Protective Role of Vitamin K3 on SARS-CoV-2 Structural Protein-Induced Inflammation and Cell Death
Abstract
1. Introduction
2. Results
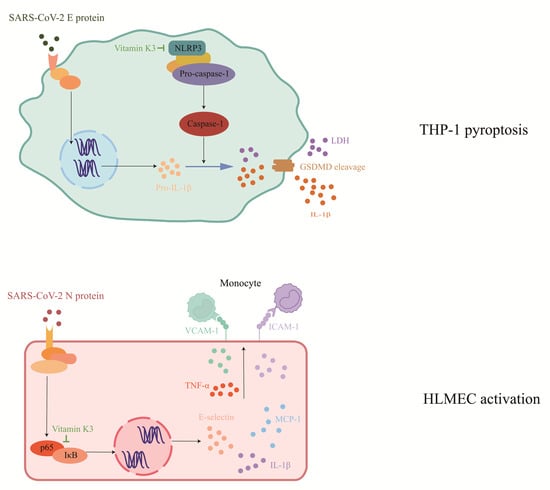
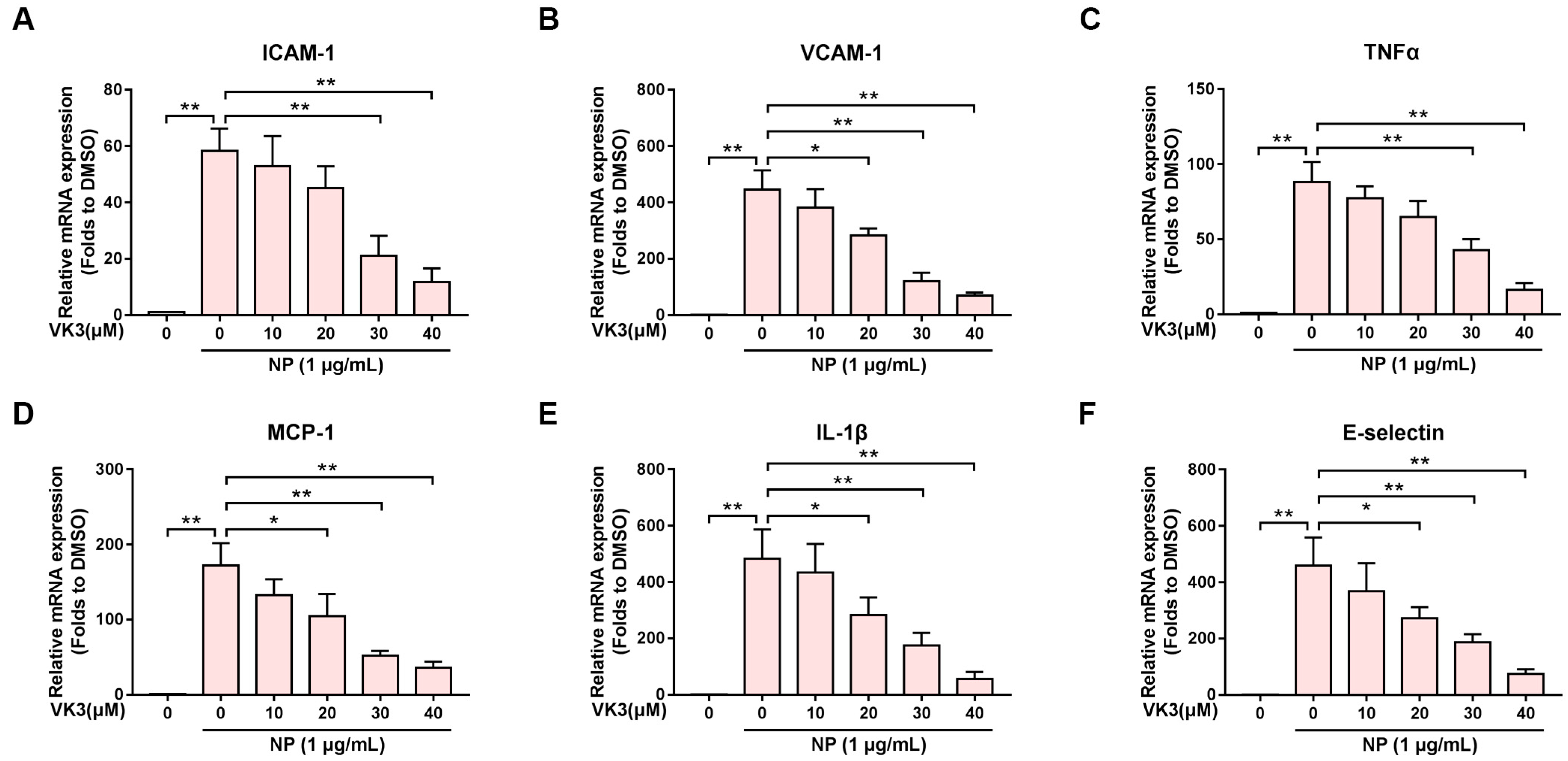
2.1. Vitamin K3 Inhibits SARS-CoV-2 N Protein-Induced Endothelial Cell Activation
2.2. Vitamin K3 Reduced the Expression of Pro-Inflammatory Cytokines after N Protein Exposure
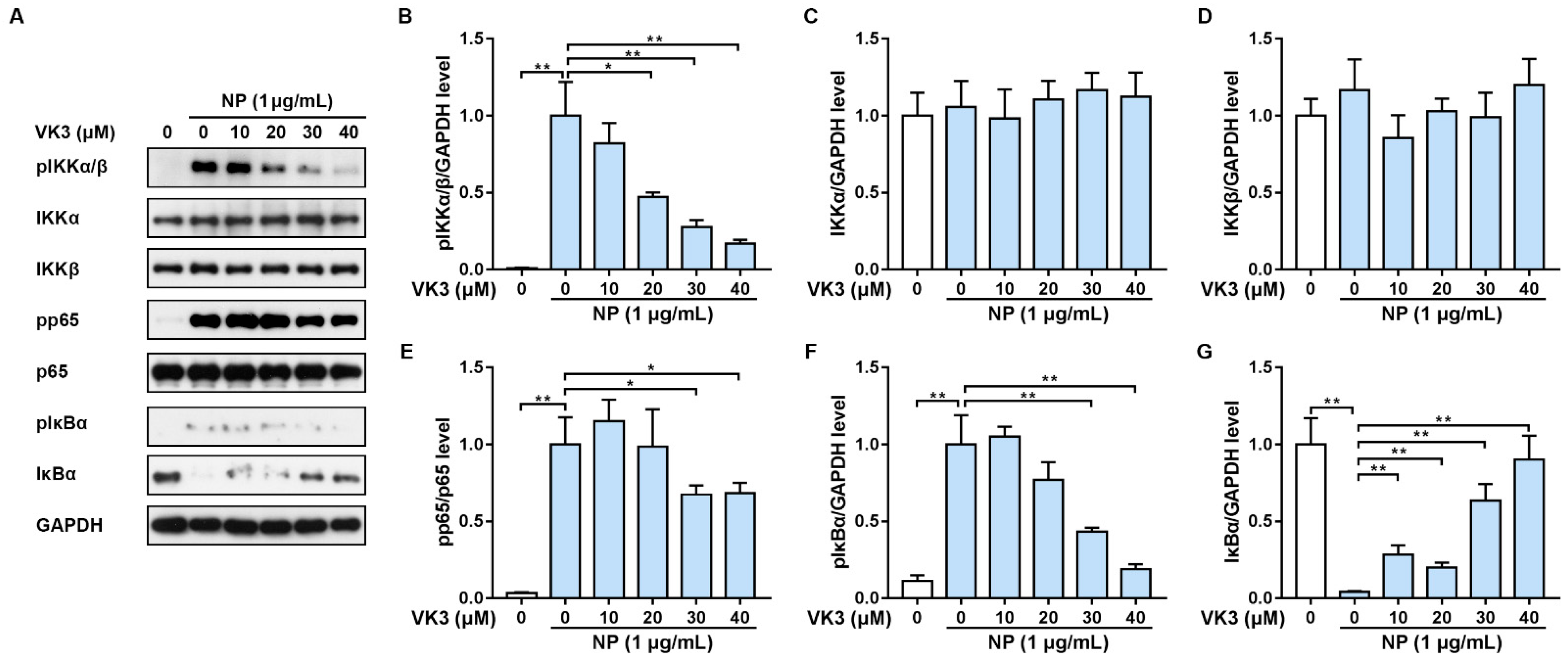
2.3. Vitamin K3 Prevents the Activation of NF-κB Signaling Pathway in HLMECs
2.4. Vitamin K3 Inhibits SARS-CoV-2 E Protein-Induced Pyroptosis in Monocytes
2.5. Vitamin K3 Decreased the Expression of Pro-Inflammatory Cytokines Induced by E Protein in THP-1
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Treatment
4.3. Monocyte Adhesion Assay
4.4. LDH Assay
4.5. MTT Assay
4.6. QPCR
4.7. Western Blot
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Safiabadi Tali, S.H.; LeBlanc, J.J.; Sadiq, Z.; Oyewunmi, O.D.; Camargo, C.; Nikpour, B.; Armanfard, N.; Sagan, S.M.; Jahanshahi-Anbuhi, S. Tools and Techniques for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)/COVID-19 Detection. Clin. Microbiol. Rev. 2021, 34. [Google Scholar] [CrossRef]
- Rahman, S.; Montero, M.T.V.; Rowe, K.; Kirton, R.; Kunik, F., Jr. Epidemiology, pathogenesis, clinical presentations, diagnosis and treatment of COVID-19: A review of current evidence. Expert. Rev. Clin. Pharmacol. 2021, 14, 601–621. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Ratchford, S.M.; Stickford, J.L.; Province, V.M.; Stute, N.; Augenreich, M.A.; Koontz, L.K.; Bobo, L.K.; Stickford, A.S.L. Vascular alterations among young adults with SARS-CoV-2. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H404–H410. [Google Scholar] [CrossRef]
- Chung, M.K.; Zidar, D.A.; Bristow, M.R.; Cameron, S.J.; Chan, T.; Harding, C.V.; Kwon, D.H.; Singh, T.; Tilton, J.C.; Tsai, E.J.; et al. COVID-19 and Cardiovascular Disease: From Bench to Bedside. Circ. Res. 2021, 128, 1214–1236. [Google Scholar] [CrossRef]
- Birnhuber, A.; Fließer, E.; Gorkiewicz, G.; Zacharias, M.; Seeliger, B.; David, S.; Welte, T.; Schmidt, J.; Olschewski, H.; Wygrecka, M.; et al. Between inflammation and thrombosis: Endothelial cells in COVID-19. Eur. Respir. J. 2021, 58, 2100377. [Google Scholar] [CrossRef]
- Pons, S.; Fodil, S.; Azoulay, E.; Zafrani, L. The vascular endothelium: The cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit. Care 2020, 24, 353. [Google Scholar] [CrossRef]
- Kruse, R.L. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Research 2020, 9, 72. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, L.; Cai, S.; Zhuang, Z.; Zhao, Z.; Jin, S.; Xie, W.; Zhou, L.; Zhang, L.; Zhao, J.; et al. RNA-induced liquid phase separation of SARS-CoV-2 nucleocapsid protein facilitates NF-κB hyper-activation and inflammation. Signal Transduct. Target. Ther. 2021, 6, 167. [Google Scholar] [CrossRef]
- Luo, L.; Li, Z.; Zhao, T.; Ju, X.; Ma, P.; Jin, B.; Zhou, Y.; He, S.; Huang, J.; Xu, X.; et al. SARS-CoV-2 nucleocapsid protein phase separates with G3BPs to disassemble stress granules and facilitate viral production. Sci Bull. 2021, 66, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- DeMarino, C.; Lee, M.-H.; Cowen, M.; Steiner, J.P.; Inati, S.; Shah, A.H.; Zaghloul, K.A.; Nath, A. Detection of SARS-CoV-2 Nucleocapsid and Microvascular Disease in the Brain: A Case Report. Neurology 2023, 100, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Lei, T.; Patel, P.S.; Lee, C.H.; Monaghan-Nichols, P.; Xin, H.-B.; Qiu, J.; Fu, M. Direct Activation of Endothelial Cells by SARS-CoV-2 Nucleocapsid Protein Is Blocked by Simvastatin. J. Virol. 2021, 95, e0139621. [Google Scholar] [CrossRef] [PubMed]
- Yalcinkaya, M.; Liu, W.; Islam, M.N.; Kotini, A.G.; Gusarova, G.A.; Fidler, T.P.; Papapetrou, E.P.; Bhattacharya, J.; Wang, N.; Tall, A.R. Modulation of the NLRP3 inflammasome by SARS-CoV-2 Envelope protein. Sci. Rep. 2021, 11, 24432. [Google Scholar] [CrossRef]
- Xia, B.; Shen, X.; He, Y.; Pan, X.; Liu, F.-L.; Wang, Y.; Yang, F.; Fang, S.; Wu, Y.; Duan, Z.; et al. SARS-CoV-2 envelope protein causes acute respiratory distress syndrome (ARDS)-like pathological damages and constitutes an antiviral target. Cell Res. 2021, 31, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Declercq, J.; De Leeuw, E.; Lambrecht, B.N. Inflammasomes and IL-1 family cytokines in SARS-CoV-2 infection: From prognostic marker to therapeutic agent. Cytokine 2022, 157, 155934. [Google Scholar] [CrossRef]
- Vora, S.M.; Lieberman, J.; Wu, H. Inflammasome activation at the crux of severe COVID-19. Nat. Reviews. Immunol. 2021, 21, 694–703. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; de Sá, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Gonçalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef]
- Huang, H.; Li, X.; Zha, D.; Lin, H.; Yang, L.; Wang, Y.; Xu, L.; Wang, L.; Lei, T.; Zhou, Z.; et al. SARS-CoV-2 E protein-induced THP-1 pyroptosis is reversed by Ruscogenin. Biochem. Cell Biol. 2023. [Google Scholar] [CrossRef]
- Lees, J.S.; Mark, P.B.; Witham, M.D. Vitamin K and vascular calcification. Curr. Opin. Nephrol. Hypertens. 2021, 30, 430–436. [Google Scholar] [CrossRef]
- Goddek, S. Vitamin D3 and K2 and their potential contribution to reducing the COVID-19 mortality rate. Int. J. Infect. Dis. 2020, 99, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.; Visser, M.P.J.; Dofferhoff, A.S.M.; Vermeer, C.; Janssens, W.; Walk, J. Vitamin K metabolism as the potential missing link between lung damage and thromboembolism in Coronavirus disease 2019. Br. J. Nutr. 2021, 126, 191–198. [Google Scholar] [CrossRef]
- Wang, R.; Hu, Q.; Wang, H.; Zhu, G.; Wang, M.; Zhang, Q.; Zhao, Y.; Li, C.; Zhang, Y.; Ge, G.; et al. Identification of Vitamin K3 and its analogues as covalent inhibitors of SARS-CoV-2 3CL(pro). Int. J. Biol. Macromol. 2021, 183, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhou, Z.; Wu, X.; Wang, Y.; Zuo, L.; Zheng, R.; Liu, Y.; Liu, Z.; Lai, X.; Zhou, L.; et al. Vitamin K3 Suppresses Pyroptosis in THP-1 Cells through Inhibition of NF-κB and JNK Signaling Pathways. Biol. Pharm. Bull. 2023, 46, 52–60. [Google Scholar] [CrossRef]
- Zhong, L.; Simard, M.J.; Huot, J. Endothelial microRNAs regulating the NF-κB pathway and cell adhesion molecules during inflammation. FASEB J. 2018, 32, 4070–4084. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Luscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020, 41, 3038–3044. [Google Scholar] [CrossRef]
- Marles, R.J.; Roe, A.L.; Oketch-Rabah, H.A. US Pharmacopeial Convention safety evaluation of menaquinone-7, a form of vitamin K. Nutr. Rev. 2017, 75, 553–578. [Google Scholar] [CrossRef]
- Mladěnka, P.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Mrštná, K.; Carazo, A.; Protti, M.; Remião, F.; Nováková, L. Vitamin K—Sources, physiological role, kinetics, deficiency, detection, therapeutic use, and toxicity. Nutr. Rev. 2022, 80, 677–698. [Google Scholar] [CrossRef]
- Wellington, K.W.; Hlatshwayo, V.; Kolesnikova, N.I.; Saha, S.T.; Kaur, M.; Motadi, L.R. Anticancer activities of vitamin K3 analogues. Investig. New Drugs 2020, 38, 378–391. [Google Scholar] [CrossRef]
- Kiely, A.; Ferland, G.; Ouliass, B.; O’Toole, P.W.; Purtill, H.; O’Connor, E.M. Vitamin K status and inflammation are associated with cognition in older Irish adults. Nutr. Neurosci. 2020, 23, 591–599. [Google Scholar] [CrossRef]
- Zheng, X.; Hou, Y.; He, H.; Chen, Y.; Zhou, R.; Wang, X.; Gong, T.; Jiang, W. Synthetic vitamin K analogs inhibit inflammation by targeting the NLRP3 inflammasome. Cell. Mol. Immunol. 2021, 18, 2422–2430. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.P.J.; Walk, J.; Vermeer, C.; Bílková, S.; Janssen, R.; Mayer, O. Enhanced vitamin K expenditure as a major contributor to vitamin K deficiency in COVID-19. Int. J. Infect. Dis. 2022, 125, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Linneberg, A.; Kampmann, F.B.; Israelsen, S.B.; Andersen, L.R.; Jørgensen, H.L.; Sandholt, H.; Jørgensen, N.R.; Thysen, S.M.; Benfield, T. The Association of Low Vitamin K Status with Mortality in a Cohort of 138 Hospitalized Patients with COVID-19. Nutrients 2021, 13, 1985. [Google Scholar] [CrossRef]
- Bai, Z.; Cao, Y.; Liu, W.; Li, J. The SARS-CoV-2 Nucleocapsid Protein and Its Role in Viral Structure, Biological Functions, and a Potential Target for Drug or Vaccine Mitigation. Viruses 2021, 13, 1115. [Google Scholar] [CrossRef]
- Hsu, S.K.; Li, C.Y.; Lin, I.L.; Syue, W.J.; Chen, Y.F.; Cheng, K.C.; Teng, Y.N.; Lin, Y.H.; Yen, C.H.; Chiu, C.C. Inflammation-related pyroptosis, a novel programmed cell death pathway, and its crosstalk with immune therapy in cancer treatment. Theranostics 2021, 11, 8813–8835. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, Y.; Shen, W.; Oladejo, A.O.; Yang, J.; Jiang, W.; Imam, B.H.; Wu, X.; Ding, X.; Yang, Y.; et al. LPS Mediates Bovine Endometrial Epithelial Cell Pyroptosis Directly Through Both NLRP3 Classical and Non-Classical Inflammasome Pathways. Front. Immunol. 2021, 12, 676088. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, J.; Shao, F. Inflammatory Caspases: Activation and Cleavage of Gasdermin-D In Vitro and During Pyroptosis. Methods Mol. Biol. 2018, 1714, 131–148. [Google Scholar] [CrossRef]
- Yap, J.K.Y.; Moriyama, M.; Iwasaki, A. Inflammasomes and Pyroptosis as Therapeutic Targets for COVID-19. J. Immunol. 2020, 205, 307–312. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gan, L.; Xu, Y.; Luo, D.; Ren, Q.; Wu, S.; Sun, C. Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-κB/GSDMD signal in mice adipose tissue. J. Pineal Res. 2017, 63, e12414. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Yi, T.; Chen, C. NF-κB-Gasdermin D (GSDMD) Axis Couples Oxidative Stress and NACHT, LRR and PYD Domains-Containing Protein 3 (NLRP3) Inflammasome-Mediated Cardiomyocyte Pyroptosis Following Myocardial Infarction. Med. Sci. Monit. 2018, 24, 6044–6052. [Google Scholar] [CrossRef]
- Miao, J.; Chard, L.S.; Wang, Z.; Wang, Y. Syrian Hamster as an Animal Model for the Study on Infectious Diseases. Front. Immunol. 2019, 10, 2329. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward Primer (5′→ 3′) | Reverse primer (5′ → 3′) |
|---|---|---|
| Human ICAM-1 | AGCTTCGTGTCCTGTATGGC | TTTTCTGGCCACGTCCAGTT |
| Human VCAM-1 | TGTTTGCAGCTTCTCAAGCTTTT | GATGTGGTCCCCTCATTCGT |
| Human TNF-α | TCTCGCACCCCGAGTGA | GGAGCTGCCCCTCAGCTT |
| Human MCP1 | CAGCCAGATGCAATCAATGCC | TGGAATCCTGAACCCACTTCT |
| Human IL-1α | AGATGCCTGAGATACCCAAAACC | CCAAGCACACCCAGTAGTCT |
| Human IL-1β | CCACAGACCTTCCAGGAGAATG | ATCCCATGTGTCGAAGAAGATAGG |
| Human IL-6 | CCTGAACCTTCCAAAGATGGC | TTCACCAGGCAAGTCTCCTCA |
| Human IL-18 | GCATCAACTTTGTGGCAAT | CCGATTTCCTTGGTCAAT |
| Human NLRP3 | CGTGAGTCCCATTAAGATGGAGT | CCCGACAGTGGATATAGAACAGA |
| Human E-Selectin | GGCAGTTCCGGGAAAGATCA | GTGGGAGCTTCACAGGTAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, Y.; Zha, D.; Lin, H.; Mao, X.; Yang, L.; Huang, H.; He, Z.; Zhou, S.; Xu, F.; Qian, Y.; et al. Protective Role of Vitamin K3 on SARS-CoV-2 Structural Protein-Induced Inflammation and Cell Death. Pharmaceuticals 2023, 16, 1101. https://doi.org/10.3390/ph16081101
Zhan Y, Zha D, Lin H, Mao X, Yang L, Huang H, He Z, Zhou S, Xu F, Qian Y, et al. Protective Role of Vitamin K3 on SARS-CoV-2 Structural Protein-Induced Inflammation and Cell Death. Pharmaceuticals. 2023; 16(8):1101. https://doi.org/10.3390/ph16081101
Chicago/Turabian StyleZhan, Yixiong, Duoduo Zha, Hongru Lin, Xianxian Mao, Lingyi Yang, Houda Huang, Zongnan He, Sheng Zhou, Fei Xu, Yisong Qian, and et al. 2023. "Protective Role of Vitamin K3 on SARS-CoV-2 Structural Protein-Induced Inflammation and Cell Death" Pharmaceuticals 16, no. 8: 1101. https://doi.org/10.3390/ph16081101
APA StyleZhan, Y., Zha, D., Lin, H., Mao, X., Yang, L., Huang, H., He, Z., Zhou, S., Xu, F., Qian, Y., & Liu, Y. (2023). Protective Role of Vitamin K3 on SARS-CoV-2 Structural Protein-Induced Inflammation and Cell Death. Pharmaceuticals, 16(8), 1101. https://doi.org/10.3390/ph16081101