Metabolomic Profile, Plasmatic Levels of Losartan and EXP3174, Blood Pressure Control in Hypertensive Patients and Their Correlation with COVID-19
Abstract
:1. Introduction
2. Results
2.1. Patients Characteristics
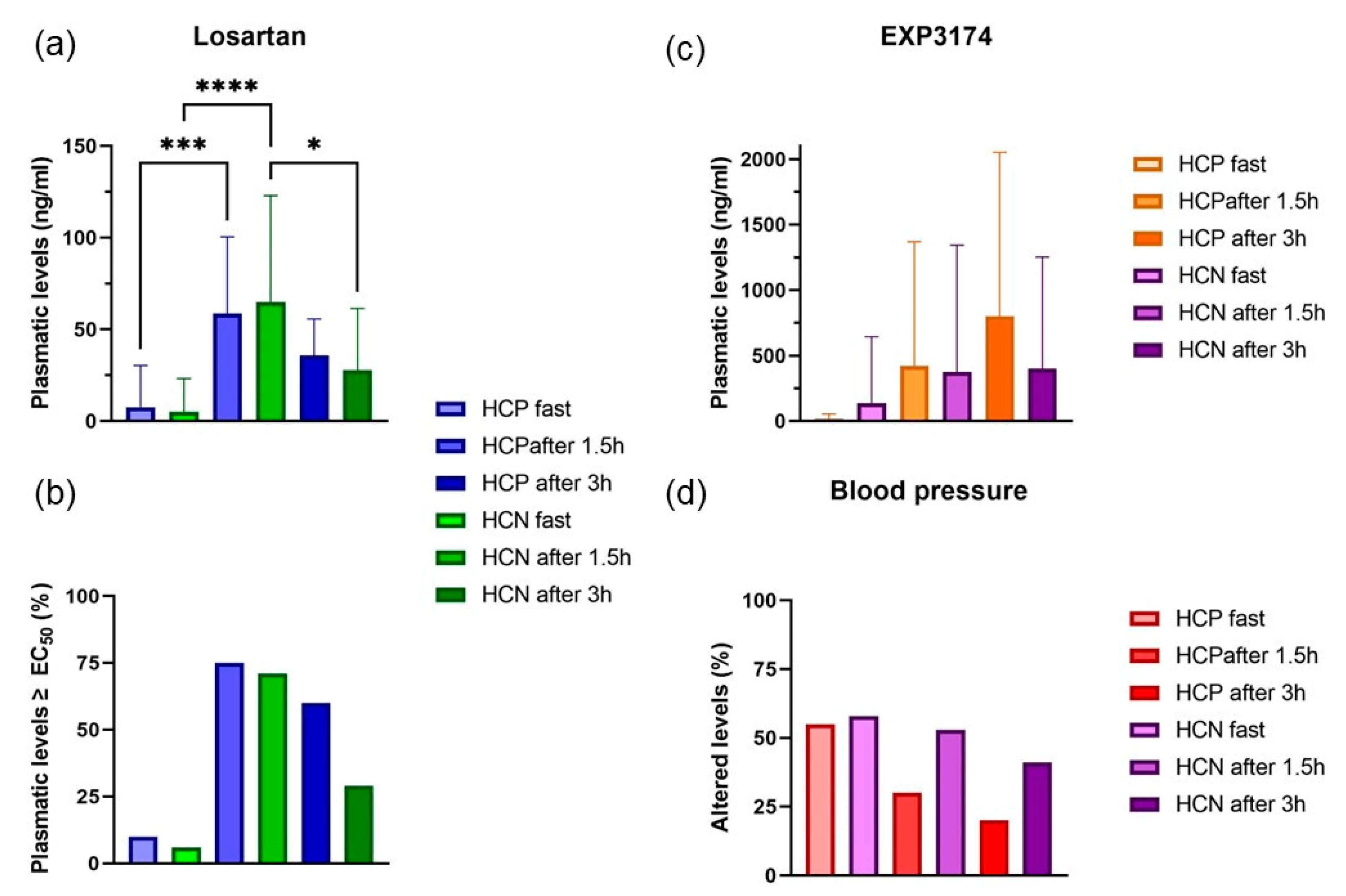
2.2. Plasmatic Quantification of Losartan and EXP3174
NMR Metabolomics
2.3. HCP Groups
2.3.1. HCP vs. NCP Groups
2.3.2. HCP 3 Months vs. 6 Months vs. 1 Year
2.3.3. HCP 3 Months vs. HCP 1 Year
2.3.4. HCP 1 Year vs. NCP 1 Year
2.3.5. HCP 6 Months vs. NCP 6 Months
2.3.6. HCP 3 Months vs. NCP 3 Months
2.4. RMN of NCP Groups
2.4.1. NCP 3 Months vs. NCP 6 Months vs. NCP 1 Year
2.4.2. NCP 3 Months vs. NCP 1 Year
2.4.3. HCN vs. NCN Groups
2.5. Correlation Maps
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Participants and Ethical Considerations
4.3. Determination of Plasmatic Levels of Losartan and EXP3174
4.4. Sample Preparation for Metabolomics and Acquisition of 1H NMR Spectra
4.5. Analysis of Clinical and Laboratory Parameters
4.6. Metabolic Pathway
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, J.; Zhou, F.; Hou, W.; Silver, Z.; Wong, C.Y.; Chang, O.; Huang, E.; Zuo, Q.K. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: A meta-analysis. Ann. N. Y. Acad. Sci. 2021, 1486, 90–111. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- South, A.M.; Diz, D.I.; Chappell, M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1084–H1090. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.-L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, L.; Cai, J.; Lei, F.; Qin, J.-J.; Xie, J.; Liu, Y.-M.; Zhao, Y.-C.; Huang, X.; Lin, L.; et al. Association of Inpatient Use of Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality among Patients with Hypertension Hospitalized with COVID-19. Circ. Res. 2020, 126, 1671–1681. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Guo, Q.; Zheng, Y.; Shi, J.; Wang, J.; Li, G.; Li, C.; Fromson, J.A.; Xu, Y.; Liu, X.; Xu, H.; et al. Immediate psychological distress in quarantined patients with COVID-19 and its association with peripheral inflammation: A mixedmethod study. Brain Behav. Immun. 2020, 88, 17–27. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Lopes, R.D.; Macedo, A.V.; Silva, P.G.d.B.E.; Moll-Bernardes, R.J.; Dos Santos, T.M.; Mazza, L.; Feldman, A.; Arruda, G.D.S.; de Albuquerque, D.C.; Camiletti, A.S.; et al. Effect of discontinuing vs. continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: A randomized clinical trial. J. Am. Med. Assoc. 2021, 325, 254–264. [Google Scholar] [CrossRef]
- Safadi, M.A.P. The intriguing features of COVID-19 in children and its impact on the pandemic. J. Pediatr. 2020, 96, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Lu, X.; Xu, C.; Sun, W.; Pan, B. Understanding of COVID-19 based on current evidence. J. Med. Virol. 2020, 92, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Wilson, I.D. Gut microorganisms, mammalian metabolism and personalized health care. Nat. Rev. Microbiol. 2005, 3, 431–438. [Google Scholar] [CrossRef]
- De Brito, V.P.; Dias, F.L.T.; Oliveira, S. Hipertensão arterial sistêmica, uso de bloqueadores dos receptores de angiotensina II e inibidores da enzima conversora da angiotensina e COVID-19: Uma revisão sistemática. InterAm. J. Med. Health 2020, 3, 1–6. [Google Scholar] [CrossRef]
- Kai, H.; Kai, M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors-lessons from available evidence and insights into COVID-19. Hypertens. Res. 2020, 43, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Cabaleiro, T.; Román, M.; Ochoa, D.; Talegón, M.; Prieto-Pérez, R.; Wojnicz, A.; López-Rodríguez, R.; Novalbos, J.; Abad-Santos, F. Evaluation of the relationship between sex, polymorphisms in CYP2C8 and CYP2C9, and pharmacokinetics of angiotensin receptor blockers. Drug Metab. Dispos. 2013, 41, 224–229. [Google Scholar] [CrossRef]
- Lo, M.-W.; Goldberg, M.R.; McCrea, J.B.; Lu, H.; Furtek, C.I.; Bjornsson, T.D. Pharmacokinetics of losartan, an angiotensin II receptor antagonist, and its active metabolite EXP3174 in humans. Clin. Pharmacol. Ther. 1995, 58, 641–649. [Google Scholar] [CrossRef]
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R.; Berl, T.; Pohl, M.A.; Lewis, J.B.; Ritz, E.; Atkins, R.C.; Rohde, R.; Raz, I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001, 345, 851–860. [Google Scholar] [CrossRef]
- Sica, D.A.; Gehr, T.W.; Ghosh, S. Clinical pharmacokinetics of losartan. Clin. Pharmacokinet. 2005, 44, 797–814. [Google Scholar] [CrossRef]
- He, C.; Liu, Y.; Wang, Y.; Tang, J.; Tan, Z.; Li, X.; Chen, Y.; Huang, Y.; Chen, X.; Ouyang, D.; et al. H NMR based pharmacometabolomics analysis of metabolic phenotype on predicting metabolism characteristics of losartan in healthy volunteers. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1095, 15–23. [Google Scholar] [CrossRef]
- Dona, A.C.; Jiménez, B.; Schäfer, H.; Humpfer, E.; Spraul, M.; Lewis, M.R.; Pearce, J.T.M.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal. Chem. 2014, 86, 9887–9894. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.S.R.; Martin-Pastor, M.; Tavares Júnior, A.G.; Queiroz, K.A.; da Silva Sólon, L.G.; Sousa, F.F.O.d. Metabolomic profile and its correlation with the plasmatic levels of losartan, EXP3174 and blood pressure control in hyper-tensive and chronic kidney disease patients. Int. J. Mol. Sci. 2023, 24, 9832. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, A.; Ghini, V.; Meoni, G.; Licari, C.; Takis, P.G.; Tenori, L.; Turano, P.; Luchinat, C. High-throughput metabolomics by 1D NMR. Angew. Chem. Int. Ed. Engl. 2019, 58, 968–994. [Google Scholar] [CrossRef] [PubMed]
- Moura, R.S.S.S.; Vasconcelos, D.F.; Freitas, E.; de Moura, F.J.D.; Rosa, T.T.; Veiga, J.P.R. Cistatina C, PCR, Log TG/HDLc e síndrome metabólica estão relacionados a microalbuminúria na hipertensão. Arq. Bras. Cardiol. 2014, 102, 54–59. [Google Scholar]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Erener, S. Diabetes, infection risk and COVID-19. Mol. Metab. 2020, 39, 101044. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alblihed, M.; Guerreiro, S.G.; Cruz-Martins, N.; Batiha, G.E.-S. COVID-19 in Relation to Hyperglycemia and Diabetes Mellitus. Front. Cardiovasc. Med. 2021, 8, 644095. [Google Scholar] [CrossRef]
- He, X.; Liu, C.; Peng, J.; Li, Z.; Li, F.; Wang, J.; Hu, A.; Peng, M.; Huang, K.; Fan, D.; et al. COVID-19 induces new-onset insulin resistance and lipid metabolic dysregulation via regulation of secreted metabolic factors. Signal Transduct. Target. Ther. 2021, 6, 427. [Google Scholar] [CrossRef]
- Rayman, G.; Lumb, A.N.; Kennon, B.; Cottrell, C.; Nagi, D.; Page, E.; Voigt, D.; Courtney, H.C.; Atkins, H.; Higgins, K.; et al. Dexamethasone therapy in COVID-19 patients: Implications and guidance for the management of blood glucose in people with and without diabetes. Diabet. Med. 2020, 38, e14378. [Google Scholar] [CrossRef]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- Angajala, A.; Lim, S.; Phillips, J.B.; Kim, J.-H.; Yates, C.; You, Z.; Tan, M. Diverse Roles of Mitochondria in Immune Responses: Novel Insights Into Immuno-Metabolism. Front. Immunol. 2018, 9, 1605. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Luo, P.; Yang, L.; Song, S.; Tan, X.; Chen, Q.; Xia, H.; Zhangm, S.; Ma, P.; et al. Plasma metabolomic profiles and clinical features in recovered COVID-19 patients without previous underlying diseases 3 months after discharge. J. Inflamm. Res. 2020, 14, 1–24. [Google Scholar]
- Xu, J.; Zhou, M.; Luo, P.; Yin, Z.; Wang, S.; Liao, T.; Yang, F.; Wang, Z.; Yang, D.; Peng, Y.; et al. Plasma metabolomic profiling of patients recovered from coronavirus disease 2019 (COVID-19) with pulmonary sequelae 3 months after discharge. Clin. Infect. Dis. 2021, 73, 2228–2239. [Google Scholar] [CrossRef]
- Felig, P. The Glucose-Alanine Cycle. Metabolism 1973, 22, 179–207. [Google Scholar] [CrossRef] [PubMed]
- Culbertson, J.Y.; Kreider, R.B.; Greenwood, M.; Cooke, M. Effects of Beta-Alanine on Muscle Carnosine and Exercise Performance: A Review of the Current Literature. Nutrients 2010, 2, 75–98. [Google Scholar] [CrossRef] [PubMed]
- Ardestani, A.; Azizi, Z. Targeting Glucose Metabolism for Treatment of COVID-19. Signal Transduct. Target. Ther. 2021, 6, 112. [Google Scholar] [CrossRef]
- Welch, C.; Greig, C.; Masud, T.; Wilson, D.; A Jackson, T. COVID-19 and Acute Sarcopenia. Aging. Dis. 2020, 11, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Feehan, J.; de Courten, M.; Apostolopoulos, V.; de Courten, B. Nutritional Interventions for COVID-19: A Role for Carnosine? Nutrients 2021, 13, 1463. [Google Scholar] [CrossRef] [PubMed]
- Munro, I.; Bernt, W.; Borzelleca, J.; Flamm, G.; Lynch, B.; Kennepohl, E.; Bär, E.; Modderman, J. Erythritol: An interpretive summary of biochemical, metabolic, toxicological and clinical data. Food Chem. Toxicol. 1998, 36, 1139–1174. [Google Scholar] [CrossRef]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef]
- Gabarre, P.; Dumas, G.; Dupont, T.; Darmon, M.; Azoulay, E.; Zafrani, L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020, 46, 1339–1348. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Chen, H.; Yan, S.; Li, D.; Li, Y.; Gong, Z. Coronavirus Disease 19 Infection Does Not Result in Acute Kidney Injury: An Analysis of 116 Hospitalized Patients from Wuhan, China. Am. J. Nephrol. 2020, 51, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Gall, W.E.; Beebe, K.; Lawton, K.A.; Adam, K.-P.; Mitchell, M.W.; Nakhle, P.J.; Ryals, J.A.; Milburn, M.V.; Nannipieri, M.; Camastra, S.; et al. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE 2010, 5, e10883. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Z.; Lu, X.; Yang, X.; Yin, P.; Kong, H.; Yu, Y.; Xu, G. Comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry for metabonomics: Biomarker discovery for diabetes mellitus. Anal. Chim. Acta 2009, 633, 257–262. [Google Scholar] [CrossRef]
- Mahrooz, A.; Muscogiuri, G.; Buzzetti, R.; Maddaloni, E. The complex combination of COVID-19 and diabetes: Pleiotropic changes in glucose metabolism. Endocrine 2021, 72, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Timm, D.E.; Titus, G.P.; Mueller, H.A.; Burgner, J.; de Córdoba, S.R.; Peñalva, M.A. Crystal structure of human homogentisate dioxygenase. Nat. Struct. Biol. 2000, 7, 542–546. [Google Scholar] [CrossRef]
- Orhan, H.; Vermeulen, N.P.; Tump, C.; Zappey, H.; Meerman, J.H. Simultaneous determination of tyrosine, phenylalanine and deoxyguanosine oxidation products by liquid chromatography-tandem mass spectrometry as non-invasive biomarkers for oxidative damage. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 799, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.M.D.; Costa, K.A. Choline: An essential nutrient for humans. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Blusztajn, J.K. Choline and human nutrition. Annu. Rev. Nutr. 1994, 14, 269–296. [Google Scholar] [CrossRef]
- Roe, A.J.; Zhang, S.; A Bhadelia, R.; Johnson, E.J.; Lichtenstein, A.H.; Rogers, G.T.; Rosenberg, I.H.; E Smith, C.; Zeisel, S.H.; Scott, T.M. Choline and its metabolites are differently associated with cardiometabolic risk factors, history of cardiovascular disease, and MRI-documented cerebrovascular disease in older adults. Am. J. Clin. Nutr. 2017, 105, 1283–1290. [Google Scholar] [CrossRef]
- Floegel, A.; Stefan, N.; Yu, Z.; Mühlenbruch, K.; Drogan, D.; Joost, H.-G.; Fritsche, A.; Häring, H.-U.; de Angelis, M.H.; Peters, A.; et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013, 62, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Junior, A.G.T. Desenvolvimento e Validação de Método por CLUE-EM/EM Para Monitoramento Plasmático de Losartana e Seu Metabólito Ativo em Pacientes Com Doença Renal Crônica. Master’s Thesis, Universidade Federal do Amapá, Macapá, Brazil, 2019. [Google Scholar]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef] [PubMed]
- López-Ibáñez, J.; Pazos, F.; Chagoyen, M. MBROLE 2.0—Functional enrichment of chemical compounds. Nucleic Acids Res. 2016, 44, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar]










| Groups | 3 Months | 6 Months | 1 Year | Total |
|---|---|---|---|---|
| HCP | n = 6 | n = 4 | n = 10 | n = 20 |
| HCN | * | * | * | n = 17 |
| NCP | n = 8 | n = 5 | n = 5 | n = 18 |
| NCN | * | * | * | n = 20 |
| Variables | HCP (n = 20) | HCN (n = 17) | NCP (n = 18) | NCN (n = 20) |
|---|---|---|---|---|
| Age | 52 ± 6.1 | 54 ± 11.1 | 44 ± 10.6 | 45 ± 9.1 |
| Gender | M = 7 F = 13 | M = 8 F = 9 | M = 9 F = 9 | M = 8 F = 12 |
| Other diseases | 8 | 7 | 7 | 4 |
| Patients with altered blood pressure 1 | ||||
| Fasting | 55% 3 | 58% 3 | 28% 3 | 0% 3 |
| After 1 h 30 m | 30% 3 | 53% 3 | * | * |
| After 3 h | 20% 3 | 41% 3 | * | * |
| Vaccinated with at least one dose | 90% | 94% | 50% | 90% |
| LDH 2 | 25% | 5.8% | a | a |
| Glucose 2 | 75% | 35% | 61.1% | 40% |
| C-Reactive protein (PCR) 2 | 55% | 23.5% | 5.5% | 20% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queiroz, K.A.; Vale, E.P.; Martín-Pastor, M.; Sólon, L.G.S.; Sousa, F.F.O. Metabolomic Profile, Plasmatic Levels of Losartan and EXP3174, Blood Pressure Control in Hypertensive Patients and Their Correlation with COVID-19. Pharmaceuticals 2023, 16, 1290. https://doi.org/10.3390/ph16091290
Queiroz KA, Vale EP, Martín-Pastor M, Sólon LGS, Sousa FFO. Metabolomic Profile, Plasmatic Levels of Losartan and EXP3174, Blood Pressure Control in Hypertensive Patients and Their Correlation with COVID-19. Pharmaceuticals. 2023; 16(9):1290. https://doi.org/10.3390/ph16091290
Chicago/Turabian StyleQueiroz, Kamila A., Everton P. Vale, Manuel Martín-Pastor, Lílian G. S. Sólon, and Francisco F. O. Sousa. 2023. "Metabolomic Profile, Plasmatic Levels of Losartan and EXP3174, Blood Pressure Control in Hypertensive Patients and Their Correlation with COVID-19" Pharmaceuticals 16, no. 9: 1290. https://doi.org/10.3390/ph16091290
APA StyleQueiroz, K. A., Vale, E. P., Martín-Pastor, M., Sólon, L. G. S., & Sousa, F. F. O. (2023). Metabolomic Profile, Plasmatic Levels of Losartan and EXP3174, Blood Pressure Control in Hypertensive Patients and Their Correlation with COVID-19. Pharmaceuticals, 16(9), 1290. https://doi.org/10.3390/ph16091290