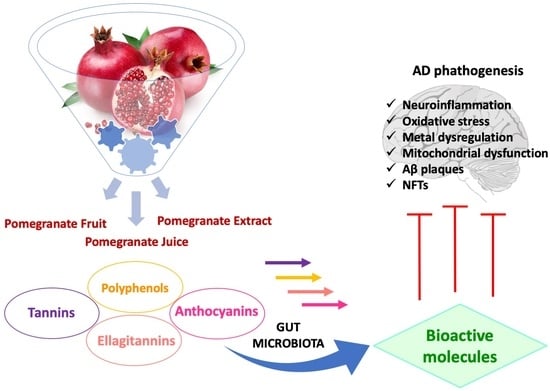
Pomegranate: A Source of Multifunctional Bioactive Compounds Potentially Beneficial in Alzheimer’s Disease
Abstract
1. Introduction
2. Pomegranate Fruit (PF)
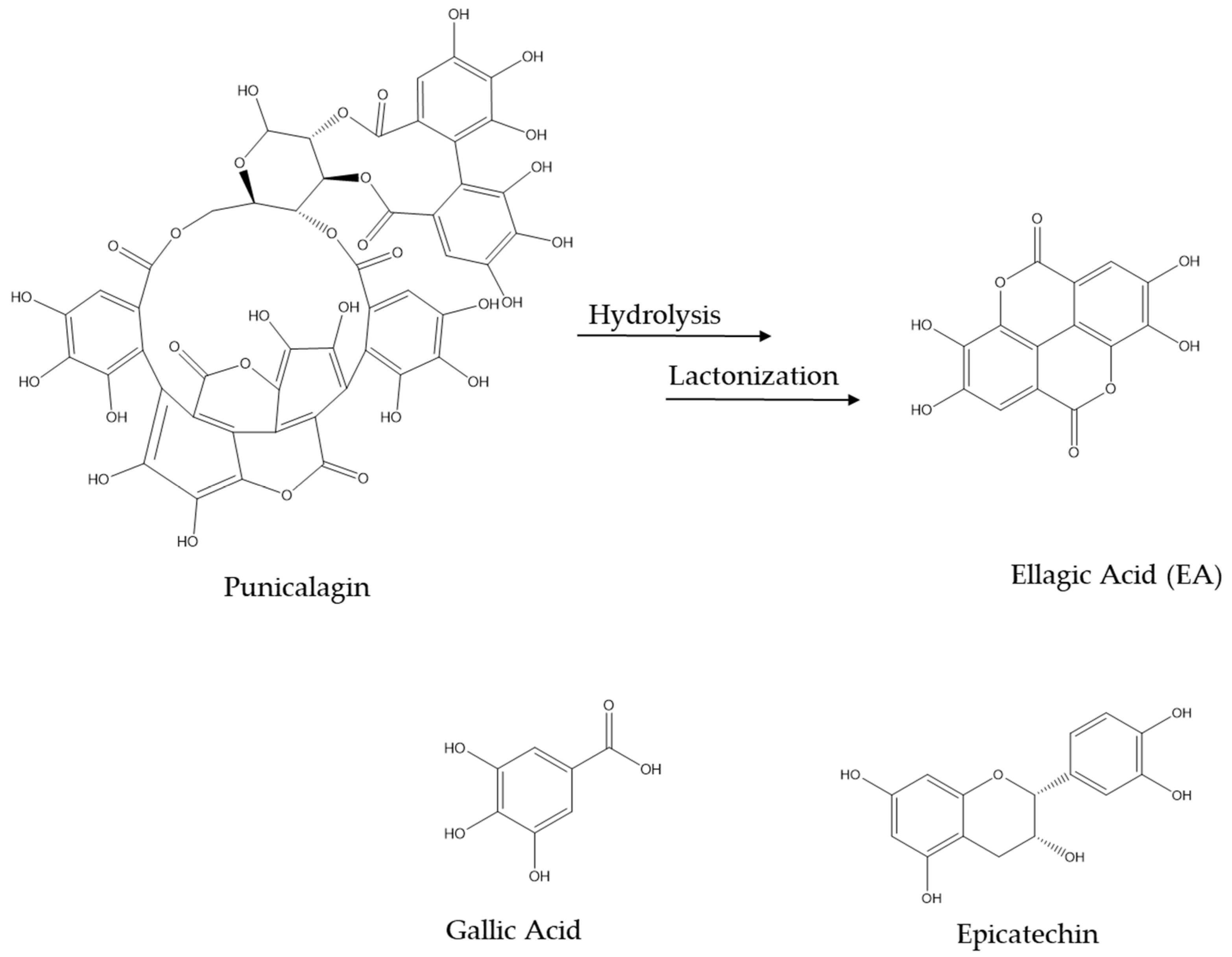
2.1. Pomegranate Peel Extract and Its Bioactive Compounds in AD
2.2. Pomegranate Juice and Extracts against AD
2.2.1. Pomegranate Protects Pups’ Mice from Brain Injury
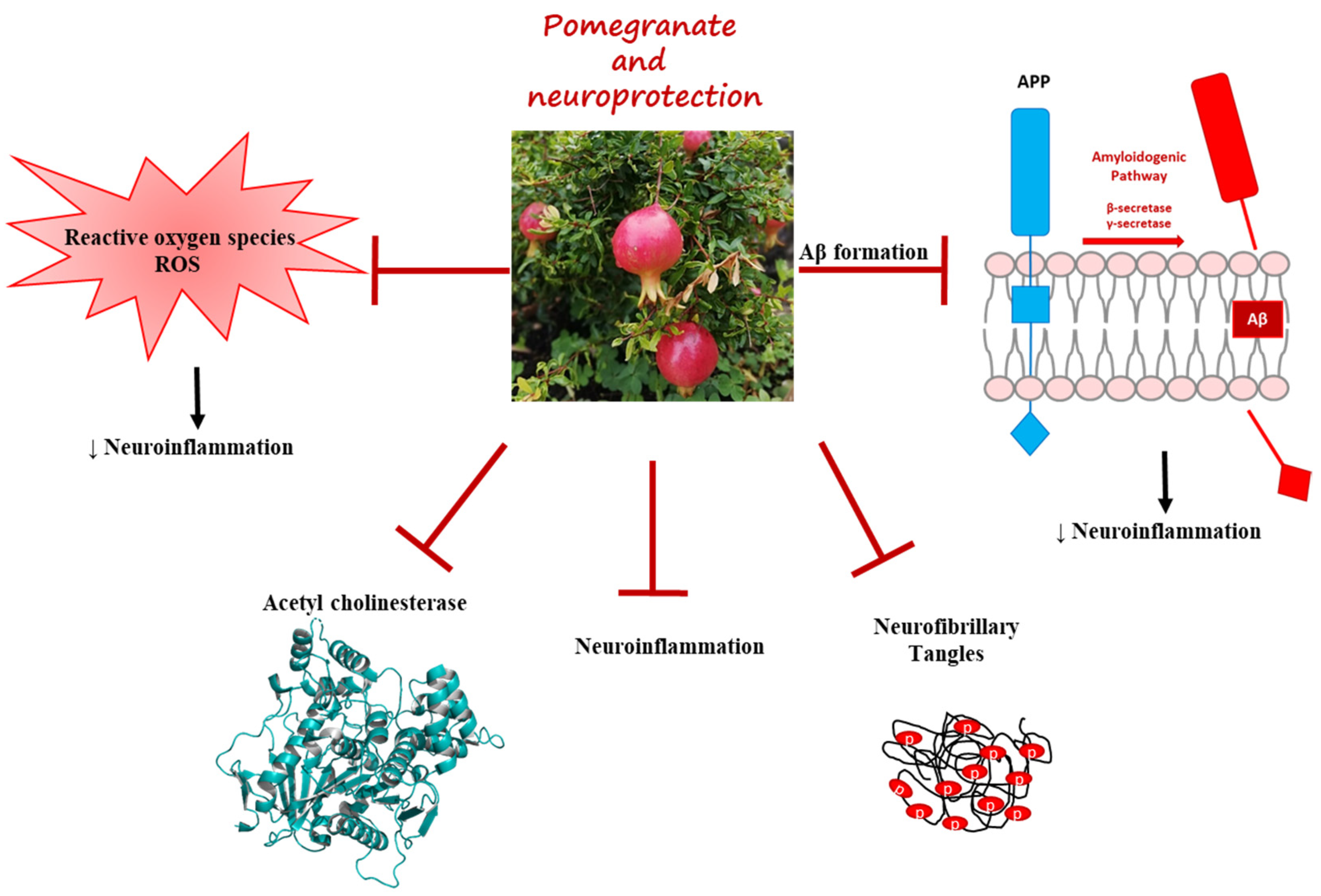
2.2.2. Pomegranate Effects in AD Model
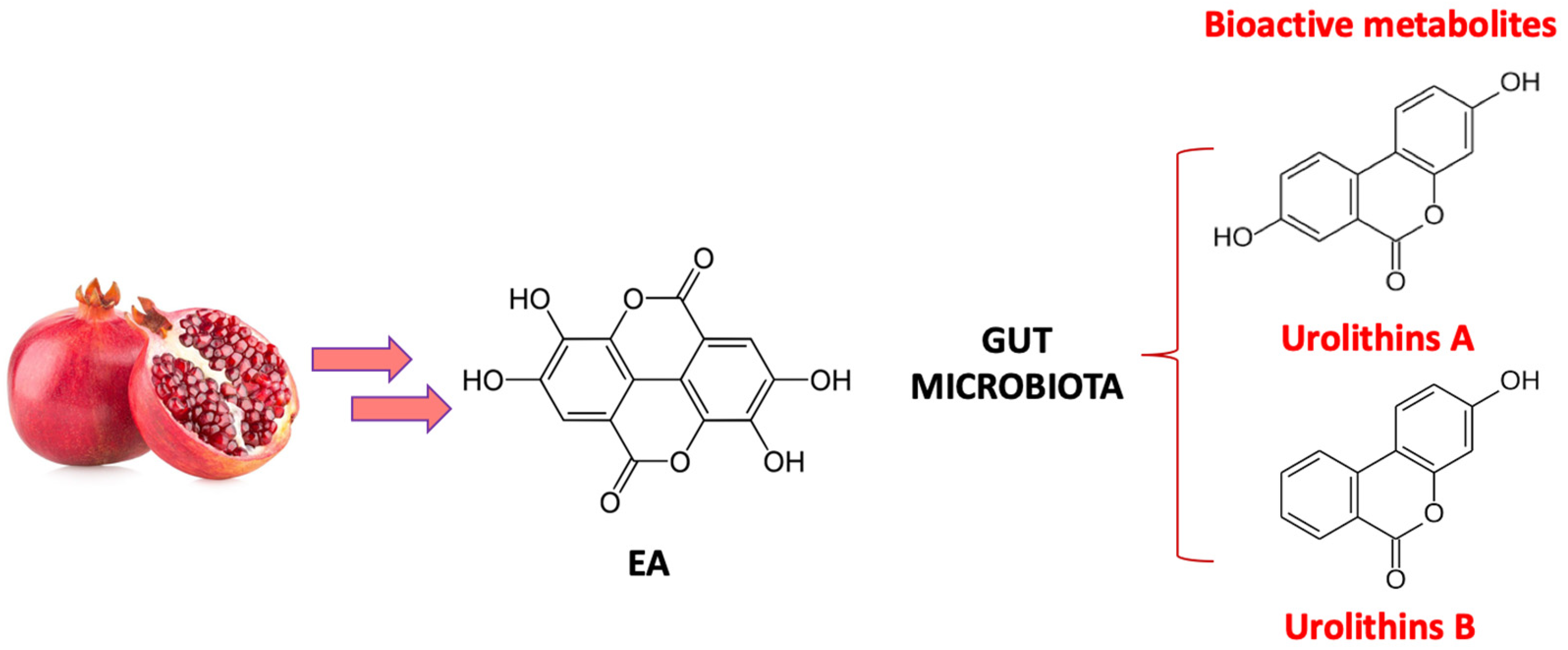
3. Biotransformation of Pomegranate and Bioactivity of Its Metabolites in AD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Voet, S.; Srinivasan, S.; Lamkanfi, M.; van Loo, G. Inflammasomes in Neuroinflammatory and Neurodegenerative Diseases. EMBO Mol. Med. 2019, 11, e10248. [Google Scholar] [CrossRef] [PubMed]
- Emerit, J.; Edeas, M.; Bricaire, F. Neurodegenerative Diseases and Oxidative Stress. Biomed. Pharmacother. 2004, 58, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Karran, E.; Mercken, M.; Strooper, B.D. The Amyloid Cascade Hypothesis for Alzheimer’s Disease: An Appraisal for the Development of Therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Yamazaki, T.; Citron, M.; Podlisny, M.B.; Koo, E.H.; Teplow, D.B.; Haass, C. The Role of APP Processing and Trafficking Pathways in the Formation of Amyloid β-Proteina. Ann. N. Y. Acad. Sci. 1996, 777, 57–64. [Google Scholar] [CrossRef]
- Delacourte, A.; Defossez, A. Alzheimer’s Disease: Tau Proteins, the Promoting Factors of Microtubule Assembly, Are Major Components of Paired Helical Filaments. J. Neurol. Sci. 1986, 76, 173–186. [Google Scholar] [CrossRef]
- Kent, S.A.; Spires-Jones, T.L.; Durrant, C.S. The Physiological Roles of Tau and Aβ: Implications for Alzheimer’s Disease Pathology and Therapeutics. Acta Neuropathol. 2020, 140, 417–447. [Google Scholar] [CrossRef]
- Delaby, C.; Hirtz, C.; Lehmann, S. Overview of the Blood Biomarkers in Alzheimer’s Disease: Promises and Challenges. Rev. Neurol. 2023, 179, 161–172. [Google Scholar] [CrossRef]
- Wang, Y.; Mandelkow, E. Tau in Physiology and Pathology. Nat. Rev. Neurosci. 2016, 17, 22–35. [Google Scholar] [CrossRef]
- Wegmann, S.; Biernat, J.; Mandelkow, E. A Current View on Tau Protein Phosphorylation in Alzheimer’s Disease. Curr. Opin. Neurobiol. 2021, 69, 131–138. [Google Scholar] [CrossRef]
- Busche, M.A.; Hyman, B.T. Synergy between Amyloid-β and Tau in Alzheimer’s Disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef]
- Ciccone, L.; Shi, C.; di Lorenzo, D.; Van Baelen, A.-C.; Tonali, N. The Positive Side of the Alzheimer’s Disease Amyloid Cross-Interactions: The Case of the Aβ 1-42 Peptide with Tau, TTR, CysC, and ApoA1. Molecules 2020, 25, 2439. [Google Scholar] [CrossRef] [PubMed]
- Tonali, N.; Nencetti, S.; Orlandini, E.; Ciccone, L. Application of PROTAC Strategy to TTR-Aβ Protein-Protein Interaction for the Development of Alzheimer’s Disease Drugs. Neural Regen. Res. 2021, 16, 1554. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, L.; Policar, C.; Stura, E.A.; Shepard, W. Human TTR Conformation Altered by Rhenium Tris-Carbonyl Derivatives. J. Struct. Biol. 2016, 195, 353–364. [Google Scholar] [CrossRef]
- Ciccone, L.; Fruchart-Gaillard, C.; Mourier, G.; Savko, M.; Nencetti, S.; Orlandini, E.; Servent, D.; Stura, E.A.; Shepard, W. Copper Mediated Amyloid- β Binding to Transthyretin. Sci. Rep. 2018, 8, 13744. [Google Scholar] [CrossRef]
- Xie, J.; Van Hoecke, L.; Vandenbroucke, R.E. The Impact of Systemic Inflammation on Alzheimer’s Disease Pathology. Front. Immunol. 2022, 12, 796867. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a Central Mechanism in Alzheimer’s Disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-H.; Geng, Z.; Yan, J.; Li, T.; Chen, Q.; Zhang, Q.-Y.; Chen, Z.-Y. Blocking GSK3β-Mediated Dynamin1 Phosphorylation Enhances BDNF-Dependent TrkB Endocytosis and the Protective Effects of BDNF in Neuronal and Mouse Models of Alzheimer’s Disease. Neurobiol. Dis. 2015, 74, 377–391. [Google Scholar] [CrossRef]
- Wilkins, H.M.; Swerdlow, R.H. Relationships Between Mitochondria and Neuroinflammation: Implications for Alzheimer’s Disease. Curr. Top. Med. Chem. 2016, 16, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Talesa, V.N. Acetylcholinesterase in Alzheimer’s Disease. Mech. Ageing Dev. 2001, 122, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Basnet, R.; Khadka, S.; Basnet, B.B.; Gupta, R. Perspective on Acetylcholinesterase: A Potential Target for Alzheimer’s Disease Intervention. Curr. Enzym. Inhib. 2020, 16, 181–188. [Google Scholar] [CrossRef]
- Folch, J.; Busquets, O.; Ettcheto, M.; Sánchez-López, E.; Castro-Torres, R.D.; Verdaguer, E.; Garcia, M.L.; Olloquequi, J.; Casadesús, G.; Beas-Zarate, C.; et al. Memantine for the Treatment of Dementia: A Review on Its Current and Future Applications. J. Alzheimer’s Dis. 2018, 62, 1223–1240. [Google Scholar] [CrossRef]
- Walsh, S.; Merrick, R.; Milne, R.; Brayne, C. Aducanumab for Alzheimer’s Disease? BMJ 2021, 374, n1682. [Google Scholar] [CrossRef] [PubMed]
- Van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Tramutola, A.; Lanzillotta, C.; Perluigi, M.; Butterfield, D.A. Oxidative Stress, Protein Modification and Alzheimer Disease. Brain Res. Bull. 2017, 133, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, G.; Chakrabarti, S.; Chatterjee, U.; Saso, L. Proteinopathy, Oxidative Stress and Mitochondrial Dysfunction: Cross Talk in Alzheimer’s Disease and Parkinson’s Disease. Drug Des. Dev. Ther. 2017, 11, 797–810. [Google Scholar] [CrossRef]
- Yan, M.H.; Wang, X.; Zhu, X. Mitochondrial Defects and Oxidative Stress in Alzheimer Disease and Parkinson Disease. Free Radic. Biol. Med. 2013, 62, 90–101. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Boyd-Kimball, D. Mitochondrial Oxidative and Nitrosative Stress and Alzheimer Disease. Antioxidants 2020, 9, 818. [Google Scholar] [CrossRef]
- Wang, X.; Su, B.; Lee, H.; Li, X.; Perry, G.; Smith, M.A.; Zhu, X. Impaired Balance of Mitochondrial Fission and Fusion in Alzheimer’s Disease. J. Neurosci. 2009, 29, 9090–9103. [Google Scholar] [CrossRef]
- Mullin, S.; Schapira, A. α-Synuclein and Mitochondrial Dysfunction in Parkinson’s Disease. Mol. Neurobiol. 2013, 47, 587–597. [Google Scholar] [CrossRef]
- Kozlowski, H.; Janicka-Klos, A.; Brasun, J.; Gaggelli, E.; Valensin, D.; Valensin, G. Copper, Iron, and Zinc Ions Homeostasis and Their Role in Neurodegenerative Disorders (Metal Uptake, Transport, Distribution and Regulation). Coord. Chem. Rev. 2009, 253, 2665–2685. [Google Scholar] [CrossRef]
- Ciccone, L.; Tonali, N.; Shepard, W.; Nencetti, S.; Orlandini, E. Physiological Metals Can Induce Conformational Changes in Transthyretin Structure: Neuroprotection or Misfolding Induction? Crystals 2021, 11, 354. [Google Scholar] [CrossRef]
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, Iron and Zinc in Alzheimer’s Disease Senile Plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Raymick, J.; Sarkar, S. Role of Metals in Alzheimer’s Disease. Metab. Brain Dis. 2021, 36, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Qiu, P.; Cui, W.; Yan, X.; Zhang, B.; He, S. Recent Advances in Multi-Target Anti-Alzheimer Disease Compounds (2013 Up to the Present). Curr. Med. Chem. 2019, 26, 5684–5710. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Nabavi, S.M.; Orhan, I.E.; Skalicka-Woźniak, K.; D’Onofrio, G.; Aiello, F. Natural Compounds and Their Derivatives as Multifunctional Agents for the Treatment of Alzheimer Disease. In Discovery and Development of Neuroprotective Agents from Natural Products; Elsevier: Amsterdam, The Netherlands, 2018; pp. 63–102. ISBN 978-0-12-809593-5. [Google Scholar]
- Singh, Y.P.; Rai, H.; Singh, G.; Singh, G.K.; Mishra, S.; Kumar, S.; Srikrishna, S.; Modi, G. A Review on Ferulic Acid and Analogs Based Scaffolds for the Management of Alzheimer’s Disease. Eur. J. Med. Chem. 2021, 215, 113278. [Google Scholar] [CrossRef] [PubMed]
- Polsinelli, I.; Nencetti, S.; Shepard, W.; Ciccone, L.; Orlandini, E.; Stura, E.A. A New Crystal Form of Human Transthyretin Obtained with a Curcumin Derived Ligand. J. Struct. Biol. 2016, 194, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A. Natural Compounds for Alzheimer’s Disease Therapy: A Systematic Review of Preclinical and Clinical Studies. Int. J. Mol. Sci. 2019, 20, 2313. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, L.; Vandooren, J.; Nencetti, S.; Orlandini, E. Natural Marine and Terrestrial Compounds as Modulators of Matrix Metalloproteinases-2 (MMP-2) and MMP-9 in Alzheimer’s Disease. Pharmaceuticals 2021, 14, 86. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Lankatillake, C.; Dias, D.A.; Docea, A.O.; Mahomoodally, M.F.; Lobine, D.; Chazot, P.L.; Kurt, B.; Boyunegmez Tumer, T.; Catarina Moreira, A.; et al. Impact of Natural Compounds on Neurodegenerative Disorders: From Preclinical to Pharmacotherapeutics. J. Clin. Med. 2020, 9, 1061. [Google Scholar] [CrossRef]
- Joseph, J.; Cole, G.; Head, E.; Ingram, D. Nutrition, Brain Aging, and Neurodegeneration. J. Neurosci. 2009, 29, 12795–12801. [Google Scholar] [CrossRef]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate Peel and Peel Extracts: Chemistry and Food Features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, W.; Wang, L.; Liu, R.; Yi, D.; Du, L. Constituents of the Flowers of Punica granatum. Fitoterapia 2006, 77, 534–537. [Google Scholar] [CrossRef]
- Wu, S.; Tian, L. Diverse Phytochemicals and Bioactivities in the Ancient Fruit and Modern Functional Food Pomegranate (Punica granatum). Molecules 2017, 22, 1606. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, L.; Nencetti, S.; Socci, S.; Orlandini, E. Neuroglobin and Neuroprotection: The Role of Natural and Synthetic Compounds in Neuroglobin Pharmacological Induction. Neural Regen. Res. 2021, 16, 2353–2358. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Santini, A. Nutraceuticals in Human Health. Foods 2020, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.S.; Lordan, R.; Horbańczuk, O.K.; Atanasov, A.G.; Chopra, I.; Horbańczuk, J.O.; Jóźwik, A.; Huang, L.; Pirgozliev, V.; Banach, M.; et al. The Current Use and Evolving Landscape of Nutraceuticals. Pharmacol. Res. 2022, 175, 106001. [Google Scholar] [CrossRef]
- Guerrero-Solano, J.A.; Jaramillo-Morales, O.A.; Velázquez-González, C.; De la O-Arciniega, M.; Castañeda-Ovando, A.; Betanzos-Cabrera, G.; Bautista, M. Pomegranate as a Potential Alternative of Pain Management: A Review. Plants 2020, 9, 419. [Google Scholar] [CrossRef]
- Vučić, V.; Grabež, M.; Trchounian, A.; Arsić, A. Composition and Potential Health Benefits of Pomegranate: A Review. Curr. Pharm. Des. 2019, 25, 1817–1827. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Shafique, B.; Wang, L.; Irfan, S.; Safdar, M.N.; Murtaza, M.A.; Nadeem, M.; Mahmood, S.; Mueen-ud-Din, G.; Nadeem, H.R. A Comprehensive Review on Phytochemistry, Bioactivity and Medicinal Value of Bioactive Compounds of Pomegranate (Punica granatum). Adv. Tradit. Med. 2023, 23, 37–57. [Google Scholar] [CrossRef]
- Tamborlin, L.; Sumere, B.R.; de Souza, M.C.; Pestana, N.F.; Aguiar, A.C.; Eberlin, M.N.; Simabuco, F.M.; Rostagno, M.A.; Luchessi, A.D. Characterization of Pomegranate Peel Extracts Obtained Using Different Solvents and Their Effects on Cell Cycle and Apoptosis in Leukemia Cells. Food Sci. Nutr. 2020, 8, 5483–5496. [Google Scholar] [CrossRef]
- Kujawska, M.; Jourdes, M.; Kurpik, M.; Szulc, M.; Szaefer, H.; Chmielarz, P.; Kreiner, G.; Krajka-Kuźniak, V.; Mikołajczak, P.Ł.; Teissedre, P.-L.; et al. Neuroprotective Effects of Pomegranate Juice against Parkinson’s Disease and Presence of Ellagitannins-Derived Metabolite—Urolithin A—In the Brain. Int. J. Mol. Sci. 2020, 21, 202. [Google Scholar] [CrossRef] [PubMed]
- George, N.; AbuKhader, M.; Al Balushi, K.; Al Sabahi, B.; Khan, S.A. An Insight into the Neuroprotective Effects and Molecular Targets of Pomegranate (Punica granatum) against Alzheimer’s Disease. Nutr. Neurosci. 2022, 25, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Ma, J.; Gao, W.; Zhang, L.; Li, J.; Li, J.; Zang, J. Pomegranate Peel as a Source of Bioactive Compounds: A Mini Review on Their Physiological Functions. Front. Nutr. 2022, 9, 887113. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.S.; Jayaprakasha, G.K.; Jena, B.S. Antioxidant and Antimutagenic Activities of Pomegranate Peel Extracts. Food Chem. 2003, 80, 393–397. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Assessment of Polyphenolic Profile and Antibacterial Activity of Pomegranate Peel (Punica granatum) Flour Obtained from Co-Product of Juice Extraction. Food Control 2016, 59, 94–98. [Google Scholar] [CrossRef]
- Ono, N.N.; Bandaranayake, P.C.G.; Tian, L. Establishment of Pomegranate (Punica granatum) Hairy Root Cultures for Genetic Interrogation of the Hydrolyzable Tannin Biosynthetic Pathway. Planta 2012, 236, 931–941. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant Activity of Pomegranate Juice and Its Relationship with Phenolic Composition and Processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Mehdi, A.; Lamiae, B.; Samira, B.; Ramchoun, M.; Abdelouahed, K.; Tamas, F.; Hicham, B. Pomegranate (Punica granatum L.) Attenuates Neuroinflammation Involved in Neurodegenerative Diseases. Foods 2022, 11, 2570. [Google Scholar] [CrossRef]
- Esther Lydia, D.; Khusro, A.; Immanuel, P.; Esmail, G.A.; Al-Dhabi, N.A.; Arasu, M.V. Photo-Activated Synthesis and Characterization of Gold Nanoparticles from Punica granatum L. Seed Oil: An Assessment on Antioxidant and Anticancer Properties for Functional Yoghurt Nutraceuticals. J. Photochem. Photobiol. B Biol. 2020, 206, 111868. [Google Scholar] [CrossRef]
- Teniente, S.L.; Flores-Gallegos, A.C.; Esparza-González, S.C.; Campos-Múzquiz, L.G.; Nery-Flores, S.D.; Rodríguez-Herrera, R. Anticancer Effect of Pomegranate Peel Polyphenols against Cervical Cancer. Antioxidants 2023, 12, 127. [Google Scholar] [CrossRef]
- Morzelle, M.C.; Salgado, J.M.; Massarioli, A.P.; Bachiega, P.; Rios, A.d.O.; Alencar, S.M.; Schwember, A.R.; Camargo, A.C. de Potential Benefits of Phenolics from Pomegranate Pulp and Peel in Alzheimer’s Disease: Antioxidant Activity and Inhibition of Acetylcholinesterase. J. Food Bioact. 2019, 5, 136–141. [Google Scholar] [CrossRef]
- Tsang, C.; Smail, N.F.; Almoosawi, S.; Davidson, I.; Al-Dujaili, E.A.S. Intake of Polyphenol-Rich Pomegranate Pure Juice Influences Urinary Glucocorticoids, Blood Pressure and Homeostasis Model Assessment of Insulin Resistance in Human Volunteers. J. Nutr. Sci. 2012, 1, e9. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wasila, H.; Liu, L.; Yuan, T.; Gao, Z.; Zhao, B.; Ahmad, I. Physicochemical Characteristics, Polyphenol Compositions and Antioxidant Potential of Pomegranate Juices from 10 Chinese Cultivars and the Environmental Factors Analysis. Food Chem. 2015, 175, 575–584. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of Antioxidant Properties of Pomegranate Peel Extract in Comparison with Pomegranate Pulp Extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Kwak, H.-M.; Jeon, S.-Y.; Sohng, B.-H.; Kim, J.-G.; Lee, J.-M.; Lee, K.-B.; Jeong, H.-H.; Hur, J.-M.; Kang, Y.-H.; Song, K.-S. β-Secretase(BACE1) Inhibitors from Pomegranate (Punica granatum) Husk. Arch. Pharm. Res. 2005, 28, 1328–1332. [Google Scholar] [CrossRef]
- Morzelle, M.C.; Salgado, J.M.; Telles, M.; Mourelle, D.; Bachiega, P.; Buck, H.S.; Viel, T.A. Neuroprotective Effects of Pomegranate Peel Extract after Chronic Infusion with Amyloid-β Peptide in Mice. PLoS ONE 2016, 11, e0166123. [Google Scholar] [CrossRef]
- Chidambara Murthy, K.N.; Jayaprakasha, G.K.; Singh, R.P. Studies on Antioxidant Activity of Pomegranate (Punica granatum) Peel Extract Using in Vivo Models. J. Agric. Food Chem. 2002, 50, 4791–4795. [Google Scholar] [CrossRef] [PubMed]
- Malviya, S.; Arvind; Jha, A.; Hettiarachchy, N. Antioxidant and Antibacterial Potential of Pomegranate Peel Extracts. J. Food Sci. Technol. 2014, 51, 4132–4137. [Google Scholar] [CrossRef]
- Harakeh, S.; Ramadan, W.S.; Muhayawi, M.S.A.; Jaouni, S.A.; Mousa, S.; Hakeem, K.R. Pomegranate Peel Extract Lessens Histopathologic Changes and Restores Antioxidant Homeostasis in the Hippocampus of Rats with Aluminium Chloride-Induced Alzheimer’s Disease. Asian Pac. J. Trop. Med. 2020, 13, 456. [Google Scholar] [CrossRef]
- Newman, R.A.; Lansky, E.P.; Block, M.L. Pomegranate: The Most Medicinal Fruit; Basic Health Publications, Inc.: Laguna Beach, CA, USA, 2007; ISBN 978-1-59120-210-3. [Google Scholar]
- Saragat, B.; Buffa, R.; Mereu, E.; Succa, V.; Cabras, S.; Mereu, R.M.; Viale, D.; Putzu, P.F.; Marini, E. Nutritional and Psycho-Functional Status in Elderly Patients with Alzheimer’s Disease. J. Nutr. Health Aging 2012, 16, 231–236. [Google Scholar] [CrossRef]
- Hu, N.; Yu, J.-T.; Tan, L.; Wang, Y.-L.; Sun, L.; Tan, L. Nutrition and the Risk of Alzheimer’s Disease. BioMed Res. Int. 2013, 2013, e524820. [Google Scholar] [CrossRef] [PubMed]
- Gentreau, M.; Chuy, V.; Féart, C.; Samieri, C.; Ritchie, K.; Raymond, M.; Berticat, C.; Artero, S. Refined Carbohydrate-Rich Diet Is Associated with Long-Term Risk of Dementia and Alzheimer’s Disease in Apolipoprotein E Ε4 Allele Carriers. Alzheimer’s Dement. 2020, 16, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Emami Kazemabad, M.J.; Asgari Toni, S.; Tizro, N.; Dadkhah, P.A.; Amani, H.; Akhavan Rezayat, S.; Sheikh, Z.; Mohammadi, M.; Alijanzadeh, D.; Alimohammadi, F.; et al. Pharmacotherapeutic Potential of Pomegranate in Age-Related Neurological Disorders. Front. Aging Neurosci. 2022, 14, 955735. [Google Scholar] [CrossRef]
- Colizzi, C. The Protective Effects of Polyphenols on Alzheimer’s Disease: A Systematic Review. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 184–196. [Google Scholar] [CrossRef]
- Loren, D.J.; Seeram, N.P.; Schulman, R.N.; Holtzman, D.M. Maternal Dietary Supplementation with Pomegranate Juice Is Neuroprotective in an Animal Model of Neonatal Hypoxic-Ischemic Brain Injury. Pediatr. Res. 2005, 57, 858–864. [Google Scholar] [CrossRef] [PubMed]
- West, T.; Atzeva, M.; Holtzman, D.M. Pomegranate Polyphenols and Resveratrol Protect the Neonatal Brain against Hypoxic-Ischemic Injury. Dev. Neurosci. 2007, 29, 363–372. [Google Scholar] [CrossRef]
- Hartman, R.E.; Shah, A.; Fagan, A.M.; Schwetye, K.E.; Parsadanian, M.; Schulman, R.N.; Finn, M.B.; Holtzman, D.M. Pomegranate Juice Decreases Amyloid Load and Improves Behavior in a Mouse Model of Alzheimer’s Disease. Neurobiol. Dis. 2006, 24, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Hartman, R.E.; Izumi, Y.; Bales, K.R.; Paul, S.M.; Wozniak, D.F.; Holtzman, D.M. Treatment with an Amyloid-β Antibody Ameliorates Plaque Load, Learning Deficits, and Hippocampal Long-Term Potentiation in a Mouse Model of Alzheimer’s Disease. J. Neurosci. 2005, 25, 6213–6220. [Google Scholar] [CrossRef]
- Gaudreault, R.; Mousseau, N. Mitigating Alzheimer’s Disease with Natural Polyphenols: A Review. Curr. Alzheimer Res. 2019, 16, 529–543. [Google Scholar] [CrossRef]
- Subash, S.; Braidy, N.; Essa, M.M.; Zayana, A.-B.; Ragini, V.; Al-Adawi, S.; Al-Asmi, A.; Guillemin, G.J. Long-Term (15 Mo) Dietary Supplementation with Pomegranates from Oman Attenuates Cognitive and Behavioral Deficits in a Transgenic Mice Model of Alzheimer’s Disease. Nutrition 2015, 31, 223–229. [Google Scholar] [CrossRef]
- Ahmed, A.; Subaiea, M.; Eid, A.; Li, L.; Seeram, P.; Zawia, H. Pomegranate Extract Modulates Processing of Amyloid-β Precursor Protein in an Aged Alzheimer’s Disease Animal Model. Curr. Alzheimer Res. 2014, 11, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Rojanathammanee, L.; Puig, K.L.; Combs, C.K. Pomegranate Polyphenols and Extract Inhibit Nuclear Factor of Activated T-Cell Activity and Microglial Activation In Vitro and in a Transgenic Mouse Model of Alzheimer Disease. J. Nutr. 2013, 143, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Essa, M.M.; Subash, S.; Akbar, M.; Al-Adawi, S.; Guillemin, G.J. Long-Term Dietary Supplementation of Pomegranates, Figs and Dates Alleviate Neuroinflammation in a Transgenic Mouse Model of Alzheimer’s Disease. PLoS ONE 2015, 10, e0120964. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Lee, J.-H.; Heo, H.J.; Cho, H.Y.; Kim, H.K.; Kim, C.-J.; Kim, M.O.; Suh, S.H.; Shin, D.-H. Punica granatum Protects Against Oxidative Stress in PC12 Cells and Oxidative Stress-Induced Alzheimer’s Symptoms in Mice. J. Med. Food 2011, 14, 695–701. [Google Scholar] [CrossRef]
- Subash, S.; Essa, M.M.; Al-Asmi, A.; Al-Adawi, S.; Vaishnav, R.; Braidy, N.; Manivasagam, T.; Guillemin, G.J. Pomegranate from Oman Alleviates the Brain Oxidative Damage in Transgenic Mouse Model of Alzheimer’s Disease. J. Tradit. Complement. Med. 2014, 4, 232–238. [Google Scholar] [CrossRef]
- Braidy, N.; Essa, M.M.; Poljak, A.; Selvaraju, S.; Al-Adawi, S.; Manivasagm, T.; Thenmozhi, A.J.; Ooi, L.; Sachdev, P.; Guillemin, G.J. Consumption of Pomegranates Improves Synaptic Function in a Transgenic Mice Model of Alzheimer’s Disease. Oncotarget 2016, 7, 64589–64604. [Google Scholar] [CrossRef]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s Disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and Microglial Activation in Alzheimer Disease: Where Do We Go from Here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Weisman, D.; Hakimian, E.; Ho, G.J. Interleukins, Inflammation, and Mechanisms of Alzheimer’s Disease. Vitam. Horm. 2006, 74, 505–530. [Google Scholar] [CrossRef]
- Lyra e Silva, N.M.; Gonçalves, R.A.; Pascoal, T.A.; Lima-Filho, R.A.S.; Resende, E.d.P.F.; Vieira, E.L.M.; Teixeira, A.L.; de Souza, L.C.; Peny, J.A.; Fortuna, J.T.S.; et al. Pro-Inflammatory Interleukin-6 Signaling Links Cognitive Impairments and Peripheral Metabolic Alterations in Alzheimer’s Disease. Transl. Psychiatry 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Shaftel, S.S.; Kyrkanides, S.; Olschowka, J.A.; Miller, J.H.; Johnson, R.E.; O’Banion, M.K. Sustained Hippocampal IL-1β Overexpression Mediates Chronic Neuroinflammation and Ameliorates Alzheimer Plaque Pathology. J. Clin. Investig. 2007, 117, 1595–1604. [Google Scholar] [CrossRef]
- Nagamoto-Combs, K.; Combs, C.K. Microglial Phenotype Is Regulated by Activity of the Transcription Factor, NFAT (Nuclear Factor of Activated T Cells). J. Neurosci. 2010, 30, 9641–9646. [Google Scholar] [CrossRef]
- Mizuma, A.; Kim, J.Y.; Kacimi, R.; Stauderman, K.; Dunn, M.; Hebbar, S.; Yenari, M.A. Microglial Calcium Release-Activated Calcium Channel Inhibition Improves Outcome from Experimental Traumatic Brain Injury and Microglia-Induced Neuronal Death. J. Neurotrauma 2019, 36, 996–1007. [Google Scholar] [CrossRef]
- Lee, S.-I.; Kim, B.-S.; Kim, K.-S.; Lee, S.; Shin, K.-S.; Lim, J.-S. Immune-Suppressive Activity of Punicalagin via Inhibition of NFAT Activation. Biochem. Biophys. Res. Commun. 2008, 371, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Rivers-Auty, J.; Mather, A.E.; Peters, R.; Lawrence, C.B.; Brough, D.; Alzheimer’s Disease Neuroimaging Initiative. Anti-Inflammatories in Alzheimer’s Disease—Potential Therapy or Spurious Correlate? Brain Commun. 2020, 2, fcaa109. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Hée, V.F.V.; Bindels, L.B.; Backer, F.D.; Cani, P.D.; Delzenne, N.M. Polyphenol-Rich Extract of Pomegranate Peel Alleviates Tissue Inflammation and Hypercholesterolaemia in High-Fat Diet-Induced Obese Mice: Potential Implication of the Gut Microbiota. Br. J. Nutr. 2013, 109, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Winand, J.; Schneider, Y.-J. The Anti-Inflammatory Effect of a Pomegranate Husk Extract on Inflamed Adipocytes and Macrophages Cultivated Independently, but Not on the Inflammatory Vicious Cycle between Adipocytes and Macrophages. Food Funct. 2014, 5, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.; Gupta, K.; Rasheed, Z.; Khan, K.A.; Haqqi, T.M. Consumption of Hydrolyzable Tannins-Rich Pomegranate Extract Suppresses Inflammation and Joint Damage in Rheumatoid Arthritis. Nutrition 2008, 24, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Madreiter-Sokolowski, C.T.; Thomas, C.; Ristow, M. Interrelation between ROS and Ca2+ in Aging and Age-Related Diseases. Redox Biol. 2020, 36, 101678. [Google Scholar] [CrossRef]
- Lyras, L.; Cairns, N.J.; Jenner, A.; Jenner, P.; Halliwell, B. An Assessment of Oxidative Damage to Proteins, Lipids, and DNA in Brain from Patients with Alzheimer’s Disease. J. Neurochem. 2002, 68, 2061–2069. [Google Scholar] [CrossRef]
- Pirinççioğlu, M.; Kızıl, G.; Kızıl, M.; Kanay, Z.; Ketani, A. The Protective Role of Pomegranate Juice against Carbon Tetrachloride–Induced Oxidative Stress in Rats. Toxicol. Ind. Health 2014, 30, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Bekir, J.; Mars, M.; Souchard, J.P.; Bouajila, J. Assessment of Antioxidant, Anti-Inflammatory, Anti-Cholinesterase and Cytotoxic Activities of Pomegranate (Punica granatum) Leaves. Food Chem. Toxicol. 2013, 55, 470–475. [Google Scholar] [CrossRef]
- Agarwal, S.; Tannenberg, R.K.; Dodd, P.R. Reduced Expression of the Inhibitory Synapse Scaffolding Protein Gephyrin in Alzheimer’s Disease. J. Alzheimer’s Dis. 2008, 14, 313–321. [Google Scholar] [CrossRef]
- Kallop, D.Y.; Meilandt, W.J.; Gogineni, A.; Easley-Neal, C.; Wu, T.; Jubb, A.M.; Yaylaoglu, M.; Shamloo, M.; Tessier-Lavigne, M.; Scearce-Levie, K.; et al. A Death Receptor 6-Amyloid Precursor Protein Pathway Regulates Synapse Density in the Mature CNS But Does Not Contribute to Alzheimer’s Disease-Related Pathophysiology in Murine Models. J. Neurosci. 2014, 34, 6425–6437. [Google Scholar] [CrossRef]
- Sotiropoulos, I.; Sousa, N. Tau as the Converging Protein between Chronic Stress and Alzheimer’s Disease Synaptic Pathology. Neurodegener. Dis. 2015, 16, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, A.; Branca, C.; Talboom, J.S.; Shaw, D.M.; Turner, D.; Ma, L.; Messina, A.; Huang, Z.; Wu, J.; Oddo, S. Reducing Ribosomal Protein S6 Kinase 1 Expression Improves Spatial Memory and Synaptic Plasticity in a Mouse Model of Alzheimer’s Disease. J. Neurosci. 2015, 35, 14042–14056. [Google Scholar] [CrossRef]
- Pollizzi, K.N.; Powell, J.D. Regulation of T Cells by MTOR: The Known Knowns and the Known Unknowns. Trends Immunol. 2015, 36, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Cassé, F.; Zhou, Y.; Zhou, M.; Xiong, Z.-Q.; Joëls, M.; Martin, S.; Krugers, H.J. MTOR Is Essential for Corticosteroid Effects on Hippocampal AMPA Receptor Function and Fear Memory. Learn. Mem. 2015, 22, 577–583. [Google Scholar] [CrossRef]
- Su, Z.-W.; Liao, J.; Zhang, H.; Zhang, T.; Wu, F.; Tian, X.-H.; Zhang, F.-T.; Sun, W.-W.; Cui, Q. Postnatal High-Protein Diet Improves Learning and Memory in Premature Rats via Activation of MTOR Signaling. Brain Res. 2015, 1611, 1–7. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Akter, R.; Afrose, A.; Rahman, M.R.; Chowdhury, R.; Nirzhor, S.S.R.; Khan, R.I.; Kabir, M.T. A Comprehensive Analysis into the Therapeutic Application of Natural Products as SIRT6 Modulators in Alzheimer’s Disease, Aging, Cancer, Inflammation, and Diabetes. Int. J. Mol. Sci. 2021, 22, 4180. [Google Scholar] [CrossRef] [PubMed]
- Pimpão, R.C.; Ventura, M.R.; Ferreira, R.B.; Williamson, G.; Santos, C.N. Phenolic Sulfates as New and Highly Abundant Metabolites in Human Plasma after Ingestion of a Mixed Berry Fruit Purée. Br. J. Nutr. 2015, 113, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.L.; Kirk, R.D.; DaSilva, N.A.; Ma, H.; Seeram, N.P.; Bertin, M.J. Polyphenol Microbial Metabolites Exhibit Gut and Blood–Brain Barrier Permeability and Protect Murine Microglia against LPS-Induced Inflammation. Metabolites 2019, 9, 78. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, W.; Zhang, L.; Wang, M.; Chang, W. The Interaction of Polyphenols and the Gut Microbiota in Neurodegenerative Diseases. Nutrients 2022, 14, 5373. [Google Scholar] [CrossRef]
- Lipińska, L.; Klewicka, E.; Sójka, M. The Structure, Occurrence and Biological Activity of Ellagitannins: A General Review. Acta Sci. Pol. Technol. Aliment. 2014, 13, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Francisco Marquez, M.; Pérez-González, A. Ellagic Acid: An Unusually Versatile Protector against Oxidative Stress. Chem. Res. Toxicol. 2014, 27, 904–918. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortés-Martín, A.; Ávila-Gálvez, M.Á.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Urolithins: A Comprehensive Update on Their Metabolism, Bioactivity, and Associated Gut Microbiota. Mol. Nutr. Food Res. 2022, 66, 2101019. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The Gut Microbiota–Brain Axis in Behaviour and Brain Disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Tow, W.-K.; Chee, P.-Y.; Sundralingam, U.; Palanisamy, U.D. The Therapeutic Relevance of Urolithins, Intestinal Metabolites of Ellagitannin-Rich Food: A Systematic Review of In Vivo Studies. Nutrients 2022, 14, 3494. [Google Scholar] [CrossRef]
- Jayatunga, D.P.W.; Hone, E.; Khaira, H.; Lunelli, T.; Singh, H.; Guillemin, G.J.; Fernando, B.; Garg, M.L.; Verdile, G.; Martins, R.N. Therapeutic Potential of Mitophagy-Inducing Microflora Metabolite, Urolithin A for Alzheimer’s Disease. Nutrients 2021, 13, 3744. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Ma, H.; Liu, W.; Niesen, D.B.; Shah, N.; Crews, R.; Rose, K.N.; Vattem, D.A.; Seeram, N.P. Pomegranate’s Neuroprotective Effects against Alzheimer’s Disease Are Mediated by Urolithins, Its Ellagitannin-Gut Microbial Derived Metabolites. ACS Chem. Neurosci. 2016, 7, 26–33. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, D.; Andreux, P.A.; Valdés, P.; Singh, A.; Rinsch, C.; Auwerx, J. Impact of the Natural Compound Urolithin A on Health, Disease, and Aging. Trends Mol. Med. 2021, 27, 687–699. [Google Scholar] [CrossRef] [PubMed]
- DaSilva, N.A.; Nahar, P.P.; Ma, H.; Eid, A.; Wei, Z.; Meschwitz, S.; Zawia, N.H.; Slitt, A.L.; Seeram, N.P. Pomegranate Ellagitannin-Gut Microbial-Derived Metabolites, Urolithins, Inhibit Neuroinflammation in Vitro. Nutr. Neurosci. 2019, 22, 185–195. [Google Scholar] [CrossRef]
- Esselun, C.; Theyssen, E.; Eckert, G.P. Effects of Urolithin A on Mitochondrial Parameters in a Cellular Model of Early Alzheimer Disease. Int. J. Mol. Sci. 2021, 22, 8333. [Google Scholar] [CrossRef] [PubMed]

| Animal Model | Diet Supplementation | Effects | Reference |
|---|---|---|---|
| APPsw/Tg2576 | Pomegranate juice | ↓ Aβ deposit ↑ cognitive function | [79] |
| APPsw/Tg2576 | Oman pomegranate | ↓ memory deficit ↓anxiety ↑ motor coordination | [82] |
| R1.40 | Pomegranate extract | ↓ Aβ deposit | [83] |
| APP/PS1 | Pomegranate extract | ↓ Aβ-stimulated ↓ TNF-α | [84] |
| APPsw/Tg2576 | Pomegranate | ↓ TNF-α ↓ IL-1β ↓ IL-6 | [85] |
| ICR mice Injected with Aβ1-42 | Pomegranate extract | ↓ neuronal cell death | [86] |
| APPsw/Tg2576 | Pomegranate juice (Oman) | ↓ MDA ↓ protein carbonyl ↓ AChE ↑ SOD ↑GPx ↑GSH ↑ GST | [87] |
| APPsw/Tg2576 | Pomegranate extract | ↑ synaptic proteins ↓TNF-α ↓IL-1β ↓IL-10β ↓iNOS ↓CCL2 ↓bcl1 ↓LC-3 | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciccone, L.; Nencetti, S.; Rossello, A.; Orlandini, E. Pomegranate: A Source of Multifunctional Bioactive Compounds Potentially Beneficial in Alzheimer’s Disease. Pharmaceuticals 2023, 16, 1036. https://doi.org/10.3390/ph16071036
Ciccone L, Nencetti S, Rossello A, Orlandini E. Pomegranate: A Source of Multifunctional Bioactive Compounds Potentially Beneficial in Alzheimer’s Disease. Pharmaceuticals. 2023; 16(7):1036. https://doi.org/10.3390/ph16071036
Chicago/Turabian StyleCiccone, Lidia, Susanna Nencetti, Armando Rossello, and Elisabetta Orlandini. 2023. "Pomegranate: A Source of Multifunctional Bioactive Compounds Potentially Beneficial in Alzheimer’s Disease" Pharmaceuticals 16, no. 7: 1036. https://doi.org/10.3390/ph16071036
APA StyleCiccone, L., Nencetti, S., Rossello, A., & Orlandini, E. (2023). Pomegranate: A Source of Multifunctional Bioactive Compounds Potentially Beneficial in Alzheimer’s Disease. Pharmaceuticals, 16(7), 1036. https://doi.org/10.3390/ph16071036