Comparative Investigation into the Roles of Imipenem:Cyclodextrin Complexation and Antibiotic Combination in Combatting Antimicrobial Resistance in Gram-Negative Bacteria
Abstract
1. Introduction
2. Results
2.1. In Silico (Molecular Docking) Studies
2.2. FTIR Spectroscopy
2.3. Bacterial Isolation and Identification
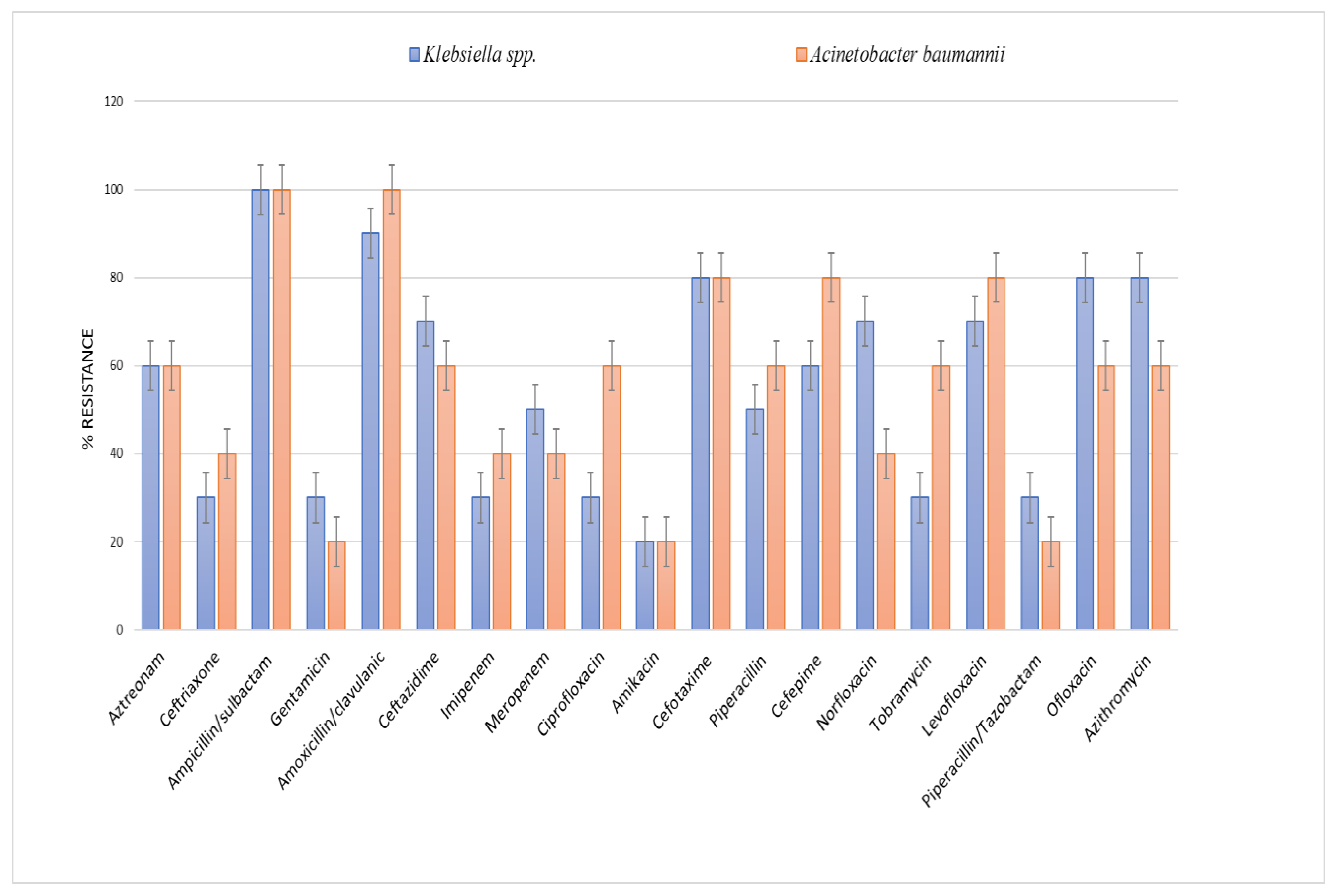
2.4. Antibiotic Susceptibility Testing
2.5. Determination of MIC for Amikacin and Imipenem for Klebsiella spp. and Acinetobacter Baumannii Isolates
2.6. Molecular Assessment of aac(6’)-Ib and bla IMP by PCR
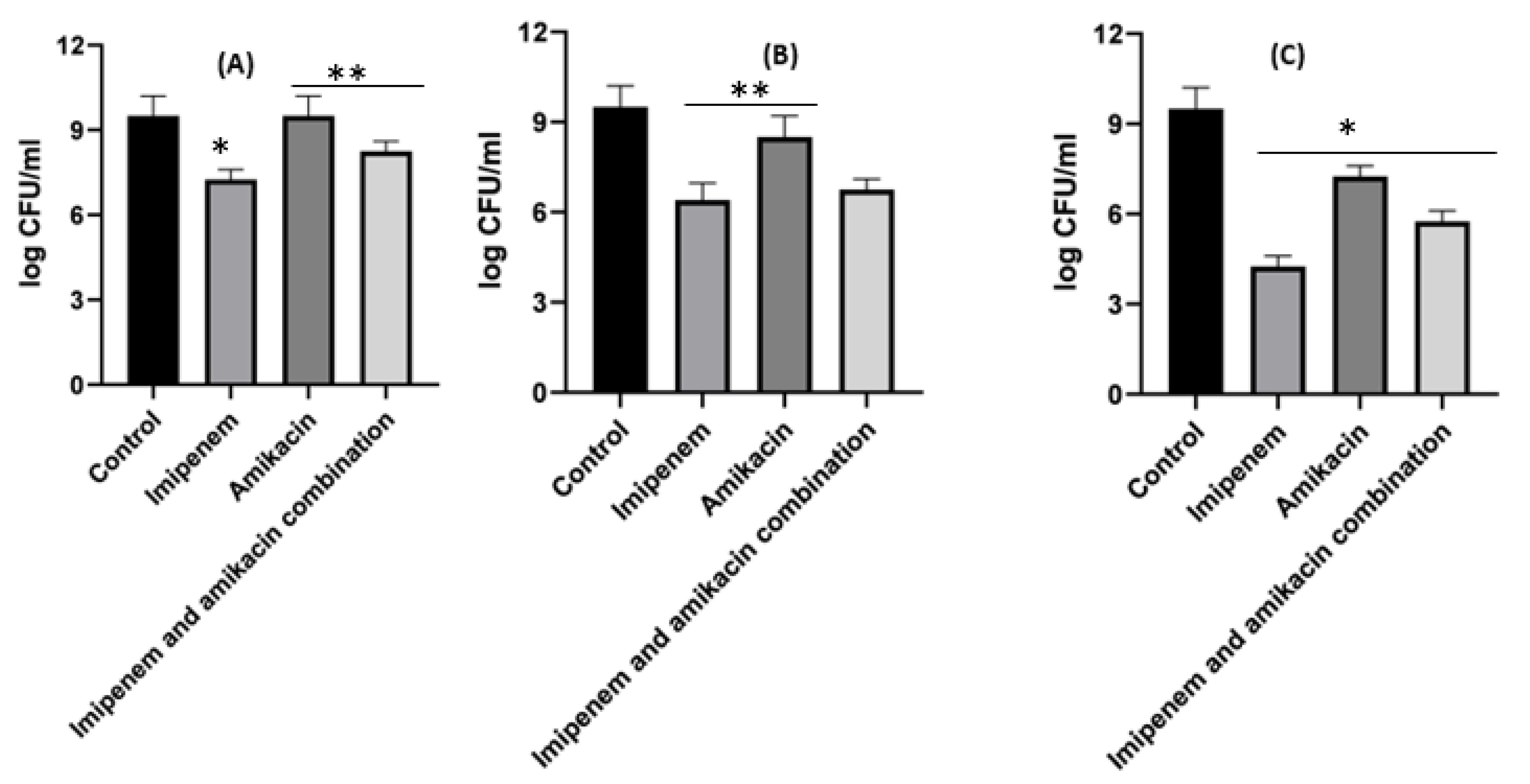
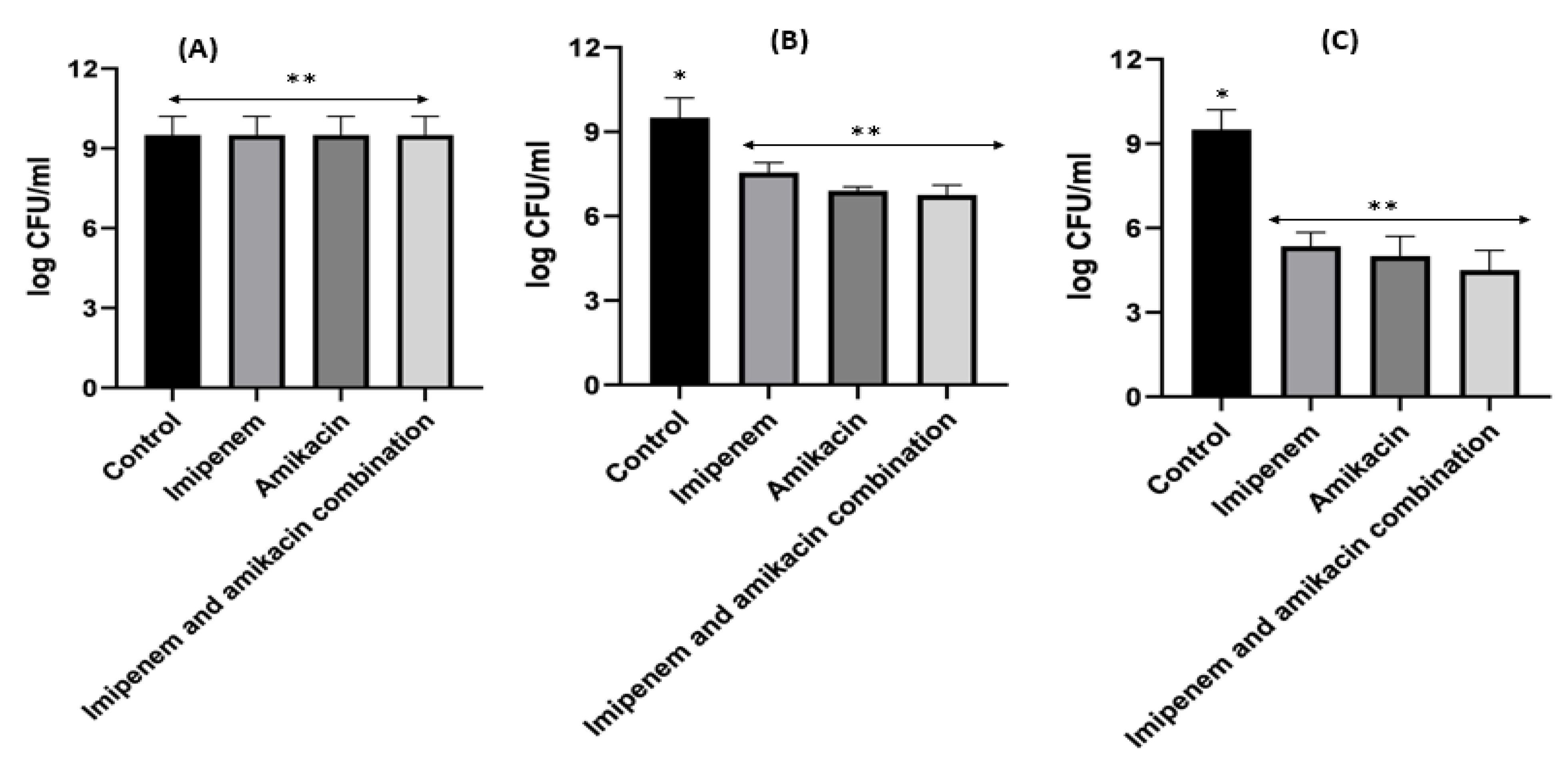
2.7. Detection of the Synergism for Amikacin and Imipenem Combination against Klebsiella pneumoniae and Acinetobacter baumannii Resistant Isolates Using the Checkerboard Technique
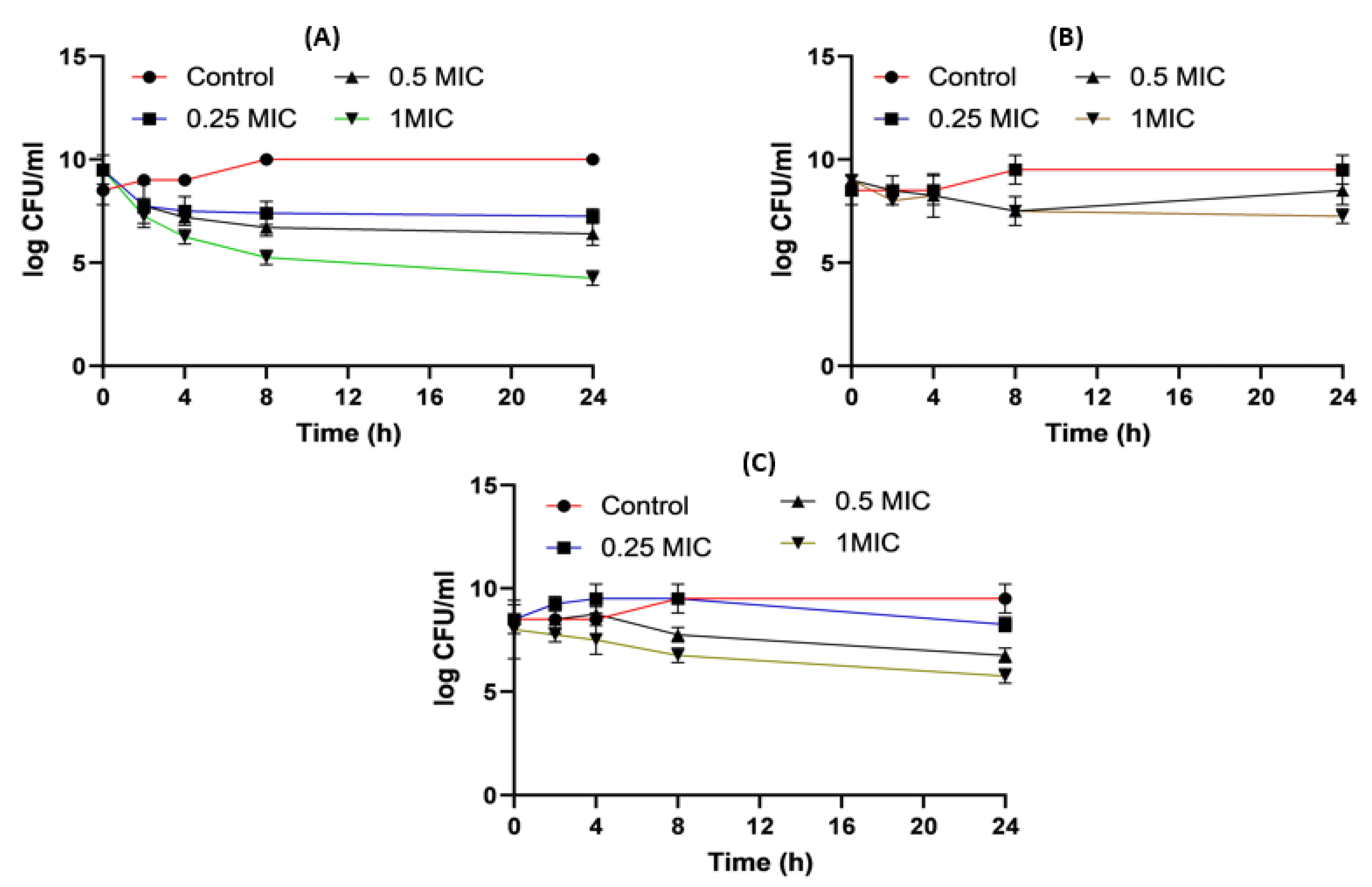
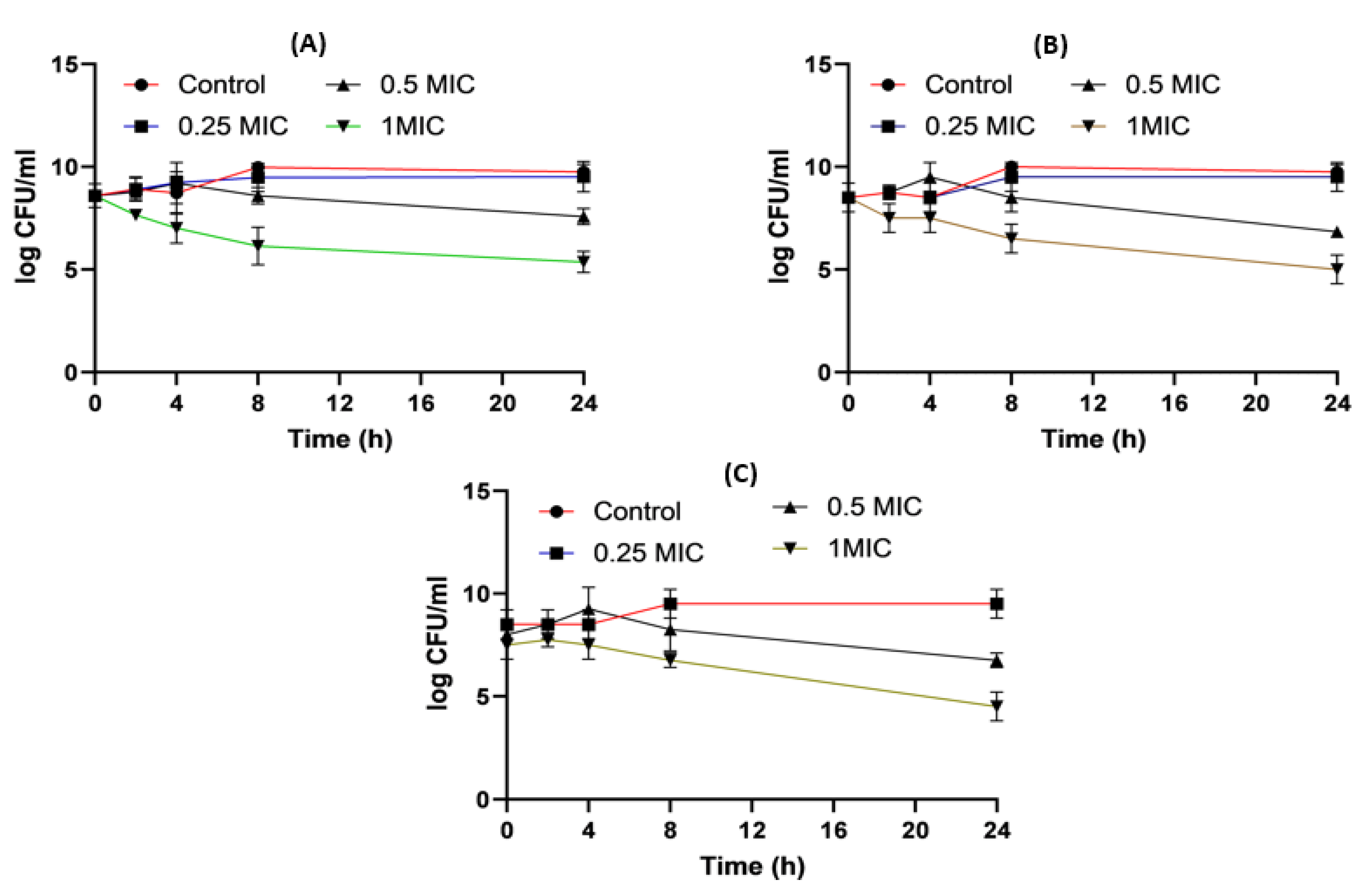
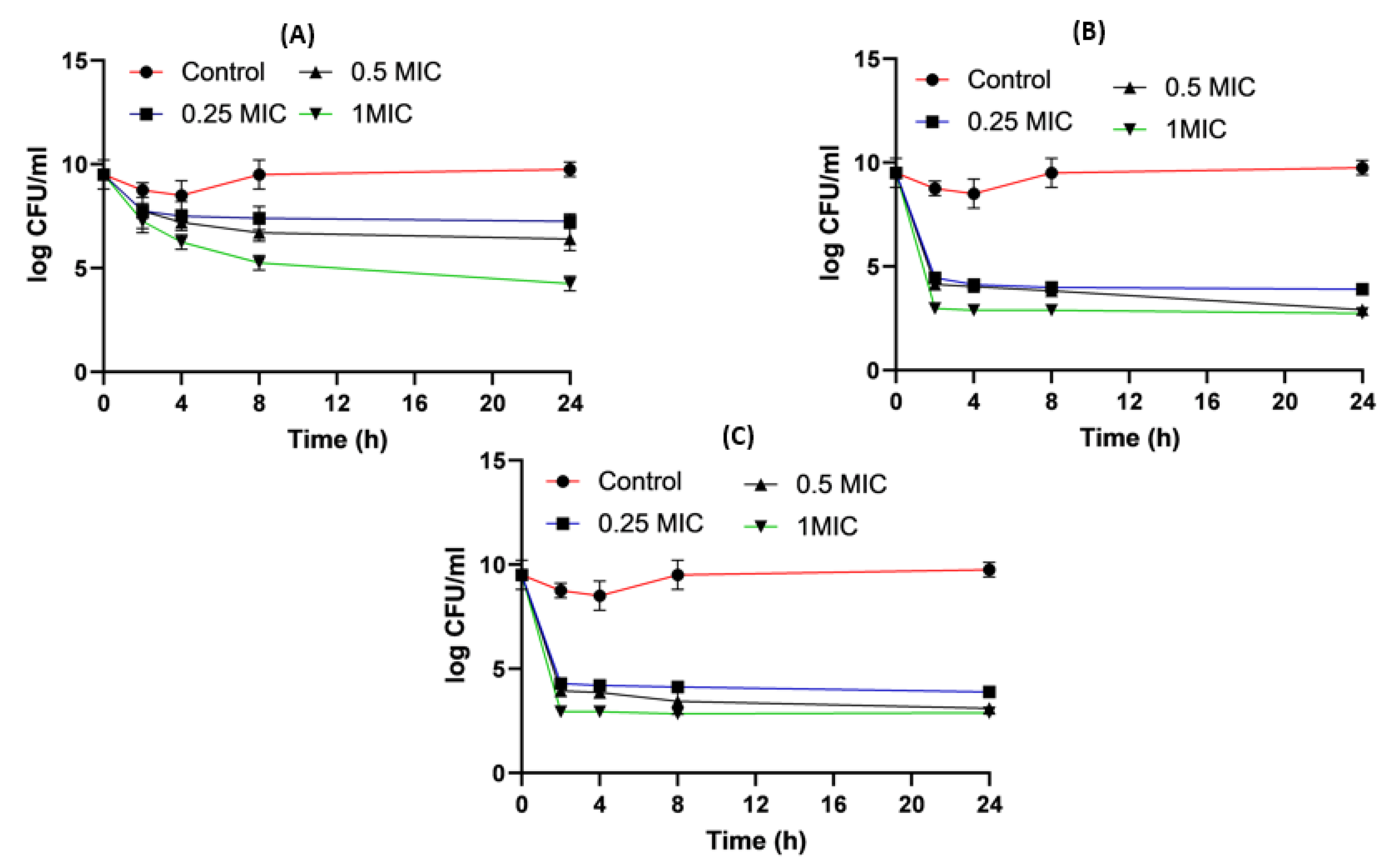
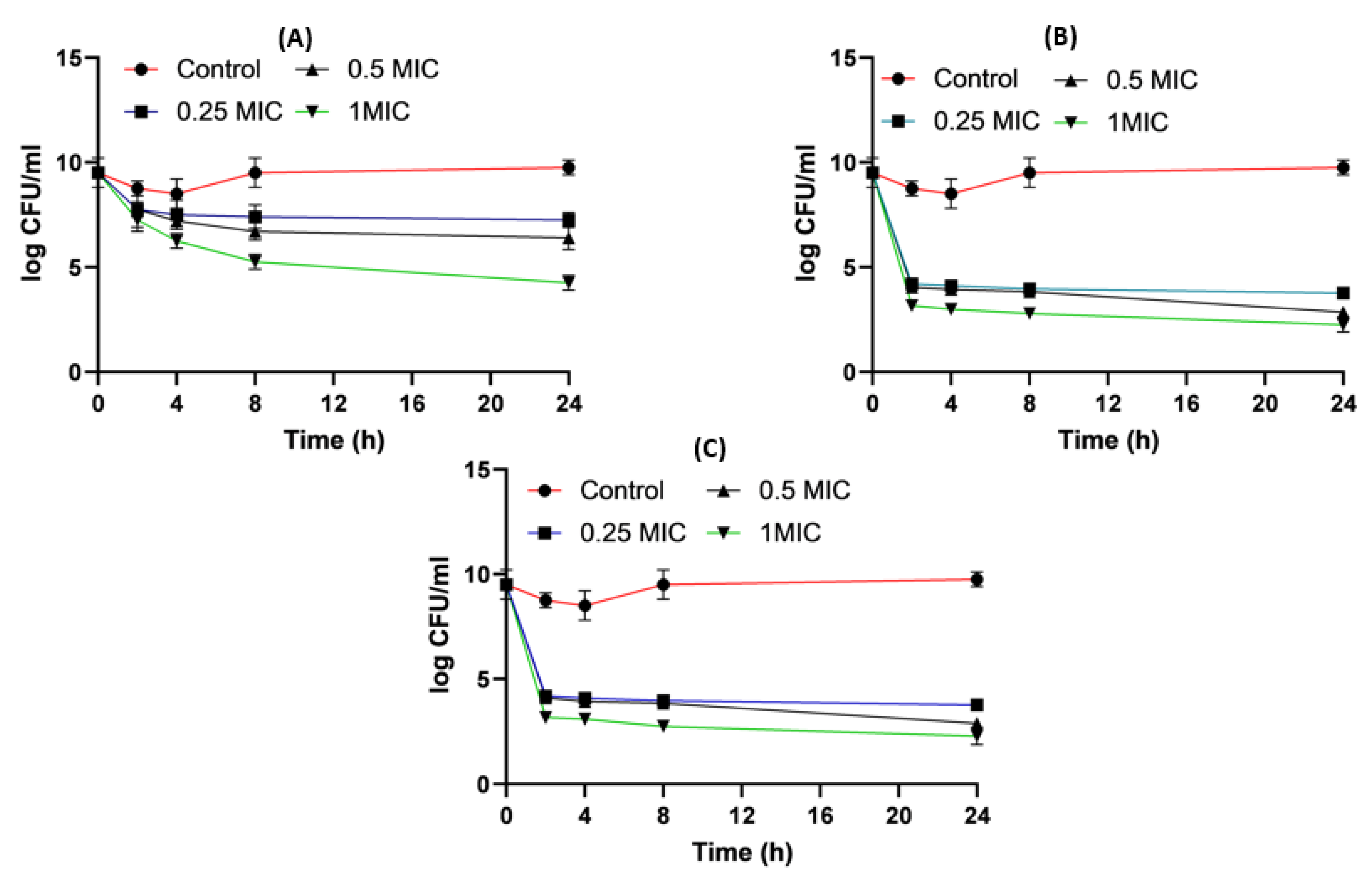
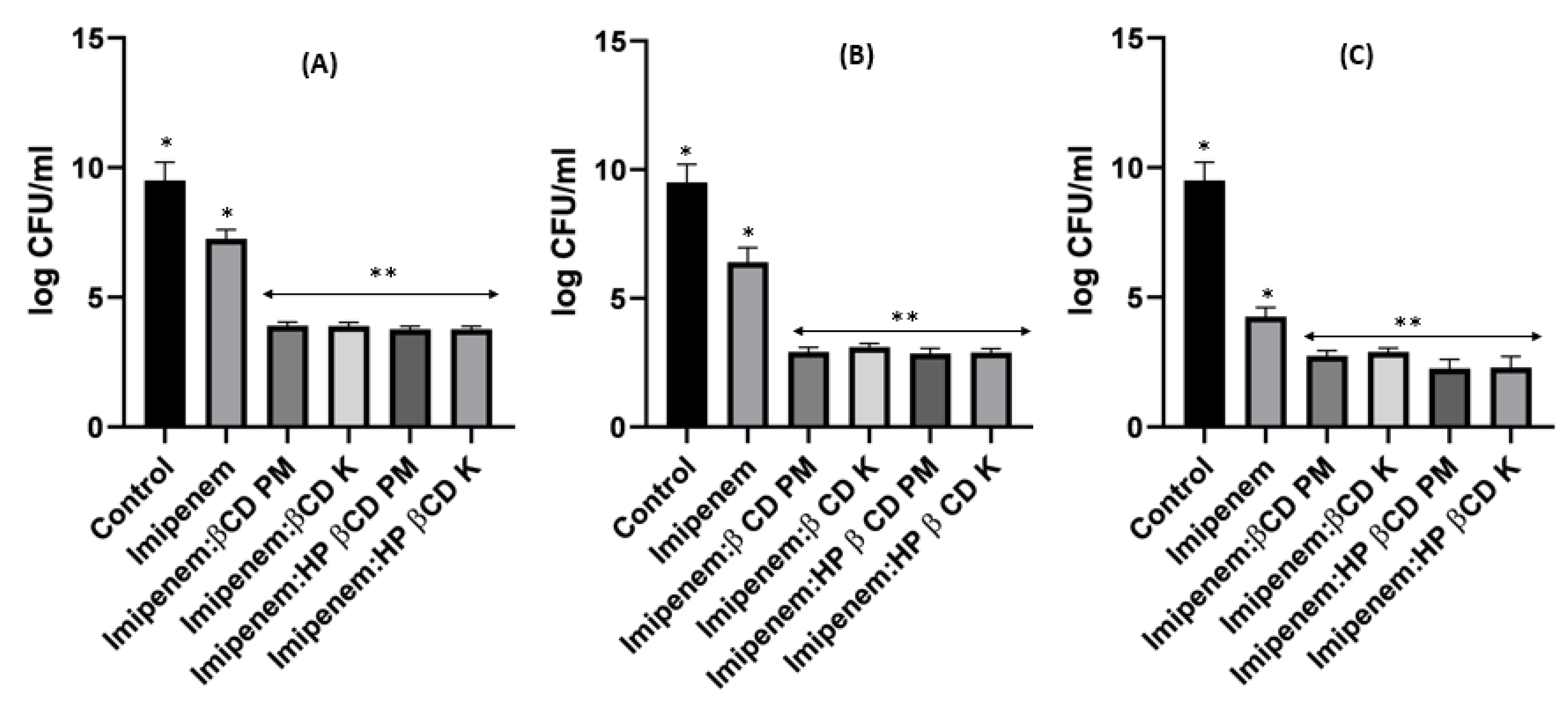
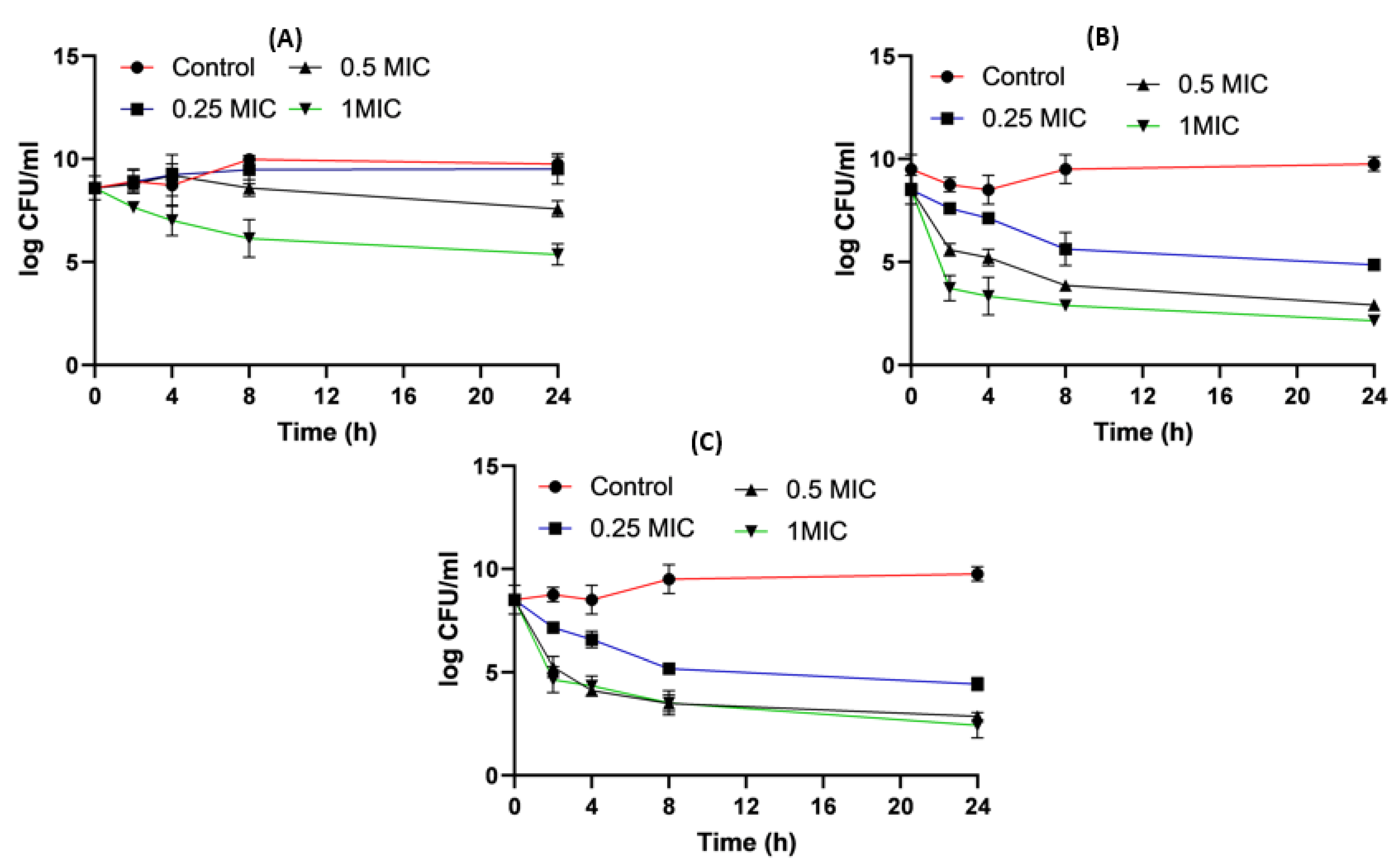
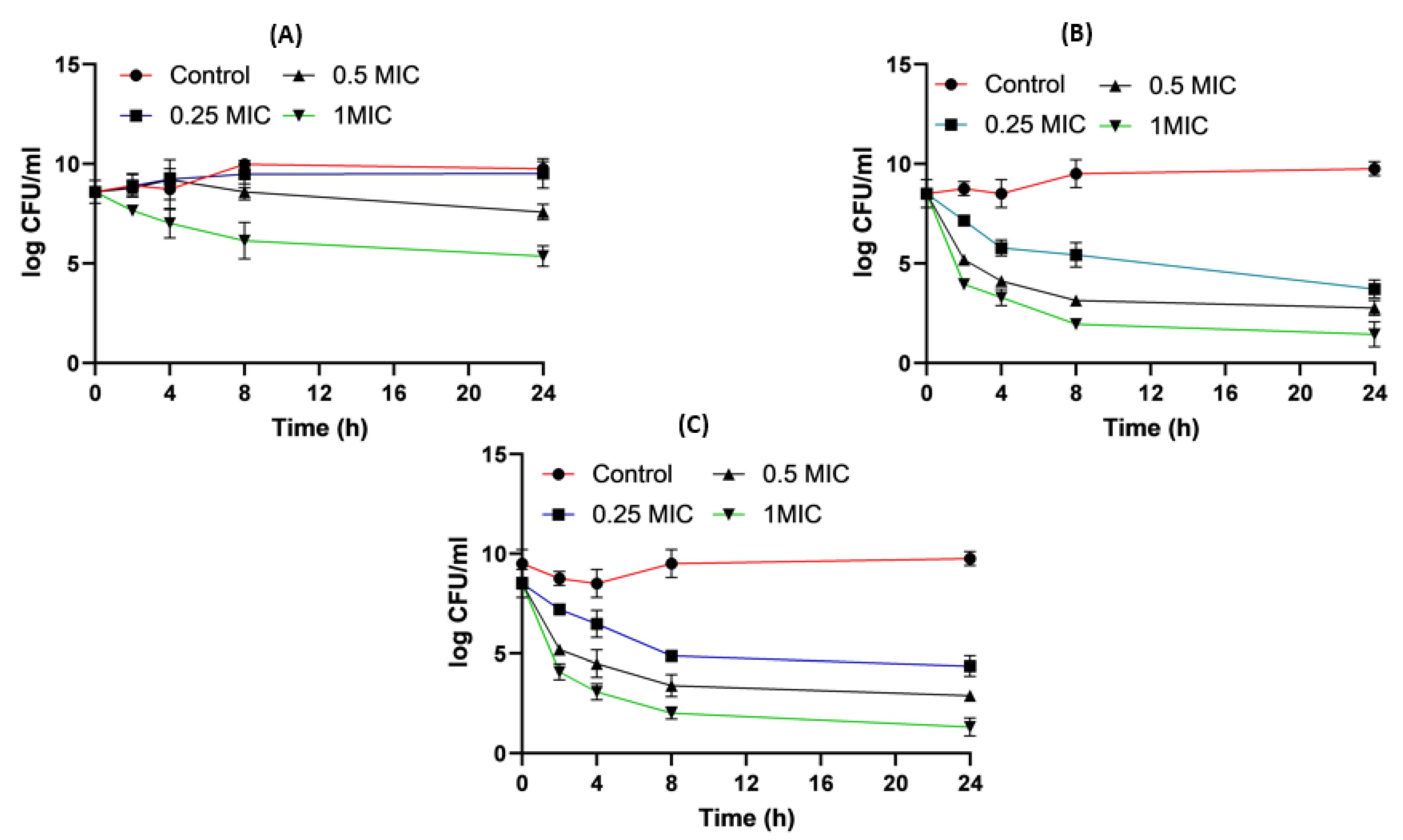
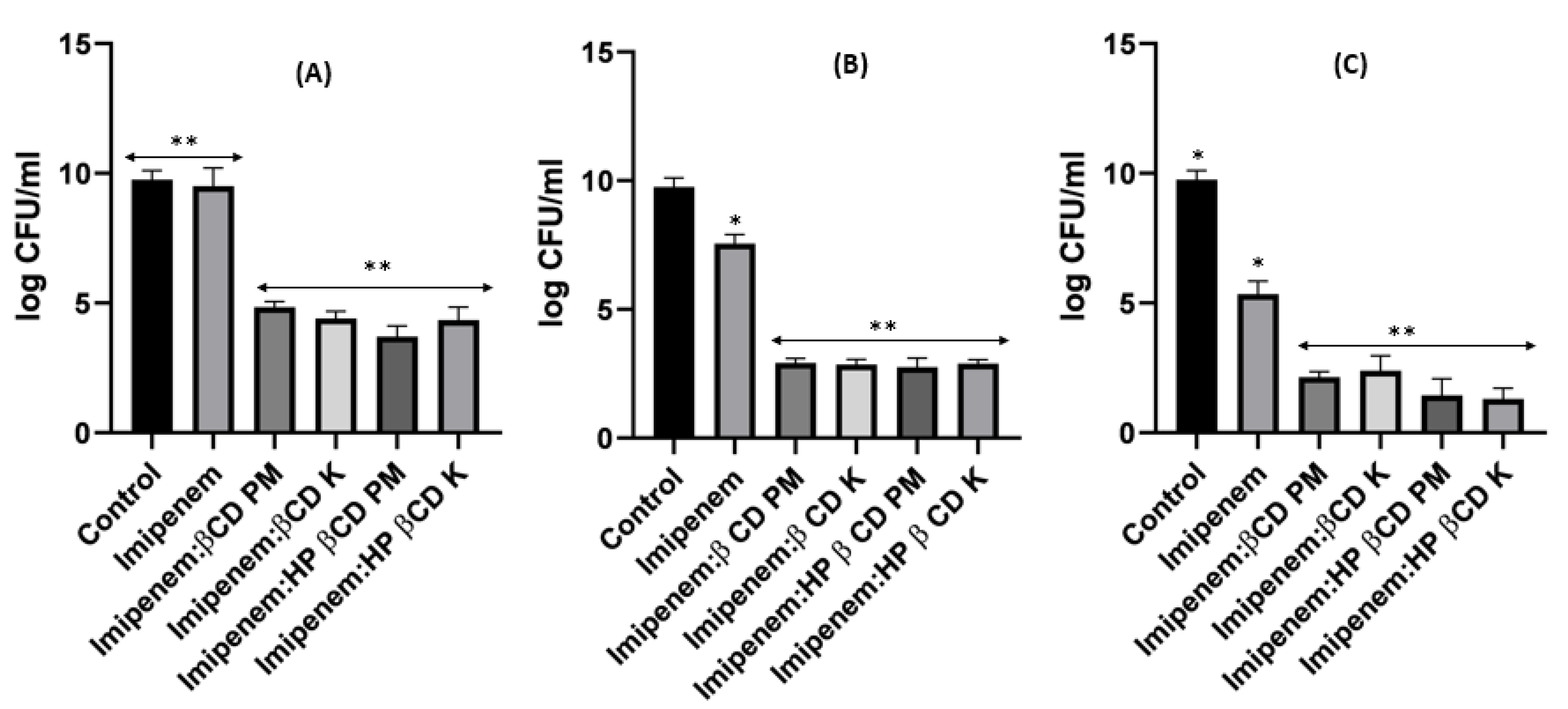
2.8. Time–Kill Studies
3. Discussion
4. Materials and Methods
4.1. Preparation of Imipenem:Cyclodextrin Physical Mixtures and Kneaded Dispersions
4.2. Molecular Docking
4.3. FTIR Spectroscopy
4.4. Bacterial Isolates
4.5. Antimicrobial Sensitivity Test
4.6. Detection of aac(6′) -Ib and bla IMP by Conventional PCR
4.7. Checkerboard Testing Method for Imipenem/Amikacin Combination
4.8. Time-Killing Assay for Impinem/Amikacin Combination and Imipenem:CD Mixtures
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, C.-I.; Song, J.-H. Antimicrobial resistance in Asia: Current epidemiology and clinical implications. Infect. Chemother. 2013, 45, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, P.M.; Jones, A.M. The changing epidemiology of resistance. J. Antimicrob. Chemother. 2009, 64, i3–i10. [Google Scholar] [CrossRef] [PubMed]
- Karaiskos, I.; Giamarellou, H. Multidrug-resistant and extensively drug-resistant Gram-negative pathogens: Current and emerging therapeutic approaches. Expert Opin. Pharmacother. 2014, 15, 1351–1370. [Google Scholar] [CrossRef]
- Mohapatra, D.P.; Debata, N.K.; Singh, S.K. Extensively drug-resistant and pandrug-resistant Gram-negative bacteria in a tertiary-care hospital in Eastern India: A 4-year retrospective study. J. Glob. Antimicrob. Resist. 2018, 15, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.; Giske, C.; Harbarth, S.; Hindler, J.; Kahlmeter, G.; Olsson-Liljequist, B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Munoz-Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Resistance: Global Report on Surveillance; WHO: Geneva, Switzerland, 2014; pp. 1–256.
- Raza, A.; Ngieng, S.; Sime, F.; Cabot, P.; Roberts, J.; Popat, A.; Kumeria, T.; Falconer, J. Oral meropenem for superbugs: Challenges and opportunities. Drug Discov. Today 2021, 26, 551–560. [Google Scholar] [CrossRef]
- Farhan, S.; Raafat, M.; Abourehab, M.; Abd El-Baky, R.; Abdalla, S.; EL-Gendy, A.; Azmy, A. Effect of imipenem and amikacin combination against multi-drug resistant Pseudomonas aeruginosa. Antibiotics 2021, 10, 1429. [Google Scholar] [CrossRef]
- Cielecka-Piontek, J.; Michalska, K.; Zalewski, P.; Jelińska, A. Recent advances in stability studies of carbapenems. Curr. Pharm. Anal. 2011, 7, 213–227. [Google Scholar] [CrossRef]
- Fonseca, A.P.; Sousa, J.C. Effect of antibiotic-induced morphological changes on surface properties, motility and adhesion of nosocomial Pseudomonas aeruginosa strains under different physiological states. J. Appl. Microbiol. 2007, 103, 1828–1837. [Google Scholar] [CrossRef]
- Lim, T.P.; Lee, W.; Tan, T.Y.; Sasikala, S.; Teo, J.; Hsu, L.Y.; Tan, T.T.; Syahidah, N.; Kwa, A.L. Effective antibiotics in combination against extreme drug-resistant Pseudomonas aeruginosa with decreased susceptibility to polymyxin B. PLoS ONE 2011, 6, e28177. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Bulitta, J.B.; Nation, R.L.; Landersdorfer, C.B. Optimization of Synergistic Combination Regimens against Carbapenem- and Aminoglycoside-Resistant Clinical Pseudomonas aeruginosa Isolates via Mechanism-Based Pharmacokinetic/Pharmacodynamic Modeling. Antimicrob. Agents Chemother. 2017, 61, e01011-16. [Google Scholar] [CrossRef]
- Kmeid, J.G.; Youssef, M.M.; Kanafani, Z.A.; Kanj, S.S. Combination therapy for Gram-negative bacteria: What is the evidence? Expert Rev. Anti-Infect. Ther. 2013, 11, 1355–1362. [Google Scholar] [CrossRef]
- Kalil, A.C. Antibiotic combination therapy for patients with gram-negative septic shock. Crit. Care Med. 2017, 45, 1933–1936. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Tandel, K.; Shete, V.; Rathi, K. Burden of extensively drug-resistant and pandrug-resistant Gram-negative bacteria at a tertiary-care centre. New Microbes New Infect. 2015, 8, 166–170. [Google Scholar] [CrossRef]
- Paczkowska, M.; Mizera, M.; Szymanowska-Powałowska, D.; Lewandowska, K.; Błaszczak, W.; Gościańska, J.; Pietrzak, R.; Cielecka-Piontek, J. β-Cyclodextrin complexation as an effective drug delivery system for meropenem. Eur. J. Pharm. Biopharm. 2016, 99, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawashdeh, N.; Al-Sadeh, K.; Al-Bitar, M. Inclusion complexes of sunscreen agents with β-cyclodextrin: Spectroscopic and molecular modeling studies. J. Spectrosc. 2013, 2013, 841409. [Google Scholar] [CrossRef]
- Bülbül, E.; Eleftheriadou, K.; Okur, N.; Siafaka, P. An update on cyclodextrins as drug vehicles for antimicrobial applications. J. Pharm. Technol. 2020, 1, 18–24. [Google Scholar] [CrossRef]
- Vandera, K.; Picconi, P.; Valero, M.; Gonzalez-Gaitano, G.; Woods, A.; Zain, N.; Bruce, K.; Clifton, L.; Skoda, M.; Rahman, K.; et al. Antibiotic-in-cyclodextrin-in-liposomes: Formulation development and interactions with model bacterial membranes. Mol. Pharm. 2020, 17, 2354–2369. [Google Scholar] [CrossRef]
- Inoue, Y.; Suzuki, R.; Murata, I.; Nomura, H.; Isshiki, Y.; Kanamoto, I. Evaluation of antibacterial activity expression of the Hinokitiol/cyclodextrin complex against bacteria. ACS Omega 2020, 5, 27180–27187. [Google Scholar] [CrossRef]
- Zaas, D.W.; Duncan, M.; Wright, J.R.; Abraham, S.N. The role of lipid rafts in the pathogenesis of bacterial infections. Biochim. Biophys. Acta Mol. Cell Res. 2005, 1746, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R. Cyclodextrins in drug delivery: An updated review. AAPS PharmSciTech 2005, 6, E329–E357. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M. Pharmaceutical applications of cyclodextrins: Effects on drug permeation through biological membranes. JPP 2011, 63, 1119–1135. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Rafe, R. Cyclodextrin: A prospective nanocarrier for the delivery of antibacterial agents against bacteria that are resistant to antibiotics. Heliyon 2023, 9, e19287. [Google Scholar] [CrossRef]
- Abdelkader, H.; Al Fatease, A.; Fathalla, Z.; Shoman, M.; Abou-Taleb, H.; Abourehab, M. Design, preparation and evaluation of supramolecular complexes with curcumin for enhanced cytotoxicity in breast cancer cell lines. Pharmaceutics 2022, 14, 2283. [Google Scholar] [CrossRef]
- Ghareeb, M.; Abdulrasool, A.; Hussein, A.; Noordin, M. Kneading Technique for Preparation of Binary Solid Dispersion of Meloxicam with Poloxamer 188. AAPSPharmSciTech 2009, 10, 1206–1215. [Google Scholar] [CrossRef]
- Zendegani, E.; Dolatabadi, S. The Efficacy of Imipenem Conjugated with Synthesized Silver Nanoparticles against Acinetobacter baumannii Clinical Isolates, Iran. Biol. Trace Elem. Res. 2020, 197, 330–340. [Google Scholar] [CrossRef]
- Shaaban, M.; Shaker, M.; Mady, F. Imipenem/cilastatin encapsulated polymeric nanoparticles for destroying carbapenem-resistant bacterial isolates. J. Nanobiotechnology 2017, 15, 29. [Google Scholar] [CrossRef]
- De Gaetano, F.; Margani, F.; Barbera, V.; D’Angelo, V.; Germanò, M.; Pistarà, V.; Ventura, C. Characterization and In Vivo Antiangiogenic Activity Evaluation of Morin-Based Cyclodextrin Inclusion Complexes. Pharmaceutics 2023, 15, 2209. [Google Scholar] [CrossRef]
- Souli, M.; Galani, I.; Giamarellou, H. Emergence of extensively drug-resistant and pandrug-resistant Gram-negative bacilli in Europe. Eurosurveillance 2008, 13, 19045. [Google Scholar] [CrossRef]
- Economou, V.; Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Rybak, L.P.; Ramkumar, V.; Mukherjea, D. Ototoxicity of non-aminoglycoside antibiotics. Front. Neurol. 2021, 12, 652674. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Fathalla, Z.; Moharram, H.; Ali, T.; Pierscionek, B. Cyclodextrin Enhances Corneal Tolerability and Reduces Ocular Toxicity Caused by Diclofenac. Oxid. Med. Cell Longev. 2018, 2018, 5260976. [Google Scholar] [CrossRef] [PubMed]
- Wareham, D.W.; Momin, M.A.; Phee, L.M.; Hornsey, M.; Standing, J.F. Cefepime/sulbactam as an enhanced antimicrobial combination therapy for the treatment of MDR Gram-negative infections. J. Antimicrob. Chemother. 2020, 75, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Aygun, F.; Aygun, F.D.; Varol, F.; Durak, C.; Çokuğraş, H.; Camcıoğlu, Y.; Çam, H. Infections with Carbapenem-Resistant Gram-Negative Bacteria are a Serious Problem Among Critically Ill Children: A Single-Centre Retrospective Study. Pathogens 2019, 8, 69. [Google Scholar] [CrossRef]
- Vena, A.; Giacobbe, D.R.; Castaldo, N.; Cattelan, A.; Mussini, C.; Luzzati, R.; De Rosa, F.G.; Puente, F.D.; Mastroianni, C.M.; Cascio, A. Clinical experience with ceftazidime-avibactam for the treatment of infections due to multidrug-resistant Gram-negative bacteria other than carbapenem-resistant Enterobacterales. Antibiotics 2020, 9, 71. [Google Scholar] [CrossRef]
- Kofteridis, D.P.; Andrianaki, A.M.; Maraki, S.; Mathioudaki, A.; Plataki, M.; Alexopoulou, C.; Ioannou, P.; Samonis, G.; Valachis, A. Treatment pattern, prognostic factors, and outcome in patients with infection due to pan-drug-resistant gram-negative bacteria. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 965–970. [Google Scholar] [CrossRef]
- Sharahi, J.Y.; Ahovan, Z.A.; Maleki, D.T.; Rad, Z.R.; Rad, Z.R.; Goudarzi, M.; Shariati, A.; Bostanghadiri, N.; Abbasi, E.; Hashemi, A. In vitro antibacterial activity of curcumin-meropenem combination against extensively drug-resistant (XDR) bacteria isolated from burn wound infections. Avicenna J. Phytomedicine 2020, 10, 3–10. [Google Scholar]
- Senda, K.; Arakawa, Y.; Ichiyama, S.; Nakashima, K.; Ito, H.; Ohsuka, S.; Shimokata, K.; Kato, N.; Ohta, M. PCR detection of metallo-beta-lactamase gene (blaIMP) in gram-negative rods resistant to broad-spectrum beta-lactams. J. Clin. Microbiol. 1996, 34, 2909–2913. [Google Scholar] [CrossRef]
- Elbadawi, H.S.; Elhag, K.M.; Mahgoub, E.; Altayb, H.N.; Ntoumi, F.; Elton, L.; McHugh, T.D.; Tembo, J.; Ippolito, G.; Osman, A.Y. Detection and characterization of carbapenem resistant Gram-negative bacilli isolates recovered from hospitalized patients at Soba University Hospital, Sudan. BMC Microbiol. 2021, 21, 136. [Google Scholar] [CrossRef]
- Manohar, P.; Leptihn, S.; Lopes, B.S.; Nachimuthu, R. Dissemination of carbapenem resistance and plasmids encoding carbapenemases in Gram-negative bacteria isolated in India. JAC-Antimicrob. Resist. 2021, 3, dlab015. [Google Scholar] [CrossRef] [PubMed]
- Gajamer, V.R.; Bhattacharjee, A.; Paul, D.; Ingti, B.; Sarkar, A.; Kapil, J.; Singh, A.K.; Pradhan, N.; Tiwari, H.K. High prevalence of carbapenemase, AmpC β-lactamase and aminoglycoside resistance genes in extended-spectrum β-lactamase-positive uropathogens from Northern India. J. Glob. Antimicrob. Resist. 2020, 20, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Firmo, E.F.; Beltrão, E.M.B.; da Silva, F.R.F.; Alves, L.C.; Brayner, F.A.; Veras, D.L.; Lopes, A.C.S. Association of blaNDM-1 with blaKPC-2 and aminoglycoside-modifying enzyme genes among Klebsiella pneumoniae, Proteus mirabilis and Serratia marcescens clinical isolates in Brazil. J. Glob. Antimicrob. Resist. 2020, 21, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Costello, S.E.; Deshpande, L.M.; Davis, A.P.; Mendes, R.E.; Castanheira, M. Aminoglycoside-modifying enzyme and 16S ribosomal RNA methyltransferase genes among a global collection of Gram-negative isolates. J. Glob. Antimicrob. Resist. 2019, 16, 278–285. [Google Scholar] [CrossRef]
- Yadav, R.; Bulitta, J.B.; Schneider, E.K.; Shin, B.S.; Velkov, T.; Nation, R.L.; Landersdorfer, C.B. Aminoglycoside concentrations required for synergy with carbapenems against Pseudomonas aeruginosa determined via mechanistic studies and modeling. Antimicrob. Agents Chemother. 2017, 61, e00722-17. [Google Scholar] [CrossRef]
- Zavascki, A.P.; Klee, B.O.; Bulitta, J.B. Aminoglycosides against carbapenem-resistant Enterobacteriaceae in the critically ill: The pitfalls of aminoglycoside susceptibility. Expert Rev. Anti-Infect. Ther. 2017, 15, 519–526. [Google Scholar] [CrossRef]
- Terbtothakun, P.; Nwabor, O.F.; Siriyong, T.; Voravuthikunchai, S.P.; Chusri, S. Synergistic Antibacterial Effects of Meropenem in Combination with Aminoglycosides against Carbapenem-Resistant Escherichia coli Harboring bla NDM-1 and bla NDM-5. Antibiotics 2021, 10, 1023. [Google Scholar] [CrossRef]
- Mathe, A.; Szabo, D.; Anderlik, P.; Rozgonyi, F.; Nagy, K. The effect of amikacin and imipenem alone and in combination against an extended-spectrum beta-lactamase-producing Klebsiella pneumoniae strain. Diagn. Microbiol. Infect. Dis. 2007, 58, 105–110. [Google Scholar] [CrossRef]
- Bliziotis, I.A.; Samonis, G.; Vardakas, K.Z.; Chrysanthopoulou, S.; Falagas, M.E. Effect of aminoglycoside and β-lactam combination therapy versus β-lactam monotherapy on the emergence of antimicrobial resistance: A meta-analysis of randomized, controlled trials. Clin. Infect. Dis. 2005, 41, 149–158. [Google Scholar] [CrossRef]
- Uddin, B.M.M.; Saha, R.; Ratan, Z.A.; Suchi, S.E.; Shamsuzzaman, S. In vitro and in vivo Evaluation of Antibiotic Combination against Imipenem Resistant Acinetobacter baumannii Strains Isolated from Bangladeshi Patients. Am. J. Infect. Dis. 2020, 8, 83–87. [Google Scholar]
- Shabayek, S.M.; El-Damasy, D.A.; Hassanin, O.M. Testing of antibiotic combinations in NDM-1-producing Nosocomial Carbapenem Resistant Acinetobacter Baumannii. Azhar Int. J. Pharm. Med. Sci. 2021, 1, 34–41. [Google Scholar] [CrossRef]
- Esadoglu, M.; Ozer, B.; Duran, N. Efficacy of Meropenem and Amikacin Combination against Metallo-Beta-Lactamase-Producing Acinetobacter Strains. Int. J. Med. Lab. Res. 2019, 4, 7–15. [Google Scholar] [CrossRef]
- Maitland, M.L.; Hudoba, C.; Snider, K.L.; Ratain, M.J. Analysis of the yield of phase II combination therapy trials in medical oncology. Clin. Cancer Res. 2010, 16, 5296–5302. [Google Scholar] [CrossRef]
- Riviere, M.K.; Le Tourneau, C.; Paoletti, X.; Dubois, F.; Zohar, S. Designs of drug-combination phase I trials in oncology: A systematic review of the literature. Ann. Oncol. 2015, 26, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska, M.; Szymanowska-Powałowska, D.; Mizera, M.; Siąkowska, D.; Błaszczak, W.; Piotrowska-Kempisty, H.; Cielecka-Piontek, J. Cyclodextrins as multifunctional excipients: Influence of inclusion into β-cyclodextrin on physicochemical and biological properties of tebipenem pivoxil. PLoS ONE 2019, 14, e0210694. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.M.; Carvalho Santana Júnior, C.; Nascimento Júnior, J.A.C.; Andrade, T.d.A.; Shanmugam, S.; Thangaraj, P.; Frank, L.A.; Serafini, M.R. Antibacterial drugs and cyclodextrin inclusion complexes: A patent review. Expert Opin. Drug Deliv. 2023, 20, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Skwarczynski, M.; Bashiri, S.; Yuan, Y.; Ziora, Z.M.; Nabil, O.; Masuda, K.; Khongkow, M.; Rimsueb, N.; Cabral, H.; Ruktanonchai, U. Antimicrobial activity enhancers: Towards smart delivery of antimicrobial agents. Antibiotics 2022, 11, 412. [Google Scholar] [CrossRef]
- Mizera, M.; Szymanowska, D.; Stasiłowicz, A.; Siąkowska, D.; Lewandowska, K.; Miklaszewski, A.; Plech, T.; Tykarska, E.; Cielecka-Piontek, J. Computer-aided design of cefuroxime axetil/cyclodextrin system with enhanced solubility and antimicrobial activity. Biomolecules 2019, 10, 24. [Google Scholar] [CrossRef]
- Benson, H.J. Microbiological Applications; A Laboratory Manual in General Microbiology; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Xia, Y.; Liang, Z.; Su, X.; Xiong, Y. Characterization of carbapenemase genes in Enterobacteriaceae species exhibiting decreased susceptibility to carbapenems in a university hospital in Chongqing, China. Ann. Lab. Med. 2012, 32, 270–275. [Google Scholar] [CrossRef]
- Kim, H.C.; Jang, J.-H.; Kim, H.; Kim, Y.-J.; Lee, K.-R.; Kim, Y.-T. Multiplex PCR for Simultaneous Detection of Aminoglycoside Resistance Genes in Escherichia coli and Klebsiella pneumoniae. Korean J. Clin. Lab. Sci. 2012, 44, 155–165. [Google Scholar]
- Tivendale, K.A.; Allen, J.L.; Ginns, C.A.; Crabb, B.S.; Browning, G.F. Association of iss and iucA, but not tsh, with plasmid-mediated virulence of avian pathogenic Escherichia coli. Infect. Immun. 2004, 72, 6554–6560. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodríguez-Hernández, M.-J.; Pachón, J.; Pichardo, C.; Cuberos, L.; Ibáñez-Martínez, J.; García-Curiel, A.; Caballero, F.J.; Moreno, I.; Jiménez-Mejías, M.E. Imipenem, doxycycline and amikacin in monotherapy and in combination in Acinetobacter baumannii experimental pneumonia. J. Antimicrob. Chemother. 2000, 45, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.J.; Lai, C.C.; Chen, C.C.; Zhang, C.C.; Weng, T.C.; Chiu, Y.H.; Toh, H.S.; Chiang, S.R.; Yu, W.L.; Ko, W.C.; et al. Colistin-sparing regimens against Klebsiella pneumoniae carbapenemase-producing K. pneumoniae isolates: Combination of tigecycline or doxycycline and gentamicin or amikacin. J. Microbiol. Immunol. Infect. 2019, 52, 273–281. [Google Scholar] [CrossRef] [PubMed]
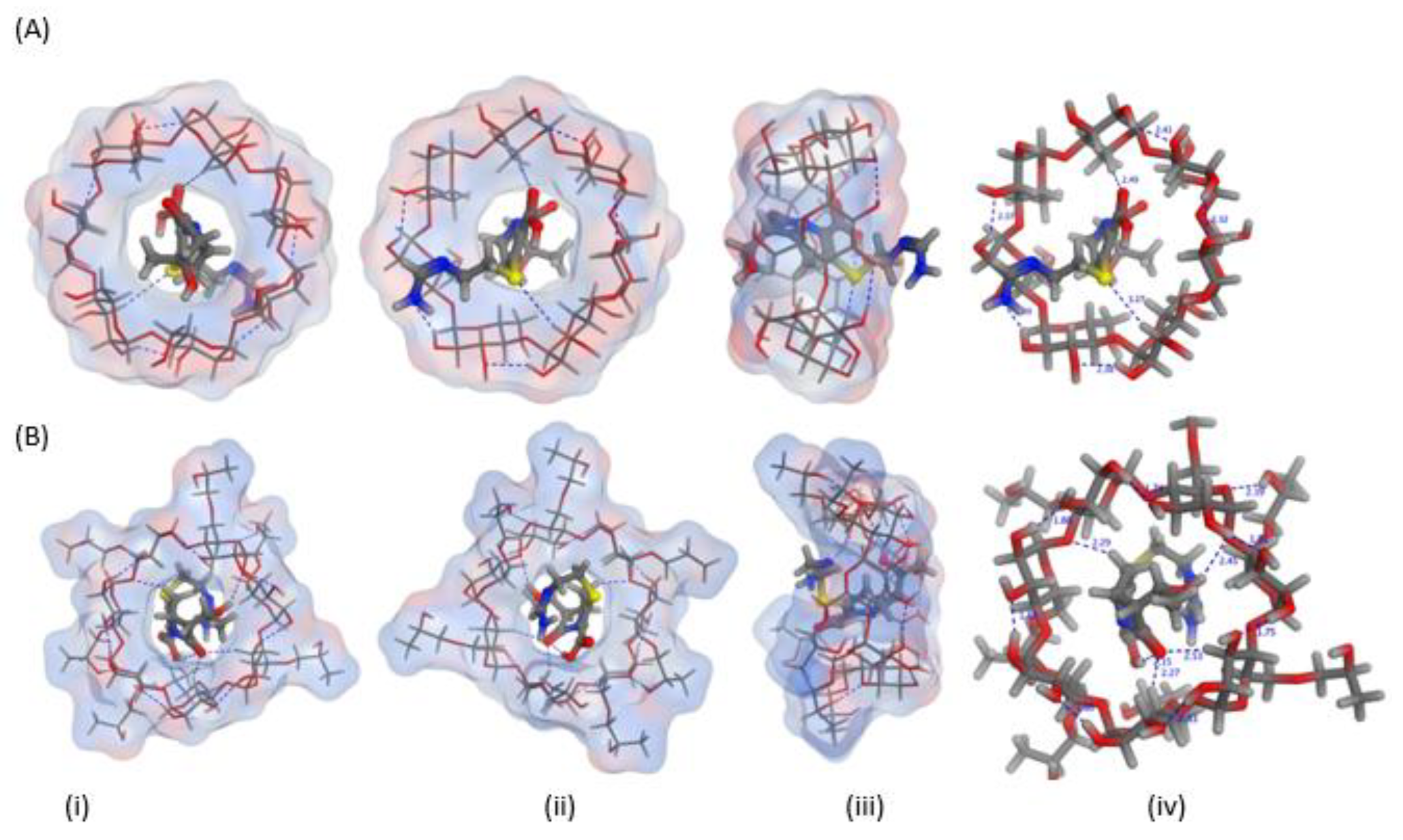
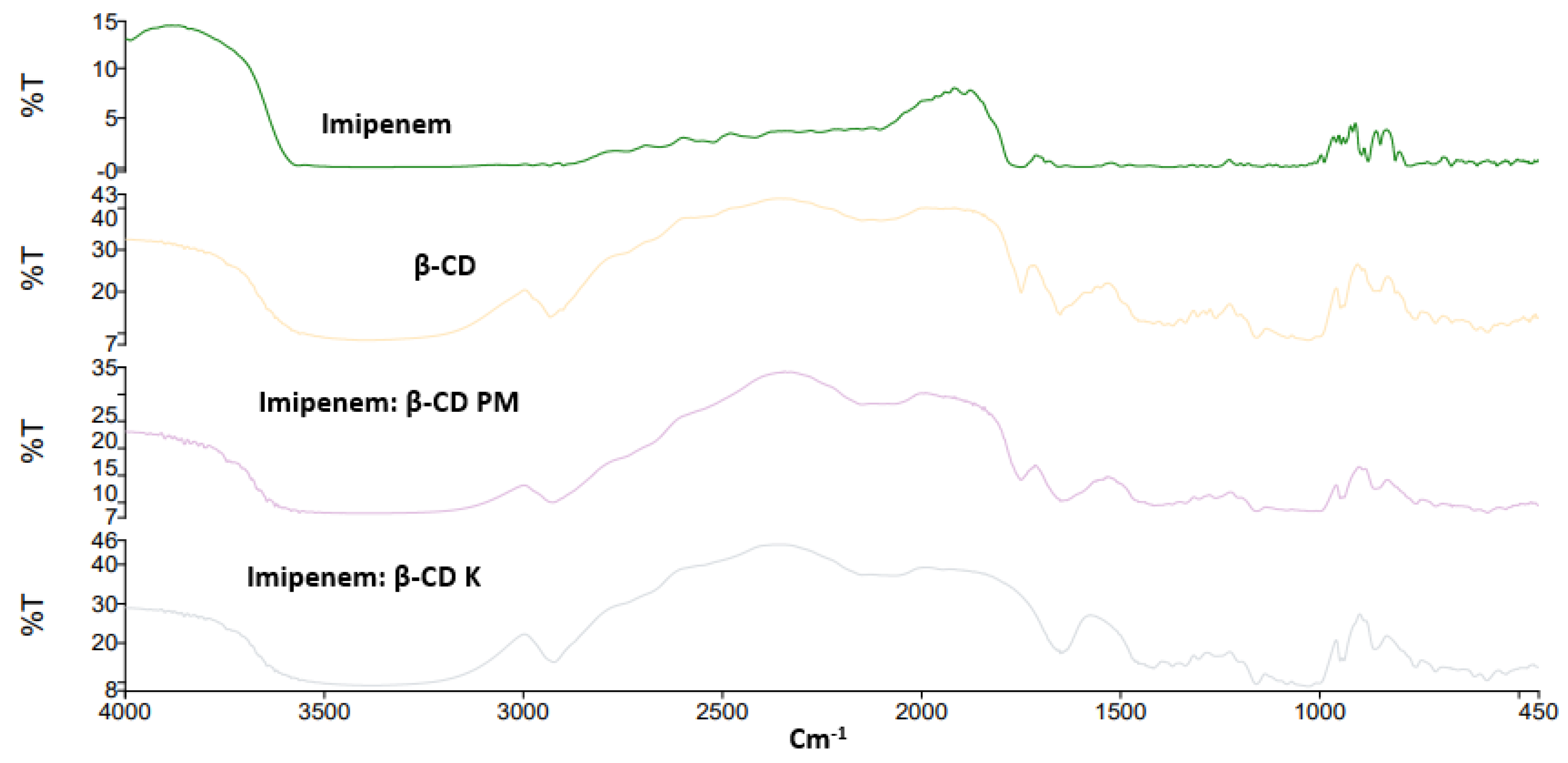
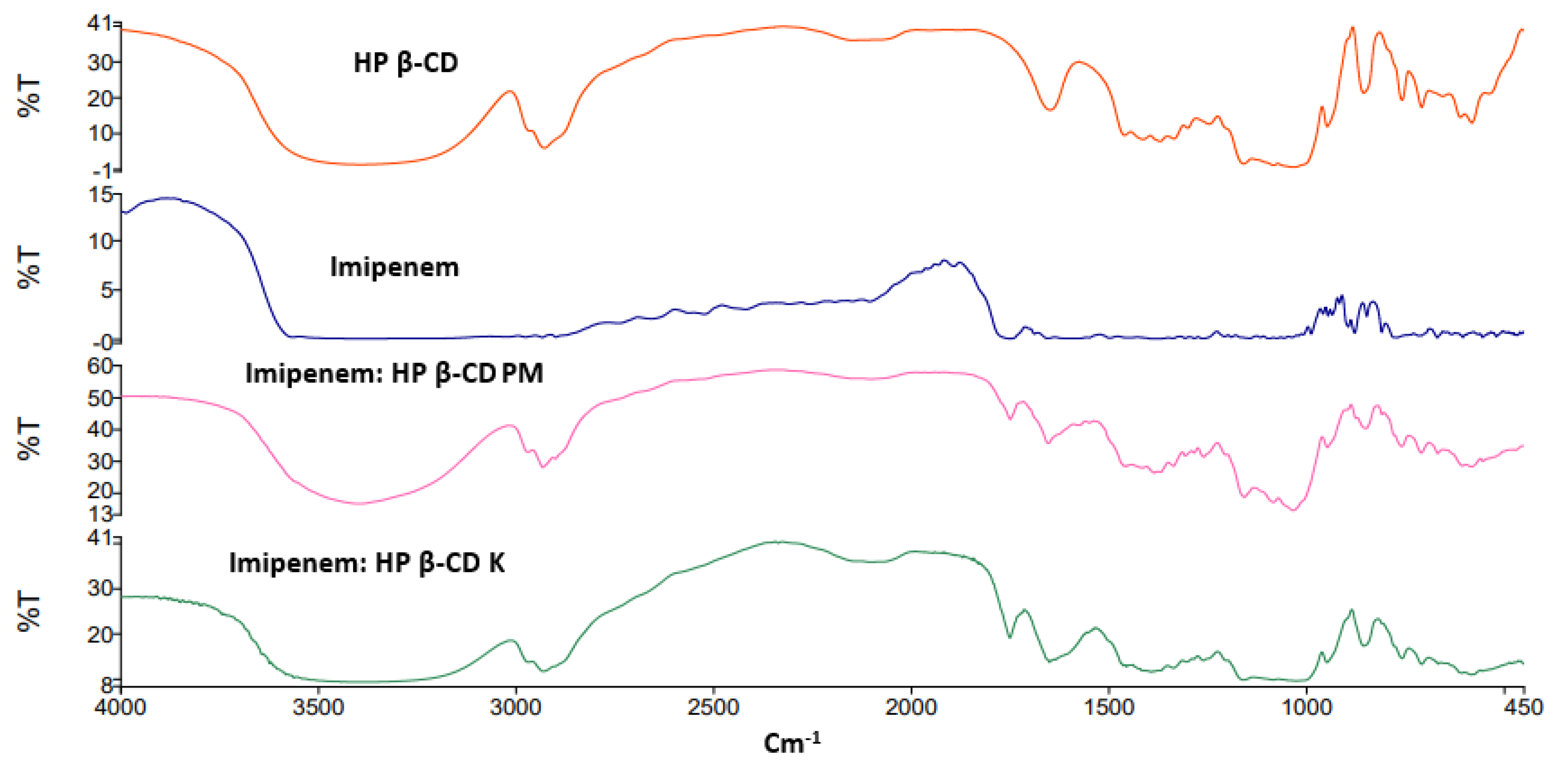
| API | Cyclodextrins | Energy Score (Kcal/mol) | Number and Types of Interactions | |
|---|---|---|---|---|
| H-Bond | Hydrophobic Attractions | |||
| Imipenem | β-cyclodextrin | −5.908 | 2 | - |
| Hydroxypropyl-β-cyclodextrin | −6.357 | 4 | - | |
| Type of the Infection | Isolate Counts | Klebsiella spp. | A. baumannii | Other Gram-Negative Bacteria |
|---|---|---|---|---|
| Skin infections | 93 | 5 | 3 | 85 |
| Ear infections | 10 | - | - | 10 |
| Chest infections | 22 | 5 | 2 | 15 |
| Urinary tract infections | 13 | - | - | 13 |
| Gastroenteritis | 12 | - | - | 12 |
| Total (%) * | 150 (100) | 10 (6.67) | 5 (3.33) | 135 (90%) |
| No. | MIC (µg/mL) | MIC90 | MIC50 | R | % | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | |||||
| Klebsiella spp. (n = 10) | 0 | 0 | 6 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 4 | 1 | 2 | 20 |
| A. baumannii (n = 5) | 0 | 0 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 8 | 2 | 1 | 20 |
| No. | MIC (µg/mL) | MIC90 | MIC50 | R | % | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | |||||
| Klebsiella spp. (n = 10) | 0 | 0 | 5 | 2 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 64 | 1 | 3 | 30 |
| A. baumannii (n = 5) | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 64 | 2 | 2 | 40 |
| Name of Organism | Source of Sample | No. of Isolates in Each Infection | Resistance Pattern of Imipenem | blaIMP | aac(6′)-Ib | ||
|---|---|---|---|---|---|---|---|
| Sensitive | Intermediate | Resistance | |||||
| Klebsiella spp. | Skin infections | 5 | 2 | 1 | 2 | 4 | - |
| Chest infections | 5 | 2 | 2 | 1 | 2 | 1 | |
| Acinetobacter baumanii | Skin infections | 3 | 2 | - | 1 | 2 | - |
| Chest infections | 2 | - | 1 | 1 | 2 | - | |
| MIC (µg/mL) | FICamikacin | FICimipenem | FICindex | Outcome | ||||
|---|---|---|---|---|---|---|---|---|
| Amikacin Alone | Imipenem Alone | Amikacin + Imipenem Combination | ||||||
| Klebsiella isolate (No. 9) | 512 | 64 | 4 | 1 | 0.0078 | 0.0156 | 0.023 | Synergistic |
| A.baumannii (No. 4) | 64 | 64 | 0.5 | 1 | 0.0078 | 0.0156 | 0.023 | Synergistic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhan, S.M.; El-Baky, R.M.A.; Ahmed, H.R.; Fathalla, Z.; Alamri, A.; Abdelkader, H.; Fatease, A.A. Comparative Investigation into the Roles of Imipenem:Cyclodextrin Complexation and Antibiotic Combination in Combatting Antimicrobial Resistance in Gram-Negative Bacteria. Pharmaceuticals 2023, 16, 1508. https://doi.org/10.3390/ph16101508
Farhan SM, El-Baky RMA, Ahmed HR, Fathalla Z, Alamri A, Abdelkader H, Fatease AA. Comparative Investigation into the Roles of Imipenem:Cyclodextrin Complexation and Antibiotic Combination in Combatting Antimicrobial Resistance in Gram-Negative Bacteria. Pharmaceuticals. 2023; 16(10):1508. https://doi.org/10.3390/ph16101508
Chicago/Turabian StyleFarhan, Sara Mahmoud, Rehab Mahmoud Abd El-Baky, Hala Rady Ahmed, Zeinab Fathalla, Ali Alamri, Hamdy Abdelkader, and Adel Al Fatease. 2023. "Comparative Investigation into the Roles of Imipenem:Cyclodextrin Complexation and Antibiotic Combination in Combatting Antimicrobial Resistance in Gram-Negative Bacteria" Pharmaceuticals 16, no. 10: 1508. https://doi.org/10.3390/ph16101508
APA StyleFarhan, S. M., El-Baky, R. M. A., Ahmed, H. R., Fathalla, Z., Alamri, A., Abdelkader, H., & Fatease, A. A. (2023). Comparative Investigation into the Roles of Imipenem:Cyclodextrin Complexation and Antibiotic Combination in Combatting Antimicrobial Resistance in Gram-Negative Bacteria. Pharmaceuticals, 16(10), 1508. https://doi.org/10.3390/ph16101508











