Advances with Lipid-Based Nanosystems for siRNA Delivery to Breast Cancers
Abstract
1. Introduction
2. Recent Development of Lipid and Lipidoid-Based NPs for siRNA Delivery for Cancer Therapy
2.1. RNAi Therapy: Challenges and Advantages
2.2. Nanoparticles
2.2.1. Exosomes
2.2.2. Liposomes
2.2.3. Solid Lipid Nanoparticles
2.2.4. Lipid-Polymer Nanoparticles
2.3. Targeted Therapy
3. Promising Lipid-Based Nanosystems for siRNA Drug Delivery to Breast Cancer
3.1. Liposomes
3.2. Lipid Nanoparticles
4. Lipid-Based Nanosystems in Clinical Development for siRNA Drug Delivery to Breast Cancer
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva-Cázares, M.B.; Saavedra-Leos, M.Z.; Jordan-Alejandre, E.; Nuñez-Olvera, S.I.; Cómpean-Martínez, I.; López-Camarillo, C. Lipid-based nanoparticles for the therapeutic delivery of non-coding RNAs in breast cancer (Review). Oncol. Rep. 2020, 44, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Statistics and Resources. Available online: https://www.bcrf.org/breast-cancer-statistics-and-resources/ (accessed on 7 June 2023).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Cronin, K.A.; Lake, A.J.; Scott, S.; Sherman, R.L.; Noone, A.M.; Howlader, N.; Henley, S.J.; Anderson, R.N.; Firth, A.U.; Ma, J.; et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018, 124, 2785–2800. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Faltus, T.; Yuan, J.; Zimmer, B.; Krämer, A.; Loibl, S.; Kaufmann, M.; Strebhardt, K. Silencing of the HER2/neu gene by siRNA inhibits proliferation and induces apoptosis in HER2/neu-overexpressing breast cancer cells. Neoplasia 2004, 6, 786–795. [Google Scholar] [CrossRef]
- Parmar, M.B.; Aliabadi, H.M.; Mahdipoor, P.; Kucharski, C.; Maranchuk, R.; Hugh, J.C.; Uludağ, H. Targeting Cell Cycle Proteins in Breast Cancer Cells with siRNA by Using Lipid-Substituted Polyethylenimines. Front. Bioeng. Biotechnol. 2015, 3, 14. [Google Scholar] [CrossRef]
- Johnson, N.; Shapiro, G.I. Cyclin-dependent kinases (cdks) and the DNA damage response: Rationale for cdk inhibitor-chemotherapy combinations as an anticancer strategy for solid tumors. Expert Opin. Ther. Targets 2010, 14, 1199–1212. [Google Scholar] [CrossRef]
- Ayoub, N.M.; Jaradat, S.K.; Al-Shami, K.M.; Alkhalifa, A.E. Targeting Angiogenesis in Breast Cancer: Current Evidence and Future Perspectives of Novel Anti-Angiogenic Approaches. Front. Pharmacol. 2022, 13, 838133. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Fukuhara, D.; Akase, D.; Aida, M.; Ui-Tei, K. siRNA Seed Region Is Divided into Two Functionally Different Domains in RNA Interference in Response to 2′-OMe Modifications. ACS Omega 2022, 7, 2398–2410. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Safe, S.; Baker, C.; Abudayyeh, A. RNAi and cancer: Implications and applications. J. RNAi Gene Silenc. 2006, 2, 136–145. [Google Scholar]
- Ewert, K.K.; Zidovska, A.; Ahmad, A.; Bouxsein, N.F.; Evans, H.M.; McAllister, C.S.; Samuel, C.E.; Safinya, C.R. Cationic liposome-nucleic acid complexes for gene delivery and silencing: Pathways and mechanisms for plasmid DNA and siRNA. Top. Curr. Chem. 2010, 296, 191–226. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Lu, Z.R. Structure and Function of Cationic and Ionizable Lipids for Nucleic Acid Delivery. Pharm. Res. 2023, 40, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.; Levins, C.G.; Cortez, C.; Langer, R.; Anderson, D.G. Lipid-based nanotherapeutics for siRNA delivery. J. Intern. Med. 2010, 267, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Kalita, T.; Dezfouli, S.A.; Pandey, L.M.; Uludag, H. siRNA Functionalized Lipid Nanoparticles (LNPs) in Management of Diseases. Pharmaceutics 2022, 14, 2520. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.N.; Lee, S.Y.; Lee, S.; Youn, H.; Im, H.J. Lipid nanoparticles for delivery of RNA therapeutics: Current status and the role of in vivo imaging. Theranostics 2022, 12, 7509–7531. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, S.; Koide, H.; Asai, T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles. Adv. Drug Deliv. Rev. 2020, 154–155, 64–78. [Google Scholar] [CrossRef]
- Sato, Y.; Hatakeyama, H.; Sakurai, Y.; Hyodo, M.; Akita, H.; Harashima, H. A pH-sensitive cationic lipid facilitates the delivery of liposomal siRNA and gene silencing activity in vitro and in vivo. J. Control. Release 2012, 163, 267–276. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Gujrati, M.; Malamas, A.; Shin, T.; Jin, E.; Sun, Y.; Lu, Z.-R. Multifunctional Cationic Lipid-Based Nanoparticles Facilitate Endosomal Escape and Reduction-Triggered Cytosolic siRNA Release. Mol. Pharm. 2014, 11, 2734–2744. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Muhammad, I.j.; Nelson, N.E.; Tran, K.T.M.; Vinikoor, T.; Chorsi, M.T.; D’Orio, E.; Nguyen, T.D. Transdermal delivery for gene therapy. Drug Deliv. Transl. Res. 2022, 12, 2613–2633. [Google Scholar] [CrossRef]
- Yang, J.P.; Huang, L. Time-dependent maturation of cationic liposome-DNA complex for serum resistance. Gene Ther. 1998, 5, 380–387. [Google Scholar] [CrossRef]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef]
- Del Pozo-Rodríguez, A.; Solinís, M.; Rodríguez-Gascón, A. Applications of lipid nanoparticles in gene therapy. Eur. J. Pharm. Biopharm. 2016, 109, 184–193. [Google Scholar] [CrossRef]
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharmacol. Rev. 2016, 68, 701–787. [Google Scholar] [CrossRef]
- Hashemi, M.; Kalalinia, F. Application of encapsulation technology in stem cell therapy. Life Sci. 2015, 143, 139–146. [Google Scholar] [CrossRef]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef]
- de la Harpe, K.M.; Kondiah, P.P.D.; Choonara, Y.E.; Marimuthu, T.; du Toit, L.C.; Pillay, V. The Hemocompatibility of Nanoparticles: A Review of Cell-Nanoparticle Interactions and Hemostasis. Cells 2019, 8, 1209. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Gao, X.; Chen, J. Leukocyte-derived biomimetic nanoparticulate drug delivery systems for cancer therapy. Acta Pharm. Sin. B 2018, 8, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Huynh, A.B.; Madu, C.O.; Lu, Y. siRNA: A Promising New Tool for Future Breast Cancer Therapy. Oncomedicine 2018, 3, 74–81. [Google Scholar] [CrossRef]
- Singh, A.; Trivedi, P.; Jain, N.K. Advances in siRNA delivery in cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 274–283. [Google Scholar] [CrossRef]
- Wang, X.; Yu, B.; Wu, Y.; Lee, R.J.; Lee, L.J. Efficient down-regulation of CDK4 by novel lipid nanoparticle-mediated siRNA delivery. Anticancer Res. 2011, 31, 1619–1626. [Google Scholar]
- Tang, J.; Howard, C.B.; Mahler, S.M.; Thurecht, K.J.; Huang, L.; Xu, Z.P. Enhanced delivery of siRNA to triple negative breast cancer cells in vitro and in vivo through functionalizing lipid-coated calcium phosphate nanoparticles with dual target ligands. Nanoscale 2018, 10, 4258–4266. [Google Scholar] [CrossRef]
- Yu, Y.H.; Kim, E.; Park, D.E.; Shim, G.; Lee, S.; Kim, Y.B.; Kim, C.W.; Oh, Y.K. Cationic solid lipid nanoparticles for co-delivery of paclitaxel and siRNA. Eur. J. Pharm. Biopharm. 2012, 80, 268–273. [Google Scholar] [CrossRef]
- Sun, T.M.; Du, J.Z.; Yao, Y.D.; Mao, C.Q.; Dou, S.; Huang, S.Y.; Zhang, P.Z.; Leong, K.W.; Song, E.W.; Wang, J. Simultaneous delivery of siRNA and paclitaxel via a “two-in-one” micelleplex promotes synergistic tumor suppression. ACS Nano 2011, 5, 1483–1494. [Google Scholar] [CrossRef]
- Yu, S.; Bi, X.; Yang, L.; Wu, S.; Yu, Y.; Jiang, B.; Zhang, A.; Lan, K.; Duan, S. Co-Delivery of Paclitaxel and PLK1-Targeted siRNA Using Aptamer-Functionalized Cationic Liposome for Synergistic Anti-Breast Cancer Effects In Vivo. J. Biomed. Nanotechnol. 2019, 15, 1135–1148. [Google Scholar] [CrossRef]
- Wang, W.; Shao, A.; Zhang, N.; Fang, J.; Ruan, J.J.; Ruan, B.H. Cationic Polymethacrylate-Modified Liposomes Significantly Enhanced Doxorubicin Delivery and Antitumor Activity. Sci. Rep. 2017, 7, 43036. [Google Scholar] [CrossRef]
- Golkar, N.; Samani, S.M.; Tamaddon, A.M. Data on cell growth inhibition induced by anti-VEGF siRNA delivered by Stealth liposomes incorporating G2 PAMAM-cholesterol versus Metafectene® as a function of exposure time and siRNA concentration. Data Brief 2016, 8, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, X.; Shao, R.; Xu, Y.; Gao, J.; Liang, W. VEGF siRNA delivered by polycation liposome-encapsulated calcium phosphate nanoparticles for tumor angiogenesis inhibition in breast cancer. Int. J. Nanomed. 2017, 12, 6075–6088. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.J.; Semple, S.C.; Klimuk, S.K.; Edwards, K.; Eisenhardt, M.L.; Leng, E.C.; Karlsson, G.; Yanko, D.; Cullis, P.R. Therapeutically optimized rates of drug release can be achieved by varying the drug-to-lipid ratio in liposomal vincristine formulations. Biochim. Biophys. Acta 2006, 1758, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; You, J.O.; Yang, J.; Jia, D.; Moses, M.A.; Auguste, D.T. Inhibiting metastatic breast cancer cell migration via the synergy of targeted, pH-triggered siRNA delivery and chemokine axis blockade. Mol. Pharm. 2014, 11, 755–765. [Google Scholar] [CrossRef]
- Sun, S.; Xu, Y.; Fu, P.; Chen, M.; Sun, S.; Zhao, R.; Wang, J.; Liang, X.; Wang, S. Ultrasound-targeted photodynamic and gene dual therapy for effectively inhibiting triple negative breast cancer by cationic porphyrin lipid microbubbles loaded with HIF1α-siRNA. Nanoscale 2018, 10, 19945–19956. [Google Scholar] [CrossRef]
- Tang, J.; Li, B.; Howard, C.B.; Mahler, S.M.; Thurecht, K.J.; Wu, Y.; Huang, L.; Xu, Z.P. Multifunctional lipid-coated calcium phosphate nanoplatforms for complete inhibition of large triple negative breast cancer via targeted combined therapy. Biomaterials 2019, 216, 119232. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef]
- Okamoto, A.; Asai, T.; Kato, H.; Ando, H.; Minamino, T.; Mekada, E.; Oku, N. Antibody-modified lipid nanoparticles for selective delivery of siRNA to tumors expressing membrane-anchored form of HB-EGF. Biochem. Biophys. Res. Commun. 2014, 449, 460–465. [Google Scholar] [CrossRef]
- Okamoto, A.; Asai, T.; Hirai, Y.; Shimizu, K.; Koide, H.; Minamino, T.; Oku, N. Systemic Administration of siRNA with Anti-HB-EGF Antibody-Modified Lipid Nanoparticles for the Treatment of Triple-Negative Breast Cancer. Mol. Pharm. 2018, 15, 1495–1504. [Google Scholar] [CrossRef]
- Yotsumoto, F.; Oki, E.; Tokunaga, E.; Maehara, Y.; Kuroki, M.; Miyamoto, S. HB-EGF orchestrates the complex signals involved in triple-negative and trastuzumab-resistant breast cancer. Int. J. Cancer 2010, 127, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, H.; Mu, C.; Wolfram, J.; Zhang, W.; Kim, H.C.; Zhu, G.; Hu, Z.; Ji, L.N.; Liu, X.; et al. Multi-step encapsulation of chemotherapy and gene silencing agents in functionalized mesoporous silica nanoparticles. Nanoscale 2017, 9, 5329–5341. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, T.; Xie, Y.; Sun, Z.; Liu, H.; Lin, J.; Liu, C.; Mao, Z.W.; Nie, S. Chitosan layered gold nanorods as synergistic therapeutics for photothermal ablation and gene silencing in triple-negative breast cancer. Acta Biomater. 2015, 25, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Parvani, J.G.; Gujrati, M.D.; Mack, M.A.; Schiemann, W.P.; Lu, Z.R. Silencing β3 Integrin by Targeted ECO/siRNA Nanoparticles Inhibits EMT and Metastasis of Triple-Negative Breast Cancer. Cancer Res. 2015, 75, 2316–2325. [Google Scholar] [CrossRef]
- Wahlgren, J.; De, L.K.T.; Brisslert, M.; Vaziri Sani, F.; Telemo, E.; Sunnerhagen, P.; Valadi, H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012, 40, e130. [Google Scholar] [CrossRef]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Q.; Zou, H.; Chou, C.K.; Chen, X. Exosome-Modified Liposomes Targeted Delivery of Thalidomide to Regulate Treg Cells for Antitumor Immunotherapy. Pharmaceutics 2023, 15, 1074. [Google Scholar] [CrossRef]
- Abuhelal, S.; Centelles, M.N.; Wright, M.; Mason, A.J.; Thanou, M. Development of Cationic Lipid LAH4-L1 siRNA Complexes for Focused Ultrasound Enhanced Tumor Uptake. Mol. Pharm. 2023, 20, 2341–2351. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Hanley, T.; Porter, C.J.; Larson, I.; Boyd, B.J. Phytantriol and glyceryl monooleate cubic liquid crystalline phases as sustained-release oral drug delivery systems for poorly water-soluble drugs II. In-vivo evaluation. J. Pharm. Pharmacol. 2010, 62, 856–865. [Google Scholar] [CrossRef]
- Karami, Z.; Hamidi, M. Cubosomes: Remarkable drug delivery potential. Drug Discov. Today 2016, 21, 789–801. [Google Scholar] [CrossRef]
- Pramanik, A.; Xu, Z.; Ingram, N.; Coletta, P.L.; Millner, P.A.; Tyler, A.I.I.; Hughes, T.A. Hyaluronic-Acid-Tagged Cubosomes Deliver Cytotoxics Specifically to CD44-Positive Cancer Cells. Mol. Pharm. 2022, 19, 4601–4611. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Xu, Z.; Shamsuddin, S.H.; Khaled, Y.S.; Ingram, N.; Maisey, T.; Tomlinson, D.; Coletta, P.L.; Jayne, D.; Hughes, T.A.; et al. Affimer Tagged Cubosomes: Targeting of Carcinoembryonic Antigen Expressing Colorectal Cancer Cells Using In Vitro and In Vivo Models. ACS Appl. Mater. Interfaces 2022, 14, 11078–11091. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.H.; Mao, C.Q.; Dou, S.; Shen, S.; Tan, Z.B.; Wang, J. Triple negative breast cancer therapy with CDK1 siRNA delivered by cationic lipid assisted PEG-PLA nanoparticles. J. Control. Release 2014, 192, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.; Dey, A.; Taliyan, R.; Puri, A.; Kharavtekar, S.; Dubey, S.K. Recent Advances in Targeted Nanocarriers for the Management of Triple Negative Breast Cancer. Pharmaceutics 2023, 15, 246. [Google Scholar] [CrossRef] [PubMed]
- Alshaer, W.; Hillaireau, H.; Vergnaud, J.; Mura, S.; Deloménie, C.; Sauvage, F.; Ismail, S.; Fattal, E. Aptamer-guided siRNA-loaded nanomedicines for systemic gene silencing in CD-44 expressing murine triple-negative breast cancer model. J. Control. Release 2018, 271, 98–106. [Google Scholar] [CrossRef]
- Mansoori, B.; Sandoghchian Shotorbani, S.; Baradaran, B. RNA interference and its role in cancer therapy. Adv. Pharm. Bull. 2014, 4, 313–321. [Google Scholar] [CrossRef]
- Mainini, F.; Eccles, M.R. Lipid and Polymer-Based Nanoparticle siRNA Delivery Systems for Cancer Therapy. Molecules 2020, 25, 2692. [Google Scholar] [CrossRef]
- Han, X.; Mitchell, M.J.; Nie, G. Nanomaterials for Therapeutic RNA Delivery. Matter 2020, 3, 1948–1975. [Google Scholar] [CrossRef]
- Kumar, A.; Singam, A.; Swaminathan, G.; Killi, N.; Tangudu, N.K.; Jose, J.; Gundloori Vn, R.; Dinesh Kumar, L. Combinatorial therapy using RNAi and curcumin nano-architectures regresses tumors in breast and colon cancer models. Nanoscale 2022, 14, 492–505. [Google Scholar] [CrossRef]
- Juliano, R.L. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016, 44, 6518–6548. [Google Scholar] [CrossRef]
- Xin, Y.; Huang, M.; Guo, W.W.; Huang, Q.; Zhang, L.Z.; Jiang, G. Nano-based delivery of RNAi in cancer therapy. Mol. Cancer 2017, 16, 134. [Google Scholar] [CrossRef] [PubMed]
- Malone, R.W.; Felgner, P.L.; Verma, I.M. Cationic liposome-mediated RNA transfection. Proc. Natl. Acad. Sci. USA 1989, 86, 6077–6081. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.C.; Klimuk, S.K.; Harasym, T.O.; Dos Santos, N.; Ansell, S.M.; Wong, K.F.; Maurer, N.; Stark, H.; Cullis, P.R.; Hope, M.J.; et al. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: Formation of novel small multilamellar vesicle structures. Biochim. Biophys. Acta BBA-Biomembr. 2001, 1510, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, M.; Ansell, S.M.; Mui, B.L.; Tam, Y.K.; Chen, J.; Du, X.; Butler, D.; Eltepu, L.; Matsuda, S.; Narayanannair, J.K.; et al. Maximizing the Potency of siRNA Lipid Nanoparticles for Hepatic Gene Silencing In Vivo. Angew. Chem. Int. Ed. 2012, 51, 8529–8533. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, G.; Tian, C.; Jiang, W.; Jin, L.; Zhang, C.; Chen, X. Exosome-mediated small RNA delivery for gene therapy. WIREs RNA 2016, 7, 758–771. [Google Scholar] [CrossRef]
- Elouahabi, A.; Ruysschaert, J.M. Formation and intracellular trafficking of lipoplexes and polyplexes. Mol. Ther. 2005, 11, 336–347. [Google Scholar] [CrossRef]
- Sarisozen, C.; Salzano, G.; Torchilin, V.P. Recent advances in siRNA delivery. Biomol. Concepts 2015, 6, 321–341. [Google Scholar] [CrossRef]
- Ozpolat, B.; Sood, A.K.; Lopez-Berestein, G. Liposomal siRNA nanocarriers for cancer therapy. Adv. Drug Deliv. Rev. 2014, 66, 110–116. [Google Scholar] [CrossRef]
- Gasco, M.R. Lipid nanoparticles: Perspectives and challenges. Adv. Drug Deliv. Rev. 2007, 59, 377–378. [Google Scholar] [CrossRef]
- Müller, R.H. Lipid nanoparticles: Recent advances. Adv. Drug Deliv. Rev. 2007, 59, 375–376. [Google Scholar] [CrossRef]
- Jorge, A.; Pais, A.; Vitorino, C. Targeted siRNA Delivery Using Lipid Nanoparticles. Methods Mol. Biol. 2020, 2059, 259–283. [Google Scholar] [CrossRef] [PubMed]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jarvis, R.; Zhu, K.; Glass, Z.; Ogurlu, R.; Gao, P.; Li, P.; Chen, J.; Yu, Y.; Yang, Y.; et al. Protein and mRNA Delivery Enabled by Cholesteryl-Based Biodegradable Lipidoid Nanoparticles. Angew. Chem. Int. Ed. Engl. 2020, 59, 14957–14964. [Google Scholar] [CrossRef]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef]
- Ball, R.L.; Hajj, K.A.; Vizelman, J.; Bajaj, P.; Whitehead, K.A. Lipid Nanoparticle Formulations for Enhanced Co-Delivery of siRNA and mRNA. Nano Lett. 2018, 18, 3814–3822. [Google Scholar] [CrossRef] [PubMed]
- Press, A.T.; Traeger, A.; Pietsch, C.; Mosig, A.; Wagner, M.; Clemens, M.G.; Jbeily, N.; Koch, N.; Gottschaldt, M.; Bézière, N.; et al. Cell type-specific delivery of short interfering RNAs by dye-functionalised theranostic nanoparticles. Nat. Commun. 2014, 5, 5565. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.W.; Su, H.; Hildebrandt, I.J.; Weber, W.A.; Davis, M.E. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivoimaging. Proc. Natl. Acad. Sci. USA 2007, 104, 15549–15554. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Gabel, M.; Knauss, A.; Fischer, D.; Neurath, M.F.; Weigmann, B. Surface Design Options in Polymer- and Lipid-Based siRNA Nanoparticles Using Antibodies. Int. J. Mol. Sci. 2022, 23, 13929. [Google Scholar] [CrossRef]
- Chadar, R.; Afsana; Kesharwani, P. Nanotechnology-based siRNA delivery strategies for treatment of triple negative breast cancer. Int. J. Pharm. 2021, 605, 120835. [Google Scholar] [CrossRef]
- Ashique, S.; Almohaywi, B.; Haider, N.; Yasmin, S.; Hussain, A.; Mishra, N.; Garg, A. siRNA-based nanocarriers for targeted drug delivery to control breast cancer. Adv. Cancer Biol.-Metastasis 2022, 4, 100047. [Google Scholar] [CrossRef]
- Hattori, Y.; Tang, M.; Torii, S.; Tomita, K.; Sagawa, A.; Inoue, N.; Yamagishi, R.; Ozaki, K.I. Optimal combination of cationic lipid and phospholipid in cationic liposomes for gene knockdown in breast cancer cells and mouse lung using siRNA lipoplexes. Mol. Med. Rep. 2022, 26, 253. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Kommineni, N.; Bryszewska, M.; Ionov, M.; Khan, W. Cationic liposomes for co-delivery of paclitaxel and anti-Plk1 siRNA to achieve enhanced efficacy in breast cancer. J. Drug Deliv. Sci. Technol. 2018, 48, 253–265. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Tang, C.; Tian, C.; Sun, Q.; Su, Z.; Xue, L.; Yin, Y.; Ju, C.; Zhang, C. Co-delivery of paclitaxel and anti-survivin siRNA via redox-sensitive oligopeptide liposomes for the synergistic treatment of breast cancer and metastasis. Int. J. Pharm. 2017, 529, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, S.; Li, Y.; Li, X.; Yang, G.; Li, M.; Xie, Y.; Su, W.; Wu, J.; Jia, L.; et al. Cationic liposomes co-deliver chemotherapeutics and siRNA for the treatment of breast cancer. Eur. J. Med. Chem. 2022, 233, 114198. [Google Scholar] [CrossRef]
- Bedi, D.; Musacchio, T.; Fagbohun, O.A.; Gillespie, J.W.; Deinnocentes, P.; Bird, R.C.; Bookbinder, L.; Torchilin, V.P.; Petrenko, V.A. Delivery of siRNA into breast cancer cells via phage fusion protein-targeted liposomes. Nanomedicine 2011, 7, 315–323. [Google Scholar] [CrossRef]
- Gote, V.; Pal, D. Octreotide-Targeted Lcn2 siRNA PEGylated Liposomes as a Treatment for Metastatic Breast Cancer. Bioengineering 2021, 8, 44. [Google Scholar] [CrossRef]
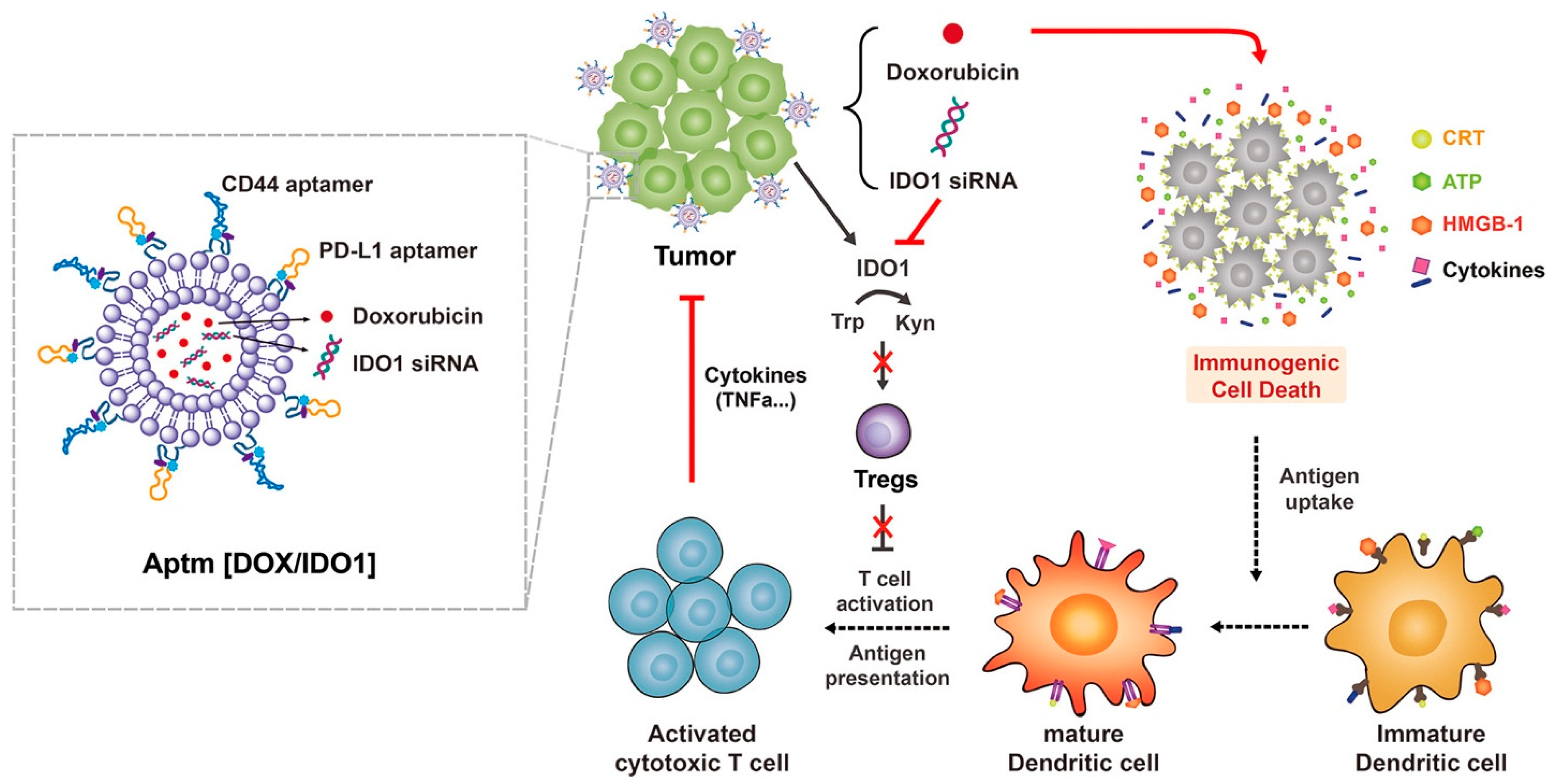
- Kim, M.; Lee, J.S.; Kim, W.; Lee, J.H.; Jun, B.-H.; Kim, K.-S.; Kim, D.-E. Aptamer-conjugated nano-liposome for immunogenic chemotherapy with reversal of immunosuppression. J. Control. Release 2022, 348, 893–910. [Google Scholar] [CrossRef]
- Li, X.; Zhou, X.; Liu, J.; Zhang, J.; Feng, Y.; Wang, F.; He, Y.; Wan, A.; Filipczak, N.; Yalamarty, S.S.K.; et al. Liposomal Co-delivery of PD-L1 siRNA/Anemoside B4 for Enhanced Combinational Immunotherapeutic Effect. ACS Appl. Mater. Interfaces 2022, 14, 28439–28454. [Google Scholar] [CrossRef]
- Monirinasab, H.; Asadi, H.; Rostamizadeh, K.; Esmaeilzadeh, A.; Khodaei, M.; Fathi, M. Novel lipid-polymer hybrid nanoparticles for siRNA delivery and IGF-1R gene silencing in breast cancer cells. J. Drug Deliv. Sci. Technol. 2018, 48, 96–105. [Google Scholar] [CrossRef]
- Yalamarty, S.S.K.; Filipczak, N.; Li, X.; Pathrikar, T.V.; Cotter, C.; Torchilin, V.P. Co-Delivery of siRNA and Chemotherapeutic Drug Using 2C5 Antibody-Targeted Dendrimer-Based Mixed Micelles for Multidrug Resistant Cancers. Pharmaceutics 2022, 14, 1470. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Mendes, L.P.; Yao, M.; Filipczak, N.; Garai, S.; Thakur, G.A.; Sarisozen, C.; Torchilin, V.P. Polyamidoamine dendrimers-based nanomedicine for combination therapy with siRNA and chemotherapeutics to overcome multidrug resistance. Eur. J. Pharm. Biopharm. 2019, 136, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Namiot, E.D.; Sokolov, A.V.; Chubarev, V.N.; Tarasov, V.V.; Schiöth, H.B. Nanoparticles in Clinical Trials: Analysis of Clinical Trials, FDA Approvals and Use for COVID-19 Vaccines. Int. J. Mol. Sci. 2023, 24, 787. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.C.; Velho, S.; Amaral, M.H. Lipid Nanoparticles Functionalized with Antibodies for Anticancer Drug Therapy. Pharmaceutics 2023, 15, 216. [Google Scholar] [CrossRef]
- Eljack, S.; David, S.; Chourpa, I.; Faggad, A.; Allard-Vannier, E. Formulation of Lipid-Based Nanoparticles for Simultaneous Delivery of Lapatinib and Anti-Survivin siRNA for HER2+ Breast Cancer Treatment. Pharmaceuticals 2022, 15, 1452. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, K. Nanoparticles-Based Strategies to Improve the Delivery of Therapeutic Small Interfering RNA in Precision Oncology. Pharmaceutics 2022, 14, 1586. [Google Scholar] [CrossRef]
- Wagner, M.J.; Mitra, R.; McArthur, M.J.; Baze, W.; Barnhart, K.; Wu, S.Y.; Rodriguez-Aguayo, C.; Zhang, X.; Coleman, R.L.; Lopez-Berestein, G.; et al. Preclinical Mammalian Safety Studies of EPHARNA (DOPC Nanoliposomal EphA2-Targeted siRNA). Mol. Cancer Ther. 2017, 16, 1114–1123. [Google Scholar] [CrossRef]
- Schultheis, B.; Strumberg, D.; Santel, A.; Vank, C.; Gebhardt, F.; Keil, O.; Lange, C.; Giese, K.; Kaufmann, J.; Khan, M.; et al. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J. Clin. Oncol. 2014, 32, 4141–4148. [Google Scholar] [CrossRef]
- Tabernero, J.; Shapiro, G.I.; LoRusso, P.M.; Cervantes, A.; Schwartz, G.K.; Weiss, G.J.; Paz-Ares, L.; Cho, D.C.; Infante, J.R.; Alsina, M.; et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013, 3, 406–417. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Papadopoulos, K.P.; Patnaik, A.; Rasco, D.W.; Martinez, D.; Wood, D.L.; Fielman, B.; Sharma, M.; Janisch, L.A.; Brown, B.D.; et al. Safety and activity of DCR-MYC, a first-in-class Dicer-substrate small interfering RNA (DsiRNA) targeting MYC, in a phase I study in patients with advanced solid tumors. J. Clin. Oncol. 2015, 33, 11006. [Google Scholar] [CrossRef]
- EphA2 siRNA in Treating Patients with Advanced or Recurrent Solid Tumors. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT01591356 (accessed on 7 June 2023).
- Phase I, Multicenter, Cohort Dose Escalation Trial to Determine the Safety, Tolerance, and Maximum Tolerated Dose of DCR-MYC, a Lipid Nanoparticle (LNP)-Formulated Small Inhibitory RNA (siRNA) Oligonucleotide Targeting MYC, in Patients with Refractory Locally Advanced or Metastatic Solid Tumor Malignancies, Multiple Myeloma, or Lymphoma; Adis International Ltd.: Auckland, New Zealand, 2017.
- Dose Escalation Trial to Evaluate the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Intravenous ALN-VSP02 in Patients with Advanced Solid Tumors with Liver Involvement. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT00882180 (accessed on 7 June 2023).
- Multi-Center, Open Label, Extension Study of ALN-VSP02 in Cancer Patients Who Have Responded to ALN-VSP02 Treatment. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT01158079 (accessed on 7 June 2023).
- El Dika, I.; Lim, H.Y.; Yong, W.P.; Lin, C.C.; Yoon, J.H.; Modiano, M.; Freilich, B.; Choi, H.J.; Chao, T.Y.; Kelley, R.K.; et al. An Open-Label, Multicenter, Phase I, Dose Escalation Study with Phase II Expansion Cohort to Determine the Safety, Pharmacokinetics, and Preliminary Antitumor Activity of Intravenous TKM-080301 in Subjects with Advanced Hepatocellular Carcinoma. Oncologist 2019, 24, 747-e218. [Google Scholar] [CrossRef] [PubMed]
- A Dose Finding Study of TKM-080301 Infusion in Neuroendocrine Tumors (NET) and Adrenocortical Carcinoma (ACC) Patients. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT01262235 (accessed on 7 June 2023).
- Mahase, E. FDA allows drugs without proven clinical benefit to languish for years on accelerated pathway. BMJ 2021, 374, n1898. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Wobst, H.J. A Decade of FDA-Approved Drugs (2010–2019): Trends and Future Directions. J. Med. Chem. 2021, 64, 2312–2338. [Google Scholar] [CrossRef]
- Lim, S.A.; Cox, A.; Tung, M.; Chung, E.J. Clinical progress of nanomedicine-based RNA therapies. Bioact. Mater. 2022, 12, 203–213. [Google Scholar] [CrossRef]
- Urits, I.; Swanson, D.; Swett, M.C.; Patel, A.; Berardino, K.; Amgalan, A.; Berger, A.A.; Kassem, H.; Kaye, A.D.; Viswanath, O. A Review of Patisiran (ONPATTRO®) for the Treatment of Polyneuropathy in People with Hereditary Transthyretin Amyloidosis. Neurol. Ther. 2020, 9, 301–315. [Google Scholar] [CrossRef]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/onpattro (accessed on 1 July 2023).
- Zhang, X.; Goel, V.; Attarwala, H.; Sweetser, M.T.; Clausen, V.A.; Robbie, G.J. Patisiran Pharmacokinetics, Pharmacodynamics, and Exposure-Response Analyses in the Phase 3 APOLLO Trial in Patients With Hereditary Transthyretin-Mediated (hATTR) Amyloidosis. J. Clin. Pharmacol. 2020, 60, 37–49. [Google Scholar] [CrossRef]
- Ebenezer, O.; Comoglio, P.; Wong, G.K.; Tuszynski, J.A. Development of Novel siRNA Therapeutics: A Review with a Focus on Inclisiran for the Treatment of Hypercholesterolemia. Int. J. Mol. Sci. 2023, 24, 4019. [Google Scholar] [CrossRef]
- Friedrich, M.; Aigner, A. Therapeutic siRNA: State-of-the-Art and Future Perspectives. BioDrugs 2022, 36, 549–571. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, J.; Zhou, J.E.; Wang, J.; Wang, Z.; Luo, S.; Wang, Y.; Zhu, S.; Yang, F.; Tang, J.; et al. Monitoring the in vivo siRNA release from lipid nanoparticles based on the fluorescence resonance energy transfer principle. Asian J. Pharm. Sci. 2023, 18, 100769. [Google Scholar] [CrossRef]
- Seo, H.; Jeon, L.; Kwon, J.; Lee, H. High-Precision Synthesis of RNA-Loaded Lipid Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2023, 12, e2203033. [Google Scholar] [CrossRef] [PubMed]
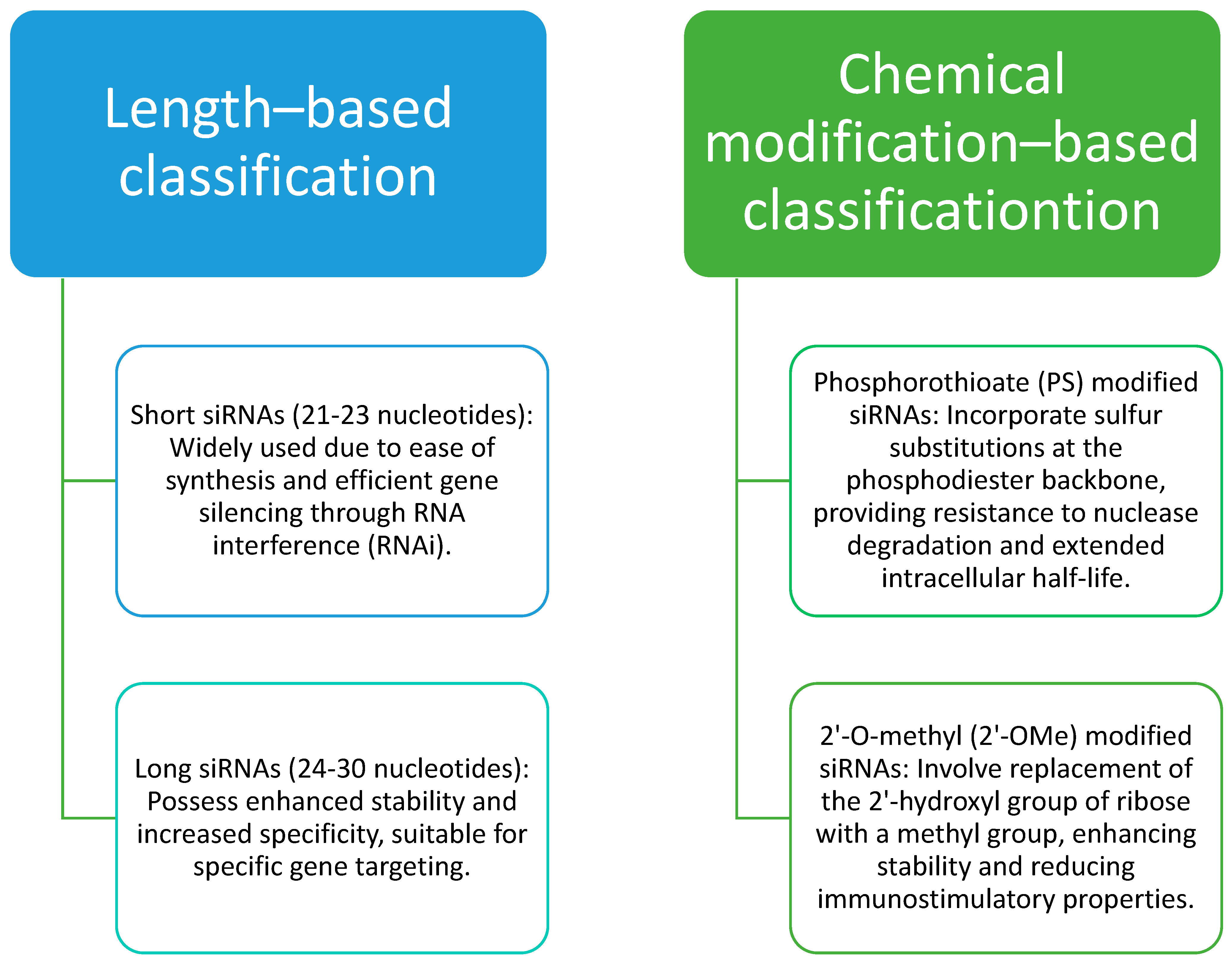

| Lipid Nanocarrier Type | Lipids Used | Active Moiety Delivered | Key Conclusions | References |
|---|---|---|---|---|
| Lipid Nanoparticles (LNPs) | Ionizable cationic lipids (e.g., DOTAP, DLin-MC3-DMA) and helper lipids (e.g., cholesterol, PEG-lipids) | siRNA (Small interfering RNA) | LNPs effectively encapsulated siRNA and protected it from degradation. The formulation demonstrated high stability, efficient cellular uptake, and endosomal escape. LNPs efficiently delivered siRNA to target cells and achieved significant gene silencing, offering promising potential for therapeutic applications. | [18,19] |
| Solid Lipid Nanoparticles (SLNs) | Solid lipids (e.g., stearic acid, glyceryl monostearate) and surfactants (e.g., Tween, Span) | siRNA | SLNs provided a stable and biocompatible platform for siRNA delivery. The formulation protected siRNA from enzymatic degradation and facilitated cellular uptake. SLNs demonstrated effective gene silencing in vitro and in vivo, highlighting their potential as siRNA delivery systems. | [20] |
| Liposomes | Cationic lipids (e.g., DOTAP, DODAB) and neutral lipids (e.g., phosphatidylcholine) | siRNA | Liposomes efficiently encapsulated siRNA and protected it from nuclease degradation. The cationic lipids facilitated cellular uptake and endosomal escape of siRNA. Liposomal siRNA delivery showed effective gene silencing in target cells, making liposomes a promising option for siRNA therapeutics. | [21,22,23] |
| Cationic Lipid-DNA Complexes (lipoplexes) | Cationic lipids (e.g., Lipofectamine, Polyethylenimine) and plasmid DNA | siRNA or gene encoding siRNA | Cationic lipids formed stable complexes with siRNA or plasmid DNA and facilitated their cellular uptake. Lipoplexes efficiently delivered siRNA or gene encoding siRNA, resulting in effective gene silencing or knockdown of the target gene. Lipoplexes showed potential for siRNA-based therapeutics and gene therapy applications. | [12,24] |
| Ethosomes | Phospholipids (e.g., phosphatidylcholine) and ethanol | siRNA | Ethosomes provided enhanced permeation of siRNA through the skin or mucosal barriers. The formulation improved siRNA stability and promoted efficient delivery into target cells. Ethosomes showed potential for transdermal or mucosal siRNA delivery, offering opportunities for local or systemic treatments. | [25] |
| Group of Lipids/Lipidoids | Examples |
|---|---|
| Cationic lipids | DOTAP, DOTMA, DC-6-14 |
| Ionizable lipids/lipidoids | A6, A18-Iso-5-2DC18, 98N12-5, DLin-MC3-DMA, Lin-DMA, DODAP, DLin-KC2-DMA, DLin-MC3-DMA, YSK05, YSK13, CL4H6. |
| Sterols | Cholesterol, DC-cholesterol, Sitosterol |
| PEG-lipid conjugates | DSPE-PEG, DMG-PEG |
| Phospholipids | DOPE, DSPC, DSPC |
| siRNA Drug | Delivery System | Target | Cancer Type | Phase, Status | Company | NCT | Ref. |
|---|---|---|---|---|---|---|---|
| siRNA-EphA2 | Liposomes | EphA2 | Advanced Solid tumors | I, Active, Not Recruiting | M.D. Anderson Cancer Center | 01591356 | [113] |
| DCR-PHXC-101 | Lipid-based NPs | Myc | Solid tumors | Terminated | Dicerna Pharmaceuticals | 02110563 | [114] |
| Atu027 | Liposomes | Protein kinase 3 | Solid tumors | I, Completed | Silence Therapeutics GmbH | 00938574 | [110] |
| ALN-VSP | Lipid-based NPs | VEGF, KSP | Solid tumors | I, completed Completed | Alnylam Pharmaceuticals | 00882180 01158079 | [115,116] |
| TKM-080301 | Lipid NPs | PLK1 | Advanced Solid tumors | II, completed | Arbutus Biopharma Corporation | 01262235 02191878 | [117,118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subhan, M.A.; Filipczak, N.; Torchilin, V.P. Advances with Lipid-Based Nanosystems for siRNA Delivery to Breast Cancers. Pharmaceuticals 2023, 16, 970. https://doi.org/10.3390/ph16070970
Subhan MA, Filipczak N, Torchilin VP. Advances with Lipid-Based Nanosystems for siRNA Delivery to Breast Cancers. Pharmaceuticals. 2023; 16(7):970. https://doi.org/10.3390/ph16070970
Chicago/Turabian StyleSubhan, Md Abdus, Nina Filipczak, and Vladimir P. Torchilin. 2023. "Advances with Lipid-Based Nanosystems for siRNA Delivery to Breast Cancers" Pharmaceuticals 16, no. 7: 970. https://doi.org/10.3390/ph16070970
APA StyleSubhan, M. A., Filipczak, N., & Torchilin, V. P. (2023). Advances with Lipid-Based Nanosystems for siRNA Delivery to Breast Cancers. Pharmaceuticals, 16(7), 970. https://doi.org/10.3390/ph16070970










