaluation in Alzheimer’s Disease: A Review of the Methods Available to Model Alzheimer’s Disease Progression. Value Health 2011, 14, 621–630. [Google Scholar] [CrossRef]
- Hori, K.; Konishi, K.; Tani, M.; Tomioka, H.; Akita, R.; Kitajima, Y.; Aoki, M.; Kikuchi, N.; Ikuse, D.; Akashi, N.; et al. Why Does the Progression of Alzheimer’s disease Accelerate? Ann. Psychiatry Ment. Health 2014, 2, 1. [Google Scholar]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward Defining the Preclinical Stages of Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimers Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Chohan, M.O.; Khatoon, S.; Iqbal, I.-G.; Iqbal, K. Involvement of in the abnormal hyperphosphorylation of tau and its reversal by Memantine. FEBS Lett. 2006, 580, 3973–3979. [Google Scholar] [CrossRef]
- Wang, L.; Bharti; Kumar, R.; Pavlov, P.F.; Winblad, B. Small molecule therapeutics for tauopathy in Alzheimer’s disease: Walking on the path of most resistance. Eur. J. Med. Chem. 2021, 209, 112915. [Google Scholar] [CrossRef]
- Mahase, E. Alzheimer’s disease: FDA approves lecanemab amid cost and safety concerns. BMJ 2023, 2, 73. [Google Scholar] [CrossRef]
- Canady, V.A. FDA approves new treatment for Alzheimer’s disease. Ment. Health Wkly. 2023, 33, 6–7. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef] [PubMed]
- Perry, V. The influence of systemic inflammation on inflammation in the brain: Implications for chronic neurodegenerative disease. Brain Behav. Immun. 2004, 18, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, X.; Sun, L.; Schultzberg, M.; Hjorth, E. Can inflammation be resolved in Alzheimer’s disease? Ther. Adv. Neurol. Disord. 2018, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.; Sharma, S.; Winston, J.; Nunez, M.; Bottini, G.; Franceschi, M.; Scarpini, E.; Frigerio, E.; Fiorentini, F.; Fernandez, M.; et al. CHF5074 Reduces Biomarkers of Neuroinflammation in Patients with Mild Cognitive Impairment: A 12-Week, Double-Blind, Placebo-Controlled Study. Curr. Alzheimer Res. 2013, 10, 742–753. [Google Scholar] [CrossRef]
- Van Eldik, L.J.; Carrillo, M.C.; Cole, P.E.; Feuerbach, D.; Greenberg, B.D.; Hendrix, J.A.; Kennedy, M.; Kozauer, N.; Margolin, R.A.; Molinuevo, J.L.; et al. The roles of inflammation and immune mechanisms in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2016, 2, 99–109. [Google Scholar] [CrossRef]
- Sporn, M.; Roberta, A.; Goodman, D. (Eds.) The Retinoids; Academic Press, Inc.: Cambridge, MA, USA, 1984; Volume 1. [Google Scholar]
- Landreth, G.E.; Cramer, P.E.; Lakner, M.M.; Cirrito, J.R.; Wesson, D.W.; Brunden, K.R.; Wilson, D.A. Response to Comments on “ApoE-Directed Therapeutics Rapidly Clear β-Amyloid and Reverse Deficits in AD Mouse Models”. Science 2013, 340, 924. [Google Scholar] [CrossRef]
- Butchart, J.; Brook, L.; Hopkins, V.; Teeling, J.; Püntener, U.; Culliford, D.; Sharples, R.; Sharif, S.; McFarlane, B.; Raybould, R.; et al. Etanercept in Alzheimer disease: A randomized, placebo-controlled, double-blind, phase 2 trial. Neurology 2015, 84, 2161–2168. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, Y.; Jiang, Z.; Qing, H. G Protein-Coupled Receptors (GPCRs) in Alzheimer’s Disease: A Focus on BACE1 Related GPCRs. Front. Aging Neurosci. 2016, 8, 58. [Google Scholar] [CrossRef]
- Garcez, M.L.; Jacobs, K.R.; Guillemin, G.J. Microbiota Alterations in Alzheimer’s Disease: Involvement of the Kynurenine Pathway and Inflammation. Neurotox. Res. 2019, 36, 424–436. [Google Scholar] [CrossRef]
- Perry, V.H.; Teeling, J. Microglia and macrophages of the central nervous system: The contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin. Immunopathol. 2013, 35, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.K.; Cho, T.; Choi, H.B.; Jantaratnotai, N.; McLarnon, J.G. Pharmacological antagonism of interleukin-8 receptor CXCR2 inhibits inflammatory reactivity and is neuroprotective in an animal model of Alzheimer’s disease. J. Neuroinflamm. 2015, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.; Akiyama, H.; Itagaki, S.; McGeer, E. Activation of the classical complement pathway in brain tissue of Alzheimer patients. Neurosci. Lett. 1989, 107, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Lue, L.-F.; Yang, L.-B.; Roher, A.; Kuo, Y.-M.; Strohmeyer, R.; Goux, W.J.; Lee, V.; Johnson, G.V.; Webster, S.D.; et al. Complement activation by neurofibrillary tangles in Alzheimer’s disease. Neurosci. Lett. 2001, 305, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Dodds, A. Which Came First, the Lectin/Classical Pathway or the Alternative Pathway of Complement? Immunobiology 2002, 205, 340–354. [Google Scholar] [CrossRef]
- Dunkelberger, J.R.; Song, W.-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.; Travers, P.; Walport, M.; Shlomchik, M. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Pub.: New York, NY, USA, 2001. [Google Scholar]
- Shah, A.; Kishore, U.; Shastri, A. Complement System in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 13647. [Google Scholar] [CrossRef]
- Klos, A.; Wende, E.; Wareham, K.J.; Monk, P.N. International Union of Basic and Clinical Pharmacology. LXXXVII. Complement Peptide C5a, C4a, and C3a Receptors. Pharmacol. Rev. 2013, 65, 500–543. [Google Scholar] [CrossRef]
- Krishnan, V.; Xu, Y.; Macon, K.; Volanakis, J.E.; Narayana, S.V.L. The structure of C2b, a fragment of complement component C2 produced during C3 convertase formation. Acta Crystallogr. Sect. D Struct. Biol. 2009, 65, 266–274. [Google Scholar] [CrossRef]
- Péterfy, H.; Tóth, G.; Pecht, I.; Erdei, A. C3a-derived peptide binds to the type I Fc R and inhibits proximal-coupling signal processes and cytokine secretion by mast cells. Int. Immunol. 2008, 20, 1239–1245. [Google Scholar] [CrossRef]
- Vickers, J.C.; China, D.; Edwardsb, A.M.; Sampsona, V.; Harperc, C.; Morrisonbd, J. Dystrophic Neurite Formation Associated with Age-Related β Amyloid Deposition in the Neocortex: Clues to the Genesis of Neurofibrillary Pathology. Exp. Neurol. 1996, 141, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schvartz, I.; Seger, D.; Shaltiel, S. Vitronectin. Int. J. Biochem. Cell Biol. 1999, 31, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Thathiah, A.; De Strooper, B. The role of G protein-coupled receptors in the pathology of Alzheimer’s disease. Nat. Rev. Neurosci. 2011, 12, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Thathiah, A.; De Strooper, B. G Protein–Coupled Receptors, Cholinergic Dysfunction, and Aβ Toxicity in Alzheimer’s Disease. Sci. Signal. 2009, 2, re8. [Google Scholar] [CrossRef]
- Nelson, C.D.; Sheng, M. GPR3 Stimulates Aβ Production via Interactions with APP and β-Arrestin2. PLoS ONE 2013, 8, e74680. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; Zeng, X.; Bossers, K.; Swaab, D.F.; Zhao, J.; Pei, G. β-Arrestin1 regulates γ-secretase complex assembly and modulates amyloid-β pathology. Cell Res. 2012, 23, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Thathiah, A.; Horré, K.; Snellinx, A.; Vandewyer, E.; Huang, Y.; Ciesielska, M.; De Kloe, G.; Munck, S.; De Strooper, B. β-arrestin 2 regulates Aβ generation and γ-secretase activity in Alzheimer’s disease. Nat. Med. 2012, 19, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Blalock, E.M.; Geddes, J.W.; Chen, K.C.; Porter, N.M.; Markesbery, W.R.; Landfield, P.W. Incipient Alzheimer’s disease: Microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc. Natl. Acad. Sci. USA 2004, 101, 2173–2178. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, Y.; Ma, Q.; Zhou, A.; Zhang, X.; Zhang, Y.-W. M1 muscarinic acetylcholine receptor interacts with BACE1 and regulates its proteosomal degradation. Neurosci. Lett. 2012, 515, 125–130. [Google Scholar] [CrossRef]
- Teng, L.; Zhao, J.; Wang, F.; Ma, L.; Pei, G. A GPCR/secretase complex regulates β- and γ-secretase specificity for Aβ production and contributes to AD pathogenesis. Cell Res. 2010, 20, 138–153. [Google Scholar] [CrossRef]
- Arendash, G.; Schleif, W.; Rezai-Zadeh, K.; Jackson, E.; Zacharia, L.; Cracchiolo, J.; Shippy, D.; Tan, J. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain β-amyloid production. Neuroscience 2006, 142, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Heese, K. P60TRP interferes with the GPCR/secretase pathway to mediate neuronal survival and synaptogenesis. J. Cell. Mol. Med. 2010, 15, 2462–2477. [Google Scholar] [CrossRef] [PubMed]
- Buggia-Prévot, V.; Fernandez, C.G.; Riordan, S.; Vetrivel, K.S.; Roseman, J.; Waters, J.; Bindokas, V.P.; Vassar, R.; Thinakaran, G. Axonal BACE1 dynamics and targeting in hippocampal neurons: A role for Rab11 GTPase. Mol. Neurodegener. 2014, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Sannerud, R.; Declerck, I.; Peric, A.; Raemaekers, T.; Menendez, G.; Zhou, L.; Veerle, B.; Coen, K.; Munck, S.; De Strooper, B.; et al. ADP ribosylation factor 6 (ARF6) controls amyloid precursor protein (APP) processing by mediating the endosomal sorting of BACE1. Proc. Natl. Acad. Sci. USA 2011, 108, E559–E568. [Google Scholar] [CrossRef] [PubMed]
- Langmead, C.J.; Watson, J.; Reavill, C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol. Ther. 2008, 117, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.W.; Kalia, L.V. Src kinases: A hub for NMDA receptor regulation. Nat. Rev. Neurosci. 2004, 5, 317–328. [Google Scholar] [CrossRef]
- Zhou, X.-B.; Wulfsen, I.; Lutz, S.; Utku, E.; Sausbier, U.; Ruth, P.; Wieland, T.; Korth, M. M2 Muscarinic Receptors Induce Airway Smooth Muscle Activation via a Dual, Gβγ-mediated Inhibition of Large Conductance Ca2+-activated K+ Channel Activity. J. Biol. Chem. 2008, 283, 21036–21044. [Google Scholar] [CrossRef]
- Odagaki, Y.; Kinoshita, M.; Toyoshima, R. Functional Activation of G-Proteins Coupled With Muscarinic Acetylcholine Receptors in Rat Brain Membranes. J. Pharmacol. Sci. 2014, 125, 157–168. [Google Scholar] [CrossRef][Green Version]
- Reisberg, B.; Ferris, S.H.; Anand, R.; Mir, P.; Geibel, V.; De Leon, M.J.; Roberts, E. Effects of Naloxone in Senile Dementia: A Double-Blind Trial. N. Engl. J. Med. 1983, 308, 721–722. [Google Scholar] [CrossRef]
- Tariot, P.N.; Sunderland, T.; Weingartner, H.; Murphy, D.L.; Cohen, M.R.; Cohen, R.M. Naloxone and Alzheimer’s Disease. Arch. Gen. Psychiatry 1986, 43, 727–732. [Google Scholar] [CrossRef]
- Henderson, V.W.; Roberts, E.; Wimer, C.; Bardolph, E.L.; Chui, H.C.; Damasio, A.R.; Eslinger, P.J.; Folstein, M.F.; Schneider, L.S.; Teng, E.L.; et al. Multicenter trial of naloxone in alzheimer’s disease. Ann. Neurol. 1989, 25, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhao, X.; Bao, G.; Zou, L.; Teng, L.; Wang, Z.; Song, M.; Xiong, J.; Bai, Y.; Pei, G. Activation of β2-adrenergic receptor stimulates γ-secretase activity and accelerates amyloid plaque formation. Nat. Med. 2006, 12, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Angulo, E.; Casadó, V.; Mallol, J.; Canela, E.I.; Viñals, F.; Ferrer, I.; Lluis, C.; Franco, R. A1 Adenosine Receptors Accumulate in Neurodegenerative Structures in Alzheimer’s Disease and Mediate Both Amyloid Precursor Protein Processing and Tau Phosphorylation and Translocation. Brain Pathol. 2003, 13, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Udayar, V.; Buggia-Prévot, V.; Guerreiro, R.L.; Siegel, G.; Rambabu, N.; Soohoo, A.L.; Ponnusamy, M.; Siegenthaler, B.; Bali, J.; Simons, M.; et al. A Paired RNAi and RabGAP Overexpression Screen Identifies Rab11 as a Regulator of β-Amyloid Production. Cell Rep. 2013, 5, 1536–1551. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Wang, Q.; Xue, Q.; Li, W.; Li, X.; Wu, Y. The Dual Role of Kinin/Kinin Receptors System in Alzheimer’s Disease. Front. Mol. Neurosci. 2019, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-S.; Hsu, J.-W.; Lin, H.-Y.; Lai, S.-W.; Huang, B.-R.; Tsai, C.-F.; Lu, D.-Y. Bradykinin B1 receptor contributes to interleukin-8 production and glioblastoma migration through interaction of STAT3 and SP-1. Neuropharmacology 2018, 144, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.; Wu, C.; Hwang, T.; Yen, M.; Parker, P.; Yang, C. BK-induced cytosolic phospholipase A2 expression via sequential PKC-δ, p42/p44 MAPK, and NF-κB activation in rat brain astrocytes. J. Cell. Physiol. 2005, 206, 246–254. [Google Scholar] [CrossRef]
- Iores-Marçal, L.M.; Viel, T.A.; Buck, H.S.; Nunes, V.A.; Gozzo, A.J.; Cruz-Silva, I.; Miranda, A.; Shimamoto, K.; Ura, N.; Araujo, M.S. Bradykinin release and inactivation in brain of rats submitted to an experimental model of Alzheimer’s disease. Peptides 2006, 27, 3363–3369. [Google Scholar] [CrossRef]
- Prediger, R.; Medeiros, R.; Pandolfo, P.; Duarte, F.; Passos, G.; Pesquero, J.; Campos, M.; Calixto, J.; Takahashi, R.N. Genetic deletion or antagonism of kinin B1 and B2 receptors improves cognitive deficits in a mouse model of Alzheimer’s disease. Neuroscience 2008, 151, 631–643. [Google Scholar] [CrossRef]
- Viel, T.A.; Caetano, A.L.; Nasello, A.G.; Lancelotti, C.L.; Nunes, V.A.; Araujo, M.S.; Buck, H.S. Increases of kinin B1 and B2 receptors binding sites after brain infusion of amyloid-beta 1–40 peptide in rats. Neurobiol. Aging 2008, 29, 1805–1814. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J. Injection of bradykinin or cyclosporine A to hippocampus induces Alzheimer-like phosphorylation of Tau and abnormal behavior in rats. Chin. Med. J. 2002, 115, 884–887. [Google Scholar]
- Amaral, F.A.; Lemos, M.T.R.; Dong, K.E.; Bittencourt, M.F.Q.P.; Caetano, A.L.; Pesquero, J.B.; Viel, T.A.; Buck, H.S. Participation of kinin receptors on memory impairment after chronic infusion of human amyloid-β 1-40 peptide in mice. Neuropeptides 2010, 44, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Passos, G.F.; Figueiredo, C.P.; Prediger, R.D.; Silva, K.A.; Siqueira, J.M.; Duarte, F.S.; Leal, P.C.; Medeiros, R.; Calixto, J.B. Involvement of phosphoinositide 3-kinase γ in the neuro-inflammatory response and cognitive impairments induced by β-amyloid 1–40 peptide in mice. Brain Behav. Immun. 2010, 24, 493–501. [Google Scholar] [CrossRef]
- Raslan, F.; Schwarz, T.; Meuth, S.G.; Austinat, M.; Bader, M.; Renné, T.; Roosen, K.; Stoll, G.; Sirén, A.-L.; Kleinschnitz, C. Inhibition of Bradykinin Receptor B1 Protects Mice from Focal Brain Injury by Reducing Blood–Brain Barrier Leakage and Inflammation. J. Cereb. Blood Flow Metab. 2010, 30, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Zipser, B.; Johanson, C.; Gonzalez, L.; Berzin, T.; Tavares, R.; Hulette, C.; Vitek, M.; Hovanesian, V.; Stopa, E. Microvascular injury and blood–brain barrier leakage in Alzheimer’s disease. Neurobiol. Aging 2007, 28, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Caetano, A.; Dong-Creste, K.; Amaral, F.; Monteiro-Silva, K.; Pesquero, J.; Araujo, M.; Montor, W.; Viel, T.; Buck, H. Kinin B2 receptor can play a neuroprotective role in Alzheimer’s disease. Neuropeptides 2015, 53, 51–62. [Google Scholar] [CrossRef] [PubMed]
- van Rossum, D.; Hanisch, U.-K. Microglia. Metab. Brain Dis. 2004, 19, 393–411. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Cunningham, C. Microglia and neurodegeneration: The role of systemic inflammation. Glia 2012, 61, 71–90. [Google Scholar] [CrossRef]
- Amor, S.; Puentes, F.; Baker, D.; Van Der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef]
- Jay, T.R.; Miller, C.M.; Cheng, P.J.; Graham, L.C.; Bemiller, S.; Broihier, M.L.; Xu, G.; Margevicius, D.; Karlo, J.C.; Sousa, G.L.; et al. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J. Exp. Med. 2015, 212, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, J.D.; Finn, M.B.; Wang, Y.; Shen, A.; Mahan, T.E.; Jiang, H.; Stewart, F.R.; Piccio, L.; Colonna, M.; Holtzman, D.M. Altered microglial response to Aβ plaques in APPPS1-21 mice heterozygous for TREM2. Mol. Neurodegener. 2014, 9, 20. [Google Scholar] [CrossRef]
- Jay, T.R.; Hirsch, A.M.; Broihier, M.L.; Miller, C.M.; Neilson, L.E.; Ransohoff, R.M.; Lamb, B.T.; Landreth, G.E. Disease Progression-Dependent Effects of TREM2 Deficiency in a Mouse Model of Alzheimer’s Disease. J. Neurosci. 2016, 37, 637–647. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, S.M.; McCray, T.J.; Allan, K.; Formica, S.V.; Xu, G.; Wilson, G.; Kokiko-Cochran, O.N.; Crish, S.D.; Lasagna-Reeves, C.A.; Ransohoff, R.M.; et al. TREM2 deficiency exacerbates tau pathology through dysregulated kinase signaling in a mouse model of tauopathy. Mol. Neurodegener. 2017, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Goldgaber, D.; Harris, H.W.; Hla, T.; Maciag, T.; Donnelly, R.J.; Jacobsen, J.S.; Vitek, M.P.; Gajdusek, D.C. Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc. Natl. Acad. Sci. USA 1989, 86, 7606–7610. [Google Scholar] [CrossRef] [PubMed]
- Plassman, B.; Havlik, R.; Steffens, D.; Helms, M.; Newman, T.; Drosdick, D.; Phillips, C.; Gau, B.; Welsh–Bohmer, K.; Burke, J.; et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology 2000, 55, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, R.A.; Orellana, D.I.; González-Billault, C.; Maccioni, R.B. Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Exp. Cell Res. 2004, 295, 245–257. [Google Scholar] [CrossRef]
- Mukaida, N. Chemokines. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef]
- Walker, D.G.; Lue, L.-F.; Beach, T.G. Gene expression profiling of amyloid beta peptide-stimulated human post-mortem brain microglia. Neurobiol. Aging 2001, 22, 957–966. [Google Scholar] [CrossRef]
- Xia, M.; Qin, S.; McNamara, M.; Mackay, C.; Hyman, B.T. Interleukin-8 receptor B immunoreactivity in brain and neuritic plaques of Alzheimer’s disease. Am. J. Pathol. 1997, 150, 1267–1274. [Google Scholar]
- Kadriu, B.; Ballard, E.D.; Henter, I.D.; Murata, S.; Gerlus, N.; Zarate, C.A. Neurobiological biomarkers of response to ketamine. Adv. Pharmacol. 2020, 89, 195–235. [Google Scholar] [CrossRef] [PubMed]
- Willette, A.A.; Pappas, C.; Hoth, N.; Wang, Q.; Klinedinst, B.; Willette, S.A.; Larsen, B.; Pollpeter, A.; Li, T.; Le, S.; et al. Inflammation, negative affect, and amyloid burden in Alzheimer’s disease: Insights from the kynurenine pathway. Brain Behav. Immun. 2021, 95, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Spalletta, G.; Caltagirone, C.; Girardi, P.; Gianni, W.; Casini, A.R.; Palmer, K. The role of persistent and incident major depression on rate of cognitive deterioration in newly diagnosed Alzheimer’s disease patients. Psychiatry Res. 2012, 198, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; Capuron, L. Inflammation-Associated Depression: Evidence, Mechanisms and Implications; Springer Science and Business Media LLC: Dordrecht, The Netherlands, 2017; ISBN 9783030310257. [Google Scholar]
- Campbell, B.M.; Charych, E.; Lee, A.W.; Möller, T. Kynurenines in CNS disease: Regulation by inflammatory cytokines. Front. Neurosci. 2014, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, G.J.; Croitoru-Lamoury, J.; Dormont, D.; Armati, P.J.; Brew, B.J. Quinolinic acid upregulates chemokine production and chemokine receptor expression in astrocytes. Glia 2003, 41, 371–381. [Google Scholar] [CrossRef]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of Depression With C-Reactive Protein, IL-1, and IL-6: A Meta-Analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef]
- Zunszain, P.A.; Anacker, C.; Cattaneo, A.; Choudhury, S.; Musaelyan, K.; Myint, A.M.; Thuret, S.; Price, J.; Pariante, C.M. Interleukin-1β: A New Regulator of the Kynurenine Pathway Affecting Human Hippocampal Neurogenesis. Neuropsychopharmacology 2011, 37, 939–949. [Google Scholar] [CrossRef]
- Von Mering, C.; Huynen, M.; Jaeggi, D.; Schmidt, S.; Bork, P.; Snel, B. STRING: A database of predicted functional associations between proteins. Nucleic Acids Res. 2003, 31, 258–261. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Kuhn, M.; Simonovic, M.; Roth, A.; Minguez, P.; Doerks, T.; Stark, M.; Muller, J.; Bork, P.; et al. The STRING database in 2011: Functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 2010, 39, D561–D568. [Google Scholar] [CrossRef]
- Snel, B. STRING: A web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 2000, 28, 3442–3444. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.; Saüch, J.; Sanz, F.; Furlong, L.I. The DisGeNET cytoscape app: Exploring and visualizing disease genomics data. Comput. Struct. Biotechnol. J. 2021, 19, 2960–2967. [Google Scholar] [CrossRef] [PubMed]
- Reimand, J.; Isserlin, R.; Voisin, V.; Kucera, M.; Tannus-Lopes, C.; Rostamianfar, A.; Wadi, L.; Meyer, M.; Wong, J.; Xu, C.; et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 2019, 14, 482–517. [Google Scholar] [CrossRef] [PubMed]
- Hogue, C.W.; Groll, M. Mcode. BMC Bioinform. 2001, 29, 1. [Google Scholar]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. S4), S11. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. An Overview on GPCRs and Drug Discovery: Structure-Based Drug Design and Structural Bi-ology on GPCRs. Methods Mol. Biol. 2009, 552, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Hashizume, M.; Yoshida, H.; Suzuki, M.; Shiina, M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. 2011, 122, 143–159. [Google Scholar] [CrossRef]
- SERPING1 Gene—Serpin Family G Member 1,” Gene Cards—The Human Gene Database. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=SERPING1 (accessed on 1 April 2023).
- Robertson, N.; Rappas, M.; Doré, A.S.; Brown, J.; Bottegoni, G.; Koglin, M.; Cansfield, J.; Jazayeri, A.; Cooke, R.M.; Marshall, F.H. Structure of the complement C5a receptor bound to the extra-helical antagonist NDT9513727. Nature 2018, 553, 111–114. [Google Scholar] [CrossRef]
- Bruchfeld, A.; Magin, H.; Nachman, P.; Parikh, S.; Lafayette, R.; Potarca, A.; Miao, S.; Bekker, P. C5a receptor inhibitor avacopan in immunoglobulin A nephropathy—An open-label pilot study. Clin. Kidney J. 2022, 15, 922–928. [Google Scholar] [CrossRef]
- Błaszczyk, J.W. Parkinson’s Disease and Neurodegeneration: GABA-Collapse Hypothesis. Front. Neurosci. 2016, 10, 269. [Google Scholar] [CrossRef]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABAA receptors: Structure, function, pharmacology, and related disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Masiulis, S.; Desai, R.; Uchański, T.; Martin, I.S.; Laverty, D.; Karia, D.; Malinauskas, T.; Zivanov, J.; Pardon, E.; Kotecha, A.; et al. GABAA receptor signalling mechanisms revealed by structural pharmacology. Nature 2019, 565, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; Agrawal, V.; Hsing, M.; Ton, A.-T.; Ban, F.; Norinder, U.; Gleave, M.E.; Cherkasov, A. Deep Docking: A Deep Learning Platform for Augmentation of Structure Based Drug Discovery. ACS Central Sci. 2020, 6, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Sente, A.; Desai, R.; Naydenova, K.; Malinauskas, T.; Jounaidi, Y.; Miehling, J.; Zhou, X.; Masiulis, S.; Hardwick, S.W.; Chirgadze, D.Y.; et al. Differential assembly diversifies GABAA receptor structures and signalling. Nature 2022, 604, 190–194. [Google Scholar] [CrossRef]
- Kim, J.J.; Gharpure, A.; Teng, J.; Zhuang, Y.; Howard, R.J.; Zhu, S.; Noviello, C.M.; Walsh, R.M., Jr.; Lindahl, E.; Hibbs, R.E. Shared structural mechanisms of general anaesthetics and benzodiazepines. Nature 2020, 585, 303–308. [Google Scholar] [CrossRef]
- Dani, N.; Barbosa, A.J.M.; Del Rio, A.; Di Girolamo, M. ADP-Ribosylated Proteins as Old and New Drug Targets for Anticancer Therapy: The Example of ARF6. Curr. Pharm. Des. 2013, 19, 624–633. [Google Scholar] [CrossRef]
- Marquer, C.; Tian, H.; Yi, J.; Bastien, J.; Dall’Armi, C.; Yang-Klingler, Y.; Zhou, B.; Chan, R.B.; Di Paolo, G. Arf6 controls retromer traffic and intracellular cholesterol distribution via a phosphoinositide-based mechanism. Nat. Commun. 2016, 7, 11919. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Zou, X. Advances and Challenges in Protein-Ligand Docking. Int. J. Mol. Sci. 2010, 11, 3016–3034. [Google Scholar] [CrossRef]
- Pagadala, N.S.; Syed, K.; Tuszynski, J. Software for molecular docking: A review. Biophys. Rev. 2017, 9, 91–102. [Google Scholar] [CrossRef]
- Scarpino, A.; Ferenczy, G.G.; Keserű, G.M. Comparative Evaluation of Covalent Docking Tools. J. Chem. Inf. Model. 2018, 58, 1441–1458. [Google Scholar] [CrossRef]
- Krass, E.; Zhai, T.; Huang, Z. Identification of Apolipoprotein E4 inhibitors for Alzheimer’s Disease Therapy through a Large-Scale Virtual Screening. IFAC-PapersOnLine 2022, 55, 27–32. [Google Scholar] [CrossRef]
- Zhang, F.; Graham, J.; Zhai, T.; Liu, Y.; Huang, Z. Discovery of MurA Inhibitors as Novel Antimicrobials through an Integrated Computational and Experimental Approach. Antibiotics 2022, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Zhang, F.; Haider, S.; Kraut, D.; Huang, Z. An Integrated Computational and Experimental Approach to Identifying Inhibitors for SARS-CoV-2 3CL Protease. Front. Mol. Biosci. 2021, 8, 661424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhai, T.; Haider, S.; Liu, Y.; Huang, Z.J. Synergistic Effect of Chlorogenic Acid and Caffeic Acid with Fosfomycin on Growth Inhibition of a Resistant Listeria monocytogenes Strain. ACS Omega 2020, 5, 7537–7544. [Google Scholar] [CrossRef]
- Lye, S.; Aust, C.E.; Griffiths, L.R.; Fernandez, F. Exploring new avenues for modifying course of progression of Alzheimer’s disease: The rise of natural medicine. J. Neurol. Sci. 2021, 422, 117332. [Google Scholar] [CrossRef]

| Abbreviation | Name | Function |
|---|---|---|
| VTN | Vitronectin | Complement system regulator: binds C5b-7, C5b-8, C5b-9 |
| C4B | Complement component C4b | Binds with C2A to form C3 convertase, opsonin |
| C1QB | B-chain of complement component C1q | C1q binds with C1r and C1s to form C1 complex, which activates classical complement pathway |
| SERPING1 | C1 inhibitor | Inhibits C1r and C1s |
| C2 | Complement component C2 | Cleaved by C1s to form C2a and C2b |
| C5AR1 | Complement C5a receptor 1 | G protein-coupled receptor for component C5a |
| CR1 | Complement receptor 1 | Increases cleavage of C3b and C4b |
| C6 | Complement component C6 | Binds with C5b, initiating cascade that results in membrane attack complex (MAC) |
| C3AR1 | Complement C3a receptor 1 | G protein-coupled receptor for component C3a |
| CFH | Complement factor H | Cofactor for C3b cleavage |
| C1QA | A chain of complement component C1q | C1q binds with C1r and C1s to form C1 complex, which activates classical complement pathway |
| CLU | Clusterin | Inhibits creation of MAC |
| C5 | Complement component C5 | Cleaved by C4bC2aC3b to form C5a and C5b |
| C4BPA | C4-binding protein alpha | Binds to component C4b |
| C1R | Complement C1r subcomponent | Serine protease involved in C1 complex |
| C4A | Complement component C4 type A | Cleaved from C4 alongside C4B, anaphylatoxin |
| CD59 | MAC-inhibitory protein | Inhibits creation of MAC |
| C1S | Complement C1s subcomponent | Serine protease involved in C1 complex |
| C3 | Complement component C3 | Cleaved by C4bC2a to form C3a and C3b |
| CD55 | Complement decay-accelerating factor | Destabilized C3 and C5 convertases |
| C1QC | C chain of complement component C1q | C1q binds with C1r and C1s to form C1 complex, which activates classical complement pathway |
| C9 | Complement component C9 | Binds with C5b-8 to form MAC |
| Abbreviation | Name | Function |
|---|---|---|
| GABRG1 | Gamma-aminobutyric acid receptor subunit gamma-1 | GPCR that inhibits neurotransmission and is involved in several signaling pathways |
| GABRG2 | Gamma-aminobutyric acid receptor subunit gamma-2 | GPCR involved in inhibiting neurotransmission, major component of GABA-A receptors |
| GABRG3 | Gamma-aminobutyric acid receptor subunit gamma-3 | GPCR involved in inhibiting neurotransmission, major component of GABA-A receptors |
| DRD2 | Dopamine receptor D2 | GPCR that inhibits adenylyl cyclase activity |
| OPRD1 | Opioid receptor delta 1 | Involved in opioid and enkephalin receptor activity and response |
| BHLHB8 | Basic helix-loop-helix domain class B, 8 | Essential for glucose homeostasis and calcium ion transmembrane transportation |
| APP | Amyloid beta precursor protein | Leads to generation of Aβ |
| RAB5A | Ras-related protein Rab-5A | Small GTPase involved in regulation of intracellular membrane transportation |
| RAB11A | Ras-related protein Rab-11A | Small GTPase involved in regulation of secretion and intracellular membrane transportation |
| RAB7A | Ras-related protein Rab-7A | Small GTPase involved in regulation of intracellular membrane transportation |
| RAB7B | Ras-related protein Rab-7B | Small GTPase involved in regulation of intracellular membrane transportation and protein degradation |
| ARF6 | ADP-ribosylation factor 6 | Binding protein involved in membrane transportation |
| BACE1 | β-site APP-cleaving enzyme 1 | Limits Aβ generation in the brain |
| Target | Crystal Structure | Clinical Trials |
|---|---|---|
| C1R | Yes | Conestat alfa, Human C1-esterase inhibitor, Palivizumab, Zinc acetate, Zinc cation |
| C1S | Yes | Sutimlimab, Conestat alfa, Copper, Human C1-esterase inhibitor, Zinc acetate |
| C4A | Yes | Human immunoglobulin G |
| C4B | Yes | Zinc cation, Copper, Human immunoglobulin G, Zinc acetate, Zinc chloride |
| C3 | Yes | Clozapine, Pegcetacoplan, Copper, Human Immunoglobulin G, Zinc acetate |
| SERPING1 | Yes | Copper, PPL-100, CINRYZE, RHUCIN |
| C2 | Not whole | Abaloparatide |
| C5AR1 | Yes | Avacopan, PMX 205, Calcium, COMPSTATIN |
| C3AR1 | No | Histamine, D-Threonine, D-Tyrosine, Serine, Calcium |
| C1QB | No | Bevacizumab, Cetuximab, Etanercept, Palivizumab, Zinc acetate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharo, C.; Zhai, T.; Huang, Z. Investigation of Potential Drug Targets Involved in Inflammation Contributing to Alzheimer’s Disease Progression. Pharmaceuticals 2024, 17, 137. https://doi.org/10.3390/ph17010137
Sharo C, Zhai T, Huang Z. Investigation of Potential Drug Targets Involved in Inflammation Contributing to Alzheimer’s Disease Progression. Pharmaceuticals. 2024; 17(1):137. https://doi.org/10.3390/ph17010137
Chicago/Turabian StyleSharo, Catherine, Tianhua Zhai, and Zuyi Huang. 2024. "Investigation of Potential Drug Targets Involved in Inflammation Contributing to Alzheimer’s Disease Progression" Pharmaceuticals 17, no. 1: 137. https://doi.org/10.3390/ph17010137
APA StyleSharo, C., Zhai, T., & Huang, Z. (2024). Investigation of Potential Drug Targets Involved in Inflammation Contributing to Alzheimer’s Disease Progression. Pharmaceuticals, 17(1), 137. https://doi.org/10.3390/ph17010137








