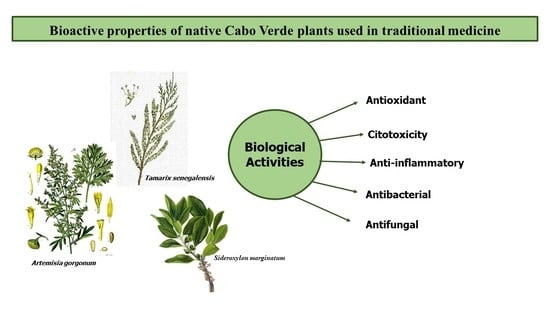
Evaluation of the Polyphenolic Composition and Bioactivities of Three Native Cabo Verde Medicinal Plants
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic Compounds
2.2. Antioxidant Activity
2.3. Cytotoxic Activity
2.4. Anti-Inflammatory Activity
3. Materials and Methods
3.1. Extract Preparation
3.2. UPLC Analysis of Phenolic Compounds
3.3. Bioactive Properties
3.3.1. Antioxidant Activity
3.3.2. Cytotoxic Activity
3.3.3. Anti-Inflammatory Activity
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lezotre, P.-L. State of Play and Review of Major Cooperation Initiatives. In International Cooperation, Convergence and Harmonization of Pharmaceutical Regulations; Elsevier Inc.: Oxford, UK, 2014; pp. 7–170. ISBN 9780128000533. [Google Scholar]
- Howes, M.J.R.; Quave, C.L.; Collemare, J.; Tatsis, E.C.; Twilley, D.; Lulekal, E.; Farlow, A.; Li, L.; Cazar, M.E.; Leaman, D.J.; et al. Molecules from Nature: Reconciling Biodiversity Conservation and Global Healthcare Imperatives for Sustainable Use of Medicinal Plants and Fungi. Plants People Planet. 2020, 2, 463–481. [Google Scholar] [CrossRef]
- Abdullahi, A.A. Trends and Challenges of Traditional Medicine in Africa. Afr. J. Trad. Complement. Altern. Med. 2011, 8, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Oladunni Balogun, F.; Omotayo Tom Ashafa, A.; Sabiu, S.; Ayokun-nun Ajao, A.; Palanisamy Perumal, C.; Idowu Kazeem, M.; Adebowale Adedeji, A. Pharmacognosy: Importance and Drawbacks. In Pharmacognosy-Medicinal Plants; IntechOpen: Rijeka, Croatia, 2019; pp. 1–19. [Google Scholar]
- Fokunang, C.N.; Ndikum, V.; Tabi, O.Y.; Jiofack, R.B.; Ngameni, B.; Guedje, N.M.; Tembe-Fokunang, E.A.; Tomkins, P.; Barkwan, S.; Kechia, F.; et al. Traditional Medicine: Past, Present and Future Research and Development Prospects and Integration in the National Health System of Cameroon. Afr. J. Trad. Complement. Altern. Med. 2011, 8, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Hooft, A.; Nabukalu, D.; Mwanga-Amumpaire, J.; Gardiner, M.A.; Sundararajan, R. Factors Motivating Traditional Healer versus Biomedical Facility Use for Treatment of Pediatric Febrile Illness: Results from a Qualitative Study in Southwestern Uganda. Am. J. Trop. Med. Hyg. 2020, 103, 501–507. [Google Scholar] [CrossRef]
- Ekor, M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Catarino, L.; Havik, P.; Romeiras, M. Medicinal Plants of Guinea-Bissau: Therapeutic Applications, Ethnic Diversity and Knowledge Transfer. J. Ethnopharmacol. 2016, 183, 71–94. [Google Scholar] [CrossRef]
- WHO. The State of Health in the WHO African Region: An Analysis of the Status of Health, Health Services and Health Systems in the Context of the Sustainable. Available online: https://apps.who.int/iris/handle/10665/275292 (accessed on 18 June 2018).
- Duarte, M.C.; Gomes, I.; Catarino, S.; Brilhante, M.; Gomes, S.; Rendall, A.; Moreno, Â.; Fortes, A.R.; Ferreira, V.S.; Baptista, I.; et al. Diversity of useful plants in Cabo Verde Islands: A biogeographic and conservation perspective. Plants 2022, 11, 1313. [Google Scholar] [CrossRef]
- Bäckström, B. Comportamentos de saúde e doença numa comunidade Cabo-Verdiana em Lisboa. Saude Soc. 2011, 20, 758–772. [Google Scholar] [CrossRef]
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef]
- Vallès, J.; Garcia, S.; Hidalgo, O.; Martín, J.; Pellicer, J.; Sanz, M.; Garnatje, T. Biology, Genome Evolution, Biotechnological Issues and Research including Applied Perspectives in Artemisia (Asteraceae). Adv. Bot. Res. 2011, 60, 349–419. [Google Scholar]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef]
- Nigam, M.; Atanassova, M.; Mishra, A.P.; Pezzani, R.; Devkota, H.P.; Plygun, S.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J. Bioactive compounds and health benefits of Artemisia species. Nat. Prod. Commun. 2019, 14, 1934578X19850354. [Google Scholar]
- Willcox, M. Artemisia species: From traditional medicines to modern antimalarials—And back again. J. Altern. Complement. Med. 2009, 15, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Ortet, R.; Prado, S.; Mouray, E.; Thomas, O.P. Sesquiterpene Lactones from the endemic Cape Verdean Artemisia gorgonum. Phytochemistry 2008, 69, 2961–2965. [Google Scholar] [CrossRef] [PubMed]
- Ortet, R.; Thomas, O.P.; Regalado, E.L.; Pino, J.A.; Filippi, J.J.; Fernández, M.D. Composition and biological properties of the volatile oil of Artemisia gorgonum Webb. Chem. Biodivers. 2010, 7, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Mignon, R.; Bastos, M.; Batista, D.; Neng, N.R.; Nogueira, J.M.F.; Vizetto-Duarte, C.; Custódio, L.; Varela, J.; Rauter, A.P. In Vitro Antitumoral Activity of Compounds Isolated from Artemisia gorgonum Webb. Phytother. Res. 2014, 28, 1329–1334. [Google Scholar] [CrossRef]
- Lima, K.; Silva, O.; Figueira, M.E.; Pires, C.; Cruz, D.; Gomes, S.; Maurício, E.M.; Duarte, M.P. Influence of the In Vitro Gastrointestinal Digestion on the Antioxidant Activity of Artemisia gorgonum Webb and Hyptis pectinata (L.) Poit. Infusions from Cape Verde. Food Res. Int. 2019, 115, 150–159. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Kalkhorani, M.; Abbas Zaidi, S.M.; Farzaei, M.H.; Rahimi, R. The genus Tamarix: Traditional Uses, Phytochemistry, and Pharmacology. J. Ethnopharmacol. 2020, 246, 112245. [Google Scholar] [CrossRef]
- Alshehri, S.A.; Wahab, S.; Saquib Abullais, S.; Das, G.; Hani, U.; Ahmad, W.; Amir, M.; Ahmad, A.; Kandasamy, G.; Vasudevan, R. Pharmacological Efficacy of Tamarix aphylla: A Comprehensive Review. Plants 2022, 11, 118. [Google Scholar] [CrossRef]
- Varela, D.; Romeiras, M.M.; Silva, L. Implications of climate change on the distribution and conservation of Cabo Verde endemic trees. Glob. Ecol. Conserv. 2022, 34, e02025. [Google Scholar] [CrossRef]
- Romeiras, M.M.; Catarino, S.; Gomes, I.; Fernandes, C.; Costa, J.C.; Caujapé-Castells, J.; Duarte, M.C. IUCN Red List assessment of the Cape Verde endemic flora: Towards a global strategy for plant conservation in Macaronesia. Bot. J. Linn. Soc. 2016, 180, 413–425. [Google Scholar] [CrossRef]
- POWO—Plants of the World Online. Available online: http://powo.science.kew.org (accessed on 18 June 2022).
- Correia, L.P.; Santana, C.P.; Medeiros, A.C.D.; Macêdo, R.O. Sideroxylon obtusifolium herbal medicine characterization using pyrolysis GC/MS, SEM and different thermoanalytical techniques. J. Therm. Anal. Calorim. 2016, 123, 993–1001. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Raith, M.; Kuster, R.M.; Rocha, L.M.; Hamburger, M.; Potterat, O. Metabolite profiling of the leaves of the Brazilian folk medicine Sideroxylon obtusifolium. Planta Med. 2012, 78, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Nunes, R.; Pasko, P.; Tyszka-Czochara, M.; Szewczyk, A.; Szlosarczyk, M.; Carvalho, I.S. Antibacterial, Antioxidant and Anti-Proliferative Properties and Zinc Content of Five South Portugal Herbs. Pharm. Biol. 2017, 55, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, V.V.; Banerjee, D. Scientific Validation of Herbal Medicine. In Herbal Medicine in India: Indigenous Knowledge, Practice, Innovation and Its Value; Spinger: Heidelberg, Germany, 2019; ISBN 978-9811372476. [Google Scholar]
- Jhariya, M.K.; Banerjee, A.; Meena, R.S.; Yadav, D.K. Agriculture, Forestry and Environmental Sustainability: A Way Forward. In Sustainable Agriculture, Forest and Environmental Management; Springer Singapore: Singapore, 2019; pp. 1–29. [Google Scholar]
- Sekkien, A.; Swilam, N.; Ebada, S.S.; Esmat, A.; El-Khatib, A.H.; Linscheid, M.W.; Singab, A.N. Polyphenols from Tamarix nilotica: LC–ESI-MSn Profiling and in Vivo Antifibrotic Activity. Molecules 2018, 23, 1411. [Google Scholar] [CrossRef] [PubMed]
- Mahfoudhi, A.; Prencipe, F.P.; Mighri, Z.; Pellati, F. Metabolite Profiling of Polyphenols in the Tunisian Plant Tamarix aphylla (L.) Karst. J. Pharm. Biomed. Anal. 2014, 99, 97–115. [Google Scholar] [CrossRef]
- .Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical Scheme for LC-MSn Identification of Chlorogenic Acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Chirikova, N.K.; Kashchenko, N.I.; Nikolaev, V.M.; Kim, S.W.; Vennos, C. Bioactive Phenolics of the genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS Profile of the Siberian Species and Their Inhibitory Potential against α-Amylase and α-Glucosidase. Front. Pharmacol. 2018, 9, 756. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Jauregui, O.; Medina-Remón, A.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M. Improved characterization of tomato polyphenols using liquid chromatography/electrospray ionization linear ion trap quadrupole Orbitrap mass spectrometry and liquid chromatography/electrospray ionization tandem mass spectrometry. RCM 2010, 24, 2986–2992. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Alves, C.T.; Silva, S.; Henriques, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antifungal activity and detailed chemical characterization of Cistus ladanifer phenolic extracts. Ind. Crops Prod. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Silva, A.R.; Ayuso, M.; Pereira, C.; Dias, M.I.; Kostić, M.; Calhelha, R.C.; Pablo, A.G.; Ferreira, I.C.F.R.; Barros, L. Evaluation of parasite and host phenolic composition and bioactivities—The Practical Case of Cytinus hypocistis (L.) L. and Halimium lasianthum (Lam.) Greuter. Ind. Crops Prod. 2022, 176, 114343. [Google Scholar] [CrossRef]
- Ali, J.S.; Riaz, N.; Mannan, A.; Latif, M.; Zia, M. Antioxidant, Antimicrobial, Enzyme Inhibition, and Cytotoxicity Guided Investigation of Sideroxylon mascatense (A.DC.) T.D. Penn. Leaves Extracts. Nat. Prod. Res. 2022, 36, 4227–4230. [Google Scholar] [CrossRef] [PubMed]
- de Aquino, P.E.A.; de Souza, T.d.F.G.; Santos, F.A.; Viana, A.F.S.C.; Louchard, B.O.; Leal, L.K.A.M.; Rocha, T.M.; Evangelista, J.S.A.M.; de Aquino, N.C.; de Alencar, N.M.N.; et al. The Wound Healing Property of N-Methyl-(2S,4R)-trans-4-Hydroxy-L-Proline from Sideroxylon obtusifolium is related to its anti-inflammatory and antioxidant actions. J. Evid.-Based Integr. Med. 2019, 24, 2515690X19865166. [Google Scholar] [CrossRef] [PubMed]
- Hassan, L.E.A.; Khadeer Ahamed, M.B.; Abdul Majid, A.S.; Baharetha, H.M.; Muslim, N.S.; Nassar, Z.D.; Abdul Majid, A.M.S. Correlation of Antiangiogenic, Antioxidant and cytotoxic activities of some Sudanese medicinal plants with phenolic and flavonoid contents. BMC Complement. Altern. Med. 2014, 14, 406. [Google Scholar] [CrossRef]
- Yusufoglu, H.S.; Alqasoumi, S.I. Anti-inflammatory and wound healing activities of herbal gel containing an antioxidant Tamarix aphylla leaf extract. Int. J. Pharmacol. 2011, 7, 829–835. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Drabu, S.; Sharma, M. Anti-inflammatory and analgesic activity of Tamarix gallica. Int. J. Pharm. Sci. 2012, 4, 653–658. [Google Scholar]
- Sayago, C.; Camargo, V.; Barbosa, F.; Gularte, C.; Pereira, G.; Miotto, S.; Cechinel Filho, V.; Luiz Puntel, R.; Folmer, V.; Mendez, A. Chemical composition and in vitro antioxidant activity of hydro-ethanolic extracts from Bauhinia forficata subsp. pruinosa and B. variegata. Acta Biol. Hung. 2013, 64, 21–33. [Google Scholar] [CrossRef]
- Wang, K.S.; Li, J.; Wang, Z.; Mi, C.; Ma, J.; Piao, L.X.; Xu, G.H.; Li, X.; Jin, X. Artemisinin inhibits inflammatory response via regulating NF-κB and MAPK Signaling Pathways. Immunopharmacol. Immunotoxicol. 2017, 39, 28–36. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Spréa, R.M.; Fernandes, Â.; Calhelha, R.C.; Pereira, C.; Pires, T.C.S.P.; Alves, M.J.; Canan, C.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical and bioactive characterization of the aromatic plant: Levisticum officinale W.D.J. Koch: A comprehensive study. Food Funct. 2020, 11, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, R.C.G.; de Souza, A.H.P.; Calhelha, R.C.; Barros, L.; Glamoclija, J.; Sokovic, M.; Peralta, R.M.; Bracht, A.; Ferreira, I.C.F.R. Bioactive formulations prepared from fruiting bodies and submerged culture mycelia of the brazilian edible mushroom Pleurotus ostreatoroseus Singer. Food Funct. 2015, 6, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
| Status | Plant (Species/Family) | Distribution | Common Name | Ecology and Conservation | Indication | Route of Administration |
|---|---|---|---|---|---|---|
| Native (non-endemic) | ||||||
| T. senegalensis (Tamaricaceae) | Arabian Peninsula, northwestern Africa, and Cabo Verde (in all the islands except in Fogo) | Tarrafe | Tree that grows in saline soil, sandy, and sea shore (Not Evaluated-IUCN) | Cold treatment | Infusion; herbal baths are also added to a tea together with a spoonful of grog (alcoholic) | |
| Endemic | ||||||
| A. gorgonum (Asteraceae) | Cabo Verde: Santo Antão, Santiago, and Fogo islands | Losna | Perennial shrub that occurs in altitude semi-arid to sub-humid zones. It is threatened by continued habitat loss (Endangered-IUCN) | Intestinal parasites, fever, uterus, and cramps | Infusion; alcoholic | |
| S. marginatum (Sapotaceae) | Cabo Verde: all the islands, except in Sal and Maio | Marmulano | Tree growing in steep escarpments and inaccessible places. It is threatened by continued habitat loss (Endangered-IUCN) | Bone fractures and pain | Infusion; alcoholic; topical |
| Peak | Rt (min) | λmax (nm) | [M − H]− (m/z) | MSn (m/z) | Tentative Identification | References |
|---|---|---|---|---|---|---|
| Tamarix senegalensis | ||||||
| 1 | 7.15 | 303 | 273 | 229 (9),193(100),178(11),153(5) | Ferulic acid sulphate derivative | [33,34] |
| 2 | 14.3 | 347 | 395 | 315(33),300(100),217(5) | Methylquercetin-sulphate (tamarixetin sulphate) | [34] |
| 3 | 15.42 | 351 | 477 | 315(12),301(100) | Methylquercetin hexoside (tamarixetin-3-O-hexoside) | [34] |
| 4 | 15.79 | 352 | 299 | 285(100),271(14) | Methylkaempferol (kaempferide) | [34] |
| 5 | 16.3 | 350 | 379 | 299(35),284(100) | Kaempferol methyl ether sulphate | [33,34] |
| 6 | 16.71 | 346 | 461 | 285(100) | Kaempferol-O-hexurunoside | [34] |
| Artemisia gorgonum | ||||||
| 7 | 5.11 | 325 | 353 | 191(100),179(45),161(5),135(5) | 3-O-Caffeoylquinic acid | Standard compound |
| 8 | 9.6 | 325 | 353 | 173(100),179(23),191(14),161(5),135(5) | 4-O-Caffeoylquinic acid | [35] |
| 9 | 10.91 | 325 | 353 | 191(100),179(8),161(7),135(4) | cis 5-O-Caffeoylquinic acid | [35] |
| 10 | 11.01 | 326 | 353 | 191(100),179(12),173(5),135(5) | trans 5-O-Caffeoylquinic acid | [35] |
| 11 | 13.11 | 334 | 593 | 431(34),341(56),311(100),313(12),283(17) | Apigenin-6-C-Glc-4”-O-Glc (isosaponarin) | [35] |
| 12 | 13.27 | 291 | 325 | 163(100) | Melilotoside | [35] |
| 13 | 14.02 | 330 | 563 | 503(25),473(98)443(100),413(5),383(35)353(32)325(5)297(5) | Apigenin-6-C-Ara-8-C-Glc (schaftoside) | [35] |
| 14 | 16.26 | 322 | 515 | MS2: 353(100); MS3: 191(23),179(54),173(100),135(19) | 1,4-di-O-Caffeoylquinic acid | [35] |
| 15 | 16.3 | 321 | 515 | MS2: 353(100); MS3: 191(100),179(51),173(13),135(5) | 3,5-di-O-Caffeoylquinic acid | [35] |
| 16 | 17.14 | 326 | 515 | MS2: 353(100); MS3: 191(34),179(42),173(100),135(11) | 4,5-di-O-Caffeoylquinic acid | [35] |
| 17 | 18.41 | 327 | 515 | MS2: 353(100); MS3: 191(98),179(88),173(100),135(14) | cis 3,4-di-O-Caffeoylquinic acid | [35] |
| 18 | 18.97 | 326 | 515 | MS2: 353(100); MS3: 191(97),179(85),173(100),135(12) | trans 3,4-di-O-Caffeoylquinic acid | [35] |
| Sideroxylon marginatum | ||||||
| 19 | 14 | 354 | 741 | 301(00) | Quercetin-O-hexosyl-deoxyhexosyl-pentoside | [36] |
| 20 | 14.49 | 354 | 595 | 301(00) | Quercetin-O-hexosyl-pentoside | [37] |
| 21 | 15.03 | 354 | 609 | 301(00) | Quercetin-O-hexosyl-deoxyhexoside | Characterization |
| 22 | 15.21 | 350 | 463 | 315(00) | Isorhamnetin derivative | DAD/MS |
| 23 | 16.87 | 347 | 447 | 301(00) | Quercetin-O-deoxyhexoside | [38] |
| Peak | Quantification (mg/g Extract) | t-Student’s Test p-Value | |
|---|---|---|---|
| Ethanolic | Infusion | ||
| Tamarix senegalensis | |||
| 1 | 1.25 ± 0.05 | 10.7 ± 0.03 | <0.001 |
| 2 | 0.63 ± 0.02 | 1.17 ± 0.02 | <0.001 |
| 3 | 0.95 ± 0.04 | 1.61 ± 0.03 | <0.001 |
| 4 | 0.78 ± 0.03 | 1.53 ± 0.01 | <0.001 |
| 5 | 1.44 ± 0.08 | 1.27 ± 0.06 | <0.001 |
| 6 | 1.59 ± 0.06 | 1.90 ± 0.08 | <0.001 |
| Total Phenolic Acids | 1.25 ± 0.05 | 10.70 ± 0.03 | <0.001 |
| Total Flavonoids | 5.4 ± 0.2 | 7.47 ± 0.03 | <0.001 |
| Total Phenolic Compounds | 13.3 ± 0.5 | 36.35 ± 0.01 | <0.001 |
| Artemisia gorgonum | |||
| 7 | 0.55 ± 0.02 | 2.23 ± 0.06 | <0.001 |
| 8 | 0.48 ± 0.01 | 2.90 ± 0.03 | <0.001 |
| 9 | 1.54 ± 0.09 | 9.5 ± 0.2 | <0.001 |
| 10 | nd | 6.0 ± 0.1 | - |
| 11 | 0.013 ± 0.001 | 0.53 ± 0.05 | <0.001 |
| 12 | 1.58 ± 0.02 | 17.8 ± 0.4 | <0.001 |
| 13 | 0.41 ± 0.01 | 4.56 ± 0.09 | <0.001 |
| 14 | 0.73 ± 0.03 | 12.66 ± 0.07 | <0.001 |
| 15 | 0.50 ± 0.02 | 15.2 ± 0.4 | <0.001 |
| 16 | 0.53 ± 0.02 | 24.3 ± 0.2 | <0.001 |
| 17 | 0.37 ± 0.01 | 1.89 ± 0.07 | <0.001 |
| 18 | 0.23 ± 0.01 | 2.07 ± 0.01 | <0.001 |
| Total Phenolic Acids | 6.48 ± 0.01 | 95 ± 1 | <0.001 |
| Total Flavonoids | 0.42 ± 0.01 | 5.1 ± 0.1 | <0.001 |
| Total Phenolic Compounds | 6.90 ± 0.01 | 100 ± 1 | <0.001 |
| Sideroxylon marginatum | |||
| 19 | 1.33 ± 0.02 | 4.5 ± 0.3 | <0.001 |
| 20 | 0.64 ± 0.01 | 1.22 ± 0.04 | <0.001 |
| 21 | 0.56 ± 0.01 | 1.36 ± 0.04 | <0.001 |
| 22 | 0.67 ± 0.01 | 1.42 ± 0.06 | <0.001 |
| 23 | 0.56 ± 001 | 0.93 ± 0.02 | <0.001 |
| Total Phenolic Compounds | 3.76 ± 0.01 | 9.5 ± 0.3 | <0.001 |
| T. senegalensis | A. gorgonum | S. marginatum | ||
|---|---|---|---|---|
| Antioxidant activity | ||||
| TBARS (EC50; mg/mL) a | Ethanolic | 0.161 ± 0.005 b | 0.149 ± 0.003 c | 0.27 ± 0.01 a |
| Infusion | 0.83 ± 0.02 a | 0.42 ± 0.02 b | 0.87 ± 0.05 a | |
| OxHLIA (EC50; µg/mL) b Δt = 60 min | Ethanolic | 39 ± 1 b | 15 ± 1 c | 99 ± 2 a |
| Infusion | 10.4 ± 0.5 c | 102 ± 4 b | 115 ± 4 a | |
| Cytotoxicity over tumor cell lines (GI50 μg/mL) c | ||||
| AGS | Ethanolic | 85 ± 2 b | 18.2 ± 0.4 c | 116 ± 12 a |
| Infusion | 208 ± 20 a | 67 ± 6 b | 75 ± 1 b | |
| CaCO2 | Ethanolic | 125 ± 4 b | 17.3 ± 0.2 c | 251 ± 12 a |
| Infusion * | >400 | 181 ± 10 | 285 ± 2 | |
| MCF-7 | Ethanolic | 149 ± 4 b | 57 ± 6 c | 201 ± 1 a |
| Infusion * | >400 | 129 ± 10 | 209 ± 4 | |
| Cytotoxicity over non-tumor cell lines (GI50 µg/mL) c | ||||
| PLP2 | Ethanolic | 178 ± 3 | >400 | >400 |
| Infusion | >400 | >400 | >400 | |
| Anti-inflammatory activity (EC50 μg/mL) d | ||||
| RAW 264.7 | Ethanolic * | 35 ± 1 | >400 | 43 ± 4 |
| Infusion | >400 | >400 | >400 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
P. Essoh, A.; Liberal, Â.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Mandim, F.; Moldão-Martins, M.; Cravo, P.; Duarte, M.P.; Moura, M.; et al. Evaluation of the Polyphenolic Composition and Bioactivities of Three Native Cabo Verde Medicinal Plants. Pharmaceuticals 2022, 15, 1162. https://doi.org/10.3390/ph15091162
P. Essoh A, Liberal Â, Fernandes Â, Dias MI, Pereira C, Mandim F, Moldão-Martins M, Cravo P, Duarte MP, Moura M, et al. Evaluation of the Polyphenolic Composition and Bioactivities of Three Native Cabo Verde Medicinal Plants. Pharmaceuticals. 2022; 15(9):1162. https://doi.org/10.3390/ph15091162
Chicago/Turabian StyleP. Essoh, Anyse, Ângela Liberal, Ângela Fernandes, Maria Inês Dias, Carla Pereira, Filipa Mandim, Margarida Moldão-Martins, Pedro Cravo, Maria Paula Duarte, Mónica Moura, and et al. 2022. "Evaluation of the Polyphenolic Composition and Bioactivities of Three Native Cabo Verde Medicinal Plants" Pharmaceuticals 15, no. 9: 1162. https://doi.org/10.3390/ph15091162
APA StyleP. Essoh, A., Liberal, Â., Fernandes, Â., Dias, M. I., Pereira, C., Mandim, F., Moldão-Martins, M., Cravo, P., Duarte, M. P., Moura, M., Romeiras, M. M., & Barros, L. (2022). Evaluation of the Polyphenolic Composition and Bioactivities of Three Native Cabo Verde Medicinal Plants. Pharmaceuticals, 15(9), 1162. https://doi.org/10.3390/ph15091162