Pharmacological and Electroceutical Targeting of the Cholinergic Anti-Inflammatory Pathway in Autoimmune Diseases
Abstract
1. Introduction
2. Interfaces between Immune Cells and Nerve Fibers
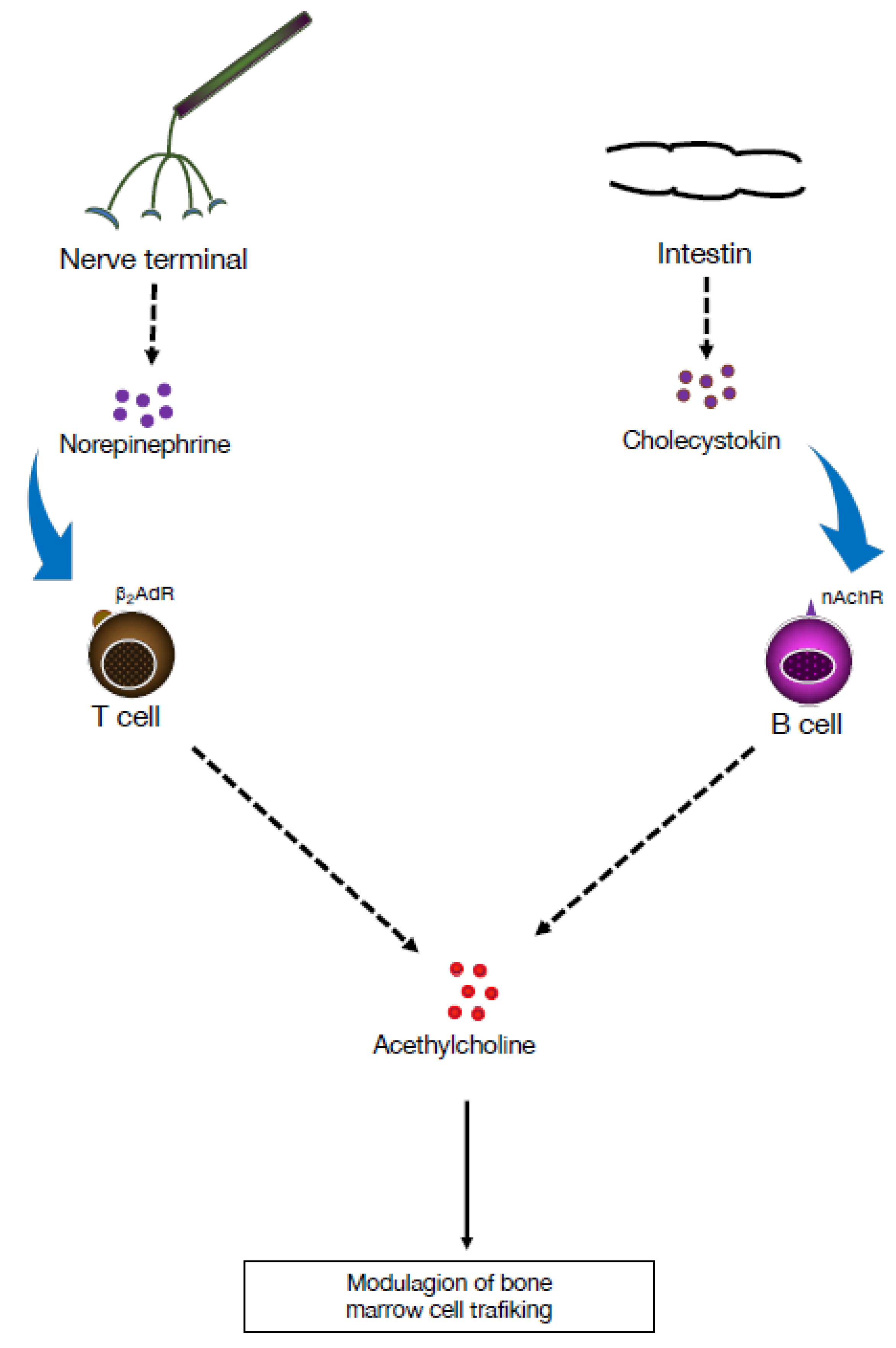
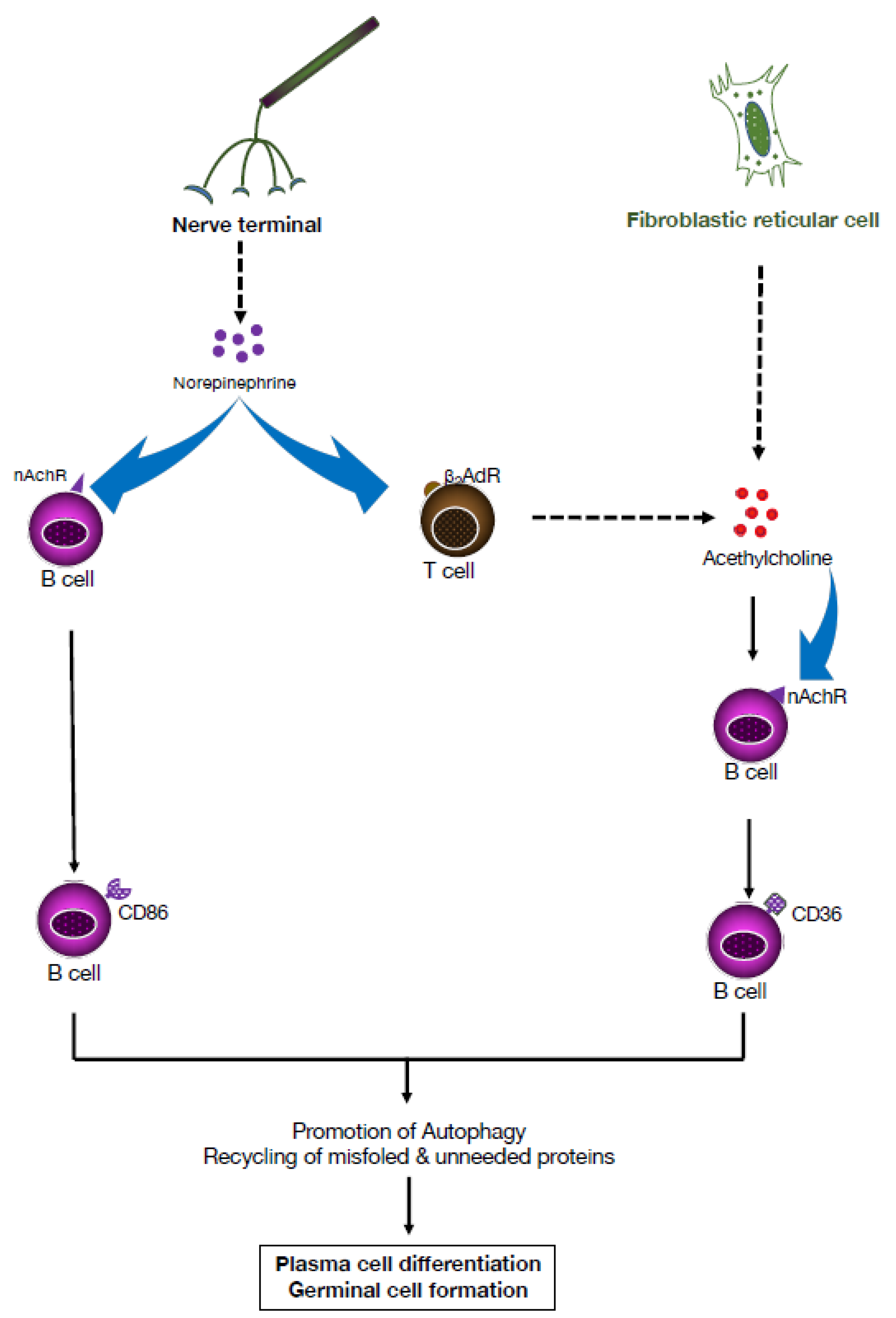
3. Cholinergic Functions of B Lymphocytes in Controlling Hematopoiesis
4. Adrenergic Modulation of the Adaptive Immune Response
5. Dominance of the Autonomic Sympathetic Nervous System in Autoimmune Diseases
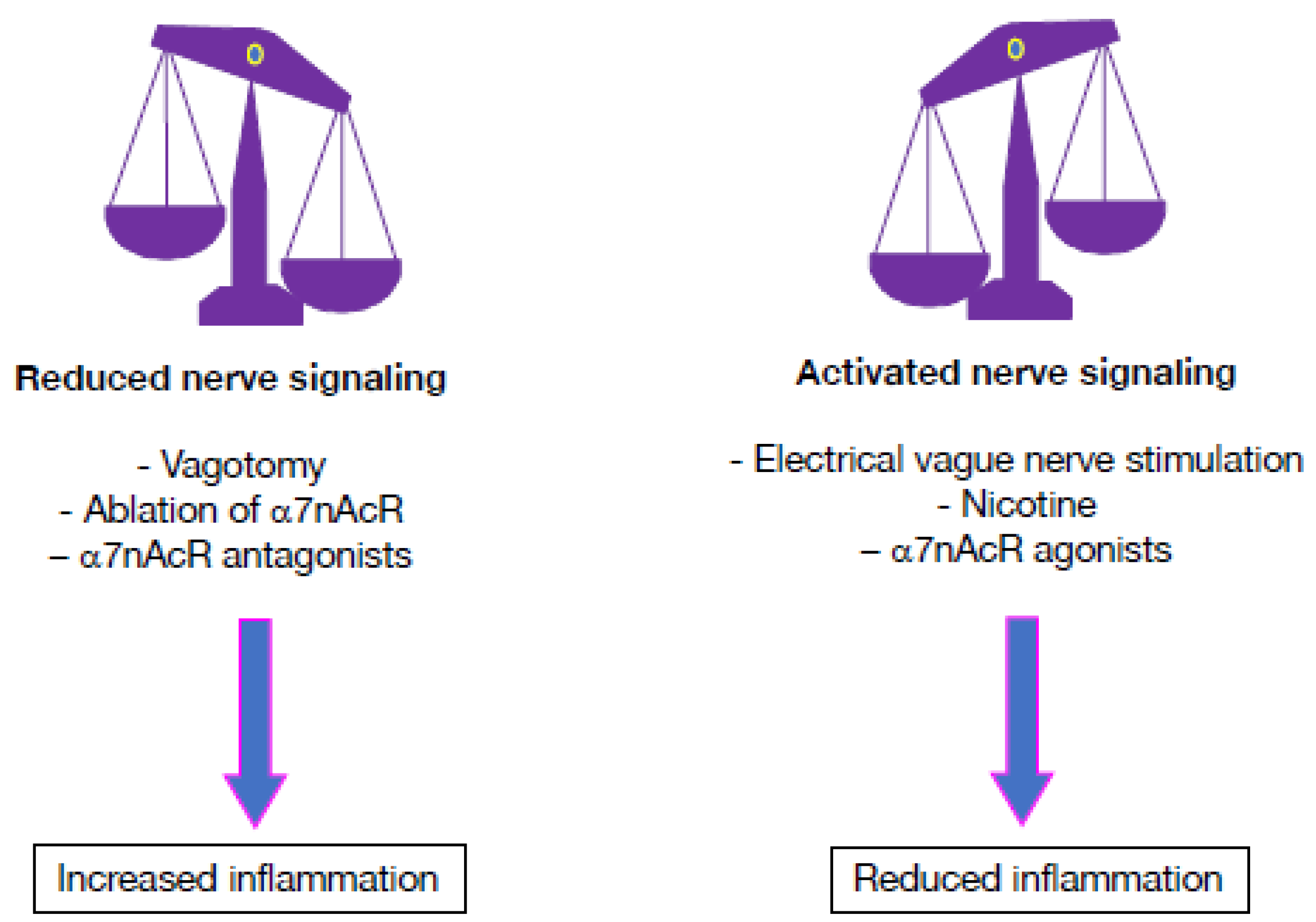
6. Targeting the Cholinergic Anti-Inflammatory Pathway Using Pharmacological Approaches
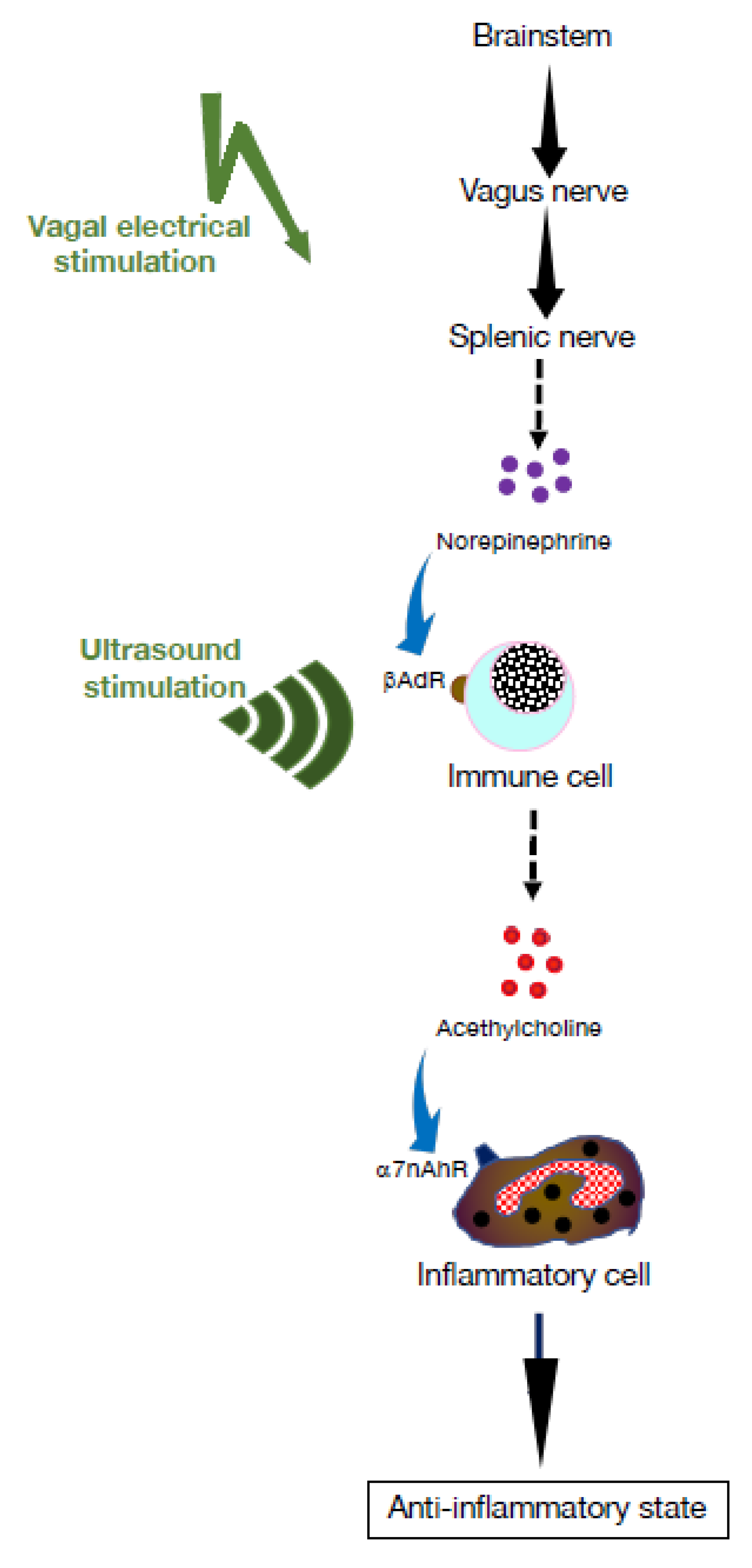
7. Dampening Inflammatory Processes Using Electrical Nervous Stimulation
8. Electroceutical Therapy for Autoimmune Disease
8.1. Rheumatoid Arthritis
8.2. Lupus
8.3. Scleroderma
8.4. Diabetes
8.5. Inflammatory Bowel Diseases
8.6. Primary Sjögren’s Syndrome
8.7. Postural Orthostatic Tachycardia Syndrome (POTS)
9. Future Prospects
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bai:, A.; Guo, Y.; Lu, N. The effect of the cholinergic anti-inflammatory pathway on experimental colitis. Scand. J Immunol. 2007, 66, 538–545. [Google Scholar] [CrossRef]
- Andersson, U.; Tracey, K.J. Reflex principles of immunological homeostasis. Annu. Rev. Immunol. 2012, 30, 313–335. [Google Scholar] [CrossRef]
- Korin, B.; Ben-Shaanan, T.L.; Schiller, M.; Dubovik, T.; Azulay-Debby, H.; Boshnak, N.T.; Koren, T.; Rolls, A. High-dimensional, single-cell characterization of the brain’s immune compartment. Nat. Neurosci. 2017, 20, 1300–1309. [Google Scholar] [CrossRef]
- Silverman, H.A.; Stiegler, A.; Tsaava, T.; Newman, J.; Steinberg, B.E.; Masi, E.B.; Robbiati, S.; Bouton, C.; Huerta, P.T.; Chavan, S.S.; et al. Standardization of methods to record Vagus nerve activity in mice. Bioelectron. Med. 2018, 4, 3. [Google Scholar] [CrossRef]
- Goehler, L.E.; Gaykema, R.P.; Hansen, M.K.; Anderson, K.; Maier, S.F.; Watkins, L.R. Vagal immune-to-brain communication: A visceral chemosensory pathway. Auton. Neurosci. 2000, 85, 49–59. [Google Scholar] [CrossRef]
- Sanders, V.M.; Straub, R.H. Norepinephrine, the beta-adrenergic receptor, and immunity. Brain Behav. Immun. 2002, 16, 290–332. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef]
- Rosas-Ballina, M.; Olofsson, P.S.; Ochani, M.; Valdes-Ferrer, S.I.; Levine, Y.A.; Reardon, C.; Tusche, M.W.; Pavlov, V.A.; Andersson, U.; Chavan, S.; et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011, 334, 98–101. [Google Scholar] [CrossRef]
- Olofsson, P.S.; Tracey, K.J. Bioelectronic medicine: Technology targeting molecular mechanisms for therapy. J. Intern. Med. 2017, 282, 3–4. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Nizri, E.; Irony-Tur-Sinai, M.; Lory, O.; Orr-Urtreger, A.; Lavi, E.; Brenner, T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J. Immunol. 2009, 183, 6681–6688. [Google Scholar] [CrossRef] [PubMed]
- Kox, M.; van Eijk, L.T.; Zwaag, J.; van den Wildenberg, J.; Sweep, F.C.; van der Hoeven, J.G.; Pickkers, P. Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 7379–7384. [Google Scholar] [CrossRef]
- Wang, H.; Liao, H.; Ochani, M.; Justiniani, M.; Lin, X.; Yang, L.; Al-Abed, Y.; Wang, H.; Metz, C.; Miller, E.J.; et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 2004, 10, 1216–1221. [Google Scholar] [CrossRef]
- Rosas-Ballina, M.; Ochani, M.; Parrish, W.R.; Ochani, K.; Harris, Y.T.; Huston, J.M.; Chavan, S.; Tracey, K.J. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl. Acad. Sci. USA 2008, 105, 11008–11013. [Google Scholar] [CrossRef]
- Baker, M.C.; Kavanagh, S.; Cohen, S.; Matsumoto, A.K.; Dikranian, A.; Tesser, J.; Kivitz, A.; Alataris, K.; Genovese, M.C. A Randomized, Double-Blind, Sham-Controlled, Clinical Trial of Auricular Vagus Nerve Stimulation for the Treatment of Active Rheumatoid Arthritis. Arthritis Rheumatol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Aranow, C.; Atish-Fregoso, Y.; Lesser, M.; Mackay, M.; Anderson, E.; Chavan, S.; Zanos, T.P.; Datta-Chaudhuri, T.; Bouton, C.; Tracey, K.J.; et al. Transcutaneous auricular vagus nerve stimulation reduces pain and fatigue in patients with systemic lupus erythematosus: A randomised, double-blind, sham-controlled pilot trial. Ann. Rheum. Dis. 2021, 80, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.L.; Wilson, C.G. A review of vagus nerve stimulation as a therapeutic intervention. J. Inflamm. Res. 2018, 11, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Ramkissoon, C.M.; Guemes, A.; Vehi, J. Overview of therapeutic applications of non-invasive vagus nerve stimulation: A motivation for novel treatments for systemic lupus erythematosus. Bioelectron. Med. 2021, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Fujii, T. Extraneuronal cholinergic system in lymphocytes. Pharmacol. Ther. 2000, 86, 29–48. [Google Scholar] [CrossRef]
- Kohm, A.P.; Mozaffarian, A.; Sanders, V.M. B cell receptor- and beta 2-adrenergic receptor-induced regulation of B7-2 (CD86) expression in B cells. J. Immunol. 2002, 168, 6314–6322. [Google Scholar] [CrossRef]
- Dimitrov, S.; Lange, T.; Born, J. Selective mobilization of cytotoxic leukocytes by epinephrine. J. Immunol. 2010, 184, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.A.; Chavan, S.S.; Tracey, K.J. Bioelectronic Medicine: From Preclinical Studies on the Inflammatory Reflex to New Approaches in Disease Diagnosis and Treatment. Cold Spring Harb. Perspect. Med. 2020, 10, a034140. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Battista, M.; Kao, W.M.; Hidalgo, A.; Peired, A.J.; Thomas, S.A.; Frenette, P.S. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006, 124, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Expression and Function of the Cholinergic System in Immune Cells. Front. Immunol. 2017, 8, 1085. [Google Scholar] [CrossRef] [PubMed]
- Reardon, C.; Duncan, G.S.; Brustle, A.; Brenner, D.; Tusche, M.W.; Olofsson, P.S.; Rosas-Ballina, M.; Tracey, K.J.; Mak, T.W. Lymphocyte-derived ACh regulates local innate but not adaptive immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 1410–1415. [Google Scholar] [CrossRef]
- Fielding, C.; Garcia-Garcia, A.; Korn, C.; Gadomski, S.; Fang, Z.; Reguera, J.L.; Perez-Simon, J.A.; Gottgens, B.; Mendez-Ferrer, S. Cholinergic signals preserve haematopoietic stem cell quiescence during regenerative haematopoiesis. Nat. Commun. 2022, 13, 543. [Google Scholar] [CrossRef]
- van der Zanden, E.P.; Snoek, S.A.; Heinsbroek, S.E.; Stanisor, O.I.; Verseijden, C.; Boeckxstaens, G.E.; Peppelenbosch, M.P.; Greaves, D.R.; Gordon, S.; De Jonge, W.J. Vagus nerve activity augments intestinal macrophage phagocytosis via nicotinic acetylcholine receptor alpha4beta2. Gastroenterology 2009, 137, 1029–1039.e4. [Google Scholar] [CrossRef] [PubMed]
- Mina-Osorio, P.; Rosas-Ballina, M.; Valdes-Ferrer, S.I.; Al-Abed, Y.; Tracey, K.J.; Diamond, B. Neural signaling in the spleen controls B-cell responses to blood-borne antigen. Mol. Med. 2012, 18, 618–627. [Google Scholar] [CrossRef]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Physiological functions of the cholinergic system in immune cells. J. Pharmacol. Sci. 2017, 134, 1–21. [Google Scholar] [CrossRef]
- Schloss, M.J.; Hulsmans, M.; Rohde, D.; Lee, I.H.; Severe, N.; Foy, B.H.; Pulous, F.E.; Zhang, S.; Kokkaliaris, K.D.; Frodermann, V.; et al. B lymphocyte-derived acetylcholine limits steady-state and emergency hematopoiesis. Nat. Immunol. 2022, 23, 605–618. [Google Scholar] [CrossRef]
- Zouali, M. B cells at the cross-roads of autoimmune diseases and auto-inflammatory syndromes. Cells 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lei, B.; Yuan, Y.; Zhang, L.; Hu, L.; Jin, S.; Kang, B.; Liao, X.; Sun, W.; Xu, F.; et al. Brain control of humoral immune responses amenable to behavioural modulation. Nature 2020, 581, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Wang, S.; Li, M.; Wang, S.; Guo, C.; Ruan, X.; Watanabe, R.; Lai, Y.; Huang, Y.; Yin, X.; et al. Spleen fibroblastic reticular cell-derived acetylcholine promotes lipid metabolism to drive autoreactive B cell responses. Cell Metab. 2023, 35, 837–854.e838. [Google Scholar] [CrossRef]
- He, C.; Wang, S.; Zhou, C.; He, M.; Wang, J.; Ladds, M.; Lianoudaki, D.; Sedimbi, S.K.; Lane, D.P.; Westerberg, L.S.; et al. CD36 and LC3B initiated autophagy in B cells regulates the humoral immune response. Autophagy 2021, 17, 3577–3591. [Google Scholar] [CrossRef] [PubMed]
- Lenschow, D.J.; Walunas, T.L.; Bluestone, J.A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 1996, 14, 233–258. [Google Scholar] [CrossRef] [PubMed]
- Pikov, V. Bioelectronic medicine for restoring autonomic balance in autoimmune diseases. Gut Microbiota. Integr. Wellness 2023, 1, 182. [Google Scholar] [CrossRef]
- Lau, P.P.; Li, L.; Merched, A.J.; Zhang, A.L.; Ko, K.W.; Chan, L. Nicotine induces proinflammatory responses in macrophages and the aorta leading to acceleration of atherosclerosis in low-density lipoprotein receptor(-/-) mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 143–149. [Google Scholar] [CrossRef]
- Jin, H.J.; Li, H.T.; Sui, H.X.; Xue, M.Q.; Wang, Y.N.; Wang, J.X.; Gao, F.G. Nicotine stimulated bone marrow-derived dendritic cells could augment HBV specific CTL priming by activating PI3K-Akt pathway. Immunol. Lett. 2012, 146, 40–49. [Google Scholar] [CrossRef]
- van Maanen, M.A.; Stoof, S.P.; Larosa, G.J.; Vervoordeldonk, M.J.; Tak, P.P. Role of the cholinergic nervous system in rheumatoid arthritis: Aggravation of arthritis in nicotinic acetylcholine receptor alpha7 subunit gene knockout mice. Ann. Rheum. Dis. 2010, 69, 1717–1723. [Google Scholar] [CrossRef]
- Koopman, F.A.; Chavan, S.S.; Miljko, S.; Grazio, S.; Sokolovic, S.; Schuurman, P.R.; Mehta, A.D.; Levine, Y.A.; Faltys, M.; Zitnik, R.; et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2016, 113, 8284–8289. [Google Scholar] [CrossRef]
- Hoover, D.B. Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacol. Ther. 2017, 179, 1–16. [Google Scholar] [CrossRef] [PubMed]
- van Maanen, M.A.; Lebre, M.C.; van der Poll, T.; LaRosa, G.J.; Elbaum, D.; Vervoordeldonk, M.J.; Tak, P.P. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 2009, 60, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Ballina, M.; Goldstein, R.S.; Gallowitsch-Puerta, M.; Yang, L.; Valdes-Ferrer, S.I.; Patel, N.B.; Chavan, S.; Al-Abed, Y.; Yang, H.; Tracey, K.J. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol. Med. 2009, 15, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Kwan, K.; Levine, Y.A.; Olofsson, P.S.; Yang, H.; Li, J.; Joshi, S.; Wang, H.; Andersson, U.; Chavan, S.S.; et al. alpha7 nicotinic acetylcholine receptor signaling inhibits inflammasome activation by preventing mitochondrial DNA release. Mol. Med. 2014, 20, 350–358. [Google Scholar] [CrossRef]
- Waltz, E. A spark at the periphery. Nat. Biotechnol. 2016, 34, 904–908. [Google Scholar] [CrossRef]
- Payne, S.C.; Furness, J.B.; Stebbing, M.J. Bioelectric neuromodulation for gastrointestinal disorders: Effectiveness and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 89–105. [Google Scholar] [CrossRef]
- Huston, J.M.; Ochani, M.; Rosas-Ballina, M.; Liao, H.; Ochani, K.; Pavlov, V.A.; Gallowitsch-Puerta, M.; Ashok, M.; Czura, C.J.; Foxwell, B.; et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 2006, 203, 1623–1628. [Google Scholar] [CrossRef]
- Meregnani, J.; Clarencon, D.; Vivier, M.; Peinnequin, A.; Mouret, C.; Sinniger, V.; Picq, C.; Job, A.; Canini, F.; Jacquier-Sarlin, M.; et al. Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton. Neurosci. 2011, 160, 82–89. [Google Scholar] [CrossRef]
- Bonaz, B.; Sinniger, V.; Hoffmann, D.; Clarencon, D.; Mathieu, N.; Dantzer, C.; Vercueil, L.; Picq, C.; Trocme, C.; Faure, P.; et al. Chronic vagus nerve stimulation in Crohn’s disease: A 6-month follow-up pilot study. Neurogastroenterol. Motil. 2016, 28, 948–953. [Google Scholar] [CrossRef]
- Mughrabi, I.T.; Hickman, J.; Jayaprakash, N.; Thompson, D.; Ahmed, U.; Papadoyannis, E.S.; Chang, Y.C.; Abbas, A.; Datta-Chaudhuri, T.; Chang, E.H.; et al. Development and characterization of a chronic implant mouse model for vagus nerve stimulation. eLife 2021, 10, e61270. [Google Scholar] [CrossRef]
- De Ferrari, G.M.; Schwartz, P.J. Vagus nerve stimulation: From pre-clinical to clinical application: Challenges and future directions. Heart Fail. Rev. 2011, 16, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Cotero, V.; Fan, Y.; Tsaava, T.; Kressel, A.M.; Hancu, I.; Fitzgerald, P.; Wallace, K.; Kaanumalle, S.; Graf, J.; Rigby, W.; et al. Noninvasive sub-organ ultrasound stimulation for targeted neuromodulation. Nat. Commun. 2019, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, T.M.; Burbach, B.J.; Shimizu, Y.; Ervasti, J.M. beta-Actin specifically controls cell growth, migration, and the G-actin pool. Mol. Biol. Cell. 2011, 22, 4047–4058. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.J.; Haqshenas, S.R.; Rothwell, J.; Saffari, N. Unmyelinated Peripheral Nerves Can Be Stimulated in Vitro Using Pulsed Ultrasound. Ultrasound. Med. Biol. 2017, 43, 2269–2283. [Google Scholar] [CrossRef] [PubMed]
- Downs, M.E.; Lee, S.A.; Yang, G.; Kim, S.; Wang, Q.; Konofagou, E.E. Non-invasive peripheral nerve stimulation via focused ultrasound in vivo. Phys. Med. Biol. 2018, 63, 035011. [Google Scholar] [CrossRef]
- Viau, M.; Zouali, M. B-lymphocytes, innate immunity, and autoimmunity. Clin. Immunol. 2005, 114, 17–26. [Google Scholar] [CrossRef]
- Goldstein, R.S.; Bruchfeld, A.; Yang, L.; Qureshi, A.R.; Gallowitsch-Puerta, M.; Patel, N.B.; Huston, B.J.; Chavan, S.; Rosas-Ballina, M.; Gregersen, P.K.; et al. Cholinergic anti-inflammatory pathway activity and High Mobility Group Box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol. Med. 2007, 13, 210–215. [Google Scholar] [CrossRef]
- Waldburger, J.M.; Boyle, D.L.; Pavlov, V.A.; Tracey, K.J.; Firestein, G.S. Acetylcholine regulation of synoviocyte cytokine expression by the alpha7 nicotinic receptor. Arthritis. Rheum. 2008, 58, 3439–3449. [Google Scholar] [CrossRef]
- Ingegnoli, F.; Buoli, M.; Antonucci, F.; Coletto, L.A.; Esposito, C.M.; Caporali, R. The Link Between Autonomic Nervous System and Rheumatoid Arthritis: From Bench to Bedside. Front. Med. 2020, 7, 589079. [Google Scholar] [CrossRef]
- Li, Z.; Hao, H.; Gao, Y.; Wang, Z.; Lu, W.; Liu, J. Expression and localization analyses of the cholinergic anti-inflammatory pathway and alpha7nAchR in different tissues of rats with rheumatoid arthritis. Acta Histochem. 2019, 121, 742–749. [Google Scholar] [CrossRef]
- Levine, Y.A.; Koopman, F.A.; Faltys, M.; Caravaca, A.; Bendele, A.; Zitnik, R.; Vervoordeldonk, M.J.; Tak, P.P. Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PLoS ONE 2014, 9, e104530. [Google Scholar] [CrossRef] [PubMed]
- Bassi, G.S.; Dias, D.P.M.; Franchin, M.; Talbot, J.; Reis, D.G.; Menezes, G.B.; Castania, J.A.; Garcia-Cairasco, N.; Resstel, L.B.M.; Salgado, H.C.; et al. Modulation of experimental arthritis by vagal sensory and central brain stimulation. Brain Behav. Immun. 2017, 64, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Gigliotti, J.C.; Huang, L.; Ye, H.; Bajwa, A.; Chattrabhuti, K.; Lee, S.; Klibanov, A.L.; Kalantari, K.; Rosin, D.L.; Okusa, M.D. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J. Am. Soc. Nephrol. 2013, 24, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Gigliotti, J.C.; Huang, L.; Bajwa, A.; Ye, H.; Mace, E.H.; Hossack, J.A.; Kalantari, K.; Inoue, T.; Rosin, D.L.; Okusa, M.D. Ultrasound Modulates the Splenic Neuroimmune Axis in Attenuating AKI. J. Am. Soc. Nephrol. 2015, 26, 2470–2481. [Google Scholar] [CrossRef]
- Zachs, D.P.; Offutt, S.J.; Graham, R.S.; Kim, Y.; Mueller, J.; Auger, J.L.; Schuldt, N.J.; Kaiser, C.R.W.; Heiller, A.P.; Dutta, R.; et al. Noninvasive ultrasound stimulation of the spleen to treat inflammatory arthritis. Nat. Commun. 2019, 10, 951. [Google Scholar] [CrossRef]
- Ben-Menachem, E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet. Neurol. 2002, 1, 477–482. [Google Scholar] [CrossRef]
- Hoffman, H.H.; Schnitzlein, H.N. The numbers of nerve fibers in the vagus nerve of man. Anat. Rec. 1961, 139, 429–435. [Google Scholar] [CrossRef]
- Bonaz, B.; Picq, C.; Sinniger, V.; Mayol, J.F.; Clarencon, D. Vagus nerve stimulation: From epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol. Motil. 2013, 25, 208–221. [Google Scholar] [CrossRef]
- Drewes, A.M.; Brock, C.; Rasmussen, S.E.; Moller, H.J.; Brock, B.; Deleuran, B.W.; Farmer, A.D.; Pfeiffer-Jensen, M. Short-term transcutaneous non-invasive vagus nerve stimulation may reduce disease activity and pro-inflammatory cytokines in rheumatoid arthritis: Results of a pilot study. Scand. J. Rheumatol. 2021, 50, 20–27. [Google Scholar] [CrossRef]
- Addorisio, M.E.; Imperato, G.H.; de Vos, A.F.; Forti, S.; Goldstein, R.S.; Pavlov, V.A.; van der Poll, T.; Yang, H.; Diamond, B.; Tracey, K.J.; et al. Investigational treatment of rheumatoid arthritis with a vibrotactile device applied to the external ear. Bioelectron. Med. 2019, 5, 4. [Google Scholar] [CrossRef]
- Bellocchi, C.; Carandina, A.; Montinaro, B.; Targetti, E.; Furlan, L.; Rodrigues, G.D.; Tobaldini, E.; Montano, N. The Interplay between Autonomic Nervous System and Inflammation across Systemic Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 2449. [Google Scholar] [CrossRef] [PubMed]
- Stojanovich, L.; Milovanovich, B.; de Luka, S.R.; Popovich-Kuzmanovich, D.; Bisenich, V.; Djukanovich, B.; Randjelovich, T.; Krotin, M. Cardiovascular autonomic dysfunction in systemic lupus, rheumatoid arthritis, primary Sjogren syndrome and other autoimmune diseases. Lupus 2007, 16, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, M.M.; Xue, X.; Duan, B.; Ochani, M.; Tracey, K.J.; Susin, M.; Metz, C.N. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int. 2008, 74, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Dowling, O.; Rochelson, B.; Way, K.; Al-Abed, Y.; Metz, C.N. Nicotine inhibits cytokine production by placenta cells via NFkappaB: Potential role in pregnancy-induced hypertension. Mol. Med. 2007, 13, 576–583. [Google Scholar] [CrossRef]
- Fairley, A.S.; Mathis, K.W. Cholinergic agonists reduce blood pressure in a mouse model of systemic lupus erythematosus. Physiol. Rep. 2017, 5, e13213. [Google Scholar] [CrossRef]
- Minagi, H.O.; Ikai, K.; Araie, T.; Sakai, M.; Sakai, T. Benefits of long-term pilocarpine due to increased muscarinic acetylcholine receptor 3 in salivary glands. Biochem. Biophys. Res. Commun. 2018, 503, 1098–1102. [Google Scholar] [CrossRef]
- Fox, R.I.; Konttinen, Y.; Fisher, A. Use of muscarinic agonists in the treatment of Sjogren’s syndrome. Clin. Immunol. 2001, 101, 249–263. [Google Scholar] [CrossRef]
- Sallam, H.; McNearney, T.A.; Doshi, D.; Chen, J.D. Transcutaneous electrical nerve stimulation (TENS) improves upper GI symptoms and balances the sympathovagal activity in scleroderma patients. Dig. Dis. Sci. 2007, 52, 1329–1337. [Google Scholar] [CrossRef]
- Tarn, J.; Legg, S.; Mitchell, S.; Simon, B.; Ng, W.F. The Effects of Noninvasive Vagus Nerve Stimulation on Fatigue and Immune Responses in Patients with Primary Sjogren’s Syndrome. Neuromodulation 2019, 22, 580–585. [Google Scholar] [CrossRef]
- George, J.A.; Bashir, G.; Qureshi, M.M.; Mohamed, Y.A.; Azzi, J.; Al-Ramadi, B.K.; Fernandez-Cabezudo, M.J. Cholinergic Stimulation Prevents the Development of Autoimmune Diabetes: Evidence for the Modulation of Th17 Effector Cells via an IFNgamma-Dependent Mechanism. Front. Immunol. 2016, 7, 419. [Google Scholar] [CrossRef]
- Guyot, M.; Simon, T.; Ceppo, F.; Panzolini, C.; Guyon, A.; Lavergne, J.; Murris, E.; Daoudlarian, D.; Brusini, R.; Zarif, H.; et al. Pancreatic nerve electrostimulation inhibits recent-onset autoimmune diabetes. Nat. Biotechnol. 2019, 37, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ji, F.; Gharibani, P.; Chen, J.D. Vagal Nerve Stimulation for Glycemic Control in a Rodent Model of Type 2 Diabetes. Obes. Surg. 2019, 29, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Dirr, E.W.; Patel, Y.; Johnson, R.D.; Otto, K.J. The effects of targeted vagus nerve stimulation on glucose homeostasis in STZ-induced diabetic rodents. Front. Neurosci. 2023, 17, 1179276. [Google Scholar] [CrossRef]
- Chow, A.K.; Gulbransen, B.D. Potential roles of enteric glia in bridging neuroimmune communication in the gut. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G145–G152. [Google Scholar] [CrossRef]
- Ha, F.; Khalil, H. Crohn’s disease: A clinical update. Therap. Adv. Gastroenterol. 2015, 8, 352–359. [Google Scholar] [CrossRef]
- Ji, H.; Rabbi, M.F.; Labis, B.; Pavlov, V.A.; Tracey, K.J.; Ghia, J.E. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol. 2014, 7, 335–347. [Google Scholar] [CrossRef]
- Salaga, M.; Blomster, L.V.; Piechota-Polanczyk, A.; Zielinska, M.; Jacenik, D.; Cygankiewicz, A.I.; Krajewska, W.M.; Mikkelsen, J.D.; Fichna, J. Encenicline, an alpha7 Nicotinic Acetylcholine Receptor Partial Agonist, Reduces Immune Cell Infiltration in the Colon and Improves Experimental Colitis in Mice. J. Pharmacol. Exp. Ther. 2016, 356, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Matteoli, G.; Gomez-Pinilla, P.J.; Nemethova, A.; Di Giovangiulio, M.; Cailotto, C.; van Bree, S.H.; Michel, K.; Tracey, K.J.; Schemann, M.; Boesmans, W.; et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 2014, 63, 938–948. [Google Scholar] [CrossRef]
- Kovacs, L.; Papos, M.; Takacs, R.; Roka, R.; Csenke, Z.; Kovacs, A.; Varkonyi, T.; Pajor, L.; Pavics, L.; Pokorny, G. Autonomic nervous system dysfunction involving the gastrointestinal and the urinary tracts in primary Sjogren’s syndrome. Clin. Exp. Rheumatol. 2003, 21, 697–703. [Google Scholar]
- Tarn, J.; Evans, E.; Traianos, E.; Collins, A.; Stylianou, M.; Parikh, J.; Bai, Y.; Guan, Y.; Frith, J.; Lendrem, D.; et al. The Effects of Noninvasive Vagus Nerve Stimulation on Fatigue in Participants with Primary Sjogren’s Syndrome. Neuromodulation 2023, 26, 681–689. [Google Scholar] [CrossRef]
- Blitshteyn, S. Is postural orthostatic tachycardia syndrome (POTS) a central nervous system disorder? J. Neurol. 2022, 269, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Ruzieh, M.; Batizy, L.; Dasa, O.; Oostra, C.; Grubb, B. The role of autoantibodies in the syndromes of orthostatic intolerance: A systematic review. Scand. Cardiovasc. J. 2017, 51, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Gunning, W.T., 3rd; Kvale, H.; Kramer, P.M.; Karabin, B.L.; Grubb, B.P. Postural Orthostatic Tachycardia Syndrome Is Associated with Elevated G-Protein Coupled Receptor Autoantibodies. J. Am. Heart. Assoc. 2019, 8, e013602. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, G.; Zhou, L.; Nuss, Z.; Beel, M.; Hines, B.; Murphy, T.; Liles, J.; Zhang, L.; Kem, D.C.; et al. Adrenergic Autoantibody-Induced Postural Tachycardia Syndrome in Rabbits. J. Am. Heart. Assoc. 2019, 8, e013006. [Google Scholar] [CrossRef] [PubMed]
- Carandina, A.; Rodrigues, G.D.; Di Francesco, P.; Filtz, A.; Bellocchi, C.; Furlan, L.; Carugo, S.; Montano, N.; Tobaldini, E. Effects of transcutaneous auricular vagus nerve stimulation on cardiovascular autonomic control in health and disease. Auton. Neurosci. 2021, 236, 102893. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, H.; Deng, J.; Zhang, G.; Fischer, H.; Stavrakis, S.; Yu, X. Low-level tragus stimulation improves autoantibody-induced hyperadrenergic postural tachycardia syndrome in rabbits. Heart Rhythm. O2 2023, 4, 127–133. [Google Scholar] [CrossRef]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef]
- Jafari, M.; Ahangari, G.; Saberi, M.; Samangoui, S.; Torabi, R.; Zouali, M. Distorted expression of dopamine receptor genes in systemic lupus erythematosus. Immunobiology 2013, 218, 979–983. [Google Scholar] [CrossRef]
- Pacheco, R.; Contreras, F.; Zouali, M. The dopaminergic system in autoimmune diseases. Front. Immunol. 2014, 5, 117. [Google Scholar] [CrossRef]
- Kelly, M.J.; Breathnach, C.; Tracey, K.J.; Donnelly, S.C. Manipulation of the inflammatory reflex as a therapeutic strategy. Cell Rep. Med. 2022, 3, 100696. [Google Scholar] [CrossRef]
- Multon, S.; Schoenen, J. Pain control by vagus nerve stimulation: From animal to man...and back. Acta. Neurol. Belg. 2005, 105, 62–67. [Google Scholar] [PubMed]
- Van Leusden, J.W.; Sellaro, R.; Colzato, L.S. Transcutaneous Vagal Nerve Stimulation (tVNS): A new neuromodulation tool in healthy humans? Front. Psychol. 2015, 6, 102. [Google Scholar] [CrossRef] [PubMed]
- Beekwilder, J.P.; Beems, T. Overview of the clinical applications of vagus nerve stimulation. J. Clin. Neurophysiol. 2010, 27, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-Eteghad, S.; Rasta, S.H.; Hamblin, M.R. Brain Photobiomodulation Therapy: A Narrative Review. Mol. Neurobiol. 2018, 55, 6601–6636. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zouali, M. Pharmacological and Electroceutical Targeting of the Cholinergic Anti-Inflammatory Pathway in Autoimmune Diseases. Pharmaceuticals 2023, 16, 1089. https://doi.org/10.3390/ph16081089
Zouali M. Pharmacological and Electroceutical Targeting of the Cholinergic Anti-Inflammatory Pathway in Autoimmune Diseases. Pharmaceuticals. 2023; 16(8):1089. https://doi.org/10.3390/ph16081089
Chicago/Turabian StyleZouali, Moncef. 2023. "Pharmacological and Electroceutical Targeting of the Cholinergic Anti-Inflammatory Pathway in Autoimmune Diseases" Pharmaceuticals 16, no. 8: 1089. https://doi.org/10.3390/ph16081089
APA StyleZouali, M. (2023). Pharmacological and Electroceutical Targeting of the Cholinergic Anti-Inflammatory Pathway in Autoimmune Diseases. Pharmaceuticals, 16(8), 1089. https://doi.org/10.3390/ph16081089





