Antiproliferative and Apoptotic Effects of Graphene Oxide @AlFu MOF Based Saponin Natural Product on OSCC Line
Abstract
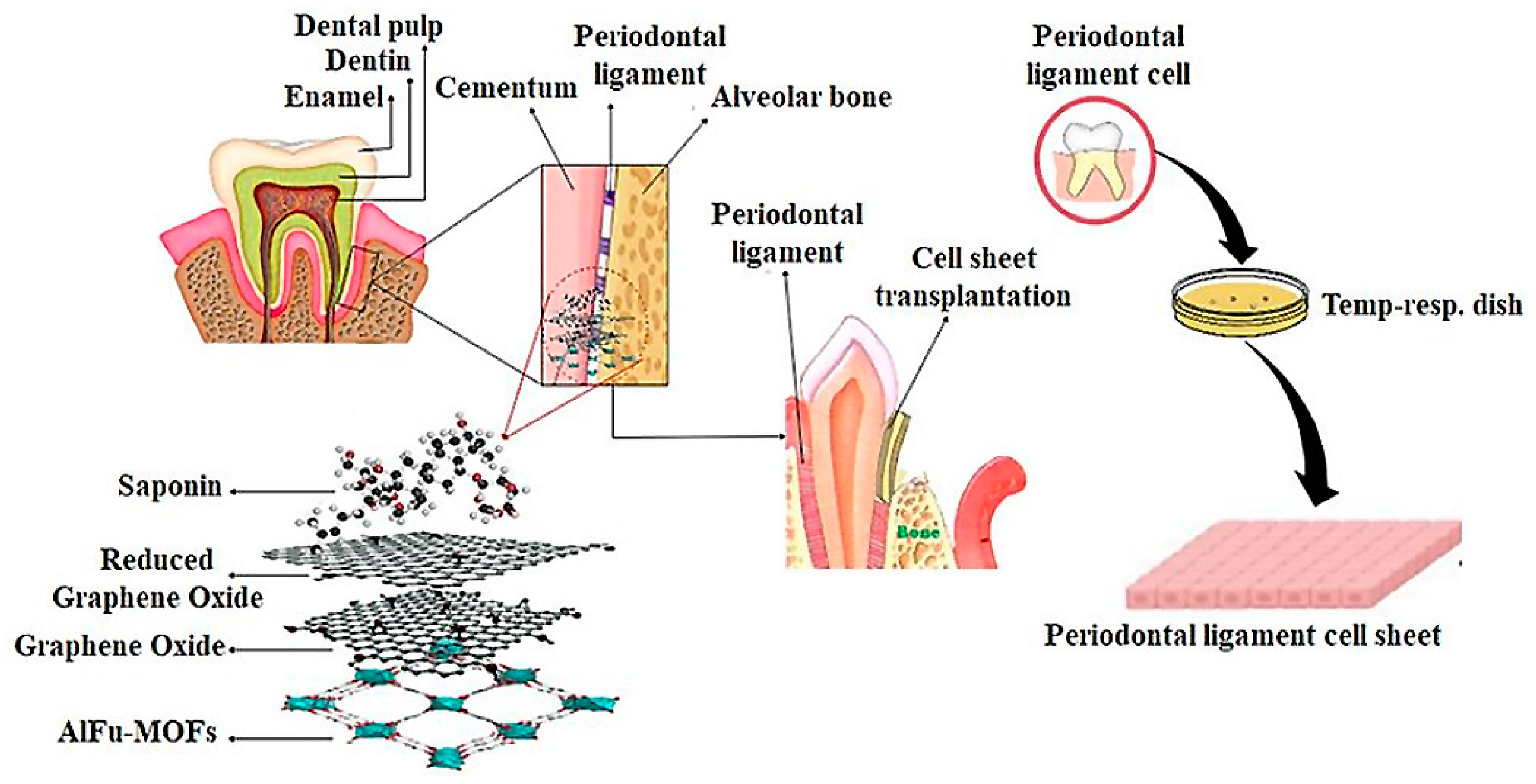
:1. Introduction
2. Results and Discussion
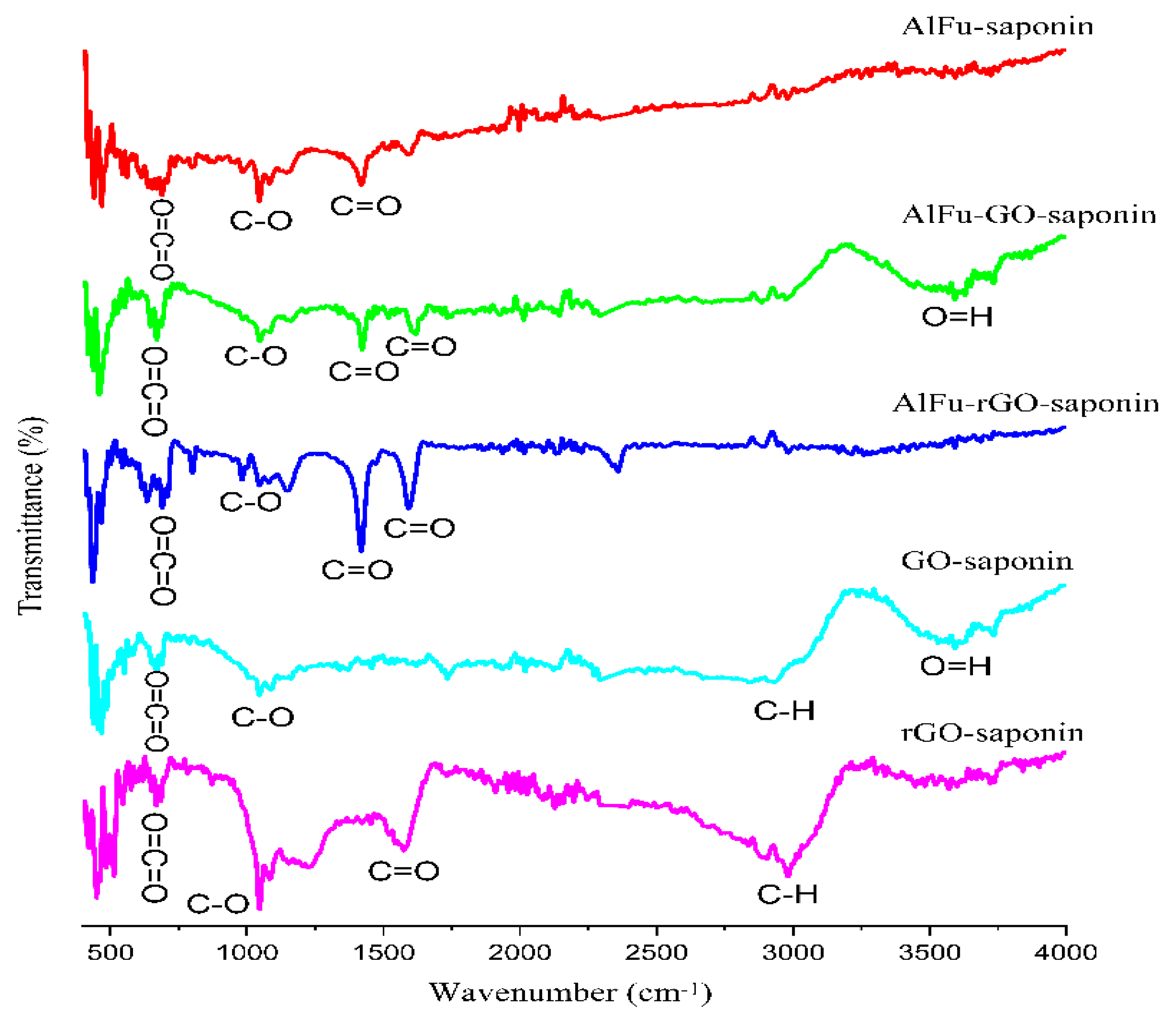
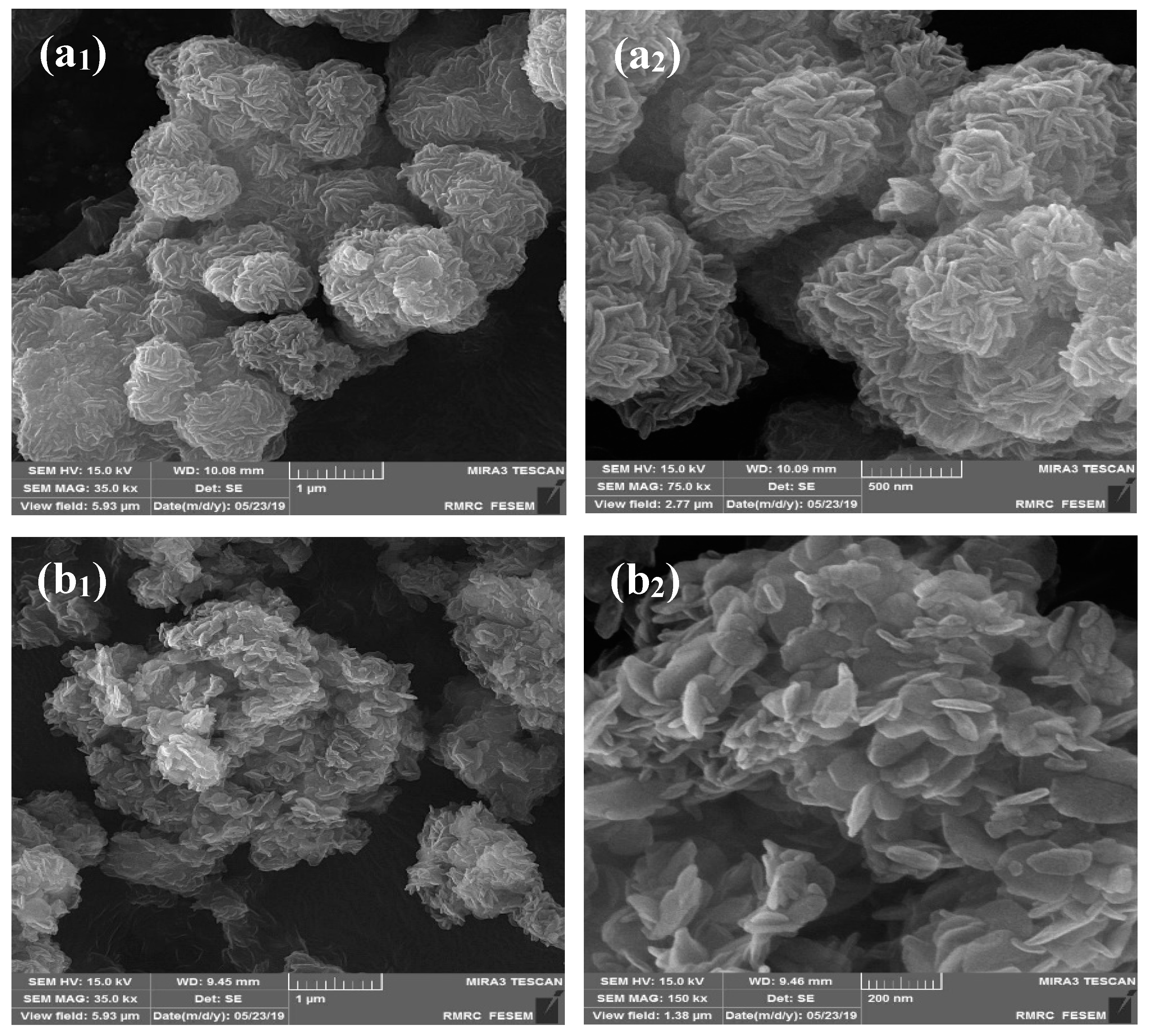
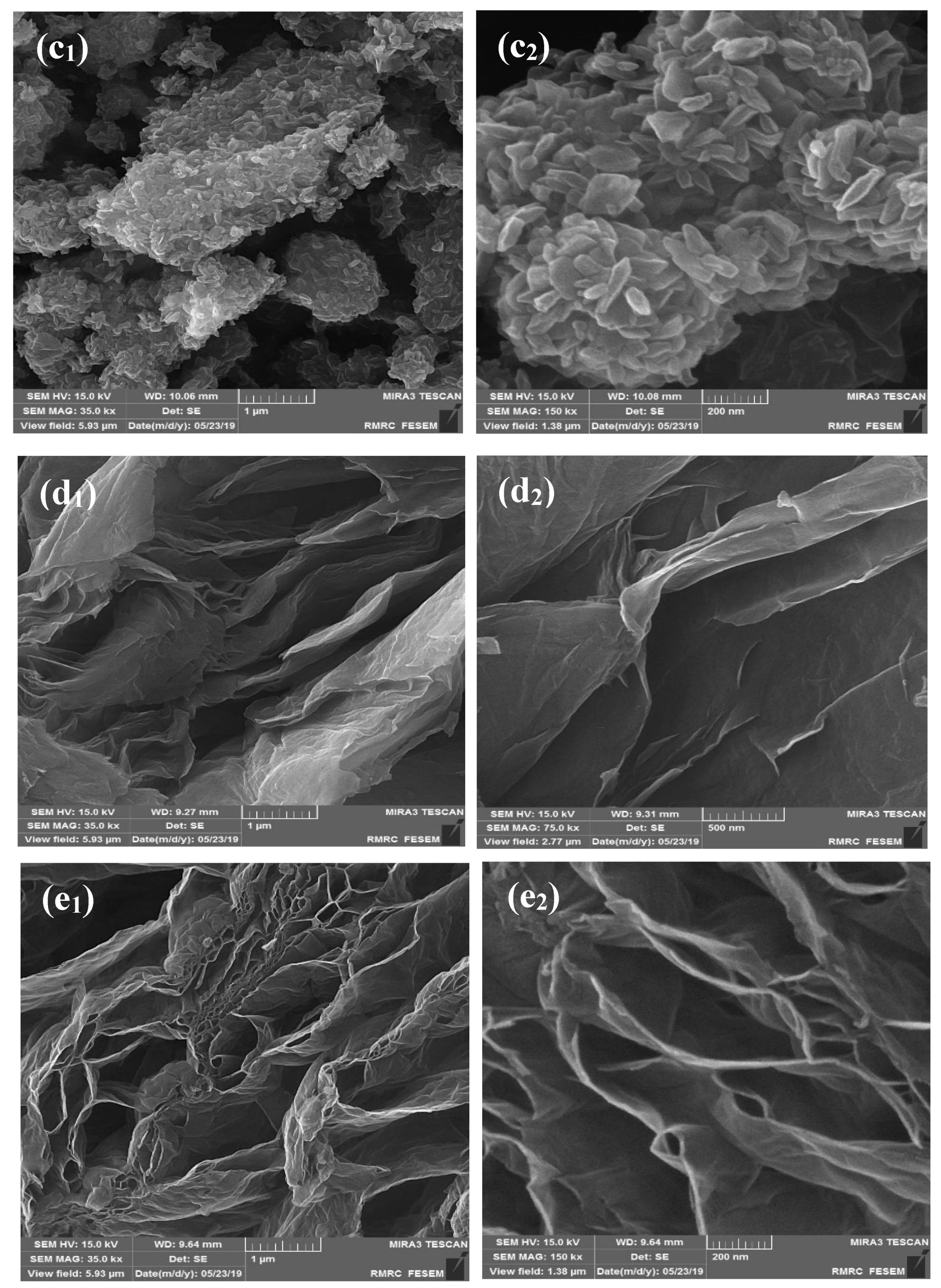
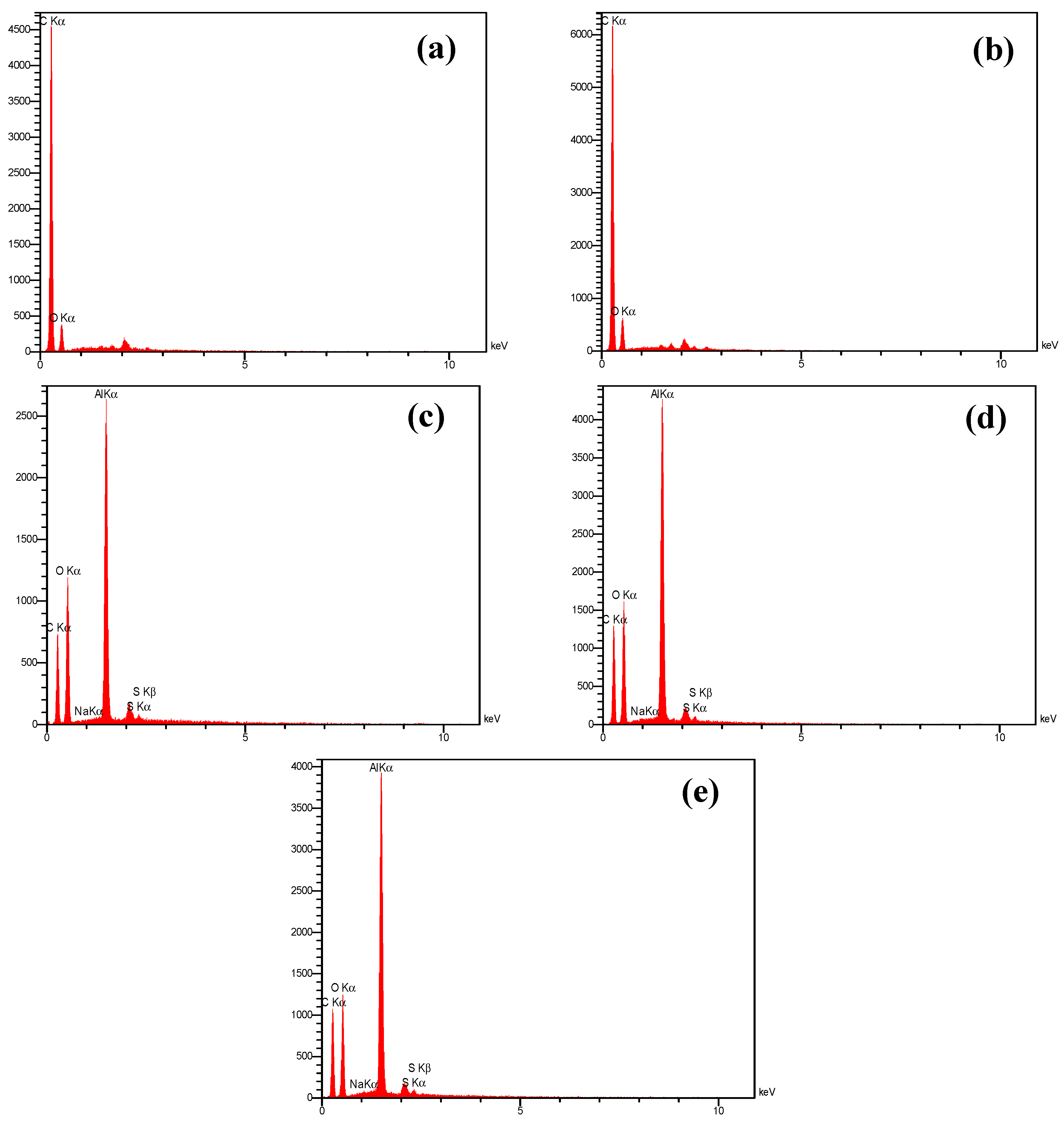
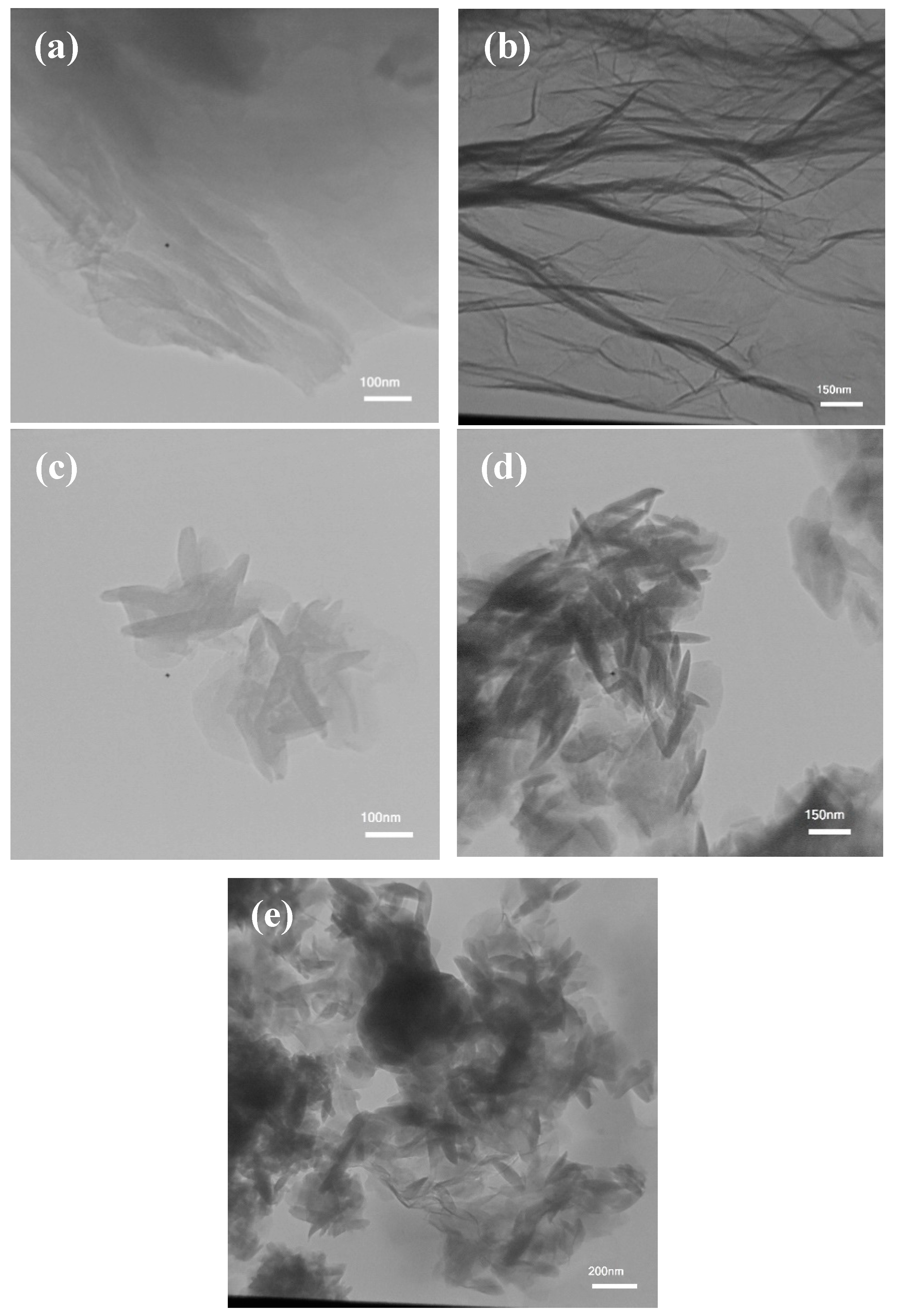
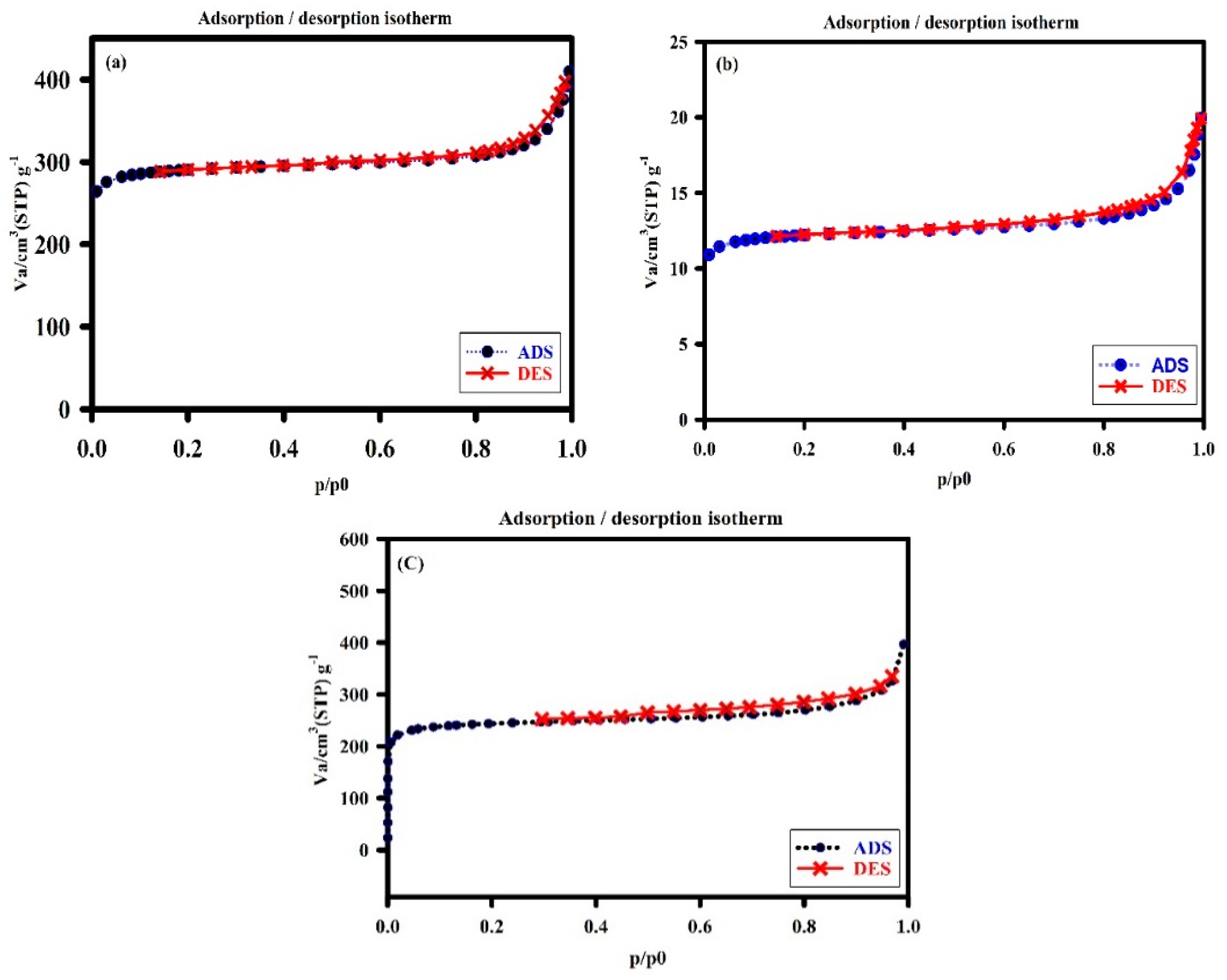
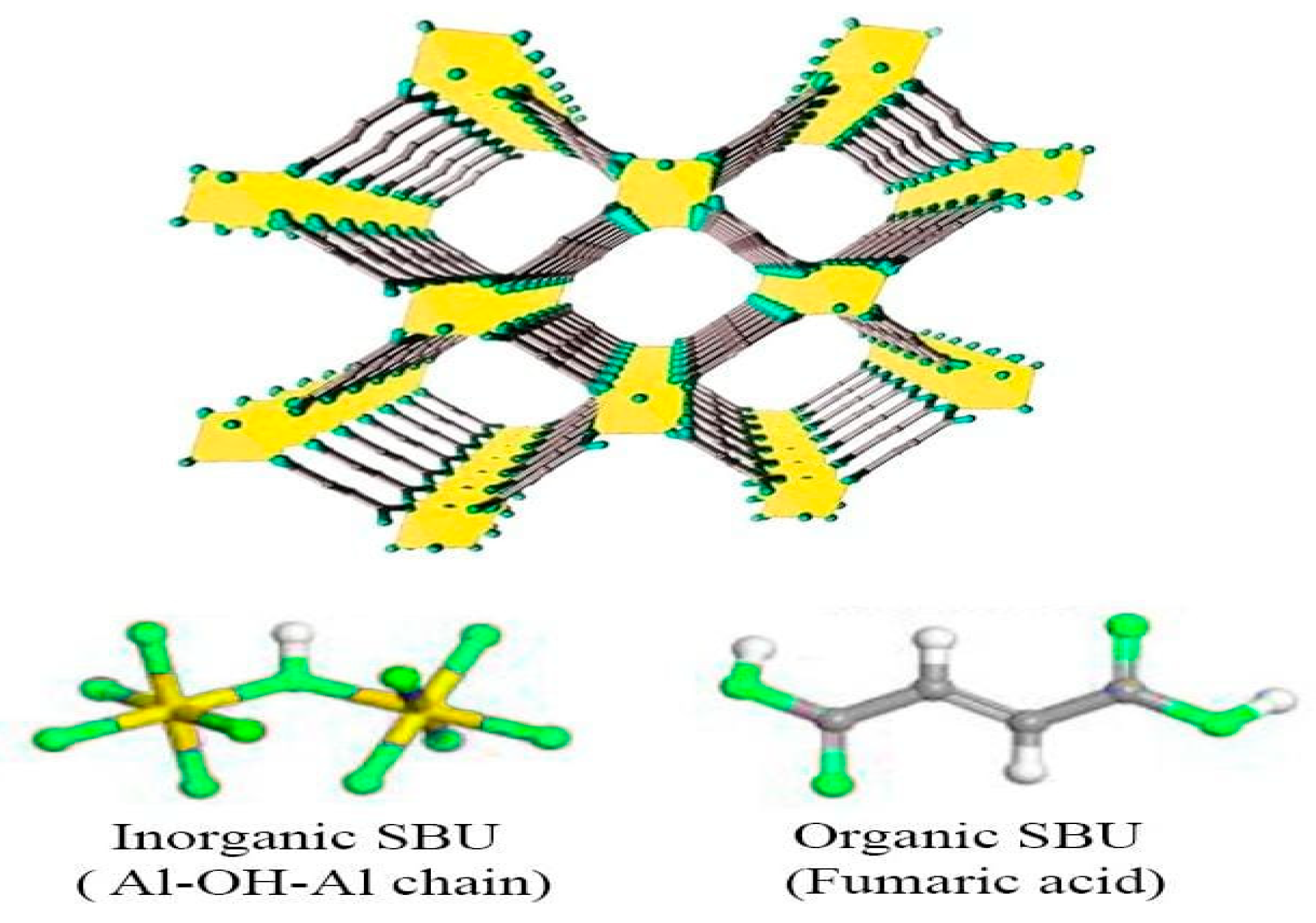
2.1. Characterization of the Developed Adsorbent
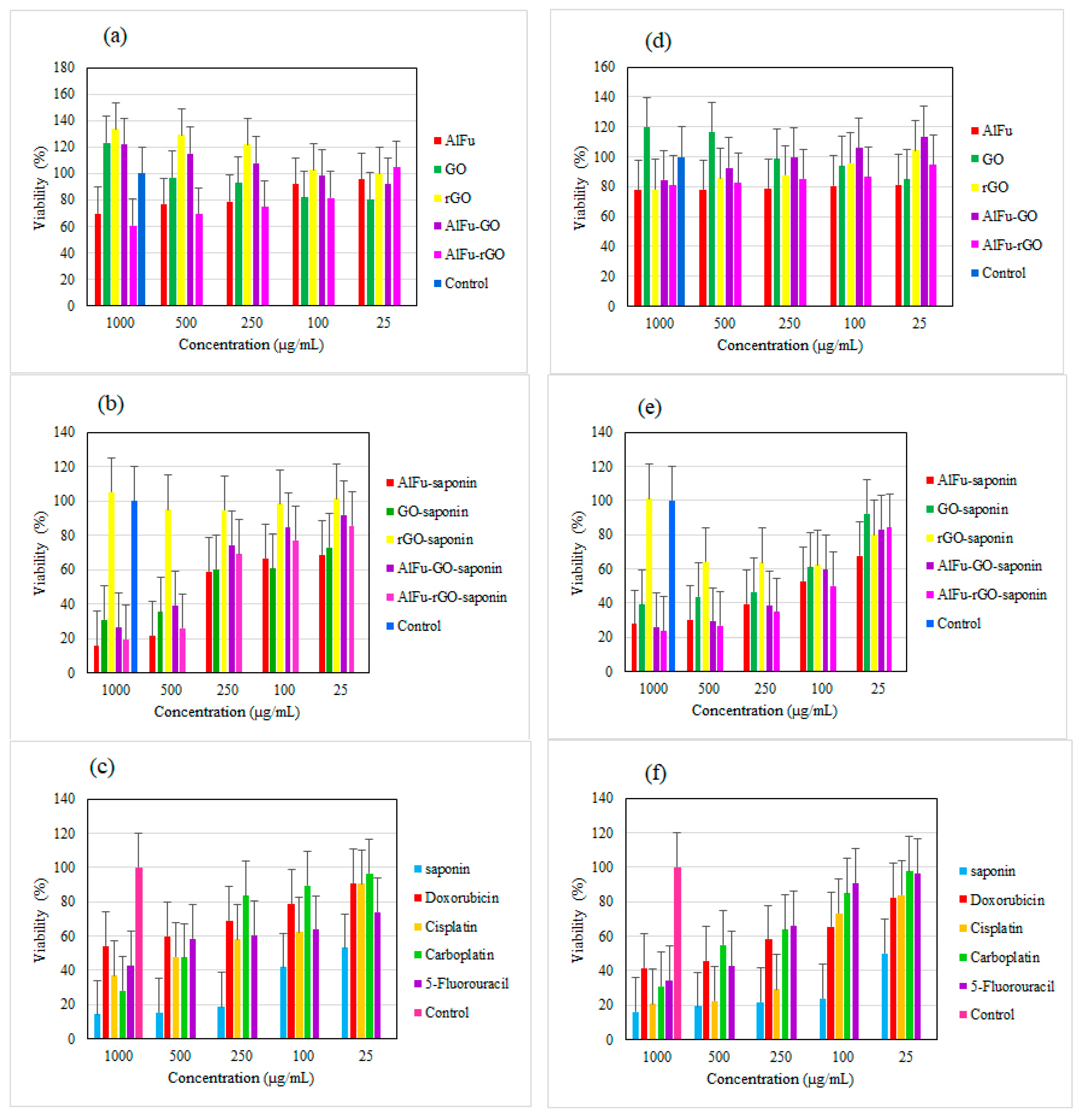
2.2. Biological Studies Results
2.3. Comparison of the Toxicity of Nanocarriers with Saponin
2.4. Flow Cytometric Detection
3. Materials and Method
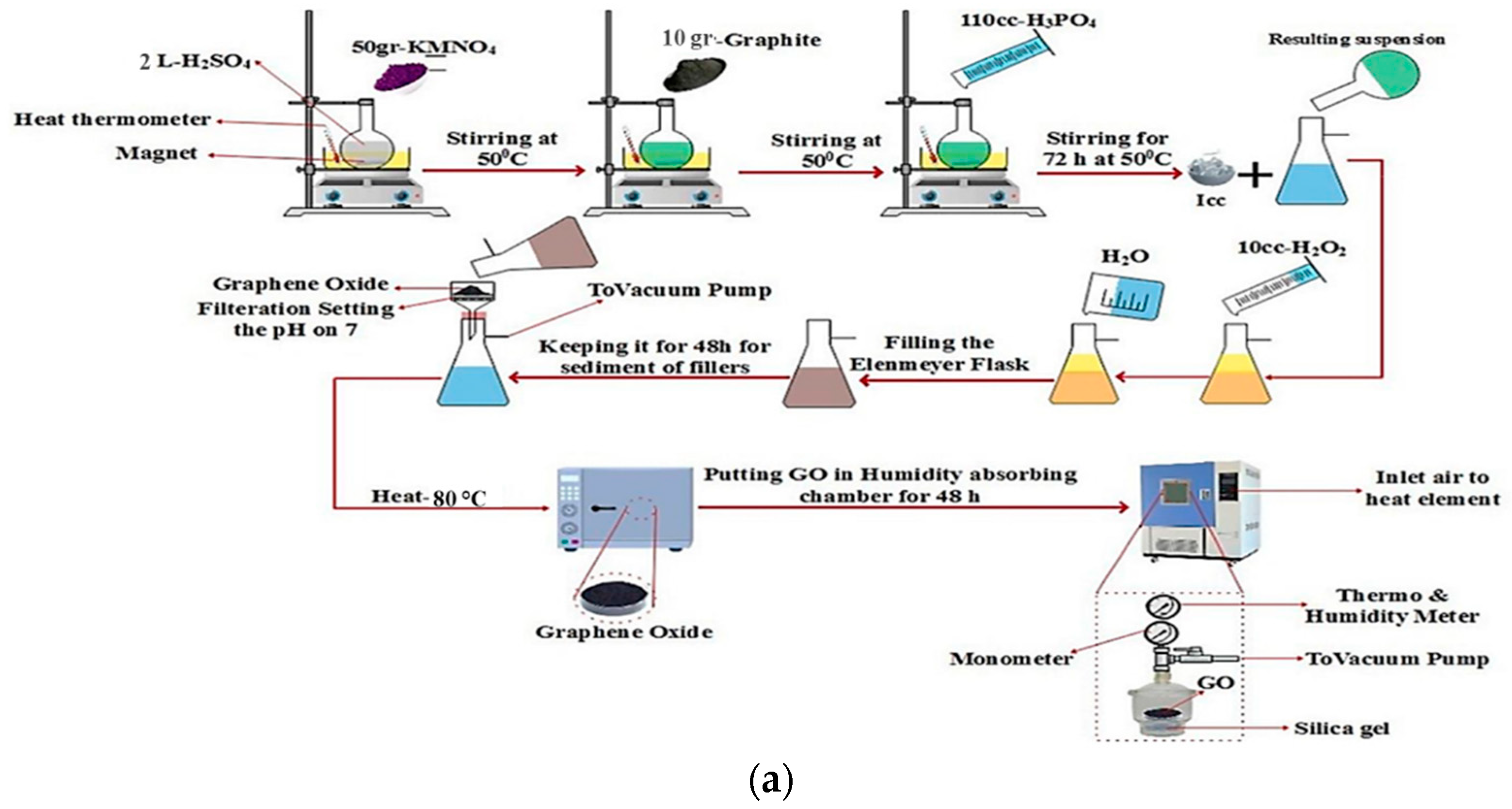
3.1. Preparation of GO and rGO
3.2. Synthesis of AlFu, AlFu–GO, and rGO Hybrid Materials
3.3. Toxicity Evaluation
3.4. Flow Cytometric Detection of Apoptosis/Necrosis
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luque-Michel, E.; Imbuluzqueta, E.; Sebastián, V.; Blanco-Prieto, M.J. Clinical advances of nanocarrier-based cancer therapy and diagnostics. Expert Opin. Drug Deliv. 2017, 14, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, F.; Gupta, S.; Li, C. Interventional nanotheranostics of pancreatic ductal adenocarcinoma. Theranostics 2016, 6, 1393. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.-Y.; Su, Y.-L.; Cheng, W.; Hu, P.-F.; Chiang, C.-S.; Chen, W.-T.; Hu, S.-H. Graphene quantum dots-mediated theranostic penetrative delivery of drug and photolytics in deep tumors by targeted biomimetic nanosponges. Nano Lett. 2018, 19, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Kalashgarani, M.Y.; Babapoor, A. Application of nano-antibiotics in the diagnosis and treatment of infectious diseases. Adv. Appl. NanoBio-Technol. 2022, 3, 22–35. [Google Scholar]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Kazemi, K.; Ghahramani, Y.; Kalashgrani, M.Y. Nano biofilms: An emerging biotechnology applications. Adv. Appl. NanoBio-Technol. 2022, 3, 8–15. [Google Scholar]
- Mousavi, S.M.; Hashemi, S.A.; Gholami, A.; Kalashgrani, M.Y.; Vijayakameswara Rao, N.; Omidifar, N.; Hsiao, W.W.-W.; Lai, C.W.; Chiang, W.-H. Plasma-Enabled Smart Nanoexosome Platform as Emerging Immunopathogenesis for Clinical Viral Infection. Pharmaceutics 2022, 14, 1054. [Google Scholar] [CrossRef]
- Sparg, S.; Light, M.; Van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Kalashgrani, M.Y.; Gholami, A.; Omidifar, N.; Babapoor, A.; Vijayakameswara Rao, N.; Chiang, W.-H. Recent Advances in Plasma-Engineered Polymers for Biomarker-Based Viral Detection and Highly Multiplexed Analysis. Biosensors 2022, 12, 286. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Yari Kalashgrani, M.; Omidifar, N.; Lai, C.W.; Vijayakameswara Rao, N.; Gholami, A.; Chiang, W.-H. The Pivotal Role of Quantum Dots-Based Biomarkers Integrated with Ultra-Sensitive Probes for Multiplex Detection of Human Viral Infections. Pharmaceuticals 2022, 15, 880. [Google Scholar] [CrossRef]
- Elekofehinti, O.O.; Iwaloye, O.; Olawale, F.; Ariyo, E.O. Saponins in cancer treatment: Current progress and future prospects. Pathophysiology 2021, 28, 250–272. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Li, Y.; Zhang, G. Cell cycle regulation and anticancer drug discovery. Cancer Biol. Med. 2017, 14, 348. [Google Scholar] [PubMed]
- Mousavi, S.M.; Hashemi, S.A.; Rahmanian, V.; Kalashgrani, M.Y.; Gholami, A.; Omidifar, N.; Chiang, W.-H. Highly sensitive flexible SERS-based sensing platform for detection of COVID-19. Biosensors 2022, 12, 466. [Google Scholar] [CrossRef] [PubMed]
- Moses, T.; Papadopoulou, K.K.; Osbourn, A. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 439–462. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Moghimipour, E.; Handali, S. Saponin: Properties, methods of evaluation and applications. Annu. Res. Rev. Biol. 2015, 5, 207–220. [Google Scholar] [CrossRef]
- Sharma, O.P.; Kumar, N.; Singh, B.; Bhat, T.K. An improved method for thin layer chromatographic analysis of saponins. Food Chem. 2012, 132, 671–674. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Yari Kalashgrani, M.; Kurniawan, D.; Gholami, A.; Rahmanian, V.; Omidifar, N.; Chiang, W.-H. Recent Advances in Inflammatory Diagnosis with Graphene Quantum Dots Enhanced SERS Detection. Biosensors 2022, 12, 461. [Google Scholar] [CrossRef]
- Vincken, J.-P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef]
- Francis, G.; Kerem, Z.; Makkar, H.P.; Becker, K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef]
- Kalashgrani, M.Y.; Javanmardi, N. Multifunctional Gold nanoparticle: As novel agents for cancer treatment. Adv. Appl. NanoBio-Technol. 2022, 3, 43–48. [Google Scholar]
- Kalashgrani, M.Y.; Nejad, F.F.; Rahmanian, V. Carbon Quantum Dots Platforms: As nano therapeutic for Biomedical Applications. Adv. Appl. NanoBio-Technol. 2022, 3, 38–42. [Google Scholar]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Florence, A.; Attwood, D. Surfactant Systems: Their Chemistry, Pharmacy and Biology; Chappmann and Hall: London, UK; New York, NY, USA, 1983. [Google Scholar]
- Dangi, J.; Vyas, S.; Dixit, V. The role of mixed micelles in drug delivery. I. Solubilization. Drug Dev. Ind. Pharm. 1998, 24, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Lehto, P.; Kortejärvi, H.; Liimatainen, A.; Ojala, K.; Kangas, H.; Hirvonen, J.; Tanninen, V.P.; Peltonen, L. Use of conventional surfactant media as surrogates for FaSSIF in simulating in vivo dissolution of BCS class II drugs. Eur. J. Pharm. Biopharm. 2011, 78, 531–538. [Google Scholar] [CrossRef]
- Sun, W.; Larive, C.K.; Southard, M.Z. A mechanistic study of danazol dissolution in ionic surfactant solutions. J. Pharm. Sci. 2003, 92, 424–435. [Google Scholar] [CrossRef]
- Jamzad, S.; Fassihi, R. Role of surfactant and pH on dissolution properties of fenofibrate and glipizide—A technical note. Aaps Pharmscitech 2006, 7, E17–E22. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.M.M.; Ahamad, F.; El-Sherbiny, M.; Ebrahim, H.A.; Eladl, M.A.; Dawood, A.F.; Khader, S.; Kavitha, K.; Teressa, D.M. An Ideal Approach for Enhancing 5-Fluorouracil Anticancer Efficacy by Nanoemulsion for Cytotoxicity against a Human Hepatoma Cell Line (HepG2 Cells). BioMed Res. Int. 2022, 2022, 4094132. [Google Scholar] [CrossRef]
- Henni-Silhadi, W.; Deyme, M.; Boissonnade, M.-M.; Appel, M.; Le Cerf, D.; Picton, L.; Rosilio, V. Enhancement of the solubility and efficacy of poorly water-soluble drugs by hydrophobically-modified polysaccharide derivatives. Pharm. Res. 2007, 24, 2317–2326. [Google Scholar] [CrossRef]
- Ménard, N.; Tsapis, N.; Poirier, C.; Arnauld, T.; Moine, L.; Gignoux, C.; Lefoulon, F.; Péan, J.-M.; Fattal, E. Novel surfactants with diglutamic acid polar head group: Drug solubilization and toxicity studies. Pharm. Res. 2012, 29, 1882–1896. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Zarei, M.; Bahrani, S.; Savardashtaki, A.; Esmaeili, H.; Lai, C.W.; Mazraedoost, S.; Abassi, M.; Ramavandi, B. Data on cytotoxic and antibacterial activity of synthesized Fe3O4 nanoparticles using Malva sylvestris. Data Brief 2020, 28, 104929. [Google Scholar] [CrossRef] [PubMed]
- Gkaniatsou, E.; Sicard, C.; Ricoux, R.; Mahy, J.-P.; Steunou, N.; Serre, C. Metal–organic frameworks: A novel host platform for enzymatic catalysis and detection. Mater. Horiz. 2017, 4, 55–63. [Google Scholar] [CrossRef]
- Yin, Z.; Wan, S.; Yang, J.; Kurmoo, M.; Zeng, M.-H. Recent advances in post-synthetic modification of metal–organic frameworks: New types and tandem reactions. Coord. Chem. Rev. 2019, 378, 500–512. [Google Scholar] [CrossRef]
- Kalashgrani, M.Y.; Harzand, F.V.; Javanmardi, N.; Nejad, F.F.; Rahmanian, V. Recent Advances in Multifunctional magnetic nano platform for Biomedical Applications: A mini review. Adv. Appl. NanoBio-Technol. 2022, 3, 31–37. [Google Scholar]
- Abazari, R.; Mahjoub, A.R.; Ataei, F.; Morsali, A.; Carpenter-Warren, C.L.; Mehdizadeh, K.; Slawin, A.M. Chitosan immobilization on bio-MOF nanostructures: A biocompatible pH-responsive nanocarrier for doxorubicin release on MCF-7 cell lines of human breast cancer. Inorg. Chem. 2018, 57, 13364–13379. [Google Scholar] [CrossRef]
- Agostoni, V.; Chalati, T.; Horcajada, P.; Willaime, H.; Anand, R.; Semiramoth, N.; Baati, T.; Hall, S.; Maurin, G.; Chacun, H. Towards an Improved anti-HIV Activity of NRTI via Metal–Organic Frameworks Nanoparticles. Adv. Healthc. Mater. 2013, 2, 1630–1637. [Google Scholar] [CrossRef]
- Alghool, S.; Slebodnick, C. One dimensional structure of Zn (II) metal organic framework (MOF) assembled rapidly at room temperature: Structural, thermal study, and luminescent properties. J. Inorg. Organomet. Polym. Mater. 2014, 24, 644–651. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Pandey, S.K.; Mehta, J.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Bioactive nano-metal–organic frameworks as antimicrobials against Gram-positive and Gram-negative bacteria. Toxicol. Res. 2018, 7, 931–941. [Google Scholar] [CrossRef]
- Huang, L.; Liu, B. Synthesis of a novel and stable reduced graphene oxide/MOF hybrid nanocomposite and photocatalytic performance for the degradation of dyes. RSC Adv. 2016, 6, 17873–17879. [Google Scholar] [CrossRef]
- Gao, M.; Han, X.; Zhan, X.; Liu, P.; Shan, Y.; Chen, Y.; Li, J.; Zhang, R.; Wang, S.; Zhang, Q. Enhancement in photoelectric performance of flexible perovskite solar cells by thermal nanoimprint pillar-like nanostructures. Mater. Lett. 2019, 248, 16–19. [Google Scholar] [CrossRef]
- Hu, L.; Peng, J.; Wang, W.; Xia, Z.; Yuan, J.; Lu, J.; Huang, X.; Ma, W.; Song, H.; Chen, W. Sequential deposition of CH3NH3PbI3 on planar NiO film for efficient planar perovskite solar cells. ACS Photonics 2014, 1, 547–553. [Google Scholar] [CrossRef]
- Hosseinpour, S.A.; Karimipour, G.; Ghaedi, M.; Dashtian, K. Use of metal composite MOF-5-Ag2O-NPs as an adsorbent for the removal of Auramine O dye under ultrasound energy conditions. Appl. Organomet. Chem. 2018, 32, e4007. [Google Scholar] [CrossRef]
- Wu, S.-C.; Yu, L.-L.; Xiao, F.-F.; You, X.; Yang, C.; Cheng, J.-H. Synthesis of aluminum-based MOF/graphite oxide composite and enhanced removal of methyl orange. J. Alloys Compd. 2017, 724, 625–632. [Google Scholar] [CrossRef]
- Yoon, S.; Calvo, J.J.; So, M.C. Removal of acid orange 7 from aqueous solution by metal-organic frameworks. Crystals 2018, 9, 17. [Google Scholar] [CrossRef]
- Dechnik, J.; Janiak, C.; De, S. Aluminium fumarate metal-organic framework: A super adsorbent for fluoride from water. J. Hazard. Mater. 2016, 303, 10–20. [Google Scholar]
- Tene, T.; Tubon Usca, G.; Guevara, M.; Molina, R.; Veltri, F.; Arias, M.; Caputi, L.S.; Vacacela Gomez, C. Toward large-scale production of oxidized graphene. Nanomaterials 2020, 10, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karmakar, S.; Bhattacharjee, S.; De, S. Aluminium fumarate metal organic framework incorporated polyacrylonitrile hollow fiber membranes: Spinning, characterization and application in fluoride removal from groundwater. Chem. Eng. J. 2018, 334, 41–53. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, X.; Chen, Y. Facile synthesis of Al-fumarate metal–organic framework nano-flakes and their highly selective adsorption of volatile organic compounds. Mater. Lett. 2017, 197, 224–227. [Google Scholar] [CrossRef]
- Azhdari, R.; Mousavi, S.M.; Hashemi, S.A.; Bahrani, S.; Ramakrishna, S. Decorated graphene with aluminum fumarate metal organic framework as a superior non-toxic agent for efficient removal of Congo Red dye from wastewater. J. Environ. Chem. Eng. 2019, 7, 103437. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, J.-J.; Cheng, F.-F.; Zheng, T.-T.; Wang, C.; Zhu, J.-J. Green and facile synthesis of highly biocompatible graphene nanosheets and its application for cellular imaging and drug delivery. J. Mater. Chem. 2011, 21, 12034–12040. [Google Scholar] [CrossRef]
- Robati, D.; Mirza, B.; Rajabi, M.; Moradi, O.; Tyagi, I.; Agarwal, S.; Gupta, V. Removal of hazardous dyes-BR 12 and methyl orange using graphene oxide as an adsorbent from aqueous phase. Chem. Eng. J. 2016, 284, 687–697. [Google Scholar] [CrossRef]
- Poorkazem, K.; Liu, D.; Kelly, T.L. Fatigue resistance of a flexible, efficient, and metal oxide-free perovskite solar cell. J. Mater. Chem. A 2015, 3, 9241–9248. [Google Scholar] [CrossRef]
- Chen, M.; Ding, Y.; Liu, Y.; Wang, N.; Yang, B.; Ma, L. Adsorptive desulfurization of thiophene from the model fuels onto graphite oxide/metal-organic framework composites. Pet. Sci. Technol. 2018, 36, 141–147. [Google Scholar] [CrossRef]
- Leung, E.; Müller, U.; Trukhan, N.; Mattenheimer, H.; Cox, G.; Blei, S. Process for Preparing Porous Metal-Organic Frameworks Based on Aluminum Fumarate. U.S. Patent US8524932B2, 3 September 2013. [Google Scholar]
- Wang, Y.; Qu, Q.; Liu, G.; Battaglia, V.S.; Zheng, H. Aluminum fumarate-based metal organic frameworks with tremella-like structure as ultrafast and stable anode for lithium-ion batteries. Nano Energy 2017, 39, 200–210. [Google Scholar] [CrossRef]
- Mousavi, S.; Zarei, M.; Hashemi, S. Polydopamine for biomedical application and drug delivery system. Med. Chem. 2018, 8, 218–229. [Google Scholar] [CrossRef]
- Wang, M.; Gustafsson, O.J.; Siddiqui, G.; Javed, I.; Kelly, H.G.; Blin, T.; Yin, H.; Kent, S.J.; Creek, D.J.; Kempe, K. Human plasma proteome association and cytotoxicity of nano-graphene oxide grafted with stealth polyethylene glycol and poly (2-ethyl-2-oxazoline). Nanoscale 2018, 10, 10863–10875. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Wan, J.; Zhang, S.; Zhang, Y.; Lee, S.-T.; Liu, Z. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS Nano 2011, 5, 516–522. [Google Scholar] [CrossRef]
- Ni, Y.; Zhang, F.; Kokot, S. Graphene oxide as a nanocarrier for loading and delivery of medicinal drugs and as a biosensor for detection of serum albumin. Anal. Chim. Acta 2013, 769, 40–48. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, J.; Zhao, Q.; Liu, L.; Zhang, Z. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Liu, Z.; Ma, Y.; Huang, Y.; Chen, Y. High-efficiency loading and controlled release of doxorubicin hydrochloride on graphene oxide. J. Phys. Chem. C 2008, 112, 17554–17558. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Du, J.; Wang, X.; Duan, A.; Gao, R.; Liu, J.; Li, B. Graphene oxide loaded with tumor-targeted peptide and anti-cancer drugs for cancer target therapy. Sci. Rep. 2021, 11, 1725. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Marín, E.; Moreno-Valenzuela, J.; Trejo-Valdez, M.; Martinez-Rivas, A.; Vargas-García, J.; Torres-Torres, C. Laser-induced electrical signal filtering by multilayer reduced graphene oxide decorated with Au nanoparticles. Opt. Express 2019, 27, 7330–7343. [Google Scholar] [CrossRef]
- Kozińska, N.; Tokarska, K.; Chudy, M.; Wojciechowski, K. Cytotoxicity of Quillaja saponaria saponins towards lung cells is higher for cholesterol-rich cells. Biophysica 2021, 1, 126–136. [Google Scholar] [CrossRef]
- Pham, H.N.T.; Sakoff, J.A.; Bond, D.R.; Vuong, Q.V.; Bowyer, M.C.; Scarlett, C.J. In vitro antibacterial and anticancer properties of Helicteres hirsuta Lour. leaf and stem extracts and their fractions. Mol. Biol. Rep. 2018, 45, 2125–2133. [Google Scholar] [CrossRef]
- Khan, M.I.; Ahhmed, A.; Shin, J.H.; Baek, J.S.; Kim, M.Y.; Kim, J.D. Green tea seed isolated saponins exerts antibacterial effects against various strains of gram positive and gram negative bacteria, a comprehensive study in vitro and in vivo. Evid.-Based Complement. Altern. Med. 2018, 2018, 3486106. [Google Scholar] [CrossRef] [PubMed]
- Aishuwariya, T.; Ramesh, S. Comparative Evaluation of Biocompatibility of Madhuca Longifolia Saponin Seed Extract with Sodium Hypochlorite-In Vitro Study. Ann. Med. Health Sci. Res. 2021, 11, 137–142. [Google Scholar]
- Liu, H.; Xi, P.; Xie, G.; Shi, Y.; Hou, F.; Huang, L.; Chen, F.; Zeng, Z.; Shao, C.; Wang, J. Simultaneous reduction and surface functionalization of graphene oxide for hydroxyapatite mineralization. J. Phys. Chem. C 2012, 116, 3334–3341. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, H.T.; Pham, T.T.; Choi, J.Y.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Development of a graphene oxide nanocarrier for dual-drug chemo-phototherapy to overcome drug resistance in cancer. ACS Appl. Mater. Interfaces 2015, 7, 28647–28655. [Google Scholar] [CrossRef]
- Rani, M.N.; Murthy, M.; Shree, N.S.; Ananda, S.; Yogesh, S.; Dinesh, R. Cuprous oxide anchored reduced graphene oxide ceramic nanocomposite using Tagetes erecta flower extract and evaluation of its antibacterial activity and cytotoxicity. Ceram. Int. 2019, 45, 25020–25026. [Google Scholar] [CrossRef]
- Poudel, K.; Banstola, A.; Tran, T.H.; Thapa, R.K.; Gautam, M.; Ou, W.; Maharjan, S.; Jeong, J.-H.; Ku, S.K.; Choi, H.-G. Hyaluronic acid wreathed, trio-stimuli receptive and on-demand triggerable nanoconstruct for anchored combinatorial cancer therapy. Carbohydr. Polym. 2020, 249, 116815. [Google Scholar] [CrossRef]
- Han, B.; Wang, Y.; Wang, L.; Shang, Z.; Wang, S.; Pei, J. Preparation of GST inhibitor nanoparticle drug delivery system and its reversal effect on the multidrug resistance in oral carcinoma. Nanomaterials 2015, 5, 1571–1587. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-M.; Jeong, Y.-I.; Kook, M.-S.; Kim, B.-H. Combinatorial effect of cold atmosphere plasma (Cap) and the anticancer drug cisplatin on oral squamous cell cancer therapy. Int. J. Mol. Sci. 2020, 21, 7646. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Mohammad, S.; Pant, A.B.; Mishra, P.R.; Pandey, G.; Gupta, S.; Farooqui, S. Co-delivery of 5-fluorouracil and curcumin nanohybrid formulations for improved chemotherapy against oral squamous cell carcinoma. J. Maxillofac. Oral Surg. 2018, 17, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, M.; Zheng, W.; Liu, Y. Platycodin D, a triterpenoid saponin from Platycodon grandiflorum, suppresses the growth and invasion of human oral squamous cell carcinoma cells via the NF-κB pathway. J. Biochem. Mol. Toxicol. 2017, 31, e21934. [Google Scholar] [CrossRef]
- Boșca, A.B.; Ilea, A.; Sorițău, O.; Tatomir, C.; Miklášová, N.; Pârvu, A.E.; Mihu, C.M.; Melincovici, C.S.; Fischer-Fodor, E. Modulatory effect of curcumin analogs on the activation of metalloproteinases in human periodontal stem cells. Eur. J. Oral Sci. 2019, 127, 304–312. [Google Scholar] [CrossRef]
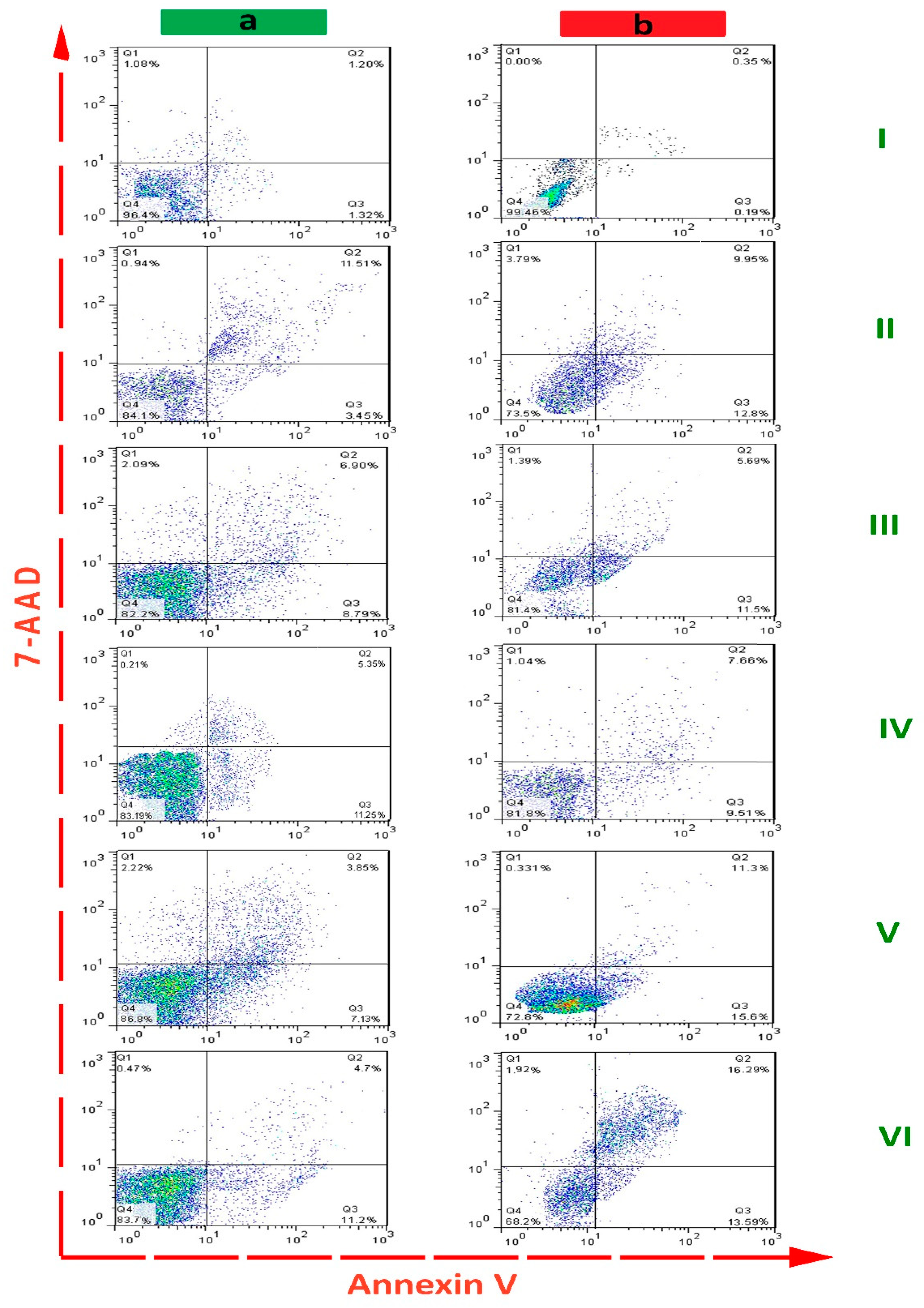

| Compound | Early Apoptosis (%) | Late Apoptosis (%) | Cumulative Apoptosis (%) | |||
|---|---|---|---|---|---|---|
| PDL cell line | OSCC cell line | PDL cell line | OSCC cell line | PDL cell line | OSCC cell line | |
| control | 1.20 ± 0.45 | 0.35 ± 0.81 | 1.32 ± 0.08 | 0195 ± 0.31 | 2.52 ± 0.78 | 1.31 ± 0.62 |
| saponin | 11.51 ± 0.14 | 9.95 ± 1.05 | 3.45 ± 0.41 | 12.80 ± 2.57 | 14.96 ± 3.04 | 22.75 ± 1.28 |
| AlFu–GO | 6.9 ± 3.39 | 5.69 ± 1.85 | 8.79 ± 0.74 | 11.5 ± 1.33 | 15.69 ± 3.93 | 17.19 ± 2.26 |
| AlFu–rGO | 5.35 ± 0.58 | 7.66 ± 0.96 | 11.25 ± 3.12 | 9.51 ± 0.51 | 16.60 ± 2.54 | 17.17 ± 2.80 |
| AlFu–GO–saponin | 3.85 ± 0.07 | 11.30 ± 0.07 | 7.13 ± 1.57 | 15.60 ± 0.16 | 10.98 ± 2.36 | 26.90 ± 3.24 |
| AlFu–rGO–saponin | 4.7 ± 1.90 | 16.29 ± 3.48 | 11.2 ± 1.43 | 13.59 ± 2.11 | 15.9 ± 4.08 | 29.88 ± 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi, S.M.; Hashemi, S.A.; Ghahramani, Y.; Azhdari, R.; Yousefi, K.; Gholami, A.; Fallahi Nezhad, F.; Vijayakameswara Rao, N.; Omidifar, N.; Chiang, W.-H. Antiproliferative and Apoptotic Effects of Graphene Oxide @AlFu MOF Based Saponin Natural Product on OSCC Line. Pharmaceuticals 2022, 15, 1137. https://doi.org/10.3390/ph15091137
Mousavi SM, Hashemi SA, Ghahramani Y, Azhdari R, Yousefi K, Gholami A, Fallahi Nezhad F, Vijayakameswara Rao N, Omidifar N, Chiang W-H. Antiproliferative and Apoptotic Effects of Graphene Oxide @AlFu MOF Based Saponin Natural Product on OSCC Line. Pharmaceuticals. 2022; 15(9):1137. https://doi.org/10.3390/ph15091137
Chicago/Turabian StyleMousavi, Seyyed Mojtaba, Seyyed Alireza Hashemi, Yasmin Ghahramani, Rouhollah Azhdari, Khadijeh Yousefi, Ahmad Gholami, Fatemeh Fallahi Nezhad, Neralla Vijayakameswara Rao, Navid Omidifar, and Wei-Hung Chiang. 2022. "Antiproliferative and Apoptotic Effects of Graphene Oxide @AlFu MOF Based Saponin Natural Product on OSCC Line" Pharmaceuticals 15, no. 9: 1137. https://doi.org/10.3390/ph15091137
APA StyleMousavi, S. M., Hashemi, S. A., Ghahramani, Y., Azhdari, R., Yousefi, K., Gholami, A., Fallahi Nezhad, F., Vijayakameswara Rao, N., Omidifar, N., & Chiang, W.-H. (2022). Antiproliferative and Apoptotic Effects of Graphene Oxide @AlFu MOF Based Saponin Natural Product on OSCC Line. Pharmaceuticals, 15(9), 1137. https://doi.org/10.3390/ph15091137









