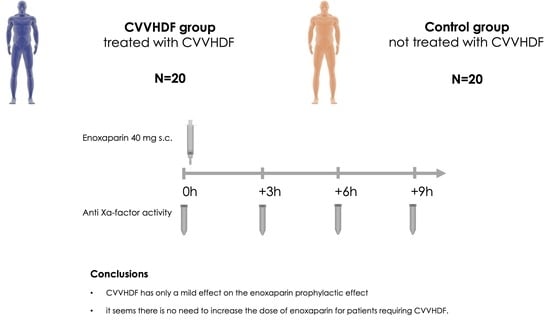
The Impact of Continuous Veno-Venous Hemodiafiltration on the Efficacy of Administration of Prophylactic Doses of Enoxaparin: A Prospective Observational Study
Abstract
:1. Introduction
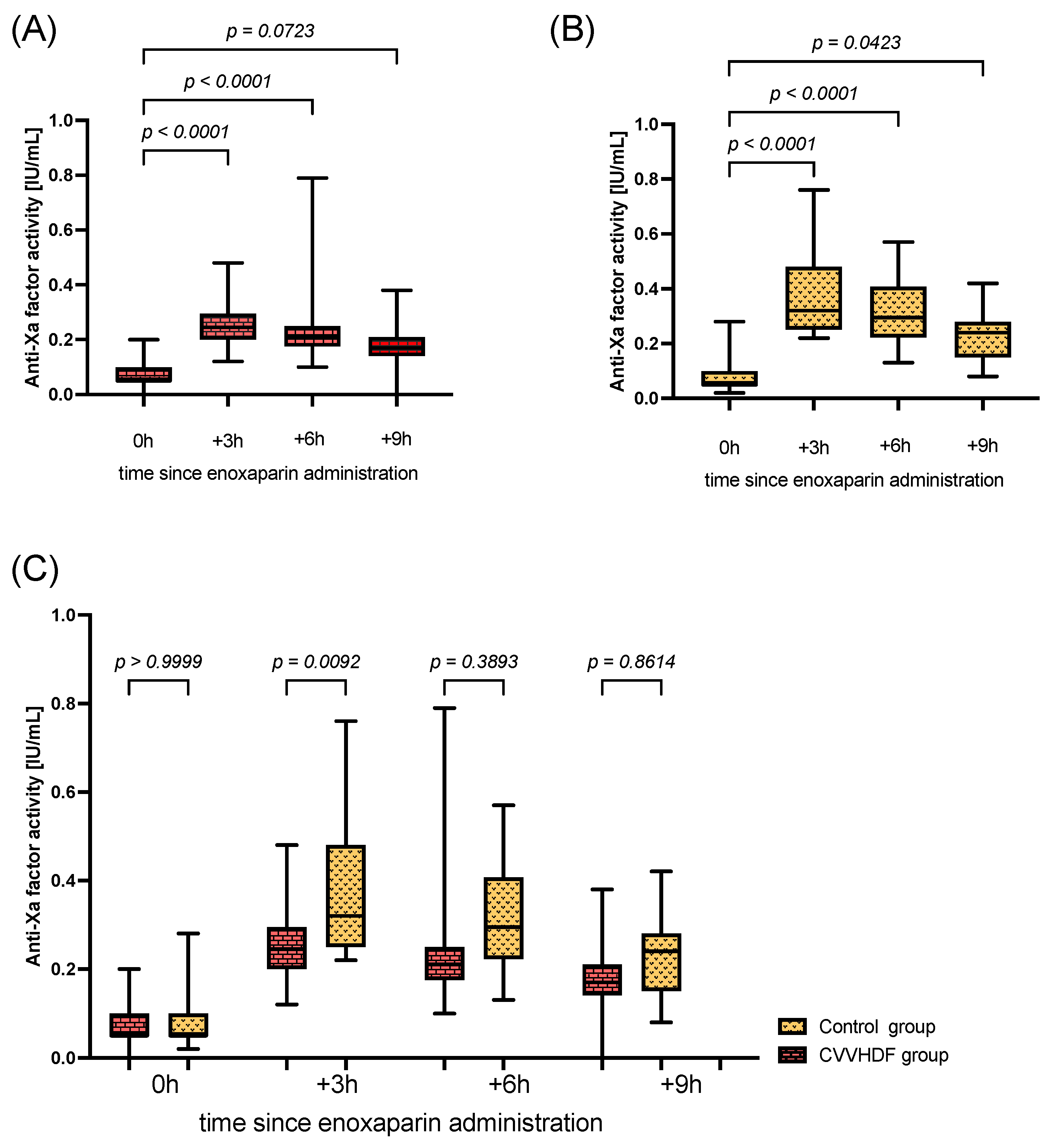
2. Results
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Study Protocol
4.3. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marik, P.E.; Andrews, L.; Maini, B. The incidence of deep venous thrombosis in ICU patients. Chest 1997, 111, 661–664. [Google Scholar] [CrossRef]
- Wahab, A.; Patnaik, R.; Gurjar, M. Use of direct oral anticoagulants in ICU patients. Part I—Applied Pharmacology. Anaesthesiol. Intensive Ther. 2021, 53, 429–439. [Google Scholar] [CrossRef]
- Boddi, M.; Peris, A. Deep vein thrombosis in intensive care. Adv. Exp. Med. Biol. 2017, 906, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Malato, A.; Dentali, F.; Siragusa, S.; Fabbiano, F.; Kagoma, Y.; Boddi, M.; Gensini, G.F.; Peris, A.; Crowther, M.; Napolitano, M. The impact of deep vein thrombosis in critically ill patients: A meta-analysis of major clinical outcomes. Blood Transfus. 2015, 13, 559–568. [Google Scholar] [PubMed]
- DeBiase, C.; Giuliano, C.A.; Doshi, M.; Ganoff, M.; Alexander Paxton, R. Enoxaparin versus unfractionated heparin for venous thromboembolism prophylaxis in renally impaired ICU patients. Pharmacotherapy 2021, 41, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Merli, G.J.; Groce, J.B. Pharmacological and clinical differences between low-molecular-weight heparins implications for prescribing practice and therapeutic interchange. Pharm. Ther. 2010, 35, 95–105. [Google Scholar]
- Benes, J.; Skulec, R.; Jobanek, J.; Cerny, V. Fixed-dose enoxaparin provides efficient DVT prophylaxis in mixed ICU patients despite low anti-Xa levels: A prospective observational cohort study. Biomed. Pap. 2022, 166, 204–210. [Google Scholar] [CrossRef]
- Helviz, Y.; Dzigivker, I.; Raveh-Brawer, D.; Hersch, M.; Zevin, S.; Einav, S. Anti-factor Xa activity of prophylactic enoxaparin regimens in critically ill patients. Isr. Med. Assoc. J. 2016, 18, 108–113. [Google Scholar]
- Vincent, P.D.; Albert, M.; Champagne, M.C.; Zikos, T.; Boulanger, I.; Blais, L.; Williamson, D.R. Factors influencing enoxaparin anti-Xa activity in surgical critically ill patients. J. Crit. Care 2011, 26, 347–351. [Google Scholar] [CrossRef]
- Walther, C.P.; Podoll, A.S.; Finkel, K.W. Summary of clinical practice guidelines for acute kidney injury. Hosp. Pract. 2014, 42, 7–14. [Google Scholar] [CrossRef]
- Koeze, J.; Keus, F.; Dieperink, W.; Van der Horst, I.C.C.; Zijlstra, J.G.; Van Meurs, M. Incidence, timing and outcome of AKI in critically ill patients varies with the definition used and the addition of urine output criteria. BMC Nephrol. 2017, 18, 70. [Google Scholar] [CrossRef] [PubMed]
- Paudel, M.S.; Wig, N.; Mahajan, S.; Pandey, R.M.; Guleria, R.; Sharma, S.K. A study of incidence of AKI in critically Ill patients. Ren. Fail. 2012, 34, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.J.; Lameire, N.H.; Vanholder, R.C.; Benoit, D.D.; Decruyenaere, J.M.A.; Colardyn, F.A. Acute renal failure in patients with sepsis in a surgical ICU: Predictive factors, incidence, comorbidity, and outcome. J. Am. Soc. Nephrol. 2003, 14, 1022–1030. [Google Scholar] [CrossRef]
- Cartin-Ceba, R.; Kashiouris, M.; Plataki, M.; Kor, D.J.; Gajic, O.; Casey, E.T. Risk factors for development of acute kidney injury in critically ill patients: A systematic review and meta-analysis of observational studies. Crit. Care Res. Pract. 2012, 2012, 691013. [Google Scholar] [CrossRef]
- Vivino, G.; Antonelli, M.; Moro, M.L.; Cottini, F.; Conti, G.; Bufi, M.; Cannata, F.; Gasparetto, A. Risk factors for acute renal failure in trauma patients. Intensive Care Med. 1998, 24, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Ricci, Z.; Cruz, D.; Ronco, C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int. 2008, 73, 538–546. [Google Scholar] [CrossRef]
- Honore, P.M.; Jacobs, R.; Joannes-Boyau, O.; De Regt, J.; Boer, W.; De Waele, E.; Collin, V.; Spapen, H.D. Septic AKI in ICU patients. Diagnosis, pathophysiology, and treatment type, dosing, and timing: A comprehensive review of recent and future developments. Ann. Intensive Care 2011, 1, 32. [Google Scholar] [CrossRef]
- Bai, M.; Zhou, M.; He, L.; Ma, F.; Li, Y.; Yu, Y.; Wang, P.; Li, L.; Jing, R.; Zhao, L.; et al. Citrate versus heparin anticoagulation for continuous renal replacement therapy: An updated meta-analysis of RCTs. Intensive Care Med. 2015, 41, 2098–2110. [Google Scholar] [CrossRef]
- Liu, C.; Mao, Z.; Kang, H.; Hu, J.; Zhou, F. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy in critically ill patients: A meta-analysis with trial sequential analysis of randomized controlled trials. Crit. Care 2016, 20, 144. [Google Scholar] [CrossRef]
- Badawy, S.S.I.; Fahmy, A. Efficacy and cardiovascular tolerability of continuous veno-venous hemodiafiltration in acute decompensated heart failure: A randomized comparative study. J. Crit. Care 2012, 27, 106.e7–106.e13. [Google Scholar] [CrossRef]
- Voigt, M.; Gebert, M.; Haug, U.; Hulko, M.; Storr, M.; Boschetti-de-Fierro, A.; Beck, W.; Krause, B. Retention of beneficial molecules and coagulation factors during haemodialysis and haemodiafiltration. Sci. Rep. 2019, 9, 6370. [Google Scholar] [CrossRef] [PubMed]
- Kusza, K.; Piechota, M. Zasady Kwalifikacji Oraz Kryteria Przyjęcia Chorych do Oddziałów Anestezjologii i Intensywnej Terapii|Anestezjologia.org.pl. Available online: https://www.anestezjologia.org.pl/artykul/zasady-kwalifikacji-oraz-kryteria-przyjecia-chorych-do-oddzialow-anestezjologii-i (accessed on 7 February 2023).
- Robinson, S.; Zincuk, A.; Strøm, T.; Larsen, T.B.; Rasmussen, B.; Toft, P. Enoxaparin, effective dosage for intensive care patients: Double-blinded, randomised clinical trial. Crit. Care 2010, 14, R41. [Google Scholar] [CrossRef] [PubMed]
- Rostas, J.W.; Brevard, S.B.; Ahmed, N.; Allen, J.; Thacker, D.; Replogle, W.H.; Gonzalez, R.P.; Frotan, A.M.; Simmons, J.D. Standard dosing of enoxaparin for venous thromboembolism prophylaxis is not sufficient for most patientswithin a trauma intensive care unit. Am. Surg. 2015, 81, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Bigos, R.; Solomon, E.; Dorfman, J.D.; Ha, M. A Weight- and Anti-Xa-Guided Enoxaparin Dosing Protocol for venous thromboembolism Prophylaxis in intensive care unit Trauma Patients. J. Surg. Res. 2021, 265, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Pluta, J.; Cieniewicz, A.; Trzebicki, J. COVID-19: Coagulation disorders and anticoagulant treatment in patients hospitalised in ICU. Anaesthesiol. Intensive Ther. 2021, 53, 153–161. [Google Scholar] [CrossRef]
- Malinoski, D.; Jafari, F.; Ewing, T.; Ardary, C.; Conniff, H.; Baje, M.; Kong, A.; Lekawa, M.E.; Dolich, M.O.; Cinat, M.E.; et al. Standard prophylactic enoxaparin dosing leads to inadequate anti-Xa levels and increased deep venous thrombosis rates in critically Ill trauma and surgical patients. J. Trauma-Inj. Infect. Crit. Care 2010, 68, 874–879. [Google Scholar] [CrossRef]
- Levine, M.N.; Planes, A.; Hirsh, J.; Goodyear, M.; Vochelle, N.; Gent, M. The relationship between anti-factor Xa level and clinical outcome in patients receiving enoxaparine low molecular weight heparin to prevent deep vein thrombosis after hip replacement. Thromb. Haemost. 1989, 62, 940–944. [Google Scholar] [CrossRef]
- Bara, L.; Planes, A.; Samama, M.M. Occurrence of thrombosis and haemorrhage, relationship with anti-Xa, anti-IIa activities, and D-dimer plasma levels in patients receiving a low molecular weight heparin, enoxaparin or tinzaparin, to prevent deep vein thrombosis after hip surgery. Br. J. Haematol. 1999, 104, 230–240. [Google Scholar] [CrossRef]
- Mayr, A.J.; Dünser, M.; Jochberger, S.; Fries, D.; Klingler, A.; Joannidis, M.; Hasibeder, W.; Schobersberger, W. Antifactor Xa activity in intensive care patients receiving thromboembolic prophylaxis with standard doses of enoxaparin. Thromb. Res. 2002, 105, 201–204. [Google Scholar] [CrossRef]
- Rojnuckarin, P.; Akkawat, B.; Juntiang, J. Stability of plasma anti-Xa activity in low-molecular-weight heparin monitoring. Clin. Appl. Thromb. 2010, 16, 313–317. [Google Scholar] [CrossRef]
- Dörffler-Melly, J.; De Jonge, E.; De Pont, A.C.; Meijers, J.; Vroom, M.B.; Büller, H.R.; Levi, M. Bioavailability of subutaneous low-molecular-weight heparin to patients on vasopressors. Lancet 2002, 359, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Meenks, S.D.; Foudraine, N.; Le Noble, J. No effect of norepinephrine dose on anti-Xa activity in critically ill patients. Artic. Int. J. Clin. Pharmacol. Ther. 2020, 58, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Priglinger, U.; Delle Karth, G.; Geppert, A.; Joukhadar, C.; Graf, S.; Berger, R.; Hülsmann, M.; Spitzauer, S.; Pabinger, I.; Heinz, G. Prophylactic anticoagulation with enoxaparin: Is the subcutaneous route appropriate in the critically ill? Crit. Care Med. 2003, 31, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Tandukar, S.; Palevsky, P.M. Continuous Renal Replacement Therapy: Who, When, Why, and How. Chest 2019, 155, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Benken, S.T.; Lizza, B.D.; Yamout, H.; Ghossein, C. Management of digoxin therapy using pharmacokinetics in a patient undergoing continuous venovenous hemofiltration. Am. J. Health Pharm. 2013, 70, 2105–2109. [Google Scholar] [CrossRef]
- Thanacoody, R.H.K. Extracorporeal elimination in acute valproic acid poisoning. Clin. Toxicol. 2009, 47, 609–616. [Google Scholar] [CrossRef]
- Oltrogge, K.M.; Peppard, W.J.; Saleh, M.; Regner, K.R.; Herrmann, D.J. Phenytoin removal by continuous venovenous hemofiltration. Ann. Pharmacother. 2013, 47, 1218–1222. [Google Scholar] [CrossRef]
- Swart, E.L.; De Jongh, J.; Zuideveld, K.P.; Danhof, M.; Thijs, L.G.; Strack Van Schijndel, R.J.M. Population pharmacokinetics of lorazepam and midazolam and their metabolites in intensive care patients on continuous venovenous hemofiltration. Am. J. Kidney Dis. 2005, 45, 360–371. [Google Scholar] [CrossRef]
- Onichimowski, D.; Nosek, K.; Ziółkowski, H.; Jaroszewski, J.; Pawlos, A.; Czuczwar, M. Adsorption of vancomycin, gentamycin, ciprofloxacin and tygecycline on the filters in continuous renal replacement therapy circuits: In full blood in vitro study. J. Artif. Organs 2021, 24, 65–73. [Google Scholar] [CrossRef]
- DelDot, M.E.; Lipman, J.; Tett, S.E. Vancomycin pharmacokinetics in critically ill patients receiving continuous venovenous haemodiafiltration. Br. J. Clin. Pharmacol. 2004, 58, 259–268. [Google Scholar] [CrossRef]
- Turner, R.B.; Kojiro, K.; Won, R.; Chang, E.; Chan, D.; Elbarbry, F. Prospective evaluation of vancomycin pharmacokinetics in a heterogeneous critically ill population. Diagn. Microbiol. Infect. Dis. 2018, 92, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Isla, A.; Gascón, A.R.; Maynar, J.; Arzuaga, A.; Corral, E.; Martín, A.; Solinís, M.Á.; Muñoz, J.L.P. In vitro and in vivo evaluation of enoxaparin removal by continuous renal replacement therapies with acrylonitrile and polysulfone membranes. Clin. Ther. 2005, 27, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- McMahon, L.P.; Chester, K.; Walker, R.G. Effects of different dialysis membranes on serum concentrations of epoetin alfa, darbepoetin alfa, enoxaparin, and iron sucrose during dialysis. Am. J. Kidney Dis. 2004, 44, 509–516. [Google Scholar] [CrossRef]
- Singer, M.; McNally, T.; Screaton, G.; Mackie, I.; Machin, S.; Cohen, S.L. Heparin clearance during continuous veno-venous haemofiltration. Intensive Care Med. 1994, 20, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Brophy, D.F.; Carr, M.E.; Martin, E.J.; Venitz, J.; Gehr, T.W.B. The pharmacokinetics of enoxaparin do not correlate with its pharmacodynamic effect in patients receiving dialysis therapies. J. Clin. Pharmacol. 2006, 46, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Parzer, S.; Balcke, P.; Mannhalter, C. Plasma protein adsorption to hemodialysis membranes: Studies in an in vitro model. J. Biomed. Mater. Res. 1993, 27, 455–463. [Google Scholar] [CrossRef]
- Vahtera, A.; Valkonen, M.; Huhtala, H.; Pettilä, V.; Kuitunen, A. Plasma anti-FXa concentration after continuous intravenous infusion and subcutaneous dosing of enoxaparin for thromboprophylaxis in critically ill patients. A randomized clinical trial. Thromb. Res. 2017, 158, 71–75. [Google Scholar] [CrossRef]
- Bergstrand, M.; Karlsson, M.O. Handling data below the limit of quantification in mixed effect models. AAPS J. 2009, 11, 371–380. [Google Scholar] [CrossRef]
- Zeghnoun, A.; Pascal, M.; Fréry, N.; Sarter, H.; Falq, G.; Focant, J.F.; Eppe, G. Epidemiological Study on Mswi Operation Dealing with the Non-Detected and Non-Quantified Data. The Example of the Serum Dioxin Data in the French Dioxin and Incinerators Study. Organohalogen Compd. 2007, 69, 2288–2291. [Google Scholar]
| CVVHDF Group (n = 20) | Control Group (n = 20) | p Value | |
|---|---|---|---|
| Female | n = 7 [35%] | n = 11 [55%] | 0.34 |
| Age (yrs.) | 59 (10.9) | 65.5 (17.5) | 0.17 |
| BMI (kg m−2) | 30.6 (6.1) | 26.1 (4.9) | 0.07 |
| Cause of admission to ICU | SShock − n = 6 [30%] MOF − n = 4 [20%] RF and AKI − n = 3 [15%] SShock and AKI − n = 3 [15%] AP − n = 1 [5%] AKI − n = 1 [5%] SShock + AP − n = 1 [5%] AKI + AP − n = 1 [5%] | RF − n = 12 [60%] Trauma − n = 4 [20%] MOF − n = 2 [10%] SAH − n = 1 [5%] AS − n = 1 [5%] | N/A |
| Time since admission to ICU (days) | 4.5 (4–7) | 7.5 (6.3–11.5) | 0.001 |
| Time since CVVHDF beginning (days) | 5 (4–8) | N/A | N/A |
| Mechanically ventilated | n = 7 [35%] | n = 9 [45%] | 0.75 |
| Vasopressors administration | Yes − n = 10 [50%] No − n = 10 [50%] | Yes − n = 10 [50%] No − n = 10 [50%] | >0.99 |
| Type of vasopressors administrated | Only NE − n = 3 [15%] NE + one other − n = 6 [30%] More than two − n= 1 [5%] | Only NE − n = 10 [50%] | 0.01 |
| Dose of norepinephrine | 0.70 (0.67–1.80) | 0.61 (0.30–1.70) | 0.46 |
| SAPS II score at admission | 45 (10) | 37 (13) | 0.08 |
| APCHE II score at admission | 20 (9) | 15 (7) | 0.03 |
| SOFA score at study day | 9 (4) | 6 (3) | 0.008 |
| Treatment outcome | Discharge to non-ICU ward n = 8 [40%] Death − n = 6 [30%] Death after readmission to ICU n = 3 [15%] Discharge to non-ICU ward and death − n = 2 [10%] Discharge to another hospital − n = 1 [5%] | Discharge to non-ICU ward n = 11 [55%] Death n = 5 [25%] Discharge to health care center n = 2 [10%] Discharge to other hospital n = 1 [5%] Discharge to non-ICU ward and death − n = 1 [5%] | 0.32 |
| CVVHDF Group (n = 20) | Control Group (n = 20) | p Value (Comparison between Control and CVVHDF Groups in Given Time Points) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-Xa factor activity (IU/mL) | 0 h | +3 h | +6 h | +9 h | 0 h | +3 h | +6 h | +9 h | 0 h | +3 h | +6 h | +9 h |
| <0.2 | n = 19 [95%] | n = 4 [20%] | n = 7 [35%] | n = 14 [70%] | n = 19 [95%] | n = 0 [0%] | n = 2 [10%] | n = 8 [40%] | >0.99 | 0.11 | 0.11 | 0.13 |
| 0.2–0.4 | n = 1 [5%] | n = 16 [80%] | n = 13 [65%] | n = 6 [30%] | n = 1 [5%] | n = 20 [100%] | n = 18 [90%] | n = 12 [60%] | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aszkiełowicz, A.; Steckiewicz, K.P.; Okrągły, M.; Wujtewicz, M.A.; Owczuk, R. The Impact of Continuous Veno-Venous Hemodiafiltration on the Efficacy of Administration of Prophylactic Doses of Enoxaparin: A Prospective Observational Study. Pharmaceuticals 2023, 16, 1166. https://doi.org/10.3390/ph16081166
Aszkiełowicz A, Steckiewicz KP, Okrągły M, Wujtewicz MA, Owczuk R. The Impact of Continuous Veno-Venous Hemodiafiltration on the Efficacy of Administration of Prophylactic Doses of Enoxaparin: A Prospective Observational Study. Pharmaceuticals. 2023; 16(8):1166. https://doi.org/10.3390/ph16081166
Chicago/Turabian StyleAszkiełowicz, Aleksander, Karol P. Steckiewicz, Michał Okrągły, Magdalena A. Wujtewicz, and Radosław Owczuk. 2023. "The Impact of Continuous Veno-Venous Hemodiafiltration on the Efficacy of Administration of Prophylactic Doses of Enoxaparin: A Prospective Observational Study" Pharmaceuticals 16, no. 8: 1166. https://doi.org/10.3390/ph16081166
APA StyleAszkiełowicz, A., Steckiewicz, K. P., Okrągły, M., Wujtewicz, M. A., & Owczuk, R. (2023). The Impact of Continuous Veno-Venous Hemodiafiltration on the Efficacy of Administration of Prophylactic Doses of Enoxaparin: A Prospective Observational Study. Pharmaceuticals, 16(8), 1166. https://doi.org/10.3390/ph16081166