Drug Discovery and Development Targeting Dementia
Abstract
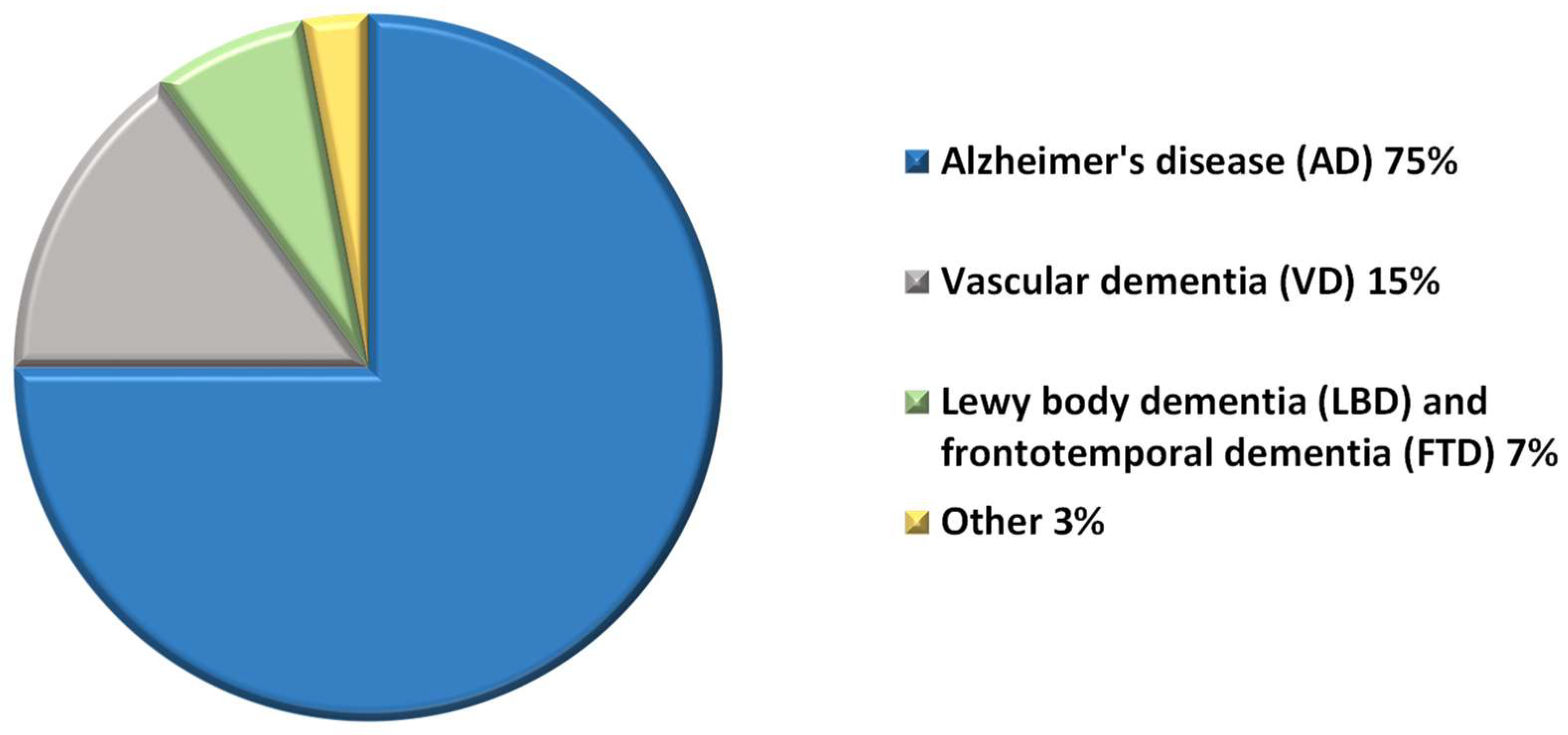
1. Introduction
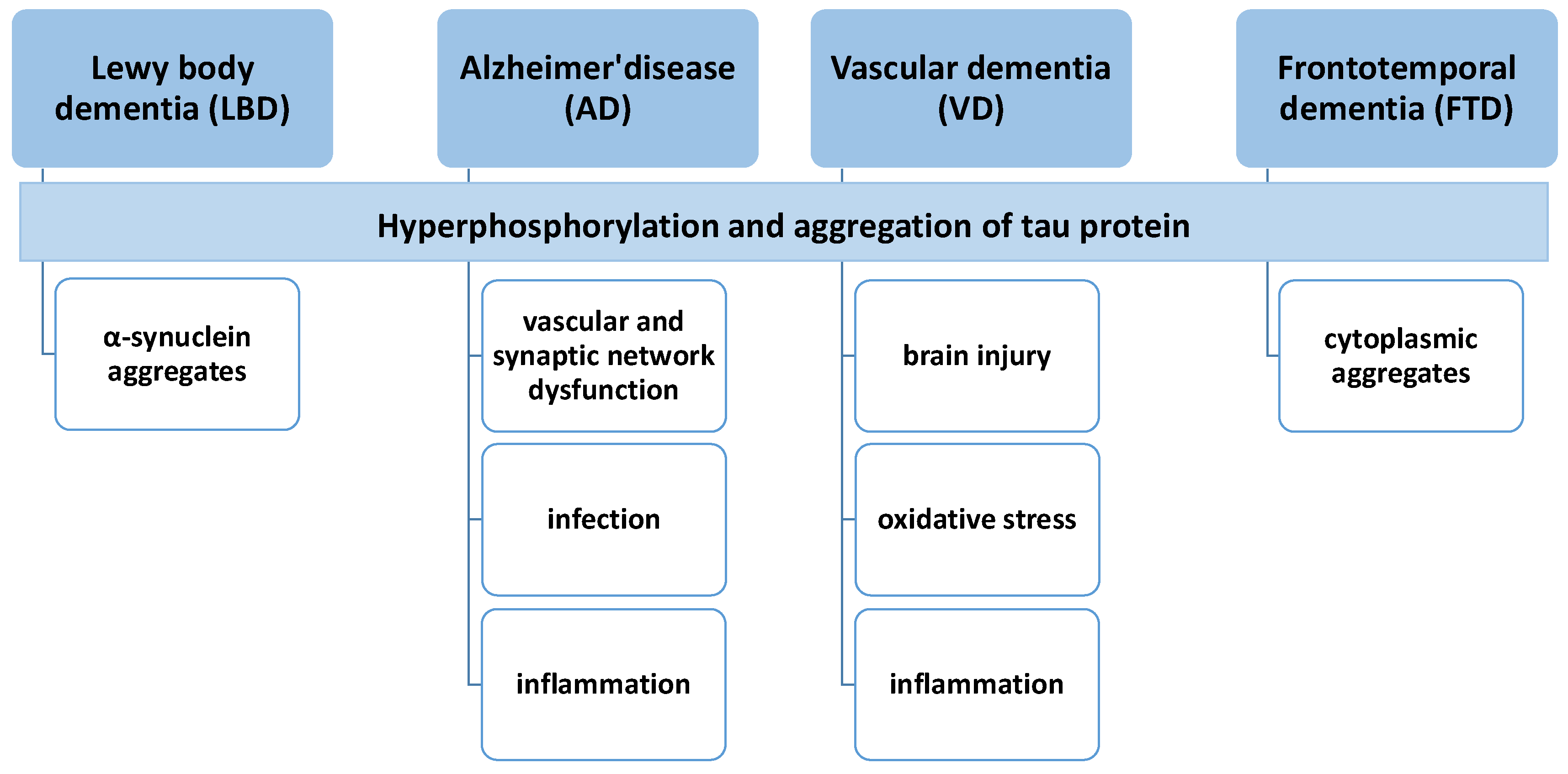
2. Etiology and Pathophysiology
3. Treatment of Dementia
4. Drug Discovery and Development for Dementia
4.1. Drug Repurposing
4.2. Small Molecules
4.3. Multi-Target-Directed Ligands (MTDLs)
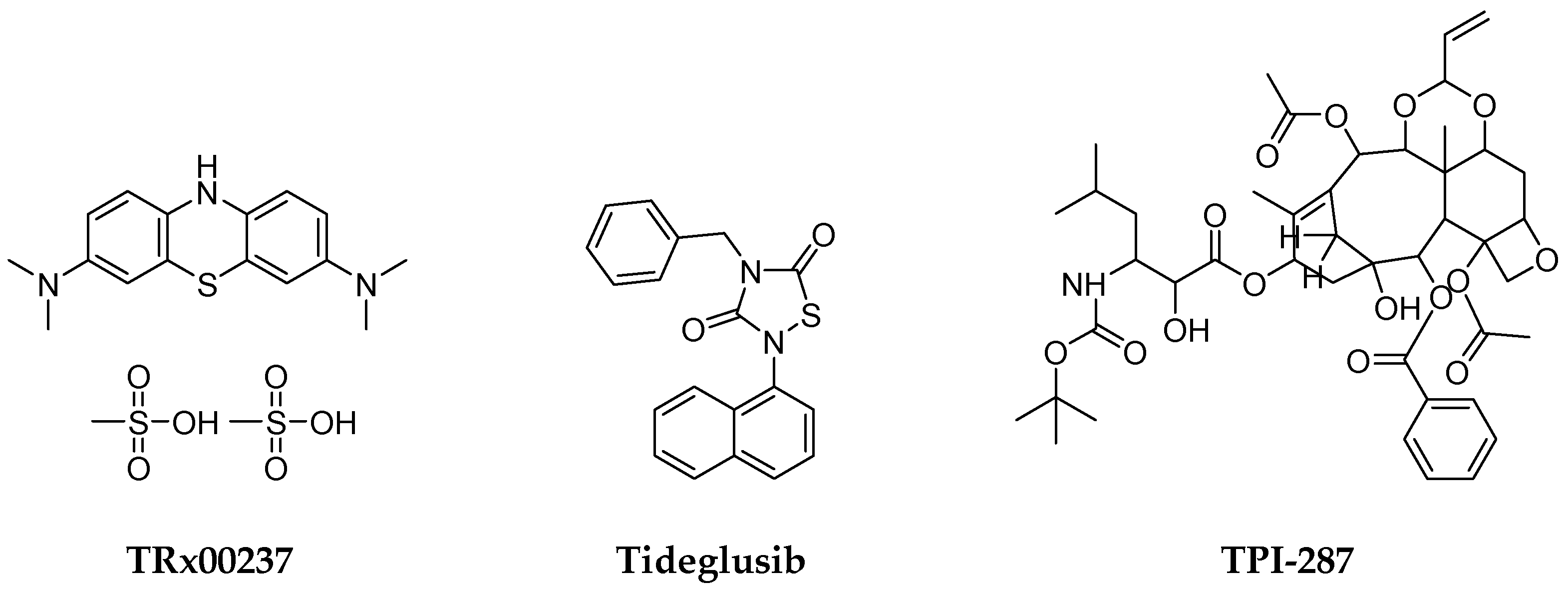
4.4. Disease-Modifying Therapies
4.5. Nanotechnology-Based Approaches
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emmady, P.D.; Tadi, P. Dementia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, January 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557444/ (accessed on 8 May 2022).
- Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 21 August 2022).
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the Global Prevalence of Dementia in 2019 and Forecasted Prevalence in 2050: An Analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Kivipelto, M.; Von Strauss, E. Epidemiology of Alzheimer’s Disease: Occurrence, Determinants, and Strategies toward Intervention. Dialogues Clin. Neurosci. 2022, 11, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Duong, S.; Patel, T.; Chang, F. Dementia: What pharmacists need to know. Can. Pharm. J. 2017, 150, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/parkinsons-disease/parkinsons-disease-and-dementia (accessed on 22 August 2022).
- Bir, S.C.; Khan, M.W.; Javalkar, V.; Toledo, E.G.; Kelley, R.E. Emerging Concepts in Vascular Dementia: A Review. J. Stroke Cerebrovasc. Dis. 2021, 30, 105864. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s Disease: The Amyloid Cascade Hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, R.H.; Khan, S.M. A “Mitochondrial Cascade Hypothesis” for Sporadic Alzheimer’s Disease. Med. Hypotheses 2004, 63, 8–20. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, C.H.; Cha, S.H.; Lee, J.H.; Lee, S.; Kim, Y.; Rah, J.C.; Jeong, S.J.; Suh, Y.H. Carboxyl-Terminal Fragment of Alzheimer’s APP Destabilizes Calcium Homeostasis and Renders Neuronal Cells Vulnerable to Excitotoxicity. FASEB J. 2000, 14, 1508–1517. [Google Scholar]
- McGeer, P.L.; Rogers, J. Anti-inflammatory Agents as a Therapeutic Approach to Alzheimer’s Disease. Neurology 1992, 42, 447. [Google Scholar] [CrossRef]
- Iadecola, C. Neurovascular Regulation in the Normal Brain and in Alzheimer’s Disease. Nat. Rev. Neurosci. 2004, 5, 347–360. [Google Scholar] [CrossRef]
- Lyubartseva, G.; Lovell, M.A. A Potential Role for Zinc Alterations in the Pathogenesis of Alzheimer’s Disease. BioFactors 2012, 38, 98–106. [Google Scholar] [CrossRef]
- Papadopoulos, Z.; Herz, J.; Kipnis, J. Meningeal Lymphatics: From Anatomy to Central Nervous System Immune Surveillance. J. Immunol. 2020, 204, 286–293. [Google Scholar] [CrossRef]
- Mullane, K.; Williams, M. Alzheimer’s Disease beyond Amyloid: Can the Repetitive Failures of Amyloid-Targeted Therapeutics Inform Future Approaches to Dementia Drug Discovery? Biochem. Pharmacol. 2020, 177, 113945. [Google Scholar] [CrossRef]
- Schneider, L. A Resurrection of Aducanumab for Alzheimer’s Disease. Lancet Neurol. 2020, 19, 111–112. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer Disease and Aducanumab: Adjusting Our Approach. Nat. Rev. Neurol. 2019, 15, 365–366. [Google Scholar] [CrossRef]
- Available online: https://www.science.org/content/blog-post/faked-beta-amyloid-data-what-does-it-mean (accessed on 22 August 2022).
- Garcia-Esparcia, P.; López-González, I.; Grau-Rivera, O.; García-Garrido, M.F.; Konetti, A.; Llorens, F.; Zafar, S.; Carmona, M.; del Rio, J.A.; Zerr, I.; et al. Dementia with Lewy Bodies: Molecular Pathology in the Frontal Cortex in Typical and Rapidly Progressive Forms. Front. Neurol. 2017, 8, 89. [Google Scholar] [CrossRef]
- Tofaris, G.K.; Razzaq, A.; Ghetti, B.; Lilley, K.S.; Spillantini, M.G. Ubiquitination of Alpha-Synuclein in Lewy Bodies Is a Pathological Event Not Associated with Impairment of Proteasome Function. J. Biol. Chem. 2003, 278, 44405–44411. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.; Ling, H.; Lashley, T.; Foley, J.A.; Strand, C.; Eid, T.M.; Holton, J.L.; Warner, T.T. Novel Clinicopathological Characteristics Differentiate Dementia with Lewy Bodies from Parkinson’s Disease Dementia. Neuropathol. Appl. Neurobiol. 2021, 47, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D. Epidemiology and Pathophysiology of Dementia-Related Psychosis. J. Clin. Psychiatry 2020, 81, 27625. [Google Scholar] [CrossRef] [PubMed]
- De Conti, L.; Borroni, B.; Baralle, M. New Routes in Frontotemporal Dementia Drug Discovery. Expert Opin. Drug Discov. 2017, 12, 659–671. [Google Scholar] [CrossRef]
- Iqbal, K.; Liu, F.; Gong, C.-X.; Grundke-Iqbal, I. Tau in Alzheimer Disease and Related Tauopathies. Curr. Alzheimer Res. 2010, 7, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef]
- Nolan, M.; Talbot, K.; Ansorge, O. Pathogenesis of FUS-Associated ALS and FTD: Insights from Rodent Models. Acta Neuropathol. Commun. 2016, 4, 99. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Rademakers, R.; Roeber, S.; Baker, M.; Kretzschmar, H.A.; MacKenzie, I.R.A. A New Subtype of Frontotemporal Lobar Degeneration with FUS Pathology. Brain 2009, 132, 2922–2931. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.L.; Topp, S.; Yang, S.; Smith, B.; Fifita, J.A.; Warraich, S.T.; Zhang, K.Y.; Farrawell, N.; Vance, C.; Hu, X.; et al. CCNF Mutations in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Nat. Commun. 2016, 7, 11253. [Google Scholar] [CrossRef] [PubMed]
- Kuret, J.; Chirita, C.N.; Congdon, E.E.; Kannanayakal, T.; Li, G.; Necula, M.; Yin, H.; Zhong, Q. Pathways of Tau Fibrillization. Biochim. Biophys. Acta 2005, 1739, 167–178. [Google Scholar] [CrossRef]
- Kalaria, R.N. Neuropathological Diagnosis of Vascular Cognitive Impairment and Vascular Dementia with Implications for Alzheimer’s Disease. Acta Neuropathol. 2016, 131, 659–685. [Google Scholar] [CrossRef]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of Acetylcholinesterase Inhibitors in Alzheimer’s Disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Blanco-Silvente, L.; Capellà, D.; Garre-Olmo, J.; Vilalta-Franch, J.; Castells, X. Predictors of Discontinuation, Efficacy, and Safety of Memantine Treatment for Alzheimer’s Disease: Meta-Analysis and Meta-Regression of 18 Randomized Clinical Trials Involving 5004 Patients. BMC Geriatr. 2018, 18, 168. [Google Scholar] [CrossRef]
- Farlow, M.R.; Miller, M.L.; Pejovic, V. Treatment Options in Alzheimer’s Disease: Maximizing Benefit, Managing Expectations. Dement Geriatr. Cogn. Disord. 2008, 25, 408–422. [Google Scholar] [CrossRef]
- Moretti, A.; Gorini, A.; Villa, R.F. Pharmacotherapy and Prevention of Vascular Dementia. CNS Neurol. Disord. Drug Targets 2011, 10, 370–390. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/ct2/show/NCT04002674 (accessed on 28 September 2022).
- George, S.; Brundin, P. Immunotherapy in Parkinson’s Disease: Micromanaging Alpha-Synuclein Aggregation. J. Park. Dis. 2015, 5, 413–424. [Google Scholar] [CrossRef]
- Schneeberger, A.; Tierney, L.; Mandler, M. Active Immunization Therapies for Parkinson’s Disease and Multiple System Atrophy. Mov. Disord. 2016, 31, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Weihofen, A.; Liu, Y.T.; Arndt, J.W.; Huy, C.; Quan, C.; Smith, B.A.; Baeriswyl, J.L.; Cavegn, N.; Senn, L.; Su, L.; et al. Development of an Aggregate-Selective, Human-Derived α-Synuclein Antibody BIIB054 That Ameliorates Disease Phenotypes in Parkinson’s Disease Models. Neurobiol. Dis. 2019, 124, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.clinicaltrials.gov/ct2/show/NCT01056965 (accessed on 29 September 2022).
- Cerejeira, J.; Lagarto, L.; Mukaetova-Ladinska, E.B. Behavioral and Psychological Symptoms of Dementia. Front. Neurol. 2012, 3, 73. [Google Scholar] [CrossRef] [PubMed]
- Tible, O.P.; Riese, F.; Savaskan, E.; Von Gunten, A. Best Practice in the Management of Behavioural and Psychological Symptoms of Dementia. Ther. Adv. Neurol. Disord. 2017, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, L.B.; Kales, H.C. Pharmacological Management of Neuropsychiatric Symptoms of Dementia. Curr. Treat Options Psychiatry 2020, 7, 489. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://aasm.org/fda-updates-insomnia-medication-info-to-include-alzheimers-study/ (accessed on 24 August 2022).
- Wang, C.; Wang, Q.; Ji, B.; Pan, Y.; Xu, C.; Cheng, B.; Bai, B.; Chen, J. The Orexin/Receptor System: Molecular Mechanism and Therapeutic Potential for Neurological Diseases. Front. Mol. Neurosci. 2018, 11, 220. [Google Scholar] [CrossRef]
- Um, Y.H.; Lim, H.K. Orexin and Alzheimer’s Disease: A New Perspective. Psychiatry Investig. 2020, 17, 616. [Google Scholar] [CrossRef]
- Geifman, N.; Kennedy, R.E.; Schneider, L.S.; Buchan, I.; Brinton, R.D. Data-Driven Identification of Endophenotypes of Alzheimer’s Disease Progression: Implications for Clinical Trials and Therapeutic Interventions. Alzheimer’s Res. Ther. 2018, 10, 4. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, P.; Wang, Q.; Chiang, C.W.; Zhou, Y.; Hou, Y.; Xu, J.; Chen, R.; Zhang, B.; Lewis, S.J.; et al. Artificial Intelligence Framework Identifies Candidate Targets for Drug Repurposing in Alzheimer’s Disease. Alzheimer’s Res. Ther. 2022, 14, 7. [Google Scholar] [CrossRef]
- Lee, C.J.; Lee, J.Y.; Han, K.; Kim, D.H.; Cho, H.; Kim, K.J.; Kang, E.S.; Cha, B.S.; Lee, Y.H.; Park, S. Blood Pressure Levels and Risks of Dementia: A Nationwide Study of 4.5 Million People. Hypertension 2022, 79, 218–229. [Google Scholar] [CrossRef]
- Hebron, M.L.; Lonskaya, I.; Moussa, C.E.H. Nilotinib Reverses Loss of Dopamine Neurons and Improves Motor Behavior via Autophagic Degradation of α-Synuclein in Parkinson’s Disease Models. Hum. Mol. Genet. 2013, 22, 3315–3328. [Google Scholar] [CrossRef] [PubMed]
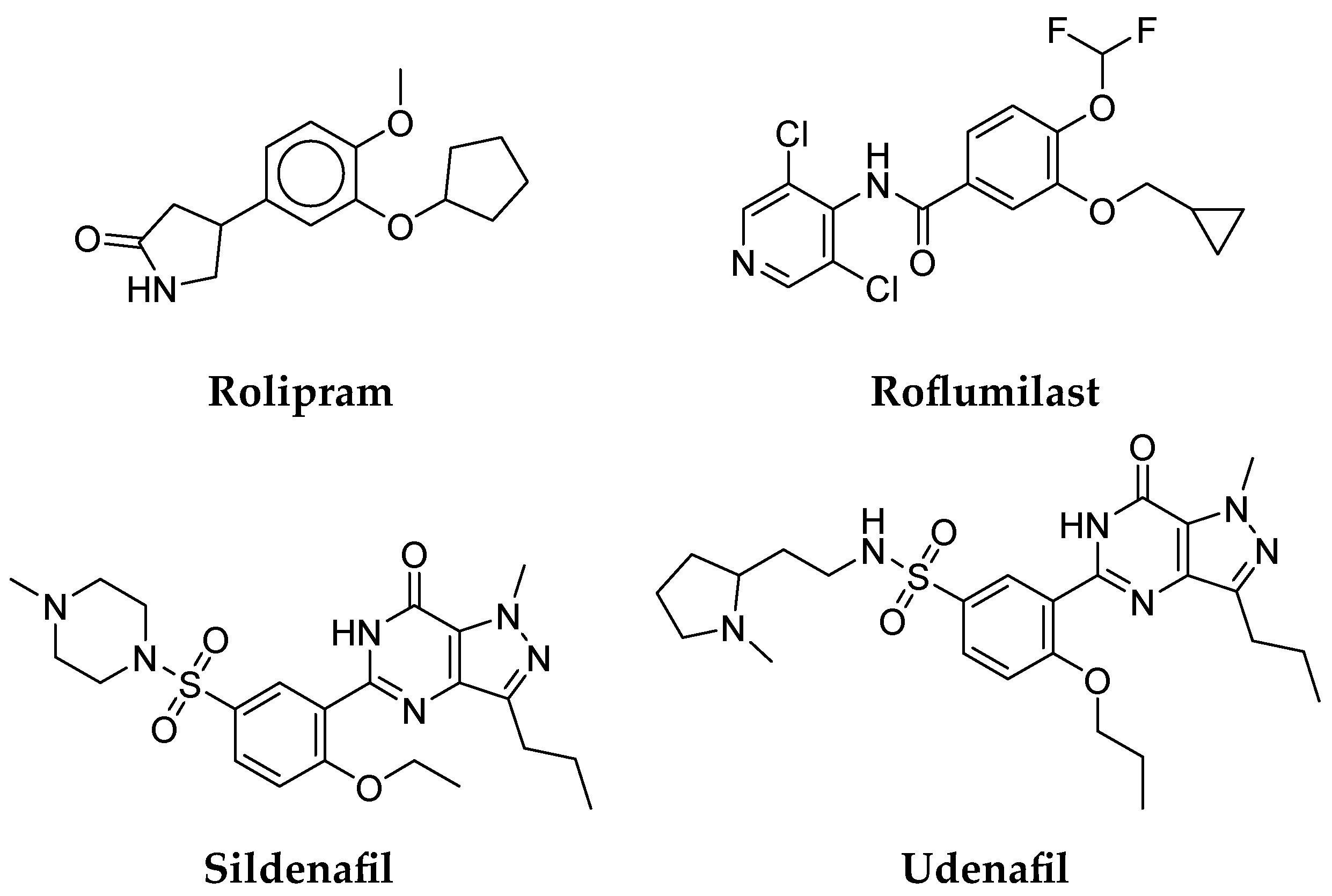
- Medina, A.E. Therapeutic Utility of Phosphodiesterase Type I Inhibitors in Neurological Conditions. Front. Neurosci. 2011, 5, 21. [Google Scholar] [CrossRef]
- Gurney, M.E.; D’Amato, E.C.; Burgin, A.B. Phosphodiesterase-4 (PDE4) Molecular Pharmacology and Alzheimer’s Disease. Neurotherapeutics 2015, 12, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Siuciak, J.A.; Chapin, D.S.; McCarthy, S.A.; Martin, A.N. Antipsychotic Profile of Rolipram: Efficacy in Rats and Reduced Sensitivity in Mice Deficient in the Phosphodiesterase-4B (PDE4B) Enzyme. Psychopharmacology 2007, 192, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Clapcote, S.J. Phosphodiesterase-4B as a Therapeutic Target for Cognitive Impairment and Obesity-Related Metabolic Diseases. Adv. Neurobiol. 2017, 17, 103–131. [Google Scholar]
- Sebastiani, G.; Morissette, C.; Lagacé, C.; Boulé, M.; Ouellette, M.J.; McLaughlin, R.W.; Lacombe, D.; Gervais, F.; Tremblay, P. The CAMP-Specific Phosphodiesterase 4B Mediates Aβ-Induced Microglial Activation. Neurobiol. Aging 2006, 27, 691–701. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, N.; Xu, W.; Ye, H.; Liu, K.; Wu, F.; Zhang, M.; Ding, Y.; Zhang, C.; Zhang, H.; et al. Inhibition of Phosphodiesterase-4 Reverses Aβ-Induced Memory Impairment by Regulation of HPA Axis Related CAMP Signaling. Front. Aging Neurosci. 2018, 10, 204. [Google Scholar] [CrossRef]
- Wang, G.; Chen, L.; Pan, X.; Chen, J.; Wang, L.; Wang, W.; Cheng, R.; Wu, F.; Feng, X.; Yu, Y.; et al. The Effect of Resveratrol on Beta Amyloid-Induced Memory Impairment Involves Inhibition of Phosphodiesterase-4 Related Signaling. Oncotarget 2016, 7, 17380. [Google Scholar] [CrossRef]
- Vanmierlo, T.; Creemers, P.; Akkerman, S.; van Duinen, M.; Sambeth, A.; de Vry, J.; Uz, T.; Blokland, A.; Prickaerts, J. The PDE4 Inhibitor Roflumilast Improves Memory in Rodents at Non-Emetic Doses. Behav. Brain Res. 2016, 303, 26–33. [Google Scholar] [CrossRef]
- Gilleen, J.; Farah, Y.; Davison, C.; Kerins, S.; Valdearenas, L.; Uz, T.; Lahu, G.; Tsai, M.; Ogrinc, F.; Reichenberg, A.; et al. An Experimental Medicine Study of the Phosphodiesterase-4 Inhibitor, Roflumilast, on Working Memory-Related Brain Activity and Episodic Memory in Schizophrenia Patients. Psychopharmacology 2021, 238, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Heckman, P.R.A.; van Duinen, M.A.; Blokland, A.; Uz, T.; Prickaerts, J.; Sambeth, A. Acute Administration of Roflumilast Enhances Sensory Gating in Healthy Young Humans in a Randomized Trial. Psychopharmacology 2018, 235, 301–308. [Google Scholar] [CrossRef]
- Sanders, O. Sildenafil for the Treatment of Alzheimer’s Disease: A Systematic Review. J. Alzheimer’s Dis. Rep. 2020, 4, 91–106. [Google Scholar] [CrossRef]
- Zuccarello, E.; Acquarone, E.; Calcagno, E.; Argyrousi, E.K.; Deng, S.X.; Landry, D.W.; Arancio, O.; Fiorito, J. Development of Novel Phosphodiesterase 5 Inhibitors for the Therapy of Alzheimer’s Disease. Biochem. Pharmacol. 2020, 176, 113818. [Google Scholar] [CrossRef]
- Grass, H.; Klotz, T.; Fathian-Sabet, B.; Berghaus, G.; Engelmann, U.; Käferstein, H. Sildenafil (Viagra®): Is There an Influence on Psychological Performance? Int. Urol. Nephrol. 2001, 32, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.C.; Cather, C.; Freudenreich, O.; Henderson, D.C.; Evins, A.E.; Culhane, M.A.; Walsh, J.P. A Placebo-Controlled Study of Sildenafil Effects on Cognition in Schizophrenia. Psychopharmacology 2009, 202, 411. [Google Scholar] [CrossRef] [PubMed]
- Safarinejad, M.R.; Taghva, A.; Shekarchi, B.; Safarinejad, S. Safety and Efficacy of Sildenafil Citrate in the Treatment of Parkinson-Emergent Erectile Dysfunction: A Double-Blind, Placebo-Controlled, Randomized Study. Int. J. Impot. Res. 2010, 22, 325–335. [Google Scholar] [CrossRef]
- Cheng, F.; Fang, J.; Zhang, P.; Zhou, Y.; Chiang, C.-W.; Pieper, A.A.; Cummings, J.L. Sildenafil Reduces the Incidence of Alzheimer’s Disease. Alzheimer’s Dement. 2021, 17, e051847. [Google Scholar] [CrossRef]
- Shim, Y.S.; Pae, C.U.; Kim, S.W.; Kim, H.W.; Kim, J.C.; Koh, J.S. Effects of Repeated Dosing with Udenafil (Zydena) on Cognition, Somatization and Erection in Patients with Erectile Dysfunction: A Pilot Study. Int. J. Impot. Res. 2011, 23, 109–114. [Google Scholar] [CrossRef]
- van Bokhoven, P.; de Wilde, A.; Vermunt, L.; Leferink, P.S.; Heetveld, S.; Cummings, J.; Scheltens, P.; Vijverberg, E.G.B. The Alzheimer’s Disease Drug Development Landscape. Alzheimer’s Res. Ther. 2021, 13, 186. [Google Scholar] [CrossRef]
- Grill, J.D.; Cummings, J.L. Novel Targets for Alzheimer’s Disease Treatment. Expert Rev. Neurother. 2010, 10, 711. [Google Scholar] [CrossRef] [PubMed]
- Loera-Valencia, R.; Cedazo-Minguez, A.; Kenigsberg, P.A.; Page, G.; Duarte, A.I.; Giusti, P.; Zusso, M.; Robert, P.; Frisoni, G.B.; Cattaneo, A.; et al. Current and Emerging Avenues for Alzheimer’s Disease Drug Targets. J. Intern. Med. 2019, 286, 398–437. [Google Scholar] [CrossRef] [PubMed]
- Loera-Valencia, R.; Piras, A.; Ismail, M.A.M.; Manchanda, S.; Eyjolfsdottir, H.; Saido, T.C.; Johansson, J.; Eriksdotter, M.; Winblad, B.; Nilsson, P. Targeting Alzheimer’s Disease with Gene and Cell Therapies. J. Intern. Med. 2018, 284, 2–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zou, Z.; Li, Q. Nicotinic Acid Supplementation Contributes to the Amelioration of Alzheimer’s Disease in Mouse Models. Ann. Transl. Med. 2022, 10, 1049. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.Z.; Zheng, X.M.; Zhou, Y.F.; Yun, L.Y.; Luo, D.M.; Hao, J.J.; Liu, P.F.; Zhang, W.K.; Xu, J.K.; Yan, Y.; et al. Cornuside Is a Potential Agent against Alzheimer’s Disease via Orchestration of Reactive Astrocytes. Nutrients 2022, 14, 3179. [Google Scholar] [CrossRef]
- Zagórska, A.; Jaromin, A. Perspectives for New and More Efficient Multifunctional Ligands for Alzheimer′s Disease Therapy. Molecules 2020, 25, 3337. [Google Scholar] [CrossRef]
- Strempfl, K.; Unger, M.S.; Flunkert, S.; Trost, A.; Reitsamer, H.A.; Hutter-Paier, B.; Aigner, L. Leukotriene Signaling as a Target in α-Synucleinopathies. Biomolecules 2022, 12, 346. [Google Scholar]
- Jellinger, K.A. Dementia with Lewy Bodies and Parkinson’s Disease-Dementia: Current Concepts and Controversies. J. Neural. Transm. 2018, 125, 615–650. [Google Scholar] [CrossRef]
- Lee, G.; Cummings, J.; Decourt, B.; Leverenz, J.B.; Sabbagh, M.N. Clinical Drug Development for Dementia with Lewy Bodies: Past and Present. Expert Opin. Investig. Drugs 2019, 28, 951–965. [Google Scholar] [CrossRef]
- Pope, E.D.; Cordes, L.; Shi, J.; Mari, Z.; Decourt, B.; Sabbagh, M.N. Dementia with Lewy Bodies: Emerging Drug Targets and Therapeutics. Expert Opin. Investig. Drugs 2021, 30, 603–609. [Google Scholar] [CrossRef]
- Khoury, R.; Liu, Y.; Sheheryar, Q.; Grossberg, G.T. Pharmacotherapy for Frontotemporal Dementia. CNS Drugs 2021, 35, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Tsai, R.M.; Boxer, A.L. Therapy and Clinical Trials in Frontotemporal Dementia: Past, Present, and Future. J. Neurochem. 2016, 138, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Lozupone, M.; Seripa, D.; Daniele, A.; Watling, M.; Giannelli, G.; Imbimbo, B.P. Development of Disease-Modifying Drugs for Frontotemporal Dementia Spectrum Disorders. Nat. Rev. Neurol. 2020, 16, 213–228. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Sudarshan, R.; Chatterjee, J. Investigating Drug–Target Interactions in Frontotemporal Dementia Using a Network Pharmacology Approach. Beni Suef. Univ. J. Basic Appl. Sci. 2021, 10, 56. [Google Scholar] [CrossRef]
- Linh, T.T.D.; Hsieh, Y.C.; Huang, L.K.; Hu, C.J. Clinical Trials of New Drugs for Vascular Cognitive Impairment and Vascular Dementia. Int. J. Mol. Sci. 2022, 23, 11067. [Google Scholar] [CrossRef]
- Lagunin, A.A.; Ivanov, S.M.; Gloriozova, T.A.; Pogodin, P.V.; Filimonov, D.A.; Kumar, S.; Goel, R.K. Combined Network Pharmacology and Virtual Reverse Pharmacology Approaches for Identification of Potential Targets to Treat Vascular Dementia. Sci. Rep. 2020, 10, 257. [Google Scholar] [CrossRef]
- Gomazkov, O.A.; Lagunin, A.A. Vascular Dementia: Molecular Targets of Neuroprotective Therapy. Biol. Bull. Rev. 2017, 7, 528–536. [Google Scholar] [CrossRef]
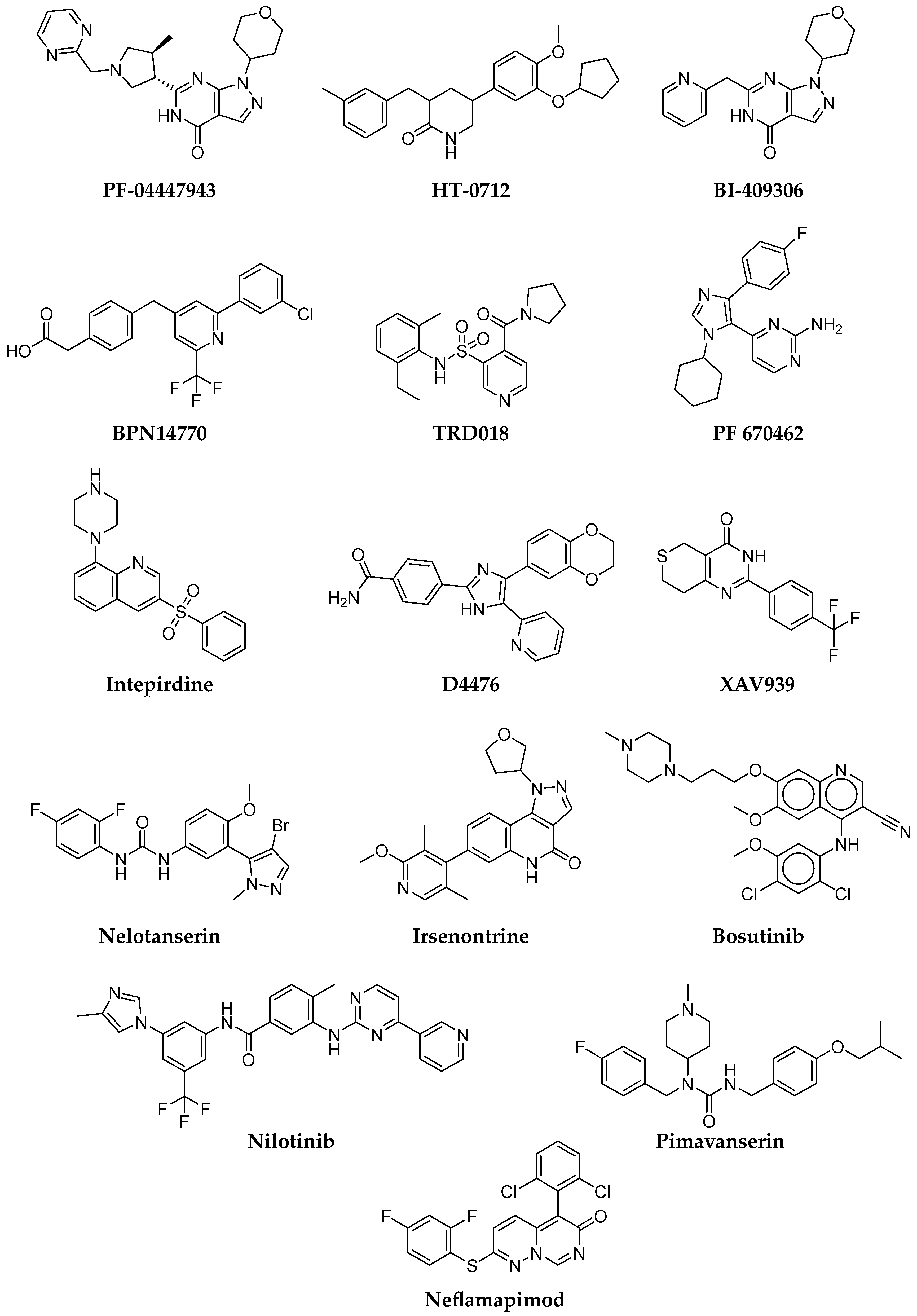
- Peters, M.; Bletsch, M.; Stanley, J.; Wheeler, D.; Scott, R.; Tully, T. The PDE4 Inhibitor HT-0712 Improves Hippocampus-Dependent Memory in Aged Mice. Neuropsychopharmacology 2014, 39, 2938–2948. [Google Scholar] [CrossRef]
- Prickaerts, J.; Heckman, P.R.A.; Blokland, A. Investigational Phosphodiesterase Inhibitors in Phase I and Phase II Clinical Trials for Alzheimer’s Disease. Expert Opin. Investig. Drugs 2017, 26, 1033–1048. [Google Scholar] [CrossRef]
- Available online: https://tetratherapeutics.com/tetra-discovery-partners-initiates-phase-2-clinical-trial-of-bpn14770-in-patients-with-early-alzheimers-disease/ (accessed on 28 November 2022).
- Charnigo, R.J.; Beidler, D.; Rybin, D.; Pittman, D.D.; Tan, B.; Howard, J.; Michelson, A.D.; Frelinger, A.L.; Clarke, N. PF-04447943, a Phosphodiesterase 9A Inhibitor, in Stable Sickle Cell Disease Patients: A Phase Ib Randomized, Placebo-Controlled Study. Clin. Transl. Sci. 2019, 12, 180–188. [Google Scholar] [CrossRef]
- Schwam, E.; Nicholas, T.; Chew, R.; Billing, C.; Davidson, W.; Ambrose, D.; Altstiel, L. A Multicenter, Double-Blind, Placebo-Controlled Trial of the PDE9A Inhibitor, PF-04447943, in Alzheimer’s Disease. Curr. Alzheimer Res. 2014, 11, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Moschetti, V.; Kim, M.; Sand, M.; Wunderlich, G.; Andersen, G.; Feifel, U.; Jang, I.J.; Timmer, W.; Rosenbrock, H.; Boland, K. The Safety, Tolerability and Pharmacokinetics of BI 409306, a Novel and Potent PDE9 Inhibitor: Overview of Three Phase I Randomised Trials in Healthy Volunteers. Eur. Neuropsychopharmacol. 2018, 28, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Frölich, L.; Wunderlich, G.; Thamer, C.; Roehrle, M.; Garcia, M.; Dubois, B. Evaluation of the Efficacy, Safety and Tolerability of Orally Administered BI 409306, a Novel Phosphodiesterase Type 9 Inhibitor, in Two Randomised Controlled Phase II Studies in Patients with Prodromal and Mild Alzheimer’s Disease. Alzheimer’s Res. Ther. 2019, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- François-Moutal, L.; Felemban, R.; Scott, D.D.; Sayegh, M.R.; Miranda, V.G.; Perez-Miller, S.; Khanna, R.; Gokhale, V.; Zarnescu, D.C.; Khanna, M. Small Molecule Targeting TDP-43’s RNA Recognition Motifs Reduces Locomotor Defects in a Drosophila Model of Amyotrophic Lateral Sclerosis (ALS). ACS Chem. Biol. 2019, 14, 2006–2013. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Shorter, J.; Wobst, H.J. Emerging Small-Molecule Therapeutic Approaches for Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Bioorg. Med. Chem. Lett. 2020, 30, 126942. [Google Scholar] [CrossRef] [PubMed]
- Hicks, D.A.; Cross, L.L.; Williamson, R.; Rattray, M. Endoplasmic Reticulum Stress Signalling Induces Casein Kinase 1-Dependent Formation of Cytosolic TDP-43 Inclusions in Motor Neuron-Like Cells. Neurochem. Res. 2020, 45, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- McGurk, L.; Gomes, E.; Guo, L.; Mojsilovic-Petrovic, J.; Tran, V.; Kalb, R.G.; Shorter, J.; Bonini, N.M. Poly(ADP-Ribose) Prevents Pathological Phase Separation of TDP-43 by Promoting Liquid Demixing and Stress Granule Localization. Mol. Cell. 2018, 71, 703–717.e9. [Google Scholar] [CrossRef]
- Pagan, F.L.; Torres-Yaghi, Y.; Hebron, M.L.; Wilmarth, B.; Turner, R.S.; Matar, S.; Ferrante, D.; Ahn, J.; Moussa, C. Safety, Target Engagement, and Biomarker Effects of Bosutinib in Dementia with Lewy Bodies. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12296. [Google Scholar] [CrossRef]
- Pophali, P.A.; Patnaik, M.M. The Role of New Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia. Cancer J. 2016, 22, 40–50. [Google Scholar] [CrossRef]
- Pagan, F.; Hebron, M.; Valadez, E.H.; Torres-Yaghi, Y.; Huang, X.; Mills, R.R.; Wilmarth, B.M.; Howard, H.; Dunn, C.; Carlson, A.; et al. Nilotinib Effects in Parkinson’s Disease and Dementia with Lewy Bodies. J. Park. Dis. 2016, 6, 503–517. [Google Scholar] [CrossRef]
- Scheltens, P.; Prins, N.; Lammertsma, A.; Yaqub, M.; Gouw, A.; Wink, A.M.; Chu, H.M.; van Berckel, B.N.M.; Alam, J. An Exploratory Clinical Study of P38α Kinase Inhibition in Alzheimer’s Disease. Ann. Clin. Transl. Neurol. 2018, 5, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Ismaili, L.; Refouvelet, B.; Benchekroun, M.; Brogi, S.; Brindisi, M.; Gemma, S.; Campiani, G.; Filipic, S.; Agbaba, D.; Esteban, G.; et al. Multitarget Compounds Bearing Tacrine- and Donepezil-like Structural and Functional Motifs for the Potential Treatment of Alzheimer’s Disease. Prog. Neurobiol. 2017, 151, 4–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Digiacomo, M.; Tu, Y.; Gu, Q.; Wang, S.; Yang, X.; Chu, J.; Chen, Q.; Han, Y.; Chen, J.; et al. Discovery of Novel Rivastigmine-Hydroxycinnamic Acid Hybrids as Multi-Targeted Agents for Alzheimer’s Disease. Eur. J. Med. Chem. 2017, 125, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lang, M.; Youdim, M.B.H.; Amit, T.; Sun, Y.; Zhang, Z.; Wang, Y.; Weinreb, O. Design, Synthesis and Evaluation of Novel Dual Monoamine-Cholinesterase Inhibitors as Potential Treatment for Alzheimer’s Disease. Neuropharmacology 2016, 109, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, B.; Lin, H.; Li, Q.; Yang, H.; Qiao, Y.; Li, Q.; Xu, Z.; Lyu, W.; Qu, W.; et al. Design, Synthesis and Evaluation of Fused Hybrids with Acetylcholinesterase Inhibiting and Nrf2 Activating Functions for Alzheimer’s Disease. Eur. J. Med. Chem. 2022, 244, 114806. [Google Scholar] [CrossRef] [PubMed]
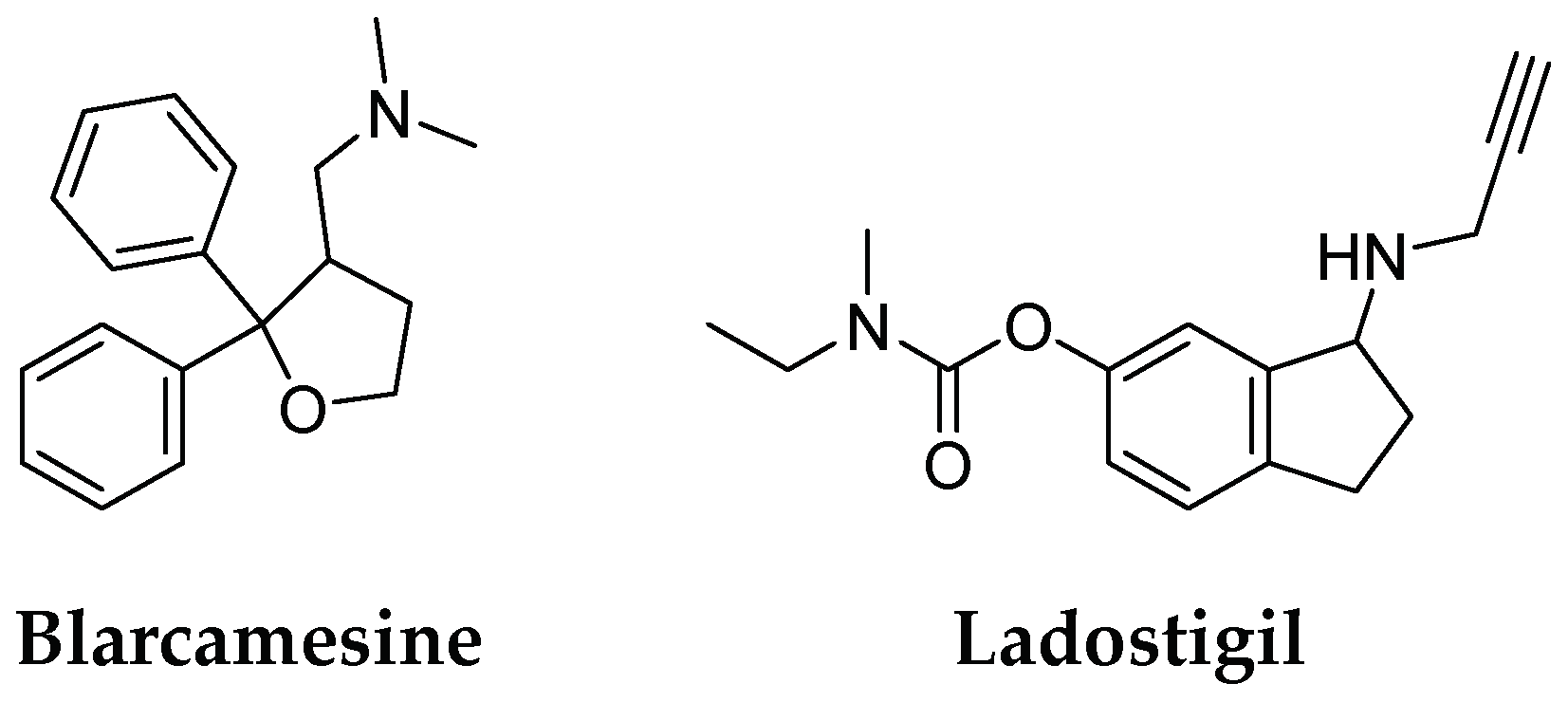
- Available online: https://www.anavex.com/post/anavex-2-73-blarcamesine-avatar-phase-3-trial-met-primary-and-secondary-efficacy-endpoints (accessed on 28 November 2022).
- Schneider, L.S.; Geffen, Y.; Rabinowitz, J.; Thomas, R.G.; Schmidt, R.; Ropele, S.; Weinstock, M. Low-Dose Ladostigil for Mild Cognitive Impairment: A Phase 2 Placebo-Controlled Clinical Trial. Neurology 2019, 93, e1474–e1484. [Google Scholar] [CrossRef] [PubMed]
- Lindström, V.; Ihse, E.; Fagerqvist, T.; Bergström, J.; Nordström, E.; Möller, C.; Lannfelt, L.; Ingelsson, M. Immunotherapy Targeting α-Synuclein, with Relevance for Future Treatment of Parkinson’s Disease and Other Lewy Body Disorders. Immunotherapy 2014, 6, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Bergström, A.L.; Kallunki, P.; Fog, K. Development of Passive Immunotherapies for Synucleinopathies. Mov. Disord. 2016, 31, 203–213. [Google Scholar] [CrossRef]
- Available online: www.alz.org/alzheimers-dementia/treatments/aducanumab (accessed on 29 November 2022).
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The Antibody Aducanumab Reduces Aβ Plaques in Alzheimer’s Disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Arndt, J.W.; Qian, F.; Smith, B.A.; Quan, C.; Kilambi, K.P.; Bush, M.W.; Walz, T.; Pepinsky, R.B.; Bussière, T.; Hamann, S.; et al. Structural and Kinetic Basis for the Selectivity of Aducanumab for Aggregated Forms of Amyloid-β. Sci. Rep. 2018, 8, 3179. [Google Scholar] [CrossRef]
- Meissner, W.G.; Traon, A.P.L.; Foubert-Samier, A.; Galabova, G.; Galitzky, M.; Kutzelnigg, A.; Laurens, B.; Lührs, P.; Medori, R.; Péran, P.; et al. A Phase 1 Randomized Trial of Specific Active A-Synuclein Immunotherapies PD01A and PD03A in Multiple System Atrophy. Mov. Disord. 2020, 35, 1957. [Google Scholar] [CrossRef]
- Goldberg, N.R.S.; Caesar, J.; Park, A.; Sedgh, S.; Finogenov, G.; Masliah, E.; Davis, J.; Blurton-Jones, M. Neural Stem Cells Rescue Cognitive and Motor Dysfunction in a Transgenic Model of Dementia with Lewy Bodies through a BDNF-Dependent Mechanism. Stem Cell Rep. 2015, 5, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Dung Nguyen, T.T.; Vo, T.K.; Tran, N.M.A.; Nguyen, M.K.; van Vo, T.; van Vo, G. Nanotechnology-Based Drug Delivery for Central Nervous System Disorders. Biomed. Pharmacother. 2021, 143, 112117. [Google Scholar] [CrossRef] [PubMed]
- Handa, M.; Tiwari, S.; Yadav, A.K.; Almalki, W.H.; Alghamdi, S.; Alharbi, K.S.; Shukla, R.; Beg, S. Therapeutic Potential of Nanoemulsions as Feasible Wagons for Targeting Alzheimer’s Disease. Drug Discov. Today 2021, 26, 2881–2888. [Google Scholar] [CrossRef] [PubMed]
- Govardhane, S.; Shende, P. Orientation of Nanocarriers in Subarachnoid Space: A Tweak in Strategic Transport for Effective CNS Delivery. J. Drug Deliv. Sci. Technol. 2022, 75, 103641. [Google Scholar] [CrossRef]
- Waris, A.; Ali, A.; Khan, A.U.; Asim, M.; Zamel, D.; Fatima, K.; Raziq, A.; Khan, M.A.; Akbar, N.; Baset, A.; et al. Applications of Various Types of Nanomaterials for the Treatment of Neurological Disorders. Nanomaterials 2022, 12, 2140. [Google Scholar] [CrossRef]
- la Barbera, L.; Mauri, E.; D’Amelio, M.; Gori, M. Functionalization Strategies of Polymeric Nanoparticles for Drug Delivery in Alzheimer’s Disease: Current Trends and Future Perspectives. Front. Neurosci. 2022, 16, 939855. [Google Scholar] [CrossRef]
- Hinge, N.S.; Kathuria, H.; Pandey, M.M. Engineering of Structural and Functional Properties of Nanotherapeutics and Nanodiagnostics for Intranasal Brain Targeting in Alzheimer’s. Appl. Mater. Today 2022, 26, 101303. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, T.D.; Nguyen, T.K.O.; Vo, T.K.; Vo, V.G. Advances in Developing Therapeutic Strategies for Alzheimer’s Disease. Biomed. Pharmacother. 2021, 139, 111623. [Google Scholar] [CrossRef]
- Shabbir, U.; Rubab, M.; Tyagi, A.; Oh, D.H. Curcumin and Its Derivatives as Theranostic Agents in Alzheimer’s Disease: The Implication of Nanotechnology. Int. J. Mol. Sci. 2020, 22, 196. [Google Scholar] [CrossRef]
- Zhang, H.; Hao, C.; Qu, A.; Sun, M.; Xu, L.; Xu, C.; Kuang, H. Light-Induced Chiral Iron Copper Selenide Nanoparticles Prevent β-Amyloidopathy In Vivo. Angew. Chem. Int. Ed. 2020, 59, 7131–7138. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.G.; Cha, M.Y.; Kim, J.I.; Hwang, T.W.; Kim, K.A.; Kim, T.H.; Song, K.C.; Kim, J.J.; Moon, M. Vitamin D-Binding Protein-Loaded PLGA Nanoparticles Suppress Alzheimer’s Disease-Related Pathology in 5XFAD Mice. Nanomedicine 2019, 17, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.K.; Ahmad, A.; Chinnathambi, S. Protein-Capped Metal Nanoparticles Inhibit Tau Aggregation in Alzheimer’s Disease. ACS Omega 2019, 4, 12833–12840. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Chu, X.; Gong, W.; Zheng, J.; Xie, X.; Wang, Y.; Yang, M.; Li, Z.; Gao, C.; Yang, Y. Neuron Tau-Targeting Biomimetic Nanoparticles for Curcumin Delivery to Delay Progression of Alzheimer’s Disease. J. Nanobiotechnology 2020, 18, 71. [Google Scholar] [CrossRef]
- Han, Y.; Chu, X.; Cui, L.; Fu, S.; Gao, C.; Li, Y.; Sun, B. Neuronal Mitochondria-Targeted Therapy for Alzheimer’s Disease by Systemic Delivery of Resveratrol Using Dual-Modified Novel Biomimetic Nanosystems. Drug Deliv. 2020, 27, 502. [Google Scholar] [CrossRef]
- Ulanova, M.; Poljak, A.; Wen, W.; Bongers, A.; Gloag, L.; Gooding, J.; Tilley, R.; Sachdev, P.; Braidy, N. Nanoparticles as Contrast Agents for the Diagnosis of Alzheimer’s Disease: A Systematic Review. Nanomedicine 2020, 15, 725–743. [Google Scholar] [CrossRef]
- Available online: https://www.medesispharma.com/clinical-stage-products/drug-to-treat-neurodegenerative-diseases/ (accessed on 9 December 2022).
- Wilson, E.N.; do Carmo, S.; Iulita, M.F.; Hall, H.; Ducatenzeiler, A.; Marks, A.R.; Allard, S.; Jia, D.T.; Windheim, J.; Cuello, A.C. BACE1 Inhibition by Microdose Lithium Formulation NP03 Rescues Memory Loss and Early Stage Amyloid Neuropathology. Transl. Psychiatry 2017, 7, e1190. [Google Scholar] [CrossRef]
- Wilson, E.N.; do Carmo, S.; Iulita, M.F.; Hall, H.; Austin, G.L.; Jia, D.T.; Malcolm, J.C.; Foret, M.K.; Marks, A.R.; Butterfield, D.A.; et al. Microdose Lithium NP03 Diminishes Pre-Plaque Oxidative Damage and Neuroinflammation in a Rat Model of Alzheimer’s-like Amyloidosis. Curr. Alzheimer Res. 2018, 15, 1220–1230. [Google Scholar] [CrossRef]
- Wilson, E.N.; do Carmo, S.; Welikovitch, L.A.; Hall, H.; Aguilar, L.F.; Foret, M.K.; Iulita, M.F.; Jia, D.T.; Marks, A.R.; Allard, S.; et al. NP03, a Microdose Lithium Formulation, Blunts Early Amyloid Post-Plaque Neuropathology in McGill-R-Thy1-APP Alzheimer-Like Transgenic Rats. J. Alzheimer’s Dis. 2020, 73, 723–739. [Google Scholar] [CrossRef]
| Drug | Structure | Therapeutic Effects |
|---|---|---|
| Donepezil | 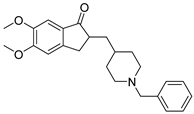 | selectively and reversibly inhibits cholinesterase, improves the cognitive and behavioral signs and symptoms of AD, neuroprotective |
| Galantamine | 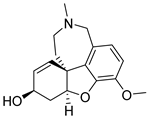 | levels decrease as the disease progresses, not considered as a disease-modifying drug |
| Rivastigmine | 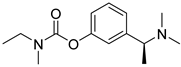 | parasympathomimetic and a reversible cholinesterase inhibitor, enhancing cholinergic function |
| Memantine | 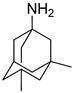 | inhibits calcium influx into cells, enhances neuronal synaptic plasticity |
| Drug | Approval | Mechanism of Action | Clinical Trials ID |
|---|---|---|---|
| Nilotinib | Chronic myelogenous leukemia | Tyrosine kinase inhibitor | NCT02947893 |
| Neflamapimod 1 | Antiarthritic/anti-inflammatory | p38 MAP kinase alpha inhibitor | NCT03402659 |
| Bexarotene | Anti-cancer | Retinoid X receptor agonist | NCT01782742 |
| Liraglutide | Anti-diabetic | Glucagon-like peptide 1 agonist | NCT01469351 |
| Rosiglitazone | Anti-diabetic | Peroxisome proliferator-activated receptor-γ agonist | NCT00265148 NCT00428090 NCT00550420 |
| Nilvadipine | Anti-hypertensive | Calcium channel blocker | NCT02017340 |
| Candesartan | Anti-hypertensive | Angiotensin receptor blocker | NCT02646982 |
| Losartan | Anti-hypertensive | Angiotensin receptor blocker | EudraCT 2012–003641–15 |
| Losartan/amlodipine | Anti-hypertensive | Angiotensin receptor blocker/Calcium channel blocker | NCT05331144 |
| Rasagiline | Parkinson’s disease | Selective monoamine oxidase B inhibitor | NCT02359552 |
| Roflumilast | Severe chronic obstructive pulmonary disease | Phosphodiesterase 4 inhibitor | NCT02051335 NCT02079844 |
| Sildenafil | Erectile dysfunction, pulmonary arterial hypertension | Phosphodiesterase 5 inhibitor | NCT05039086 |
| Small Molecule | Mechanism of Action | Clinical Trials ID | Condition or Disease |
|---|---|---|---|
| HT-0712 | Phosphodiesterase 4 inhibitor | NCT02013310 | age-associated memory impairment |
| BPN14770 | Phosphodiesterase 4 inhibitor | NCT02648672 NCT0303010 NCT02840279 | safety, tolerability, and pharmacokinetic profile |
| PF-04447943 | Phosphodiesterase 9 inhibitor | NCT00988598 NCT00930059 | cognitive and behavioral symptoms of AD |
| BI 409306 | Phosphodiesterase 9 inhibitor | NCT02392468 NCT02337907 | cognitive impairment due to AD |
| Intepirdine | Selective 5-HT6 receptor antagonist | NCT02586909 | long-term safety and tolerability in LBD |
| Nelotanserin | 5-HT2A inverse agonist | NCT02871427 | long-term safety and tolerability in LBD |
| Ramelteon | Melatonin receptors MT1 and MT2 selective agonist | NCT00325728 | mild-to-moderate AD |
| Bosutinib | Tyrosine kinases Abl/Src dual inhibitor | NCT03888222 | safety, tolerability, biomarkers in LBD |
| Nilotinib | Abl tyrosine kinase inhibitor | NCT04002674 | safety, tolerability, biomarkers in LBD |
| Irsenontrine | Phosphodiesterase 9 inhibitor | NCT03467152 | safety, tolerability, biomarkers in LBD |
| Pimavanserin | 5-HT2A inverse agonist | NCT03325556 | dementia-related psychosis |
| Neflamapimod | p38 mitogen-activated protein (MAP) kinase inhibitor | NCT04001517 | cognitive effects in LBD |
| Name | Description | Administration | Clinical Trials ID |
|---|---|---|---|
| NanoLithium® NP03 | Proof-of-concept study to assess safety, tolerance, and efficacy of NanoLithium® NP03 in patients with mild-to-severe AD | Depositing in the gingivo-jugal groove of each cheek | NCT05423522 |
| APH-1105 | Study to assess the safety, tolerability, and efficacy of intranasal delivery of APH-1105 for the treatment of mild-to-moderate AD in adults | Intranasal | NCT03806478 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagórska, A.; Czopek, A.; Fryc, M.; Jaromin, A.; Boyd, B.J. Drug Discovery and Development Targeting Dementia. Pharmaceuticals 2023, 16, 151. https://doi.org/10.3390/ph16020151
Zagórska A, Czopek A, Fryc M, Jaromin A, Boyd BJ. Drug Discovery and Development Targeting Dementia. Pharmaceuticals. 2023; 16(2):151. https://doi.org/10.3390/ph16020151
Chicago/Turabian StyleZagórska, Agnieszka, Anna Czopek, Monika Fryc, Anna Jaromin, and Ben J. Boyd. 2023. "Drug Discovery and Development Targeting Dementia" Pharmaceuticals 16, no. 2: 151. https://doi.org/10.3390/ph16020151
APA StyleZagórska, A., Czopek, A., Fryc, M., Jaromin, A., & Boyd, B. J. (2023). Drug Discovery and Development Targeting Dementia. Pharmaceuticals, 16(2), 151. https://doi.org/10.3390/ph16020151







