Non-Anticoagulant Activities of Low Molecular Weight Heparins—A Review
Abstract
1. Introduction
2. The Structure of LMWHs Varies Depending on the Preparation Process
2.1. Nitrite Degradation
2.2. Chemical β-Elimination
2.3. Enzymatic β-Elimination
2.4. Peroxide Degradation
2.5. Novel Methods for Heparin Depolymerization
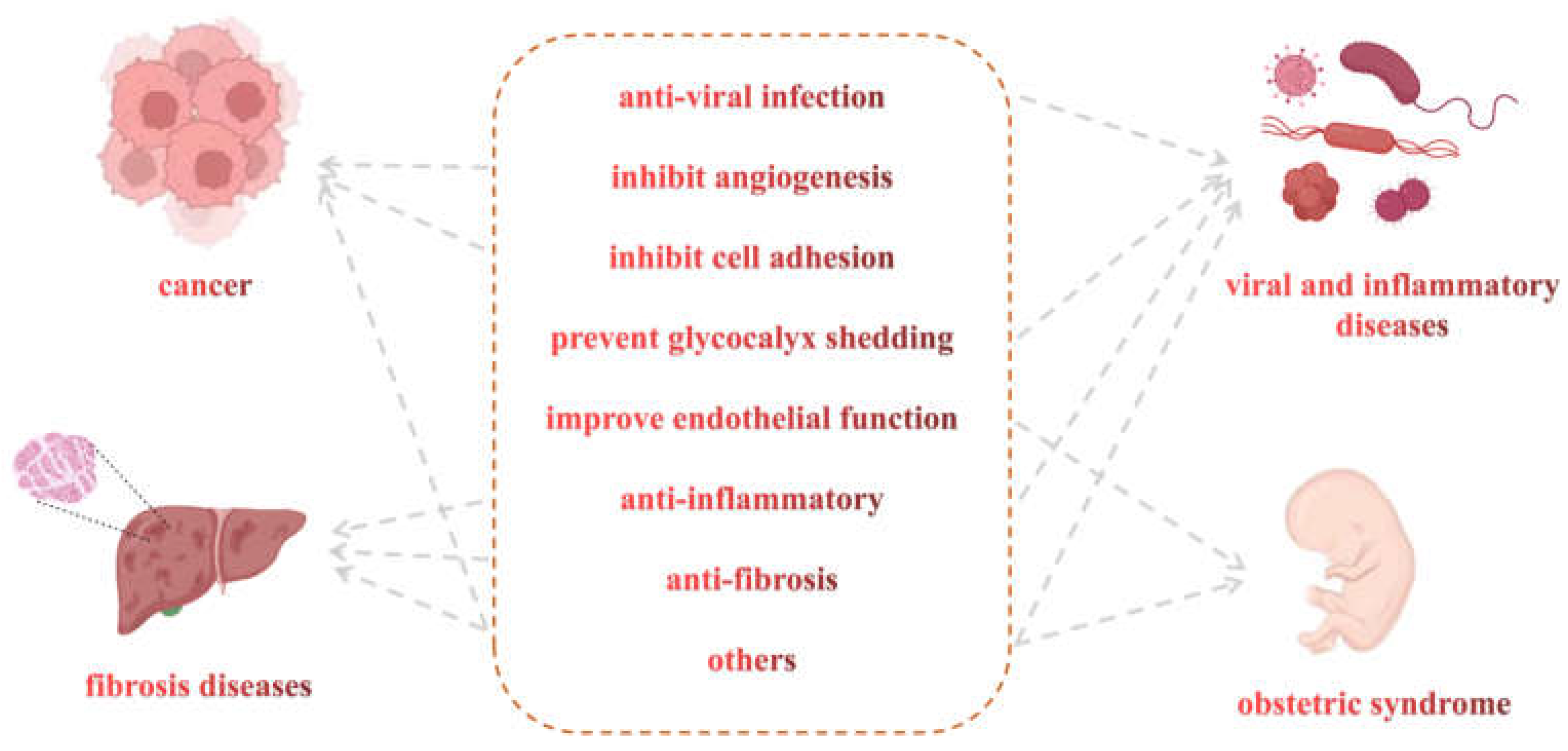
3. Non-Anticoagulant Activities of LMWHs
3.1. Potential Effects of LMWHs in Some Viral and Inflammatory Diseases
3.1.1. Preventing Glycocalyx Shedding
3.1.2. Anti-Inflammatory
3.1.3. Reducing Virus Entry into Cells
3.2. Potential Effects of LMWHs in Cancer
3.2.1. Inhibiting Angiogenesis
3.2.2. Inhibiting Cell Adhesion
3.3. Potential Effects of LMWHs in Fibrotic Diseases
3.4. Potential Effects of LMWHs in Obstetrical Diseases
3.5. Potential Effects of LMWHs in Other Diseases
4. Various Heparin Conjugates with Non-Coagulant Effects
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raul Alaez-Verson, C.; Lantero, E.; Fernandez-Busquets, X. Heparin: New life for an old drug. Nanomedicine 2017, 12, 1727–1744. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Xu, H.; Yu, L.; Zhang, L. Heparin: An essential drug for modern medicine. In Glycans and Glycosaminoglycans as Clinical Biomarkers and Therapeutics; Zhang, L., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 163, pp. 1–19. [Google Scholar]
- Warkentin, T.E.; Levine, M.N.; Hirsh, J.; Horsewood, P.; Roberts, R.S.; Gent, M.; Kelton, J.G. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N. Engl. J. Med. 1995, 332, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Gaitanidis, A.; Breen, K.A.; Christensen, M.A.; Saillant, N.N.; Kaafarani, H.M.A.; Velmahos, G.C.; Mendoza, A.E. Low-Molecular Weight Heparin is Superior to Unfractionated Heparin for Elderly Trauma Patients. J. Surg. Res. 2021, 268, 432–439. [Google Scholar] [CrossRef]
- Linhardt, R.J.; Gunay, N.S. Production and chemical processing of low molecular weight heparins. Semin. Thromb. Hemost. 1999, 25 (Suppl. S3), 5–16. [Google Scholar] [PubMed]
- Gray, E.; Mulloy, B.; Barrowcliffel, T.W. Heparin and low-molecular-weight heparin. Annu. Rev. Immunol. 2008, 99, 807–818. [Google Scholar] [CrossRef]
- Hirsh, J.; Warkentin, T.E.; Shaughnessy, S.G.; Anand, S.S.; Halperin, J.L.; Raschke, R.; Granger, C.; Ohman, E.M.; Dalen, J.E. Heparin and low-molecular-weight heparin—Mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest 2001, 119, 64S–94S. [Google Scholar] [CrossRef]
- Merli, G.J.; Vanscoy, G.J.; Rihn, T.L.; Groce Iii, J.B.; McCormick, W. Applying Scientific Criteria to Therapeutic Interchange: A Balanced Analysis of Low-Molecular-Weight Heparins. J. Thromb. Thrombolys. 2001, 11, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Guan, Y.; Zhu, P.; Li, F.; Yu, M.; Linhardt, R.J.; Chi, L.; Jin, L. Preparation of low molecular weight heparins from bovine and ovine heparins using nitrous acid degradation. Carbohydr. Polym. 2018, 197, 83–91. [Google Scholar] [CrossRef]
- Li, D.; Chi, L.; Jin, L.; Xu, X.; Du, X.; Ji, S.; Chi, L. Mapping of low molecular weight heparins using reversed phase ion pair liquid chromatography-mass spectrometry. Carbohydr. Polym. 2014, 99, 339–344. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, X.; Liu, H.; Pan, Q.; Chen, Y. Photoswitchable Heparinase III for Enzymatic Preparation of Low Molecular Weight Heparin. Org. Lett. 2018, 20, 48–51. [Google Scholar] [CrossRef]
- Achour, O.; Poupard, N.; Bridiau, N.; Juchereau, S.B.; Sannier, F.; Piot, J.M.; Arnaudin, I.F.; Maugard, T. Anti-heparanase activity of ultra-low-molecular-weight heparin produced by physicochemical depolymerization. Carbohydr. Polym. 2016, 135, 316–323. [Google Scholar] [CrossRef]
- Vismara, E.; Pierini, M.; Guglieri, S.; Liverani, L.; Mascellani, G.; Torri, G. Structural modification induced in heparin by a Fenton-type depolymerization process. Semin. Thromb. Hemost. 2007, 33, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; Hosoyama, S.; Ohno, A.; Masuko, S.; Yang, B.; Sterner, E.; Wang, Z.; Linhardt, R.J.; Toida, T. Photochemical preparation of a novel low molecular weight heparin. Carbohydr. Polym. 2012, 87, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Z.; Li, J.; Chen, J.; Li, S.; Cheng, H.; Liu, D.; Ye, X.; Linhardt, R.J.; Chen, S. Preparation of low molecular weight heparin using an ultrasound-assisted Fenton-system. Ultrason. Sonochem. 2019, 52, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Fu, L.; He, P.; Xia, K.; Varghese, S.; Dordick, J.; Wang, H.; Zhang, F.; Linhardt, R.J. Enzymatic synthesis of low molecular weight heparins from N-sulfo heparosan depolymerized by heparanase or heparin lyase. Carbohydr. Polym. 2022, 295, 119825. [Google Scholar] [CrossRef]
- Zhao, D.; Sang, Q.; Cui, H. Preparation and evaluation a new generation of low molecular weight heparin. Biomed. Pharmacother. 2016, 79, 194–200. [Google Scholar] [CrossRef]
- Achour, O.; Bridiau, N.; Godhbani, A.; Le Joubioux, F.; Juchereau, S.B.; Sannier, F.; Piot, J.-M.; Arnaudin, I.F.; Maugard, T. Ultrasonic-assisted preparation of a low molecular weight heparin (LMWH) with anticoagulant activity. Carbohydr. Polym. 2013, 97, 684–689. [Google Scholar] [CrossRef]
- Wang, P.; Chi, L.; Zhang, Z.; Zhao, H.; Zhang, F.; Linhardt, R.J. Heparin: An old drug for new clinical applications. Carbohydr. Polym. 2022, 295, 119818. [Google Scholar] [CrossRef]
- Huo, L.; Liu, C.F.; Yuan, Y.J.; Liu, X.Y.; Cao, Q.J. Pharmacological inhibition of ferroptosis as a therapeutic target for sepsis-associated organ damage. Eur. J. Med. Chem. 2023, 257, 115438. [Google Scholar] [CrossRef]
- Lu, L.; Liu, L.P.; Gui, R.; Dong, H.; Su, Y.R.; Zhou, X.H.; Liu, F.X. Discovering common pathogenetic processes between COVID-19 and sepsis by bioinformatics and system biology approach. Front. Immunol. 2022, 13, 975848. [Google Scholar] [CrossRef]
- Oud, L.; Garza, J. The Impact of COVID-19 on Sepsis-Related Mortality in the United States. J. Clin. Med. Res. 2023, 15, 328–331. [Google Scholar] [CrossRef]
- Poli, D.; Antonucci, E.; Ageno, W.; Prandoni, P.; Palareti, G.; Marcucci, R.; Investigators, S.-C. Low in-hospital mortality rate in patients with COVID-19 receiving thromboprophylaxis: Data from the multicentre observational START-COVID Register. Intern. Emerg. Med. 2022, 17, 1013–1021. [Google Scholar] [CrossRef]
- Thachil, J. The versatile heparin in COVID-19. J. Thromb. Haemost. 2020, 18, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Abbadi, A.; Loftis, J.; Wang, A.M.; Yu, M.J.; Wang, Y.; Shakya, S.; Li, X.X.; Maytin, E.; Hascall, V. Heparin inhibits proinflammatory and promotes anti-inflammatory macrophage polarization under hyperglycemic stress. J. Biol. Chem. 2020, 295, 4849–4857. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, X. The role of heparin in sepsis: Much more than just an anticoagulant. Br. J. Haematol. 2017, 179, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Weinbaum, S.; Tarbell, J.M.; Damiano, E.R. The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 2007, 9, 121–167. [Google Scholar] [CrossRef]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.M.J.; Egbrink, M.G.A.O. The endothelial glycocalyx: Composition, functions, and visualization. Pflug. Arch. Eur. J. Phy. 2007, 454, 345–359. [Google Scholar] [CrossRef]
- Haraldsson, B.; Nystroem, J.; Deen, W.M. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol. Rev. 2008, 88, 451–487. [Google Scholar] [CrossRef]
- LaRiviere, W.B.; Schmidt, E.P. The Pulmonary Endothelial Glycocalyx in ARDS: A Critical Role for Heparan Sulfate. In Membranes in Pulmonary Vascular Disease; Belvitch, P., Dudek, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 82, pp. 33–52. [Google Scholar]
- Potje, S.R.; Costa, T.J.; Fraga-Silva, T.F.C.; Martins, R.B.; Benatti, M.N.; Almado, C.E.L.; de Sa, K.S.G.; Bonato, V.L.D.; Arruda, E.; Louzada-Junior, P.; et al. Heparin prevents in vitro glycocalyx shedding induced by plasma from COVID-19 patients. Life Sci. 2021, 276, 119376. [Google Scholar] [CrossRef]
- Bar-Ner, M.; Eldor, A.; Wasserman, L.; Matzner, Y.; Cohen, I.R.; Fuks, Z.; Vlodavsky, I. Inhibition of heparanase-mediated degradation of extracellular matrix heparan sulfate by non-anticoagulant heparin species. Blood 1987, 70, 551–557. [Google Scholar] [CrossRef]
- Buijsers, B.; Yanginlar, C.; de Nooijer, A.; Grondman, I.; Maciej-Hulme, M.L.; Jonkman, I.; Janssen, N.A.F.; Rother, N.; de Graaf, M.; Pickkers, P.; et al. Increased Plasma Heparanase Activity in COVID-19 Patients. Front. Immunol. 2020, 11, 575047. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; Speciality, H.L.H.A. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, T.; Cai, D.; Hu, Z.; Chen, J.a.; Liao, H.; Zhi, L.; Wei, H.; Zhang, Z.; Qiu, Y.; et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020, 53, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China (vol 579, pg 265, 2020). Nature 2020, 580, E7. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, M.; Zhang, S.L.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.M.; et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N. Engl. J. Med. 2020, 382, e38. [Google Scholar] [CrossRef]
- Mughal, M.S.; Kaur, I.P.; Jaffery, A.R.; Wang, C.; Asif, M.; Ricca, A.J.; Du, D.T.; Patton, C.D.; Granet, K. The Potential Role of Therapeutic Dose of Low Molecular Weight Heparin (LWMH) to Attenuate Hyper-Inflammatory State in Hospitalized COVID-19 Patients. Blood 2020, 136, S11–S12. [Google Scholar] [CrossRef]
- Kuschert, G.S.V.; Coulin, F.; Power, C.A.; Proudfoot, A.E.I.; Hubbard, R.E.; Hoogewerf, A.J.; Wells, T.N.C. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry 1999, 38, 12959–12968. [Google Scholar] [CrossRef]
- Rops, A.L.; van den Hoven, M.J.; Baselmans, M.M.; Lensen, J.F.; Wijnhoven, T.J.; van den Heuvel, L.P.; van Kuppevelt, T.H.; Berden, J.H.; van der Vlag, J. Heparan sulfate domains on cultured activated glomerular endothelial cells mediate leukocyte trafficking. Kidney Int. 2008, 73, 52–62. [Google Scholar] [CrossRef][Green Version]
- Buijsers, B.; Yanginlar, C.; Maciej-Hulme, M.L.; de Mast, Q.; van der Vlag, J. Beneficial non-anticoagulant mechanisms underlying heparin treatment of COVID-19 patients. Ebiomedicine 2020, 59, 102969. [Google Scholar] [CrossRef]
- Shi, C.; Wang, C.; Wang, H.; Yang, C.; Zhang, Y.U. Clinical Observations of Low Molecular Weight Heparin in Relieving Inflammation in COVID-19 Patients: A Retrospective Cohort Study; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2020. [Google Scholar]
- Weiler, J.M.; Edens, R.E.; Linhardt, R.J.; Kapelanski, D.P. Heparin and modified heparin inhibit complement activation in vivo. J. Immunol. 1992, 148, 3210–3215. [Google Scholar] [CrossRef]
- Walls, A.C.; Tortorici, M.A.; Snijder, J.; Xiong, X.L.; Bosch, B.J.; Rey, F.A.; Veesler, D. Tectonic conformational changes of a coronavirus spike glycoprotein promote membrane fusion. Proc. Natl. Acad. Sci. USA 2017, 114, 11157–11162. [Google Scholar] [CrossRef]
- Tandon, R.; Sharp, J.S.; Zhang, F.M.; Pomin, V.H.; Ashpole, N.M.; Mitra, D.; Jin, W.H.; Liu, H.; Sharma, P.; Linhardt, R.J.; et al. Effective Inhibition of SARS-CoV-2 Entry by Heparin and Enoxaparin Derivatives. J. Virol. 2021, 95, e01987-20. [Google Scholar] [CrossRef]
- Tree, J.A.; Turnbull, J.E.; Buttigieg, K.R.; Elmore, M.J.; Coombes, N.; Hogwood, J.; Mycroft-West, C.J.; Lima, M.A.; Skidmore, M.A.; Karlsson, R.; et al. Unfractionated heparin inhibits live wild type SARS-CoV-2 cell infectivity at therapeutically relevant concentrations. Br. J. Pharm. 2021, 178, 626–635. [Google Scholar] [CrossRef]
- Jin, Z.M.; Du, X.Y.; Xu, Y.C.; Deng, Y.Q.; Liu, M.Q.; Zhao, Y.; Zhang, B.; Li, X.F.; Zhang, L.K.; Peng, C.; et al. Structure of M-pro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Li, J.W.; Zhang, Y.T.; Pang, H.M.; Li, S.J. Heparin interacts with the main protease of SARS-CoV-2 and inhibits its activity. Spectrochim. Acta A 2022, 267, 120595. [Google Scholar] [CrossRef]
- Ma, C.L.; Sacco, M.D.; Hurst, B.; Townsend, J.A.; Hu, Y.M.; Szeto, T.; Zhang, X.J.; Tarbet, B.; Marty, M.T.; Chen, Y.; et al. Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 2020, 30, 678–692. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Korc, M.; Friesel, R.E. The Role of Fibroblast Growth Factors in Tumor Growth. Curr. Cancer Drug Tar. 2009, 9, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Quarto, N.; Amalric, F. Heparan sulfate proteoglycans as transducers of FGF-2 signalling. J. Cell Sci. 1994, 107 Pt 11, 3201–3212. [Google Scholar] [CrossRef] [PubMed]
- Merli, G.J.; Groce, J.B. Pharmacological and clinical differences between low-molecular-weight heparins: Implications for prescribing practice and therapeutic interchange. J. Pharm. Ther. 2010, 35, 95–105. [Google Scholar]
- Braga, V.; Harwood, A.J. Super glue. Nat. Cell Biol. 2001, 3, E168–E170. [Google Scholar] [CrossRef]
- Huang, S.; Ingber, D.E. The structural and mechanical complexity of cell-growth control. Nat. Cell Biol. 1999, 1, E131–E138. [Google Scholar] [CrossRef]
- Ejaz, U.; Akhtar, F.; Xue, J.B.; Wan, X.Y.; Zhang, T.; He, S.Y. Review: Inhibitory potential of low molecular weight Heparin in cell adhesion; emphasis on tumor metastasis. Eur. J. Pharmacol. 2021, 892, 173778. [Google Scholar] [CrossRef]
- Borsig, L. Antimetastatic activities of heparins and modified heparins. Experimental evidence. Thromb. Res. 2010, 125, S66–S71. [Google Scholar] [CrossRef]
- Yu, C.J.; Ye, S.J.; Feng, Z.H.; Ou, W.J.; Zhou, X.K.; Li, L.D.; Mao, Y.Q.; Zhu, W.; Wei, Y.Q. Effect of Fraxiparine, a type of low molecular weight heparin, on the invasion and metastasis of lung adenocarcinoma A549 cells. Oncol. Lett. 2010, 1, 755–760. [Google Scholar] [CrossRef]
- Hostettler, N.; Naggi, A.; Torri, G.; Ishai-Michaeli, R.; Casu, B.; Vlodavsky, I.; Borsig, L. P-selectin- and heparanase-dependent antimetastatic activity of non-anticoagulant heparins. FASEB J. 2007, 21, 3562–3572. [Google Scholar] [CrossRef]
- Springer, T.A. Structural basis for selectin mechanochemistry. Proc. Natl. Acad. Sci. USA 2009, 106, 91–96. [Google Scholar] [CrossRef]
- Borsig, L. Selectins in cancer immunity. Glycobiology 2018, 28, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Borsig, L.; Wong, R.; Feramisco, J.; Nadeau, D.R.; Varki, N.M.; Varki, A. Heparin and cancer revisited: Mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc. Natl. Acad. Sci. USA 2001, 98, 3352–3357. [Google Scholar] [CrossRef] [PubMed]
- Varki, N.M.; Varki, A. Heparin inhibition of selectin-mediated interactions during the hematogenous phase of carcinoma metastasis: Rationale for clinical studies in humans. Semin. Thromb. Hemost. 2002, 28, 53–66. [Google Scholar] [CrossRef]
- Gout, S.; Tremblay, P.L.; Huot, J. Selectins and selectin ligands in extravasation of cancer cells and organ selectivity of metastasis. Clin. Exp. Metastasis 2008, 25, 335–344. [Google Scholar] [CrossRef] [PubMed]
- St Hill, C.A. Interactions between endothelial selectins and cancer cells regulate metastasis. Front. Biosci. Landmrk 2011, 16, 3233–3251. [Google Scholar] [CrossRef] [PubMed]
- Fritzsche, J.; Simonis, D.; Bendas, G. Melanoma cell adhesion can be blocked by heparin in vitro: Suggestion of VLA-4 as a novel target for antimetastatic approaches. Thromb. Haemost. 2008, 100, 1166–1175. [Google Scholar] [CrossRef]
- Schlesinger, M.; Roblek, M.; Ortmann, K.; Naggi, A.; Torri, G.; Borsig, L.; Bendas, G. The role of VLA-4 binding for experimental melanoma metastasis and its inhibition by heparin. Thromb. Res. 2014, 133, 855–862. [Google Scholar] [CrossRef]
- Alyahya, R.; Sudha, T.; Racz, M.; Stain, S.C.; Mousa, S.A. Anti-metastasis efficacy and safety of non-anticoagulant heparin derivative versus low molecular weight heparin in surgical pancreatic cancer models. Int. J. Oncol. 2015, 46, 1225–1231. [Google Scholar] [CrossRef]
- Lurje, I.; Gaisa, N.T.; Weiskirchen, R.; Tacke, F. Mechanisms of organ fibrosis: Emerging concepts and implications for novel treatment strategies. Mol. Asp. Med. 2023, 92, 101191. [Google Scholar] [CrossRef]
- Guillot, A.; Tacke, F. Liver Macrophages: Old Dogmas and New Insights. Hepatol. Commun. 2019, 3, 730–743. [Google Scholar] [CrossRef]
- Bartneck, M.; Schrammen, P.L.; Mockel, D.; Govaere, O.; Liepelt, A.; Krenkel, O.; Ergen, C.; McCain, M.V.; Eulberg, D.; Luedde, T.; et al. The CCR2(+) Macrophage Subset Promotes Pathogenic Angiogenesis for Tumor Vascularization in Fibrotic Livers. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Sutti, S.; Bruzzi, S.; Heymann, F.; Liepelt, A.; Krenkel, O.; Toscani, A.; Ramavath, N.N.; Cotella, D.; Albano, E.; Tacke, F. CX(3)CR1 Mediates the Development of Monocyte-Derived Dendritic Cells during Hepatic Inflammation. Cells 2019, 8, 1099. [Google Scholar] [CrossRef]
- Pardo, A.; Cabrera, S.; Maldonado, M.; Selman, M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir. Res. 2016, 17, 23. [Google Scholar] [CrossRef]
- Travis, M.A.; Sheppard, D. TGF-beta Activation and Function in Immunity. Annu. Rev. Immunol. 2014, 32, 51–82. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kotani, T.; Suzuka, T.; Matsuda, S.; Takeuchi, T.; Sato, T. Adipose-derived stem/stromal cells with heparin-enhanced anti- inflammatory and antifibrotic effects mitigate induced pulmonary fibrosis in mice. Biochem. Biophys. Res. Commun. 2022, 629, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Hao, J.H.; Ren, W.H.; Zhu, J.R. Effects of heparin on liver fibrosis in patients with chronic hepatitis B. World J. Gastroenterol. 2003, 9, 1611–1614. [Google Scholar] [CrossRef]
- Saito, T.; Tabata, Y. Preparation of gelatin hydrogels incorporating low-molecular-weight heparin for anti-fibrotic therapy. Acta Biomater. 2012, 8, 646–652. [Google Scholar] [CrossRef]
- Jinnin, M.; Ihn, H.; Mimura, Y.; Asano, Y.; Yamane, K.; Tamaki, K. Effects of hepatocyte growth factor on the expression of type I collagen and matrix metalloproteinase-1 in normal and scleroderma dermal fibroblasts. J. Investig. Dermatol. 2005, 124, 324–330. [Google Scholar] [CrossRef]
- Li, W.C.; Zhang, J.S.; Huang, Q.C.; Zhu, H.G.; Zhang, X.R. Long-term administering low anticoagulant activity heparin can lessen rat hepatic fibrosis induced by either CCl4 or porcine serum injection. Hepatol. Res. 2006, 36, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209. [Google Scholar] [CrossRef]
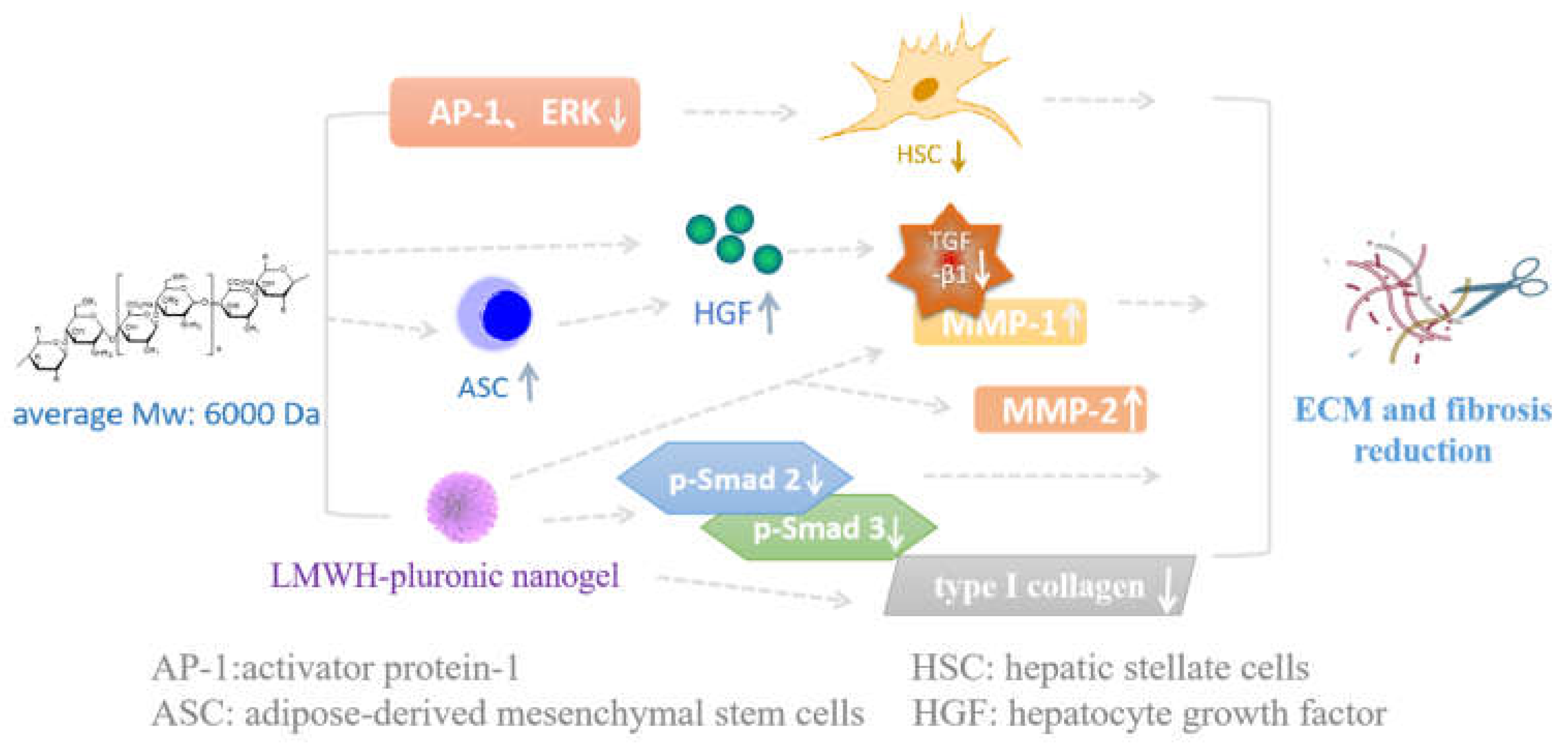
- Lee, J.H.; Lee, H.; Joung, Y.K.; Jung, K.H.; Choi, J.H.; Lee, D.H.; Park, K.D.; Hong, S.S. The use of low molecular weight heparin-pluronic nanogels to impede liver fibrosis by inhibition the TGF-b/Smad signaling pathway. Biomaterials 2011, 32, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.W.M.; Groot, P.G.D.; Koike, T.; Meroni, P.L. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2010, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Kemp, M.; Thomas, W. Antiphospholipid syndrome in obstetrics. Lupus 2018, 27, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, R. First trimester utero-placental circulation: Maternal-fetal interaction. J. Perinat. Med. 1998, 26, 168–174. [Google Scholar] [PubMed]
- Brenner, B. Enoxaparin use in pregnancy: State of the art. Womens Health 2007, 3, 9–14. [Google Scholar] [CrossRef]
- Walker, I.D. Arterial thromboembolism in pregnancy. Best Pract. Res. Clin. Haematol. 2003, 16, 297–310. [Google Scholar] [CrossRef]
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and function of the normal human placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef]
- Farquharson, R.G.; Quenby, S.; Greaves, M. Antiphospholipid syndrome in pregnancy: A randomized, controlled trial of treatment—To the editor—In reply. Obstet. Gynecol. 2002, 100, 408–413. [Google Scholar] [CrossRef]
- Sugi, T.; Mcintyre, J.A. Antiphospholipid antibodies (APA) and recurrent pregnancy loss: Treating a unique APA positive population. Hum. Reprod. 2003, 18, 1553–1554. [Google Scholar] [CrossRef][Green Version]
- Greer, I.A. Venous thromboembolism and anticoagulant therapy in pregnancy. Gend. Med. 2005, 2 (Suppl. A), S10–S17. [Google Scholar] [CrossRef]
- Cruz-Lemini, M.; Vázquez, J.C.; Ullmo, J.; Llurba, E. Low-molecular-weight heparin for prevention of preeclampsia and other placenta-mediated complications: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021, 226, S1126–S1144. [Google Scholar] [CrossRef] [PubMed]
- Mclaughlin, K.; Baczyk, D.; Potts, A.; Hladunewich, M.; Parker, J.D.; Kingdom, J.C.P. Low Molecular Weight Heparin Improves Endothelial Function in Pregnant Women at High Risk of Preeclampsia. Hypertension 2017, 69, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Benck, U.; Haeckel, S.; Clorius, J.H.; van der Woude, F.J. Proteinuria-lowering effect of heparin therapy in diabetic nephropathy without affecting the renin-angiotensin-aldosterone system. Clin. J. Am. Soc. Nephrol. 2007, 2, 58–67. [Google Scholar] [CrossRef]
- Lewis, E.J.; Xu, X. Abnormal glomerular permeability characteristics in diabetic nephropathy: Implications for the therapeutic use of low-molecular weight heparin. Diabetes Care 2008, 31 (Suppl. S2), S202–S207. [Google Scholar] [CrossRef]
- Ceol, M.; Gambaro, G.; Sauer, U.; Baggio, B.; Schleicher, E.D. Glycosaminoglycan Therapy Prevents TGF-β1 Overexpression and Pathologic Changes in Renal Tissue of Long-Term Diabetic Rats. J. Am. Soc. Nephrol. 2000, 11, 2324–2336. [Google Scholar] [CrossRef]
- Chung, S.W.; Bae, S.M.; Lee, M.; Al-Hilal, T.A.; Lee, C.K.; Kim, J.K.; Kim, I.-S.; Kim, S.Y.; Byun, Y. LHT7, a chemically modified heparin, inhibits multiple stages of angiogenesis by blocking VEGF, FGF2 and PDGF-B signaling pathways. Biomaterials 2015, 37, 271–278. [Google Scholar] [CrossRef]
- Fang, Z.; Lin, L.; Li, Z.; Gu, L.; Pan, D.; Li, Y.; Chen, J.; Ding, H.; Tian, X.; Gong, Q.; et al. Stimuli-responsive heparin-drug conjugates co-assembled into stable nanomedicines for cancer therapy. Acta Biomater. 2023, 164, 422–434. [Google Scholar] [CrossRef]
- Hwang, H.H.; Jeong, H.J.; Yun, S.; Byun, Y.; Okano, T.; Kim, S.W.; Lee, D.Y. Anticancer Effect of Heparin-Taurocholate Conjugate on Orthotopically Induced Exocrine and Endocrine Pancreatic Cancer. Cancers 2021, 13, 5775. [Google Scholar] [CrossRef]
- Lee, J.-H.; Yang, S.-B.; Lee, J.-H.; Lim, H.; Lee, S.; Kang, T.-B.; Lim, J.-H.; Kim, Y.J.; Park, J. Doxorubicin covalently conjugated heparin displays anti-cancer activity as a self-assembled nanoparticle with a low-anticoagulant effect. Carbohydr. Polym. 2023, 314, 120930. [Google Scholar] [CrossRef]
- Choi, J.U.; Zhang, X.; Hasan, M.M.; Karim, M.; Chung, S.W.; Alam, F.; Alqahtani, F.; Reddy, S.Y.; Kim, I.-S.; Al-Hilal, T.A.; et al. Targeting angiogenic growth factors using therapeutic glycosaminoglycans on doppel-expressing endothelial cells for blocking angiogenic signaling in cancer. Biomaterials 2022, 283, 121423. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.H.; Kim, H.S.; Lee, D.Y. Gastrointestinally absorbable lactoferrin-heparin conjugate with anti-angiogenic activity for treatment of brain tumor. J. Control. Release 2023, 355, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Min, K.; Jeon, S.H.; Kwon, K.; Tae, G. Self-assembled hemin-conjugated heparin with dual-enzymatic cascade reaction activities for acute kidney injury. Carbohydr. Polym. 2023, 316, 121088. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Park, D.; Kim, T.H.; Chung, J.J.; Jung, Y.; Kim, S.H. Substance P/Heparin-Conjugated PLCL Mitigate Acute Gliosis on Neural Implants and Improve Neuronal Regeneration via Recruitment of Neural Stem Cells. Adv. Healthc. Mater. 2021, 10, 2100107. [Google Scholar] [CrossRef] [PubMed]
- Boothello, R.S.; Sankaranarayanan, N.V.; Sistla, J.C.; Nagarajan, B.; Sharon, C.; Chittum, J.E.; Niyaz, R.Y.; Roy, S.; Nandi, A.; O’Hara, C.P.; et al. Glycan Modulation of Insulin-like Growth Factor-1 Receptor. Angew. Chem. Int. Ed. 2022, 61, e202211320. [Google Scholar] [CrossRef]
- Patel, N.J.; Sharon, C.; Baranwal, S.; Boothello, R.S.; Desai, U.R.; Patel, B.B. Heparan sulfate hexasaccharide selectively inhibits cancer stem cells self-renewal by activating p38 MAP kinase. Oncotarget 2016, 7, 84608–84622. [Google Scholar] [CrossRef]
- Ostapoff, K.T.; Awasthi, N.; Kutluk Cenik, B.; Hinz, S.; Dredge, K.; Schwarz, R.E.; Brekken, R.A. PG545, an angiogenesis and heparanase inhibitor, reduces primary tumor growth and metastasis in experimental pancreatic cancer. Mol. Cancer Ther. 2013, 12, 1190–1201. [Google Scholar] [CrossRef]
- Edward, H.; Haynes, N.M.; Carleen, C.; Brennan, T.V.; Darryn, B.; Paul, H.; Tomislav, K.; Fleur, L.; Liwen, L.; Yiping, Y. Immunomodulatory activities of pixatimod: Emerging nonclinical and clinical data, and its potential utility in combination with PD-1 inhibitors. J. Immunother. Cancer 2018, 6, 54. [Google Scholar]
- Hao, C.; Sun, M.J.; Wang, H.M.; Zhang, L.J.; Wang, W. Low molecular weight heparins and their clinical applications. Glycans Glycosaminoglycans Clin. Biomark. Ther. 2019, 163, 21–39. [Google Scholar]
- Meyer, G.; Besse, B.; Doubre, H.; Charles-Nelson, A.; Aquilanti, S.; Izadifar, A.; Azarian, R.; Monnet, I.; Lamour, C.; Descourt, R.; et al. Anti-tumour effect of low molecular weight heparin in localised lung cancer: A phase III clinical trial. Eur. Respir. J. 2018, 52, 1801220. [Google Scholar] [CrossRef]
- Han, A.H.; Yu, G.Q.; Yin, H.Z. Clinical effects of low-molecular-weight heparin combined with ulinastatin in children with acute pancreatitis. Trop. J. Pharm. Res. 2016, 15, 1787–1792. [Google Scholar] [CrossRef][Green Version]
- Wen, J.; Zhang, X.; Li, C. Clinical Effect of Low Molecular Weight Heparin Sodium Combined with Magnesium Sulfate in the Treatment of Patients with Severe Preeclampsia. JCPSP J. Coll. Physicians Surg. Pak. 2019, 29, 119–122. [Google Scholar] [CrossRef]
- Martinelli, I.; Ciavarella, A.; Abbattista, M.; Aliberti, S.; De Zan, V.; Folli, C.; Panigada, M.; Gori, A.; Artoni, A.; Ierardi, A.M.; et al. Increasing dosages of low-molecular-weight heparin in hospitalized patients with Covid-19. Intern. Emerg. Med. 2021, 16, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zheng, C.; Xiong, B.; Xia, X. Efficacy and safety of heparin plus dexamethasone after partial splenic embolization for liver cirrhosis with massive splenomegaly. BMC Gastroenterol. 2022, 22, 470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.G.; Tang, X.H.; Shen, J. The efficacy and safety of low-molecular-weight heparin calcium combined with Xueshuantong injections in the treatment of elderly acute deep venous thrombosis patients. Am. J. Transl. Res. 2021, 13, 3120–3128. [Google Scholar] [PubMed]
- Dotan, I.; Hallak, A.; Arber, N.; Santo, M.; Alexandrowitz, A.; Knaani, Y.; Hershkoviz, R.; Brazowski, E. Low-dose low-molecular weight heparin (enoxaparin) is effective as adjuvant treatment in active ulcerative colitis—An open trial. Dig. Dis. Sci. 2001, 46, 2239–2244. [Google Scholar] [CrossRef]
- Tian, M.; Liu, C.H. Heparin calcium treated Henoch-Schonlein purpura nephritis in children through inhibiting hyperfibrinolysis. Ren. Fail. 2015, 37, 1100–1104. [Google Scholar] [CrossRef]
- Luo, A.; Liu, Y. The effect of low-molecular-weight heparin combined with amikacin on the coagulation function and bacterial clearance in the treatment of patients with severe senile pneumonia. Pak. J. Med. Sci. 2023, 39, 172–176. [Google Scholar] [CrossRef]
- Yang, M.; Xu, Y.; Chen, H.; Xu, Z.; Luo, F. Benefits and risks of low molecular weight heparin in patients with acute exacerbation of chronic obstructive pulmonary disease: A meta-analysis of randomized controlled trials. Inflammopharmacology 2020, 28, 451–462. [Google Scholar] [CrossRef]
- Surbone, A.; Fuso, L.; Passera, R.; Ferrero, A.; Marchese, C.; Martino, C.; Luchin, A.; Renzo, M.F.D.; Zola, P. Daily administration of low molecular weight heparin increases Hepatocyte Growth Factor serum levels in gynaecological patients: Pharmacokinetic parameters and clinical implications. BMC Res. Notes 2012, 5, 517–524. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, T.; Ren, D.; Zhou, J.; Liu, L.; Li, J.; Fu, S.; Ni, T.; Xu, W.; Yang, Y.; et al. Low-molecular-weight heparin therapy reduces 28-day mortality in patients with sepsis-3 by improving inflammation and coagulopathy. Front. Med. 2023, 10, 1157775. [Google Scholar] [CrossRef] [PubMed]
- Kovacsovics, T.J.; Mims, A.; Salama, M.E.; Pantin, J.; Rao, N.; Kosak, K.M.; Ahorukomeye, P.; Glenn, M.J.; Deininger, M.W.N.; Boucher, K.M.; et al. Combination of the low anticoagulant heparin CX-01 with chemotherapy for the treatment of acute myeloid leukemia. Blood Adv. 2018, 2, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Gao, Y.; Williams, G.R. Core/shell poly(ethylene oxide)/Eudragit fibers for site-specific release. Int. J. Pharm. 2017, 523, 376–385. [Google Scholar] [CrossRef]
- Rüzgar Özemre, G.; Kara, A.A.; Pezik, E.; Tort, S.; Vural, İ.; Acartürk, F. Preparation of nanodelivery systems for oral administration of low molecular weight heparin. J. Drug Deliv. Sci. Technol. 2023, 79, 104068. [Google Scholar] [CrossRef]
- Eder, J.; Bermejo-Jambrina, M.; Vlaming, K.E.; Kaptein, T.M.; Zaderer, V.; Kemper, E.M.; Wilflingseder, D.; Reitsma, S.; de Bree, G.J.; Cohn, D.M.; et al. Inhalation of Low Molecular Weight Heparins as Prophylaxis against SARS-CoV-2. Mbio 2022, 13, e02558-22. [Google Scholar] [CrossRef] [PubMed]
- Shute, J.K. Heparin, Low Molecular Weight Heparin, and Non-Anticoagulant Derivatives for the Treatment of Inflammatory Lung Disease. Pharmaceuticals 2023, 16, 584. [Google Scholar] [CrossRef] [PubMed]



| Preparation Methods | Essence | Advantages | Mw/Da | Ref. |
|---|---|---|---|---|
| Preparation from bovine and ovine heparins | nitrous acid cleavage | expanding the animal source of heparin preparation | average: 5971 (bovine), 6131 (ovine) | [9] |
| Enzymatic Preparation with photoswitchable heparinase III | enzymatic β-elimination | artificially controlled enzymatic degradation; well-distributed molecular weight; high anticoagulant activity | [11] | |
| Preparation of a new NG-LMWH | chemical β-elimination | the same activity as heparin in anti-FXa and anti-FIIa; high safety by neutralizing protamine | 5400~9000 | [17] |
| Ultrasonic-assisted preparation | free radical oxidation | fewer chemical impurities; high anticoagulant activity | ~6300 | [18] |
| Preparation from the ultrasound-assisted Fenton system | free radical oxidation | efficient and convenient; high anticoagulant activity | ~4870 | [15] |
| Enzymatic synthesis from n-sulfo heparosan depolymerized by heparinase or heparin lyase | synthesis | not dependent on animal sources; the same activity as enoxaparin in Anti-FXa and anti-FIIa; high purity | ~4000 | [16] |
| Medicines | Applicable Disease | Mechanisms | Results | Ref. |
|---|---|---|---|---|
| LMWH and Ulinastatin | acute pancreatitis in children | improve the coagulation function | enhanced the efficacy of conventional treatment and reduced mortality | [114] |
| LMWH and Magnesium sulfate | severe pre-eclampsia | reduce the infiltration ability of cytotrophoblasts and regulate endothelial cell function | improved neonatal survival rate | [115] |
| enoxaparin | prevention of venous thromboembolism in COVID-19 | reduced mortality, clinical deterioration, and venous thromboembolism | [116] | |
| LMWH and Dexamethasone | partial splenic embolization in cirrhotic patients with massive splenomegaly | improve the coagulation function | reduced the incidence of complications (such as post-embolization syndrome, portal vein thrombosis, refractory ascites) | [117] |
| LMWH calcium and Xueshuantong injection | elderly acute deep venous thrombosis patients | improve the coagulation function and inhibit thrombosis | effectively treated acute deep venous thrombosis patients | [118] |
| enoxaparin | active ulcerative colitis | anti-inflammatory | significantly improved ulcerative colitis | [119] |
| LMWH calcium | henoch–schonlein purpura nephritis | inhibit high fibrinolysis to exert anticoagulant effects | showed good curative effect on proteinuria, alleviated the renal injury and protected the kidney function | [120] |
| LMWH and Amikacin Sulfate | severe senile pneumonia | improve the coagulation function and inhibit pathogen adhesion | promoted the recovery of patients | [121] |
| LMWH | acute exacerbation of chronic obstructive pulmonary disease | improve the coagulation function and anti-inflammatory | significantly reduced the risk of thrombosis | [122] |
| nadroparin calcium | epithelial cell ovarian carcinoma | increase HGF serum concentration | enhancers for ovarian cancer chemotherapy drugs | [123] |
| LMWH | sepsis | improve inflammation and coagulation function | significantly reduced the 28-day mortality rate in patients with sepsis | [124] |
| LMWH | liver fibrosis in patients with chronic hepatitis B | inhibit collagen proliferation in liver tissues | inhibited liver fibrosis and used as anti-fibrosis drug | [79] |
| tinzaparin | localized lung cancer | no significant effect on overall survival and recurrence-free survival | [113] | |
| Cytarabine and Idarubicin with heparin derivative | acute myeloid leukemia | block the CXCL12/CXCR4 axis; inhibits platelet factor-4 | made the human body have good tolerance and enhanced the therapeutic effect | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, K.; Wang, K.; Zhou, Y.; Xue, H.; Wang, F.; Jin, H.; Zhao, W. Non-Anticoagulant Activities of Low Molecular Weight Heparins—A Review. Pharmaceuticals 2023, 16, 1254. https://doi.org/10.3390/ph16091254
Feng K, Wang K, Zhou Y, Xue H, Wang F, Jin H, Zhao W. Non-Anticoagulant Activities of Low Molecular Weight Heparins—A Review. Pharmaceuticals. 2023; 16(9):1254. https://doi.org/10.3390/ph16091254
Chicago/Turabian StyleFeng, Ke, Kaixuan Wang, Yu Zhou, Haoyu Xue, Fang Wang, Hongzhen Jin, and Wei Zhao. 2023. "Non-Anticoagulant Activities of Low Molecular Weight Heparins—A Review" Pharmaceuticals 16, no. 9: 1254. https://doi.org/10.3390/ph16091254
APA StyleFeng, K., Wang, K., Zhou, Y., Xue, H., Wang, F., Jin, H., & Zhao, W. (2023). Non-Anticoagulant Activities of Low Molecular Weight Heparins—A Review. Pharmaceuticals, 16(9), 1254. https://doi.org/10.3390/ph16091254






