Damoctocog Alfa Pegol for Hemophilia A Prophylaxis: An Italian Multicenter Survey
Abstract
1. Introduction
2. Results
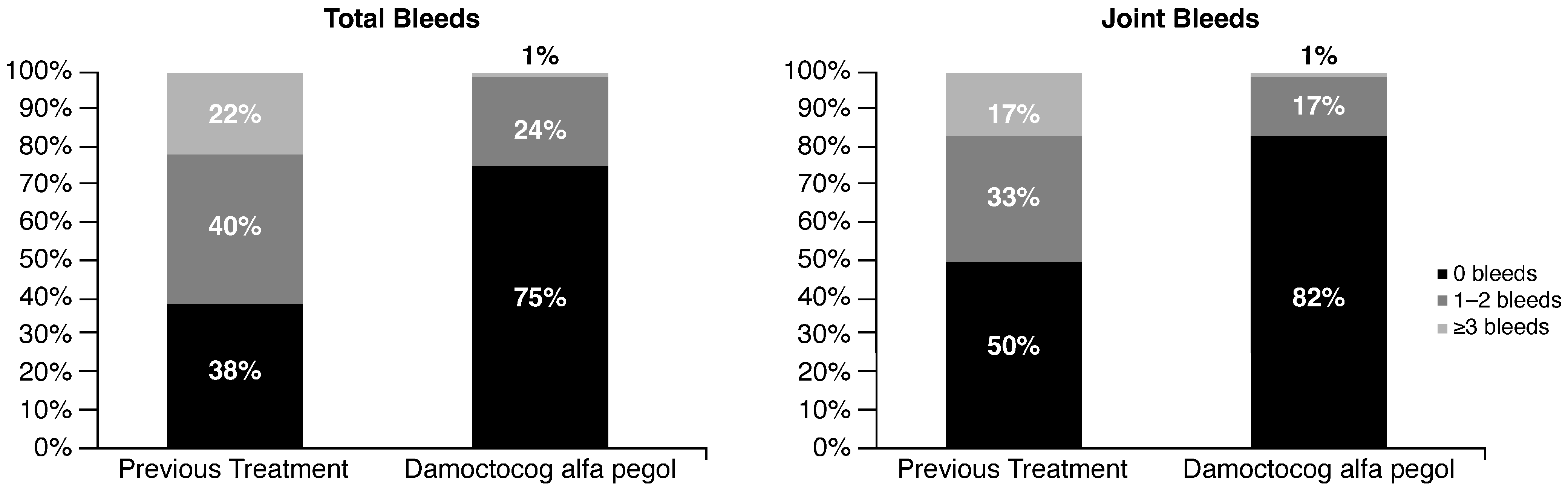
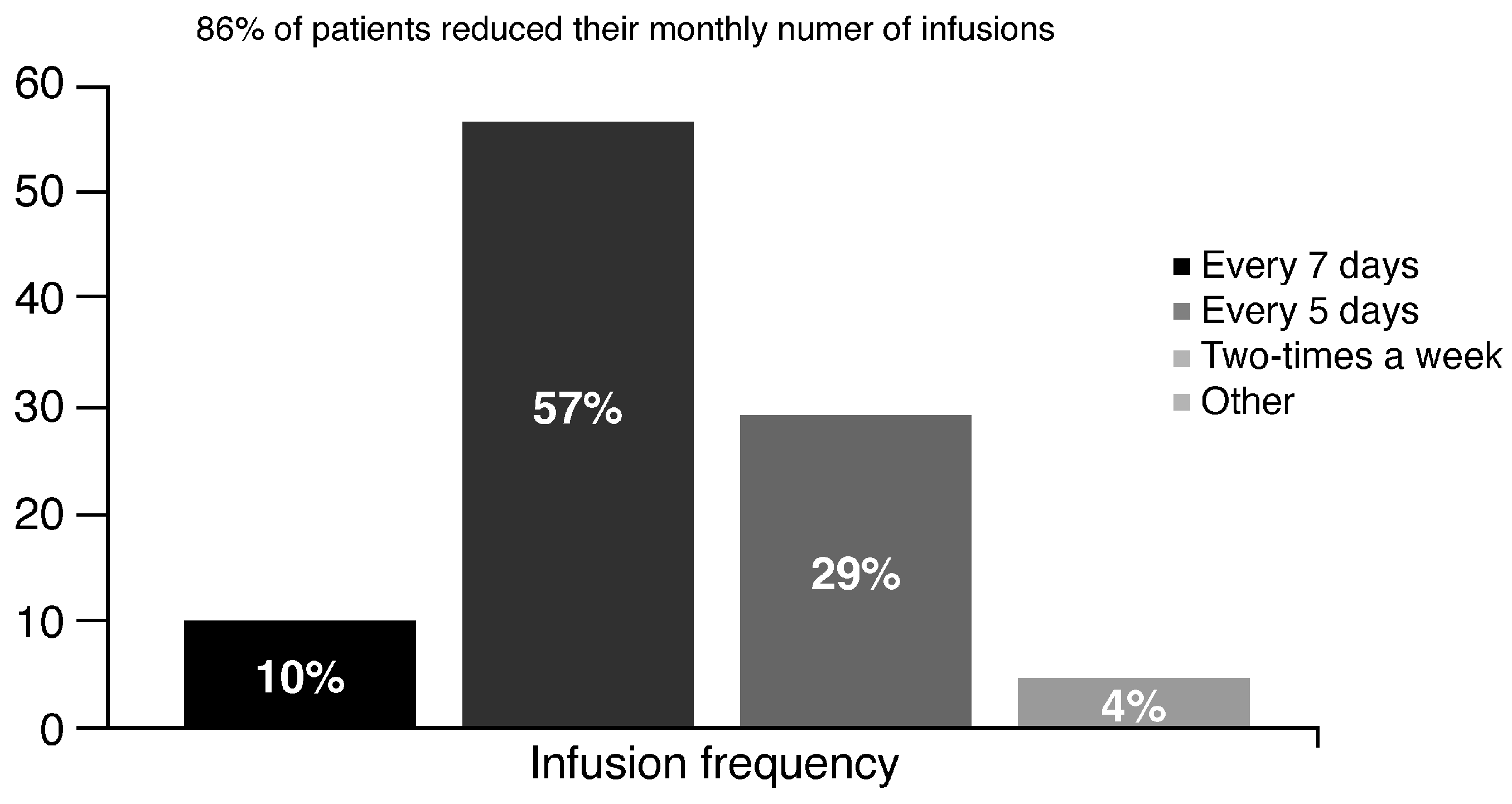
2.1. Switch to Damoctocog Alfa Pegol
2.2. Prophylaxis with Damoctocog Alfa Pegol: Clinicians’ Perception
3. Discussion
4. Materials and Methods
Patients Treated with Damoctocog Alfa Pegol: Characteristics
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schramm, W. The history of haemophilia—A short review. Thromb. Res. 2014, 134 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Mannucci, P.M. The history of hemophilia. Semin. Thromb. Hemost. 2014, 40, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Ingram, G.I.C. The history of haemophilia. J. Clin. Pathol. 1976, 29, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Rosendaal, F.R.; Smit, C.; Briët, E. Hemophilia treatment in historical perspective: A review of medical and social developments. Ann. Hematol. 1991, 62, 5–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hopff, F. Ueber die Hämophilie oder die erbliche Anlage zu tödtlichen Blutungen. In Inaugural-Abhandlung; Würzburg: Becker, CW, USA, 1828. [Google Scholar]
- Schönlein, J.L. Allgemeine und Specielle Pathologie und Therapie, 4th ed.; Litteratur-Comptoir: St. Gallen, Switzerland, 1839. [Google Scholar]
- Larsson, S.A. Life expectancy of Swedish haemophiliacs, 1831–1980. Br. J. Haematol. 1985, 59, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Lane, S. Haemorrhagic diathesis. Successful transfusion of blood. Lancet 1840, 35, 185–188. [Google Scholar] [CrossRef]
- Landsteiner, K. Über Agglutinationserscheinungen normalen menschlichen Blutes. Wien. Klin. Wochenschr. 1901, 14, 1132–1134. [Google Scholar]
- Bendien, W.M.; van Creveld, S. Investigations of haemophilia. Acta Brevia Neerl. Physiol. Pharmacol. Microbiol. E A 1935, 5, 135–138. [Google Scholar]
- Pool, J.G.; Shannon, A.E. Production of high-potency concentrates of antihemophilic globulin in a closed-bag system. N. Engl. J. Med. 1965, 273, 1443–1447. [Google Scholar] [CrossRef]
- Mannucci, P.M.; Ruggeri, Z.M.; Pareti, F.I.; Capitanio, A. 1-Deamino-8-d-arginine vasopressin: A new pharmacological approach to the management of haemophilia and von Willebrands’ diseases. Lancet 1977, 1, 869–872. [Google Scholar] [CrossRef]
- Nilsson, I.M.; Berntorp, E.; Löfqvist, T.; Pettersson, H. Twenty-five years’ experience of prophylactic treatment in severe haemophilia A and B. J. Intern. Med. 1992, 232, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, P.M. Back to the future: A recent history of haemophilia treatment. Haemophilia 2008, 14, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Schimpf, K.; Mannucci, P.M.; Kreuz, W.; Brackmann, H.H.; Auerswald, G.; Ciavarella, N.; Mösseler, J.; DeRosa, V.; Kraus, B.; Brueckmann, C.; et al. Absence of hepatitis after treatment with a pasteurized factor VIII concentrate in patients with haemophilia and no previous transfusions. N. Engl. J. Med. 1987, 316, 918–922. [Google Scholar] [CrossRef]
- Schimpf, K.; Brackmann, H.H.; Kreuz, W.; Kraus, B.; Haschke, F.; Schramm, W.; Moesseler, J.; Auerswald, G.; Sutor, A.H.; Koehler, K.; et al. Absence of anti-human immunodeficiency virus types 1 and 2 seroconversion after the treatment of hemophilia A or von Willebrand’s disease with pasteurized factor VIII concentrate. N. Engl. J. Med. 1989, 321, 1148–1152. [Google Scholar] [CrossRef]
- Pipe, S.W. Recombinant clotting factors. Thromb. Haemost. 2008, 99, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Mahlangu, J.; Young, G.; Hermans, C.; Blanchette, V.; Berntorp, E.; Santagostin, E. Defining extended half-life rFVIII-A critical review of the evidence. Haemophilia 2018, 24, 348–358. [Google Scholar] [CrossRef]
- Iorio, A.; Stonebraker, J.S.; Chambost, H.; Makris, M.; Coffin, D.; Herr, C.; Germini, F. Data and Demographics Committee of the World Federation of Hemophilia. Establishing the Prevalence and Prevalence at Birth of Hemophilia in Males: A Meta-analytic Approach Using National Registries. Ann. Intern. Med. 2019, 171, 540–546. [Google Scholar] [CrossRef]
- Paik, J.; Deeks, E.D. Damoctocog alfa pegol: A review in haemophilia A. Drugs 2019, 79, 1147–1156. [Google Scholar] [CrossRef]
- Shah, A.; Coyle, T.; Lalezari, S.; Fischer, K.; Kohlstaedde, B.; Delesen, H.; Radke, S.; Michaels, L.A. BAY 94-9027, a PEGylated recombinant factor VIII, exhibits a prolonged half-life and higher area under the curve in patients with severe haemophilia A: Comprehensive pharmacokinetic assessment from clinical studies. Haemophilia 2018, 24, 733–740. [Google Scholar] [CrossRef]
- Reding, M.T.; Ng, H.J.; Poulsen, L.H.; Eyster, M.E.; Pabinger, I.; Shin, H.J.; Walsch, R.; Lederman, M.; Wang, M.; Hardtke, M.; et al. Safety and efficacy of BAY 94-9027, a prolonged-half-life factor VIII. J. Thromb. Haemost. 2017, 15, 411–419. [Google Scholar] [CrossRef]
- Reding, M.T.; Pabinger, I.; Holme, P.A.; Maas Enriquez, M.; Mancuso, M.E.; Lalezari, S.; Miesbach, W.; Di Minno, G.; Klamroth, R.; Hermans, C. Efficacy and safety of damoctocog alfa pegol prophylaxis in patients ≥40 years with severe haemophilia A and comorbidities: Post hoc analysis from the PROTECT VIII study. Ther. Adv. Hematol. 2023, 14, 20406207231166779. [Google Scholar] [CrossRef] [PubMed]
- Jivi (Antihemophilic Factor-Recombinant Pegylated-Aucl Kit). DailyMed. 2018. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f04e5bd5-d7e2-453b-a407-2616d81a695d (accessed on 1 June 2023).
- Sanabria, M.; Álvarez Román, M.T.; Castaman, G.; Janbain, M.; Matsushita, T.; Meijer, K.; Oldenburg, J.; Friedl, S.; Reding, M.T. Design of the HEM-POWR study: A prospective, observational study of real-world treatment with damoctocog alfa pegol in patients with haemophilia A. BMJ Open 2021, 11, e044997. [Google Scholar] [CrossRef] [PubMed]
- Lieuw, K. Many factor VIII products available in the treatment of hemophilia A: An embarrassment of riches? J. Blood Med. 2017, 8, 67–73. [Google Scholar] [CrossRef]
- Von Mackensen, S.; Kalnins, W.; Krucker, J.; Weiss, J.; Miesbach, W.; Albisetti, M.; Pabinger, I.; Oldenburg, J. Haemophilia patients’ unmet needs and their expectations of the new extended half-life factor concentrates. Haemophilia 2017, 23, 566–574. [Google Scholar] [CrossRef]
- Manco-Johnson, M.J.; Abshire, T.C.; Shapiro, A.D.; Riske, B.; Hacker, M.R.; Kilcoyne, R.; Ingram, J.D.; Manco-Johnson, M.L.; Funk, S.; Jacobson, L.; et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N. Engl. J. Med. 2007, 357, 535–544. [Google Scholar] [CrossRef]
- Srivastava, A.; Santagostino, E.; Dougall, A.; Kitchen, S.; Sutherland, M.; Pipe, S.W.; Carcao, M.; Mahlangu, J.; Ragni, M.V.; Windyga, J.; et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia 2020, 26, 1–158. [Google Scholar] [CrossRef]
- Zanon, E.; De Cristofaro, R.; Franchini, M.; Morfini, M.; Pasut, G.; Molinari, A.C.; Santoro, C.; Santoro, R.C.; Coppola, A.; Rocino, A. Bioequivalence of recombinant factor VIII products: A position paper from the Italian Association of Hemophilia Centers. Blood Trans. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Versloot, O.; Kemler, E.; Blokzijl, J.; Timmer, M.; Schuuring, M.; van Galen, K.P.M.; Kremer Hovinga, I.C.L.; van der Valk, P.R.; van Vulpen, L.F.D.; Schutgens, R.E.G.; et al. Clotting factor activity levels and bleeding risk in people with haemophilia playing sports. Haemophilia 2023. online ahead of print. [Google Scholar] [CrossRef]
- Núñez, R.; Álvarez-Román, M.T.; Bonanad, S.; González-Porras, J.R.; De La Corte-Rodriguez, H.; Berrueco, R.; Jiménez-Yuste, V. The limitations and unmet needs of the five cornerstones to guarantee lifelong optimization of prophylaxis in hemophilia patients. TH Open 2022, 6, e365–e377. [Google Scholar] [CrossRef]
- Reding, M.T.; Pabinger, I.; Holme, P.A.; Poulsen, L.; Negrier, C.; Chalasani, P.; Maas Enriquez, M.; Wang, M.; Meijer, K.; Mancuso, M.E.; et al. Confirmed long-term safety and efficacy of prophylactic treatment with BAY 94-9027 in severe haemophilia A: Final results of the PROTECT VIII extension study. Haemophilia 2021, 27, e347–e356. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, M.E.; Reding, M.T.; Negrier, C.; Kerlin, B.A.; Rangarajan, S.; Simpson, M.L. Decreased bleeding rates in patients with hemophilia a switching from standard-half-life FVIII to BAY 94-9027 prophylaxis. Thromb. Haemost. 2021, 121, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Vashi, P.; Batt, K.; Klamroth, R.; Mancuso, M.E.; Majewska, R.; Tiede, A.; Mantovani, L.G. Indirect treatment comparison of damoctocog alfa pegol versus turoctocog alfa pegol as prophylactic treatment in patients with hemophilia A. J. Blood Med. 2021, 12, 935–943. [Google Scholar] [CrossRef]
- Mokhtar, F.M.; Sathar, J.; Huri, H.Z. Medication adherence for haemophilia patients: Outcome of prophylaxis treatment intervention. Healthcare 2021, 9, 1702. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, N.; Salinas-Luna, V.; Acharya, S.; Sharathkumar, A. Perceptions about the efficacy of extended half-life (EHL) factor products in persons with haemophilia (PWH): A national survey of haemophilia providers from haemophilia treatment centres (HTCs) in the United States. Hemophilia 2021, 27, e780–e783. [Google Scholar] [CrossRef]
- Lambert, T.; Benson, G.; Dolan, G.; Hermans, C.; Jiménez-Yuste, V.; Ljung, R.; Morfini, M.; Zupančić-Šalek, S.; Santagostino, E. Practical aspects of extended half-life products for the treatment of haemophilia. Ther. Adv. Hematol. 2018, 9, 295–308. [Google Scholar] [CrossRef]
- Sergunova, V.; Leesment, S.; Kozlov, A.; Inozemtsev, V.; Platitsina, P.; Lyapunova, S.; Onufrievich, A.; Polyako, V.; Sherstyukova, E. Investigation of Red Blood Cells by Atomic Force Microscopy. Sensors 2022, 22, 2055. [Google Scholar] [CrossRef]
- Magazzù, A.; Marcuello, C. Investigation of Soft Matter Nanomechanics by Atomic Force Microscopy and Optical Tweezers: Comprehensive Review. Nanomaterials 2023, 13, 963. [Google Scholar] [CrossRef]
- Lostao, A.; Lim, K.; Pallarés, M.C.; Ptak, A.; Marcuello, C. Recent advances in sensing the inter-biomolecular interactions at the nanoscale—A comprehensive review of AFM-based force spectroscopy. Int. J. Biol. Macromol. 2023, 238, 12408. [Google Scholar] [CrossRef]
| 30–40 IU/kg Two-Times a Week | 45–60 IU/kg Every 5 Days | 60 IU/kg Every 7 Days |
|---|---|---|
| Patient characteristics: Active lifestyle (80%) 12–18 years (67%) Severe bleeding phenotype (67%) Playing sports (53%) Presence of synovitis (40%) | Patient characteristics: 19–65 years (60%) Active lifestyle (40%) | Patient characteristics: Over 65 (53%) Inactive or sedentary lifestyle (53%) Mild bleeding phenotype (53%) Poor autonomy (40%) Difficult venous access (33%) Comorbidities (27%) |
| Previous FVIII Concentrate | Damoctocog Alfa Pegol Treatment | Difference (Δ) | |
|---|---|---|---|
| IU/kg/month | 389 | 321 | −68 |
| IU/kg/year | 4673 | 3848 | −825 |
| IU/month (e.g., patient weight 70 kg) | 27,230 | 22,470 | −4760 |
| IU/year (e.g., patient weight 70 kg) | 326,760 | 269,640 | −57,120 |
| Baseline Characteristics | n (%) |
|---|---|
| Patients | 164 (100) |
| Age 12–18 years 19–40 years 41–65 years >65 years | 25 (15) 63 (38) 60 (37) 16 (10) |
| Hemophilia A degree: Mild Moderate Severe | 3 (2) 24 (15) 137 (83) |
| School or work activities: Full-time work Part-time work High school/university Retired No work/no school | 71 (43) 24 (15) 40 (24) 16 (10) 13 (8) |
| Autonomy: Low Medium High | 12 (7) 49 (30) 103 (63) |
| Comorbidities: Cardiovascular diseases Metabolic diseases Liver diseases Others | 87 (53) 33 (20) 66 (40) 66 (40) |
| Sports activity: None Occasionally Habitually | 58 (35) 57 (35) 49 (30) |
| Attitude: Pessimistic/disheartened Unresponsive/indifferent Proactive/seeking continuous improvement | 12 (7) 22 (13) 130 (79) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanon, E. Damoctocog Alfa Pegol for Hemophilia A Prophylaxis: An Italian Multicenter Survey. Pharmaceuticals 2023, 16, 1195. https://doi.org/10.3390/ph16091195
Zanon E. Damoctocog Alfa Pegol for Hemophilia A Prophylaxis: An Italian Multicenter Survey. Pharmaceuticals. 2023; 16(9):1195. https://doi.org/10.3390/ph16091195
Chicago/Turabian StyleZanon, Ezio. 2023. "Damoctocog Alfa Pegol for Hemophilia A Prophylaxis: An Italian Multicenter Survey" Pharmaceuticals 16, no. 9: 1195. https://doi.org/10.3390/ph16091195
APA StyleZanon, E. (2023). Damoctocog Alfa Pegol for Hemophilia A Prophylaxis: An Italian Multicenter Survey. Pharmaceuticals, 16(9), 1195. https://doi.org/10.3390/ph16091195





