Local Delivery of Therapeutics to the Cochlea Using Nanoparticles and Other Biomaterials
Abstract
:1. Introduction
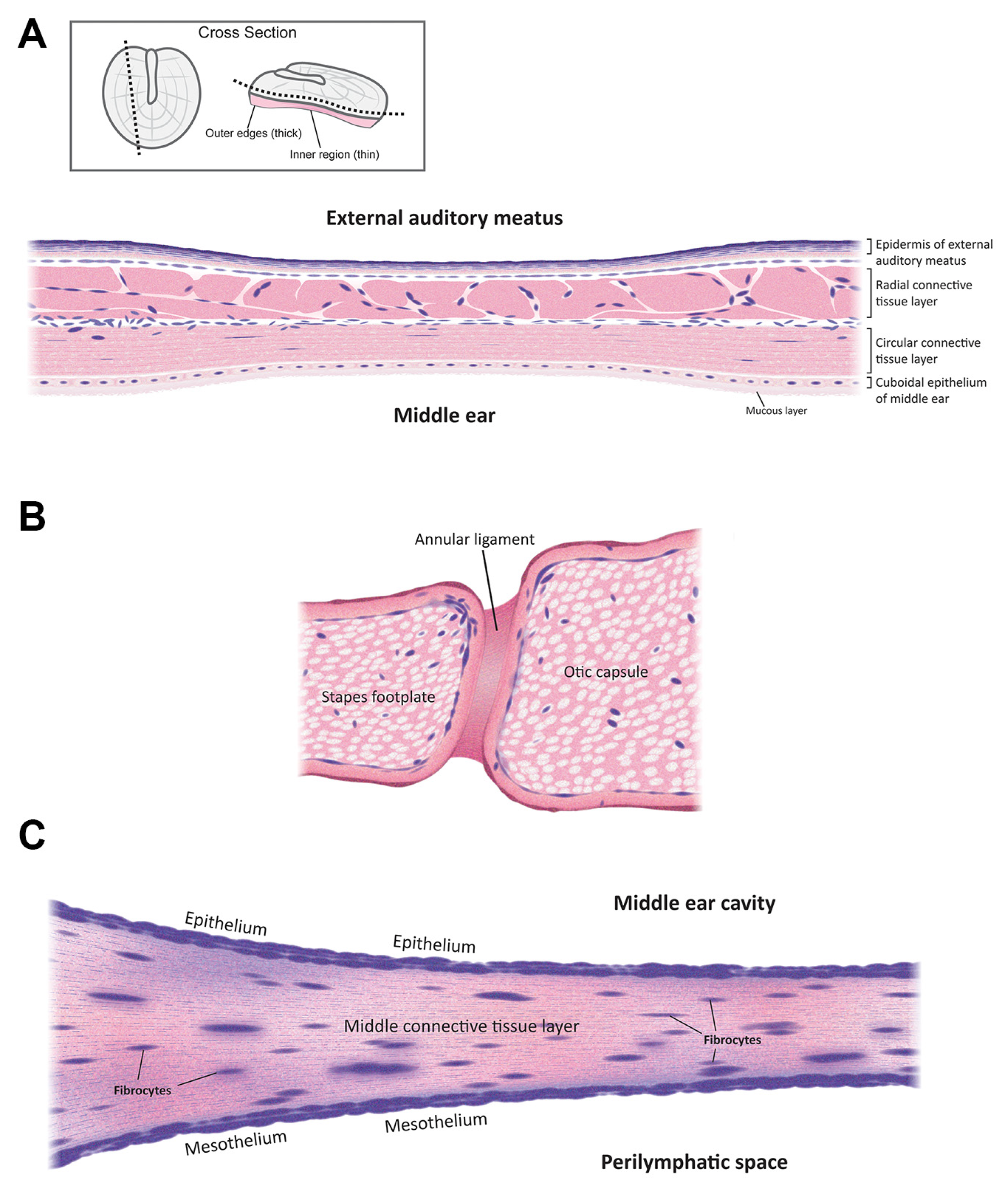
2. Anatomy
3. Delivery Routes
3.1. Systemic Delivery
3.2. Topical Delivery via the External Auditory Meatus
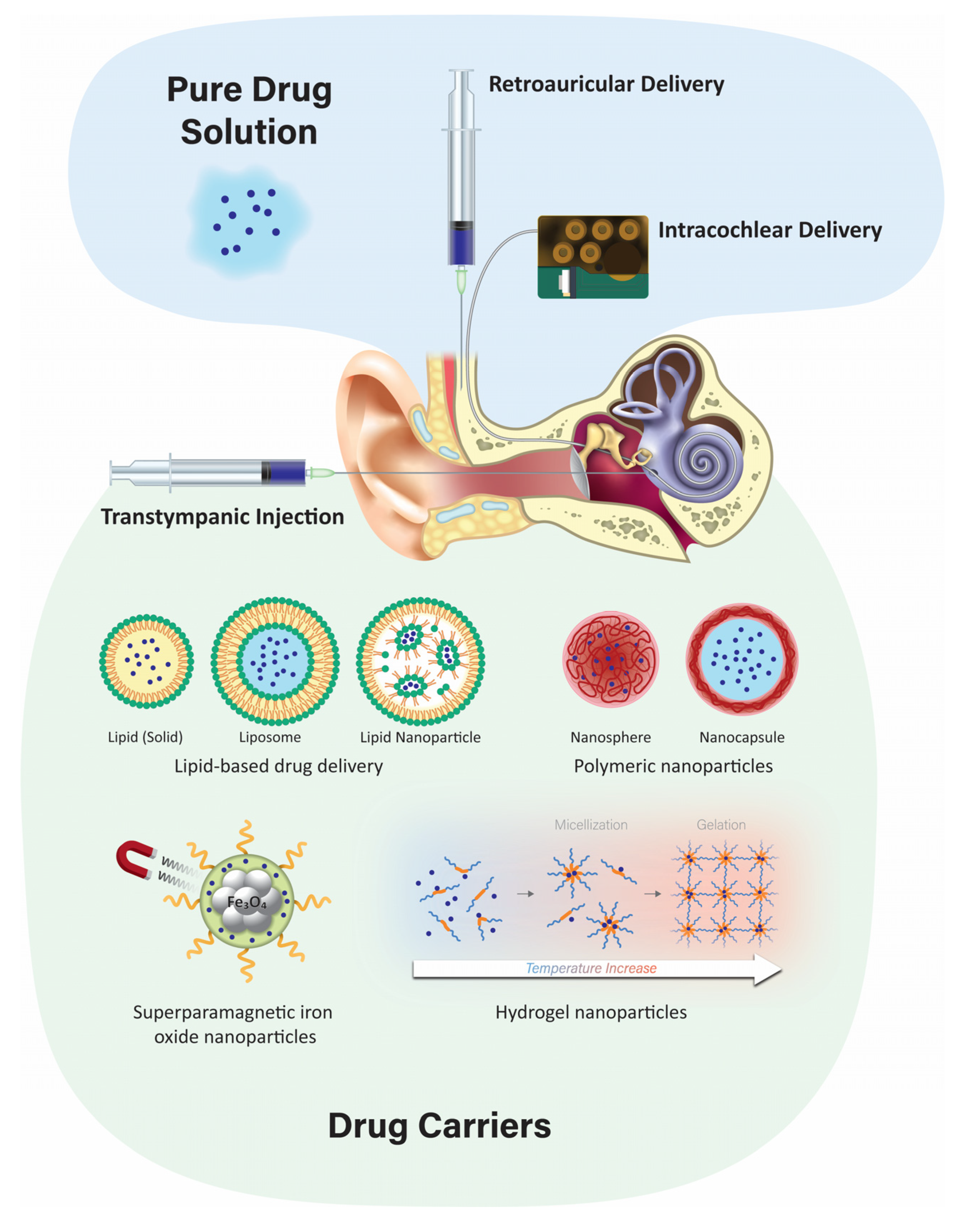
3.3. Transtympanic Delivery
3.4. Retroauricular Delivery
3.5. Intracochlear Application
4. Localized Inner Ear Delivery Methods
4.1. Developing Different Injectable Solutions like Hydrogels
4.2. Poloxamer 407 and Its Mechanism
4.3. Nanoparticulate Injection Systems
4.3.1. Polymeric Nanoparticles
4.3.2. Solid Lipid Nanoparticles
4.3.3. Liposomes
4.3.4. Superparamagnetic Iron Oxide Nanoparticles (SPION)
4.4. Advantages and Disadvantages of the Nanoparticulate Injection System
4.5. Positively-Charged Biomaterials for Local Drug Delivery
Advantages and Disadvantages of Positively-Charged Biomaterials
4.6. Negatively-Charged Biomaterials for Local Drug Delivery
Advantages and Disadvantages of Negatively-Charged Biomaterials
5. Pharmacokinetics and Pharmacodynamics of the Drugs in the Inner Ear
6. Quantification of Drugs in the Inner Ear
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernabei, R.; Bonuccelli, U.; Maggi, S.; Marengoni, A.; Martini, A.; Memo, M.; Pecorelli, S.; Peracino, A.P.; Quaranta, N.; Stella, R.; et al. Hearing loss and cognitive decline in older adults: Questions and answers. Aging Clin. Exp. Res. 2014, 26, 567–573. [Google Scholar] [CrossRef]
- Daniel, E. Noise and hearing loss: A review. J. Sch. Health 2007, 77, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.R.; Yaffe, K.; Xia, J.; Xue, Q.L.; Harris, T.B.; Purchase-Helzner, E.; Satterfield, S.; Ayonayon, H.N.; Ferrucci, L.; Simonsick, E.M. Hearing Loss and Cognitive Decline in Older Adults. JAMA Intern. Med. 2013, 173, 293–299. [Google Scholar] [CrossRef] [PubMed]
- McCall, A.A.; Swan, E.E.L.; Borenstein, J.T.; Sewell, W.F.; Kujawa, S.G.; McKenna, M.J. Drug delivery for treatment of inner ear disease: Current state of knowledge. Ear Hear. 2010, 31, 156–165. [Google Scholar] [CrossRef]
- Kros, C.J.; Steyger, P.S. Aminoglycoside- and Cisplatin-Induced Ototoxicity: Mechanisms and Otoprotective Strategies. Cold Spring Harb. Perspect. Med. 2019, 9, a033548. [Google Scholar] [CrossRef]
- Swan, E.E.L.; Mescher, M.J.; Sewell, W.F.; Tao, S.L.; Borenstein, J.T. Inner ear drug delivery for auditory applications. Adv. Drug Deliv. Rev. 2008, 60, 1583–1599. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Agrahari, V.; Mitra, A.K. Inner ear targeted drug delivery: What does the future hold? Ther. Deliv. 2017, 8, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Van der Jeught, S.; Dirckx, J.J.J.; Aerts, J.R.M.; Bradu, A.; Podoleanu, A.G.; Buytaert, J.A.N. Full-Field Thickness Distribution of Human Tympanic Membrane Obtained with Optical Coherence Tomography. J. Assoc. Res. Otolaryngol. 2013, 14, 483. [Google Scholar] [CrossRef]
- Szymanski, A.; Toth, J.; Ogorevc, M.; Geiger, Z. Anatomy, Head and Neck, Ear Tympanic Membrane; StatPearls Publishing: Tampa, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK448117/ (accessed on 30 July 2022).
- Gyo, K.; Aritomo, H.; Goode, R.L. Measurement of the ossicular vibration ratio in human temporal bones by use of a video measuring system. Acta Oto Laryngol. 1987, 103, 87–95. [Google Scholar] [CrossRef]
- Zdilla, M.J.; Skrzat, J.; Kozerska, M.; Leszczyński, B.; Tarasiuk, J.; Wroński, S. Oval window size and shape: A micro-CT anatomical study with considerations for stapes surgery. Otol. Neurotol. 2018, 39, 558. [Google Scholar] [CrossRef]
- Mancheño, M.; Aristegui, M.; Sañudo, J.R. Round and Oval Window Anatomic Variability: Its Implication for the Vibroplasty Technique. Otol. Neurotol. 2017, 38, e50–e57. [Google Scholar] [CrossRef]
- Goycoolea, M.V.; Lundman, L. Round window membrane. Structure function and permeability: A review. Microsc. Res. Tech. 1997, 36, 201–211. Available online: https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/(SICI)1097-0029(19970201)36:3%3C201::AID-JEMT8%3E3.0.CO;2-R (accessed on 23 September 2021). [CrossRef]
- Liu, H.; Hao, J.; Li, K.S. Current strategies for drug delivery to the inner ear. Acta Pharm. Sin. B 2013, 3, 86–96. [Google Scholar] [CrossRef]
- Zhang, X.; Gan, R.Z. Dynamic Properties of Human Round Window Membrane in Auditory Frequencies. Med. Eng. Phys. 2013, 35, 310. [Google Scholar] [CrossRef]
- Szeto, B.; Chiang, H.; Valentini, C.; Yu, M.; Kysar, J.W.; Lalwani, A.K. Inner ear delivery: Challenges and opportunities. Laryngoscope Investig. Otolaryngol. 2020, 5, 122. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Landegger, L.D.; Stankovic, K.M. Gene therapy for human sensorineural hearing loss. Front. Cell. Neurosci. 2019, 13, 323. [Google Scholar] [CrossRef]
- Peppi, M.; Marie, A.; Belline, C.; Borenstein, J.T. Intracochlear drug delivery systems: A novel approach whose time has come. Expert Opin. Drug Deliv. 2018, 15, 319–324. [Google Scholar] [CrossRef]
- Chae, R.; Rodriguez Rubio, R. Anatomy of petrous face. Handb. Clin. Neurol. 2020, 170, 143–156. [Google Scholar] [CrossRef]
- Sakamoto, T.; Hiraumi, H. Anatomy of the inner ear. In Regenerative Medicine for the Inner Ear; Springer: Tokyo, Japan, 2014; pp. 3–13. [Google Scholar] [CrossRef]
- Nayak, G.; Lee, S.I.; Yousaf, R.; Edelmann, S.E.; Trincot, C.; van Itallie, C.M.; Sinha, G.P.; Rafeeq, M.; Jones, S.M.; Belyantseva, I.A.; et al. Tricellulin deficiency affects tight junction architecture and cochlear hair cells. J. Clin. Investig. 2013, 123, 4036. [Google Scholar] [CrossRef] [Green Version]
- Hibino, H.; Nin, F.; Tsuzuki, C.; Kurachi, Y. How is the highly positive endocochlear potential formed? The specific architecture of the Stria vascularis and the roles of the ion-transport apparatus. Pflug. Arch. Eur. J. Physiol. 2010, 459, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Echteler, S.M.; Fay, R.R.; Popper, A.N. Structure of the mammalian cochlea. In Comparative Hearing: Mammals; Fay, R.R., Popper, A.N., Eds.; Springer: New York, NY, USA, 1994; pp. 134–171. [Google Scholar] [CrossRef]
- Nyberg, S.; Joan Abbott, N.; Shi, X.; Steyger, P.S.; Dabdoub, A. Delivery of therapeutics to the inner ear: The challenge of the blood-labyrinth barrier. Sci. Transl. Med. 2019, 11, eaao0935. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte—Endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Koo, J.W.; Quintanilla-Dieck, L.; Jiang, M.; Liu, J.; Urdang, Z.D.; Allensworth, J.J.; Cross, C.P.; Li, H.; Steyger, P.S. Endotoxemia-mediated inflammation potentiates aminoglycoside-induced ototoxicity. Sci. Transl. Med. 2015, 7, 298ra118. [Google Scholar] [CrossRef]
- Marcotti, W.; van Netten, S.M.; Kros, C.J. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J. Physiol. 2005, 567, 505. [Google Scholar] [CrossRef]
- Karasawa, T.; Wang, Q.; Fu, Y.; Cohen, D.M.; Steyger, P.S. TRPV4 enhances the cellular uptake of aminoglycoside antibiotics. J. Cell Sci. 2008, 121, 2871–2879. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, Q.; Karasawa, T.; Koo, J.W.; Li, H.; Steyger, P.S. Sodium-Glucose Transporter-2 (SGLT2; SLC5A2) Enhances Cellular Uptake of Aminoglycosides. PLoS ONE 2014, 9, e108941. [Google Scholar] [CrossRef]
- Sojo-Dorado, J.; Rodríguez-Baño, J. Gentamicin. In Kucer’s the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs, 7th ed.; Grayson, M.L., Cosgrove, S.E., Crowe, S., Hope, W., McCarthy, J.S., Mills, J., Mouton, J.W., Paterson, D.L., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 964–991. [Google Scholar] [CrossRef]
- Piu, F.; Bishop, K.M. Local drug delivery for the treatment of neurotology disorders. Front. Cell. Neurosci. 2019, 13, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoskison, E.; Daniel, M.; Al-Zahid, S.; Shakesheff, K.M.; Bayston, R.; Birchall, J.P. Drug delivery to the ear. Ther. Deliv. 2013, 4, 115–124. [Google Scholar] [CrossRef]
- Wooltorton, E. Health and Drug Alerts: Ototoxic effects from gentamicin ear drops. Can. Med. Assoc. J. 2002, 167, 56. Available online: https://pmc/articles/PMC116645/ (accessed on 24 April 2022).
- Macfadyen, C.A.; Acuin, J.M.; Gamble, C.L. Topical antibiotics without steroids for chronically discharging ears with underlying eardrum perforations. Cochrane Database Syst. Rev. 2005, 2005, CD004618. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Feng, L.; Tolia, G.; Liddell, M.R.; Hao, J.; Li, S.K. Evaluation of intratympanic formulations for inner ear delivery: Methodology and sustained release formulation testing. Drug Dev. Ind. Pharm. 2014, 40, 896. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hartsock, J.J.; Dai, C.; Salt, A.N. Permeation Enhancers for Intratympanically-Applied Drugs studied using Fluorescent Dexamethasone as a Marker. Otol. Neurotol. 2018, 39, 639. [Google Scholar] [CrossRef]
- Yoda, S.; Cureoglu, S.; Shimizu, S.; Morita, N.; Fukushima, H.; Sato, T.; Harada, T.; Paparella, M.M. Round window membrane in Ménière’s disease: A human temporal bone study. Otol. Neurotol. 2011, 32, 147–151. [Google Scholar] [CrossRef] [PubMed]
- King, E.B.; Salt, A.N.; Kel, G.E.; Eastwood, H.T.; O’Leary, S.J. Gentamicin administration on the stapes footplate causes greater hearing loss and vestibulotoxicity than round window administration in guinea pigs. Hear. Res. 2013, 304, 159–166. [Google Scholar] [CrossRef]
- King, E.B.; Shepherd, R.K.; Brown, D.J.; Fallon, J.B. Gentamicin Applied to the Oval Window Suppresses Vestibular Function in Guinea Pigs. J. Assoc. Res. Otolaryngol. 2017, 18, 291–299. [Google Scholar] [CrossRef]
- Cros, O.; Borga, M.; Pauwels, E.; Dirckx, J.J.J.; Gaihede, M. Micro-channels in the mastoid anatomy. Indications of a separate blood supply of the air cell system mucosa by micro-CT scanning. Hear. Res. 2013, 301, 60–65. [Google Scholar] [CrossRef]
- Gaihede, M. Treatment of Otitis Media with Retroauricular Steroid Injection—Aalborg University’s Research Portal. 2015. Available online: https://vbn.aau.dk/en/publications/treatment-of-otitis-media-with-retroauricular-steroid-injection (accessed on 21 July 2021).
- Fooken Jensen, P.V.; Gaihede, M. Congestion of mastoid mucosa and influence on middle ear pressure—Effect of retroauricular injection of adrenaline. Hear. Res. 2016, 340, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, Z.; Zhou, Q.; Chen, Y.; Yang, L.; Tan, J.; Zeng, X.; Li, P. Impacts of different methylprednisolone administration routes in patients with sudden hearing loss or Meniere’s disease. J. Otol. 2020, 15, 149. [Google Scholar] [CrossRef]
- Chen, A.; Liu, W.; Xu, L.; Hou, Z.; Fan, Z.; Wang, H.; Wang, M. Comparison of the Pathway to the Inner Ear Between Postauricular and Intramuscular Injection of Dexamethasone in Guinea Pigs. Front. Neurol. 2022, 13, 399. [Google Scholar] [CrossRef]
- Plontke, S.K.; Hartsock, J.J.; Gill, R.M.; Salt, A.N. Intracochlear Drug Injections through the Round Window Membrane: Measures to Improve Drug Retention. Audiol. Neurotol. 2016, 21, 72–79. [Google Scholar] [CrossRef]
- Manrique-Huarte, R.; de Linera-Alperi, M.A.; Parilli, D.; Rodriguez, J.A.; Borro, D.; Dueck, W.F.; Smyth, D.; Salt, A.; Manrique, M. Inner ear drug delivery through a cochlear implant: Pharmacokinetics in a Macaque experimental model. Hear. Res. 2021, 404, 108228. [Google Scholar] [CrossRef]
- Pararas, E.E.L.; Borkholder, D.A.; Borenstein, J.T. Microsystems Technologies for Drug Delivery to the Inner Ear. Adv. Drug Deliv. Rev. 2012, 64, 1650. [Google Scholar] [CrossRef] [PubMed]
- Shimogori, H.; Yamashita, H. Efficacy of intracochlear administration of betamethasone on peripheral vestibular disorder in the guinea pig. Neurosci. Lett. 2000, 294, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Abaamrane, L.; Raffin, F.; Schmerber, S.; Sendowski, I. Intracochlear perfusion of leupeptin and z-VAD-FMK: Influence of antiapoptotic agents on gunshot-induced hearing loss. Eur. Arch. Oto-Rhino-Laryngol. 2011, 268, 987–993. [Google Scholar] [CrossRef]
- Tandon, V.; Kang, W.S.; Robbins, T.A.; Spencer, A.J.; Kim, E.S.; McKenna, M.J.; Kujawa, S.G.; Fiering, J.; Pararas, E.E.L.; Mescher, M.J.; et al. Microfabricated reciprocating micropump for intracochlear drug delivery with integrated drug/fluid storage and electronically controlled dosing. Lab Chip 2016, 16, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Plontke, S.K.; Götze, G.; Rahne, T.; Liebau, A. Intracochlear drug delivery in combination with cochlear implants: Current aspects. HNO 2017, 65 (Suppl. 1), 19–28. [Google Scholar] [CrossRef]
- Boisvert, I.; Reis, M.; Au, A.; Cowan, R.; Dowell, R.C. Cochlear implantation outcomes in adults: A scoping review. PLoS ONE 2020, 15, e0232421. [Google Scholar] [CrossRef]
- Benet, L.Z.; Hosey, C.M.; Ursu, O.; Oprea, T.I. BDDCS, the Rule of 5 and drugability. Adv. Drug Deliv. Rev. 2016, 101, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Barich, D.H.; Zell, M.T.; Munson, E.J. Physicochemical properties, formulation, and drug delivery. In Drug Delivery: Principles and Applications, 2nd ed.; Wang, B., Siahaan, T.J., Soltero, R., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2016; pp. 35–48. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Doak, B.C.; Over, B.; Giordanetto, F.; Kihlberg, J. Oral druggable space beyond the rule of 5: Insights from drugs and clinical candidates. Chem. Biol. 2014, 21, 1115–1142. [Google Scholar] [CrossRef]
- Hao, J.; Li, S.K. Inner ear drug delivery: Recent advances, challenges, and perspective. Eur. J. Pharm. Sci. 2019, 126, 82–92. [Google Scholar] [CrossRef]
- Kanzaki, S. Gene Delivery into the Inner Ear and Its Clinical Implications for Hearing and Balance. Molecules 2018, 23, 2507. [Google Scholar] [CrossRef]
- Rathnam, C.; Chueng, S.T.D.; Ying YL, M.; Lee, K.B.; Kwan, K. Developments in Bio-Inspired Nanomaterials for Therapeutic Delivery to Treat Hearing Loss. Front. Cell. Neurosci. 2019, 13, 493. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Szczupak, M.; Rajguru, S.; Balaban, C.; Hoffer, M.E. Inner ear therapeutics: An overview of middle ear delivery. Front. Cell. Neurosci. 2019, 13, 261. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Fariba, G.; Farahani, S.V. Theoretical Description of Hydrogel Swelling: A Review. Iran. Polym. J. 2020, 19, 375–398. Available online: https://www.sid.ir/en/Journal/ViewPaper.aspx?ID=171784 (accessed on 30 July 2022).
- El Kechai, N.; Agnely, F.; Mamelle, E.; Nguyen, Y.; Ferrary, E.; Bochot, A. Recent advances in local drug delivery to the inner ear. Int. J. Pharm. 2015, 494, 83–101. [Google Scholar] [CrossRef]
- Lajud, S.A.; Nagda, D.A.; Qiao, P.; Tanaka, N.; Civantos, A.; Gu, R.; Cheng, Z.; Tsourkas, A.; O’Malley, B.W.; Li, D. A Novel Chitosan-Hydrogel-Based Nanoparticle Delivery System for Local Inner Ear Application. Otol. Neurotol. 2015, 36, 341. [Google Scholar] [CrossRef] [PubMed]
- Hütten, M.; Dhanasingh, A.; Hessler, R.; Stöver, T.; Esser, K.H.; Möller, M.; Lenarz, T.; Jolly, C.; Groll, J.; Scheper, V. In Vitro and In Vivo Evaluation of a Hydrogel Reservoir as a Continuous Drug Delivery System for Inner Ear Treatment. PLoS ONE 2014, 9, e104564. [Google Scholar] [CrossRef]
- Gausterer, J.C.; Saidov, N.; Ahmadi, N.; Zhu, C.; Wirth, M.; Reznicek, G.; Arnoldner, C.; Gabor, F.; Honeder, C. Intratympanic application of poloxamer 407 hydrogels results in sustained N-acetylcysteine delivery to the inner ear. Eur. J. Pharm. Biopharm. 2020, 150, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Borden, R.C.; Saunders, J.E.; Berryhill, W.E.; Krempl, G.A.; Thompson, D.M.; Queimado, L. Hyaluronic Acid Hydrogel Sustains the Delivery of Dexamethasone across the Round Window Membrane. Audiol. Neurotol. 2011, 16, 1–11. [Google Scholar] [CrossRef]
- Yu, D.; Sun, C.; Zheng, Z.; Wang, X.; Chen, D.; Wu, H.; Wang, X.; Shi, F. Inner ear delivery of dexamethasone using injectable silk-polyethylene glycol (PEG) hydrogel. Int. J. Pharm. 2016, 503, 229–237. [Google Scholar] [CrossRef]
- Shibata, S.B.; Cortez, S.R.; Wiler, J.A.; Swiderski, D.L.; Raphael, Y. Hyaluronic Acid Enhances Gene Delivery into the Cochlea. Hum. Gene Ther. 2012, 23, 302. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef]
- Fakhari, A.; Corcoran, M.; Schwarz, A. Thermogelling properties of purified poloxamer 407. Heliyon 2017, 3, e00390. [Google Scholar] [CrossRef]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Wang, X.; Dellamary, L.; Fernandez, R.; Harrop, A.; Keithley, E.M.; Harris, J.P.; Ye, Q.; Lichter, J.; Lebel, C.; Piu, F. Dose-dependent sustained release of dexamethasone in inner ear cochlear fluids using a novel local delivery approach. Audiol. Neuro Otol. 2009, 14, 393–401. [Google Scholar] [CrossRef]
- Dickey, D.T.; Muldoon, L.L.; Kraemer, D.F.; Neuwelt, E.A. Protection against cisplatin-induced ototoxicity by N-acetylcysteine in a rat model. Hear. Res. 2004, 193, 25–30. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos EV, R.; Rodriguez-Torres MD, P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnology 2018, 16, 71. [Google Scholar] [CrossRef]
- Mirza, A.Z.; Siddiqui, F.A.; Mirza, A.Z.; Siddiqui, F.A. Nanomedicine and drug delivery: A mini review. Int. Nano Lett. 2014, 4, 94. [Google Scholar] [CrossRef]
- Lu, H.; Wang, J.; Wang, T.; Zhong, J.; Bao, Y.; Hao, H. Recent Progress on Nanostructures for Drug Delivery Applications. J. Nanomater. 2016, 2016, 5762431. [Google Scholar] [CrossRef]
- Seymour, L.W.; Ulbrich, K.; Steyger, P.S.; Brereton, M.; Subr, V.; Strohalm, J.; Duncan, R. Tumour tropism and anti-cancer efficacy of polymer-based doxorubicin prodrugs in the treatment of subcutaneous murine B16F10 melanoma. Br. J. Cancer 1994, 70, 636. [Google Scholar] [CrossRef]
- Yu, Z.; Yu, M.; Zhang, Z.; Hong, G.; Xiong, Q. Bovine serum albumin nanoparticles as controlled release carrier for local drug delivery to the inner ear. Nanoscale Res. Lett. 2014, 9, 343. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Yang, K.J.; Kim, D.E.; Lee, K.Y.; Park, S.N.; Kim, D.K.; Kim, J.D. Intratympanic delivery of oligoarginine-conjugated nanoparticles as a gene (or drug) carrier to the inner ear. Biomaterials 2015, 73, 243–253. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Yang, K.J.; Park, S.N.; Kim, D.K.; Kim, J.D. The effect of dexamethasone/cell-penetrating peptide nanoparticles on gene delivery for inner ear therapy. Int. J. Nanomed. 2016, 11, 6123–6134. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Hannula, M.; Misra, S.; Feng, H.; Labrador, R.H.; Aula, A.S.; Hyttinen, J.; Pyykkö, I. Micro CT visualization of silver nanoparticles in the middle and inner ear of rat and transportation pathway after transtympanic injection. J. Nanobiotechnol. 2015, 13, 5. [Google Scholar] [CrossRef]
- Lin, Y.C.; Shih, C.P.; Chen, H.C.; Chou, Y.L.; Sytwu, H.K.; Fang, M.C.; Lin, Y.Y.; Kuo, C.Y.; Su, H.H.; Hung, C.L.; et al. Ultrasound Microbubble–Facilitated Inner Ear Delivery of Gold Nanoparticles Involves Transient Disruption of the Tight Junction Barrier in the Round Window Membrane. Front. Pharmacol. 2021, 12, 1623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, G.; Wen, L.; Yang, F.; Shao, A.L.; Li, X.; Long, W.; Mu, L. Novel multiple agents loaded PLGA nanoparticles for brain delivery via inner ear administration: In vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2013, 48, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wen, X.; Wen, L.; Tirelli, N.; Zhang, X.; Zhang, Y.; Su, H.; Yang, F.; Chen, G. Enhanced local bioavailability of single or compound drugs delivery to the inner ear through application of PLGA nanoparticles via round window administration. Int. J. Nanomed. 2014, 9, 5591. [Google Scholar] [CrossRef]
- Kim, D.-H.; Nguyen, T.N.; Han, Y.-M.; Tran, P.; Rho, J.; Lee, J.-Y.; Son, H.-Y.; Park, J.-S. Local drug delivery using poly(lactic-co-glycolic acid) nanoparticles in thermosensitive gels for inner ear disease treatment. Drug Deliv. 2021, 28, 2268–2277. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.W.; Levy, M.; Kao, A.; Noh, S.H.; Bozovic, D.; Cheon, J. Magnetic nanoparticles for ultrafast mechanical control of inner ear hair cells. ACS Nano 2014, 8, 6590–6598. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, W.; Poe, D.; Qin, J.; Fornara, A.; Zhang, Y.; Ramadan, U.A.; Muhammed, M.; Pyykkö, I. MRI manifestation of novel superparamagnetic iron oxide nanoparticles in the rat inner ear. Nanomedicine 2010, 5, 739–754. [Google Scholar] [CrossRef]
- Thaler, M.; Roy, S.; Fornara, A.; Bitsche, M.; Qin, J.; Muhammed, M.; Salvenmoser, W.; Rieger, G.; Fischer, A.S.; Glueckert, R. Visualization and analysis of superparamagnetic iron oxide nanoparticles in the inner ear by light microscopy and energy filtered TEM. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Ostrovsky, S.; Israel, L.L.; Feng, H.; Kettunen, M.I.; Lellouche, J.P.M.; Pyykkö, I. Efficient penetration of ceric ammonium nitrate oxidant-stabilized gamma-maghemite nanoparticles through the oval and round windows into the rat inner ear as demonstrated by MRI. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1883–1891. [Google Scholar] [CrossRef]
- Zou, J.; Sood, R.; Ranjan, S.; Poe, D.; Ramadan, U.A.; Kinnunen, P.K.J.; Pyykkö, I. Manufacturing and in vivo inner ear visualization of MRI traceable liposome nanoparticles encapsulating gadolinium. J. Nanobiotechnol. 2010, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Sadabad, R.K.; Xia, A.; Benkafadar, N.; Faniku, C.; Preciado, D.; Yang, S.; Valdez, T.A. Topical Delivery of Elastic Liposomal Vesicles for Treatment of Middle and Inner Ear Disease. bioRxiv 2022. [Google Scholar] [CrossRef]
- Zou, J.; Sood, R.; Zhang, Y.; Kinnunen, P.K.J.; Pyykkö, I. Pathway and morphological transformation of liposome nanocarriers after release from a novel sustained inner-ear delivery system. Nanomedicine 2014, 9, 2143–2155. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; Cirillo, G.; Amato, R.; Guidotti, L.; Amantea, D.; de Luca, M.; Nicoletta, F.P.; Iemma, F.; Garcia-Gil, M. Encapsulation of Alpha-Lipoic Acid in Functional Hybrid Liposomes: Promising Tool for the Reduction of Cisplatin-Induced Ototoxicity. Pharmaceuticals 2022, 15, 394. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-Hydrogel: A Hybrid Biomaterial System for Localized Drug Delivery. Ann. Biomed. Eng. 2016, 44, 2049. [Google Scholar] [CrossRef]
- Cervantes, B.; Arana, L.; Murillo-Cuesta, S.; Bruno, M.; Alkorta, I.; Varela-Nieto, I. Solid Lipid Nanoparticles Loaded with Glucocorticoids Protect Auditory Cells from Cisplatin-Induced Ototoxicity. J. Clin. Med. 2019, 8, 1464. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Hou, S.-X.; Hu, P. In vitro dexamethasone release from nanoparticles and its pharmacokinetics in the inner ear after administration of the drug-loaded nanoparticles via the round window. Nan Fang Yi Ke Da Xue Xue Bao 2008, 28, 1022–1024. Available online: https://pubmed.ncbi.nlm.nih.gov/18583254/ (accessed on 1 August 2021).
- Yang, K.J.; Son, J.; Jung, S.Y.; Yi, G.; Yoo, J.; Kim, D.K.; Koo, H. Optimized phospholipid-based nanoparticles for inner ear drug delivery and therapy. Biomaterials 2018, 171, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Pyykko, I.; Zou, J.; Zhang, Y.; Zhang, W.; Feng, H.; Kinnunen, P. Nanoparticle based inner ear therapy. World J. Otorhinolaryngol. 2013, 3, 114–133. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Y.; Cao, W.; Xie, S.; Wen, L.; Chen, G. Understanding the translocation mechanism of PLGA nanoparticles across round window membrane into the inner ear: A guideline for inner ear drug delivery based on nanomedicine. Int. J. Nanomed. 2018, 13, 479. [Google Scholar] [CrossRef]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.T.; Passirani, C.; Saulnier, P.; Benoit, J.P. Lipid nanocapsules: A new platform for nanomedicine. Int. J. Pharm. 2009, 379, 201–209. [Google Scholar] [CrossRef]
- Li, L.; Chao, T.; Brant, J.; O’Malley, B.; Tsourkas, A.; Li, D. Advances in Nano-based Inner Ear Delivery Systems for the Treatment of Sensorineural Hearing Loss. Adv. Drug Deliv. Rev. 2017, 108, 2. [Google Scholar] [CrossRef] [PubMed]
- Scheper, V.; Wolf, M.; Scholl, M.; Kadlecova, Z.; Perrier, T.; Klok, H.A.; Saulnier, P.; Lenarz, T.; Stöver, T. Potential novel drug carriers for inner ear treatment: Hyperbranched polylysine and lipid nanocapsules. Nanomedicine 2009, 4, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Pena, S.A.; Zhu, A.; Eshraghi, N.; Fesharaki, A.; Horesh, E.J.; Mittal, J.; Eshraghi, A.A. Nanoparticle-based drug delivery in the inner ear: Current challenges, limitations and opportunities. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Wareing, M.; Mhatre, A.N.; Pettis, R.; Han, J.J.; Haut, T.; Pfister, M.H.F.; Hong, K.; Zheng, W.W.; Lalwani, A.K. Cationic liposome mediated transgene expression in the guinea pig cochlea. Hear. Res. 1999, 128, 61–69. [Google Scholar] [CrossRef]
- Okano, T.; Nakagawa, T.; Kita, T.; Endo, T.; Ito, J. Cell-gene delivery of brain-derived neurotrophic factor to the mouse inner ear. Mol. Ther. 2006, 14, 866–871. [Google Scholar] [CrossRef]
- Ge, X.; Jackson, R.L.; Liu, J.; Harper, E.A.; Hoffer, M.E.; Wassel, R.A.; Dormer, K.J.; Kopke, R.D.; Balough, B.J. Distribution of PLGA nanoparticles in chinchilla cochleae. Otolaryngol. Head Neck Surg. 2007, 137, 619–623. [Google Scholar] [CrossRef]
- Shimoji, M.; Ramaswamy, B.; Shukoor, M.I.; Benhal, P.; Broda, A.; Kulkarni, S.; Malik, P.; McCaffrey, B.; Lafond, J.F.; Nacev, A.; et al. (2019). Toxicology study for magnetic injection of prednisolone into the rat cochlea. Eur. J. Pharm. Sci. 2019, 126, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic Nanoparticles as MRI Contrast Agents. Top. Curr. Chem. 2020, 378, 40. [Google Scholar] [CrossRef] [PubMed]
- Kopke, R.D.; Wassel, R.A.; Mondalek, F.; Grady, B.; Chen, K.; Liu, J.; Gibson, D.; Dormer, K.J. Magnetic Nanoparticles: Inner Ear Targeted Molecule Delivery and Middle Ear Implant. Audiol. Neurotol. 2006, 11, 123–133. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, X.; Yang, F.; Mu, L. Disposition of nanoparticle-based delivery system via inner ear administration. Curr. Drug Metab. 2010, 11, 886–897. [Google Scholar] [CrossRef]
- Buckiová, D.; Ranjan, S.; Newman, T.A.; Johnston, A.H.; Sood, R.; Kinnunen, P.K.J.; Popelář, J.; Chumak, T.; Syka, J. Minimally invasive drug delivery to the cochlea through application of nanoparticles to the round window membrane. Nanomedicine 2012, 7, 1339–1354. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Saulnier, P.; Perrier, T.; Zhang, Y.; Manninen, T.; Toppila, E.; Pyykkö, I. Distribution of lipid nanocapsules in different cochlear cell populations after round window membrane permeation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Ding, S.; Cai, H.; Wang, J.; Wen, L.; Yang, F.; Chen, G. Nanomedicine strategy for optimizing delivery to outer hair cells by surface-modified poly(lactic/glycolic acid) nanoparticles with hydrophilic molecules. Int. J. Nanomed. 2016, 11, 5959–5969. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.S.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef] [PubMed]
- Musazzi, U.M.; Franzé, S.; Cilurzo, F. Innovative pharmaceutical approaches for the management of inner ear disorders. Drug Deliv. Transl. Res. 2018, 8, 436–449. [Google Scholar] [CrossRef]
- Lee, J.J.; Jang, J.H.; Choo, O.S.; Lim, H.J.; Choung, Y.H. Steroid intracochlear distribution differs by administration method: Systemic versus intratympanic injection. Laryngoscope 2018, 128, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Young, C.C.; Vedadghavami, A.; Bajpayee, A.G. Bioelectricity for Drug Delivery: The Promise of Cationic Therapeutics. Bioelectricity 2020, 2, 68. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y. Otological significance of the round window. Adv. Otorhinolaryngol. 1984, 33, 1–162. [Google Scholar] [PubMed]
- Zhang, R.; Qin, X.; Kong, F.; Chen, P.; Pan, G. Improving cellular uptake of therapeutic entities through interaction with components of cell membrane. Drug Deliv. 2019, 26, 328–342. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Jafari, S. Cell penetrating peptides: A concise review with emphasis on biomedical applications. Biomed. Pharmacother. 2018, 108, 1090–1096. [Google Scholar] [CrossRef]
- Wei, X.; Shao, B.; He, Z.; Ye, T.; Luo, M.; Sang, Y.; Liang, X.; Wang, W.; Luo, S.; Yang, S.; et al. Cationic nanocarriers induce cell necrosis through impairment of Na+/K+-ATPase and cause subsequent inflammatory response. Cell Res. 2015, 25, 237–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, X.; Liu, Y.; Dong, L.; Luo, Y.; Zhu, G.; Qian, X.; Chen, J.; Lu, L.; Wang, J.; et al. Linear polyethylenimine-plasmid DNA nanoparticles are ototoxic to the cultured sensory epithelium of neonatal mice. Mol. Med. Rep. 2015, 11, 4381–4388. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Liang, Z.; Huang, W.; Wen, L.; Chen, G. Engineering PLGA nano-based systems through understanding the influence of nanoparticle properties and cell-penetrating peptides for cochlear drug delivery. Int. J. Pharm. 2017, 532, 55–65. [Google Scholar] [CrossRef]
- Dash-Wagh, S.; Jacob, S.; Lindberg, S.; Fridberger, A.; Langel, Ü.; Ulfendahl, M. Intracellular Delivery of Short Interfering RNA in Rat Organ of Corti Using a Cell-penetrating Peptide PepFect6. Mol. Ther. Nucleic Acids 2012, 1, e61. [Google Scholar] [CrossRef]
- Youm, I.; Musazzi, U.M.; Gratton, M.A.; Murowchick, J.B.; Youan, B.B.C. Label-Free Ferrocene-Loaded Nanocarrier Engineering for In Vivo Cochlear Drug Delivery and Imaging. J. Pharm. Sci. 2016, 105, 3162–3171. [Google Scholar] [CrossRef]
- Iwai, K.; Nakagawa, T.; Endo, T.; Matsuoka, Y.; Kita, T.; Kim, T.S.; Tabata, Y.; Ito, J. Cochlear protection by local insulin-like growth factor-1 application using biodegradable hydrogel. Laryngoscope 2006, 116, 529–533. [Google Scholar] [CrossRef]
- Xu, X.; Lin, K.; Wang, Y.; Xu, K.; Sun, Y.; Yang, X.; Yang, M.; He, Z.; Zhang, Y.; Zheng, H.; et al. A metal–Organic framework based inner ear delivery system for the treatment of noise-induced hearing loss. Nanoscale 2020, 12, 16359–16365. [Google Scholar] [CrossRef]
- Milton Harris, J.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef]
- Salt, A.N.; Hirose, K. Communication pathways to and from the inner ear and their contributions to drug delivery. Hear. Res. 2018, 362, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Salt, A.N.; Plontke, S.K. Pharmacokinetic principles in the inner ear: Influence of drug properties on intratympanic applications. Hear. Res. 2018, 368, 28–40. [Google Scholar] [CrossRef]
- King, E.B.; Salt, A.N.; Eastwood, H.T.; O’Leary, S.J. Direct entry of gadolinium into the vestibule following intratympanic applications in Guinea pigs and the influence of cochlear implantation. J. Assoc. Res. Otolaryngol. 2011, 12, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Rybak, L.P.; Dhukhwa, A.; Mukherjea, D.; Ramkumar, V. Local Drug Delivery for Prevention of Hearing Loss. Front. Cell. Neurosci. 2019, 13, 300. [Google Scholar] [CrossRef]
- El Kechai, N.; Mamelle, E.; Nguyen, Y.; Huang, N.; Nicolas, V.; Chaminade, P.; Yen-Nicolaÿ, S.; Gueutin, C.; Granger, B.; Ferrary, E.; et al. Hyaluronic acid liposomal gel sustains delivery of a corticoid to the inner ear. J. Control. Release 2016, 226, 248–257. [Google Scholar] [CrossRef]
- Salt, A.N.; Plontke, S.K. Principles of Local Drug Delivery to the Inner Ear. Audiol. Neurotol. 2009, 14, 350. [Google Scholar] [CrossRef]
- Guan, L.; Yang, H.; Cai, Y.; Sun, L.; Di, P.; Li, W.; Liu, G.; Tang, Y. ADMET-score—A comprehensive scoring function for evaluation of chemical drug-likeness. MedChemComm 2019, 10, 148. [Google Scholar] [CrossRef]
- Abughazaleh, R.D.; Tracy, T.S. Therapeutic Index. Wiley StatsRef Stat. Ref. Online 2014. [Google Scholar] [CrossRef]
- Sawamura, S.; Ogata, G.; Asai, K.; Razvina, O.; Ota, T.; Zhang, Q.; Madhurantakam, S.; Akiyama, K.; Ino, D.; Kanzaki, S.; et al. Analysis of Pharmacokinetics in the Cochlea of the Inner Ear. Front. Pharmacol. 2021, 12, 633505. [Google Scholar] [CrossRef]
- Parnes, L.S.; Sun, A.H.; Freeman, D.J. Corticosteroid pharmacokinetics in the inner ear fluids: An animal study followed by clinical application. Laryngoscope 1999, 109, 1–17. [Google Scholar] [CrossRef]
- Plontke, S.K.; Biegner, T.; Kammerer, B.; Delabar, U.; Salt, A.N. Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otol. Neurotol. 2008, 29, 401–406. [Google Scholar] [CrossRef]
- Hellberg, V.; Wallin, I.; Ehrsson, H.; Laurell, G. Cochlear pharmacokinetics of cisplatin: An in vivo study in the guinea pig. Laryngoscope 2013, 123, 3172–3177. [Google Scholar] [CrossRef]
- Wang, Y.; Han, L.; Diao, T.; Jing, Y.; Wang, L.; Zheng, H.; Ma, X.; Qi, J.; Yu, L. A comparison of systemic and local dexamethasone administration: From perilymph/cochlea concentration to cochlear distribution. Hear. Res. 2018, 370, 1–10. [Google Scholar] [CrossRef]
- Grondin, Y.; Cotanche, D.A.; Manneberg, O.; Molina, R.; Treviño-Villarreal, J.H.; Sepulveda, R.; Clifford, R.; Bortoni, M.E.; Forsberg, S.; LaBrecque, B.; et al. Pulmonary delivery of d-methionine is associated with an increase in ALCAR and glutathione in cochlear fluids. Hear. Res. 2013, 298, 93–103. [Google Scholar] [CrossRef]
- Taylor, A.E.; Keevil, B.; Huhtaniemi, I.T. Mass spectrometry and immunoassay: How to measure steroid hormones today and tomorrow. Eur. J. Endocrinol. 2015, 173, D1–D12. [Google Scholar] [CrossRef] [PubMed]
- Plontke, S.K.; Mynatt, R.; Gill, R.M.; Borgmann, S.; Salt, A.N. Concentration gradient along the scala tympani after local application of gentamicin to the round window membrane. Laryngoscope 2007, 117, 1191–1198. [Google Scholar] [CrossRef]
- Hahn, H.; Salt, A.N.; Schumacher, U.; Plontke, S.K. Gentamicin concentration gradients in scala tympani perilymph following systemic applications. Audiol. Neuro Otol. 2013, 18, 383. [Google Scholar] [CrossRef]
- Roehm, P.; Hoffer, M.; Balaban, C.D. Gentamicin uptake in the chinchilla inner ear. Hear. Res. 2007, 230, 43–52. [Google Scholar] [CrossRef]
- Huisken, J.; Stainier, D.Y.R. Selective plane illumination microscopy techniques in developmental biology. Development 2009, 136, 1963–1975. [Google Scholar] [CrossRef]
- McDonnell, L.A.; Heeren, R.M.A. Imaging mass spectrometry. Mass Spectrom. Rev. 2007, 26, 606–643. [Google Scholar] [CrossRef] [PubMed]
- Bagotsky, V.S. Fundamentals of Electrochemistry, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2006; pp. 51–60. [Google Scholar] [CrossRef]
- Jackowska, K.; Krysinski, P. New trends in the electrochemical sensing of dopamine. Anal. Bioanal. Chem. 2013, 405, 3753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Yu, P.; Lin, Y.; Zhou, N.; Li, T.; Ma, F.; Mao, L. In vivo electrochemical monitoring of the change of cochlear perilymph ascorbate during salicylate-induced tinnitus. Anal. Chem. 2012, 84, 5433–5438. [Google Scholar] [CrossRef] [PubMed]
| Administration Route | Evaluation of Uptake | References | |
|---|---|---|---|
| Natural Protein Nanoparticles | |||
| Bovine serum albumin nanoparticles | Transtympanic injection | Fluorescence microscopy and SEM | [80] |
| Poly (2-hydroxyethyl l-aspartamide; PHEA) nanoparticles | Transtympanic injection | Fluorescence microscopy | [81] |
| PHEA-g-C18-Arg8 (PCA) nanoparticles | In vitro | TEM | [82] |
| Metallic Nanoparticles | |||
| Polyvinylpyrrolidone silver nanoparticles | Transtympanic injection | Micro CT imaging | [83] |
| Gold nanoparticles | Microbubbles and intratympanic injection | SEM, TEM, ABR, confocal microscopy and mass spectrometry | [84] |
| Polymeric Nanoparticles | |||
| PLGA nanoparticles | Transtympanic injection | HPLC analysis | [85] |
| PLGA nanoparticles | Transtympanic injection | HPLC analysis | [86] |
| PLGA nanoparticles | In vitro | HPLC analysis | [87] |
| PEG-conjugated magnetic nanoparticles | Ex-vivo | Optical microscopy | [88] |
| Inorganic Nanoparticles | |||
| SPION | Transtympanic and intracochlear injection | MRI and TEM | [89] |
| SPION | Organotypic culture | Light microscopy and TEM | [90] |
| SPION | Transtympanic injection | MRI | [91] |
| Liposomes | |||
| Liposomes | Transtympanic injection | MRI | [92] |
| Liposomes | Drops to the tympanic membrane | Confocal microscopy | [93] |
| Liposomes | Intracochlear osmotic pump | MRI and cryo-TEM | [94] |
| Liposomes | In vitro | Confocal microscopy | [95] |
| Lipid Nanoparticles | |||
| Solid lipid nanoparticles | Transtympanic injection | ABR and light microscopy | [96] |
| Solid lipid nanoparticles | In vitro | Confocal microscopy and flow cytometry | [97] |
| Solid lipid nanoparticles | Transtympanic injection | HPLC analysis | [98] |
| Phospholipid nanoparticles | Transtympanic injection | ABR and confocal imaging | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dash, S.; Zuo, J.; Steyger, P.S. Local Delivery of Therapeutics to the Cochlea Using Nanoparticles and Other Biomaterials. Pharmaceuticals 2022, 15, 1115. https://doi.org/10.3390/ph15091115
Dash S, Zuo J, Steyger PS. Local Delivery of Therapeutics to the Cochlea Using Nanoparticles and Other Biomaterials. Pharmaceuticals. 2022; 15(9):1115. https://doi.org/10.3390/ph15091115
Chicago/Turabian StyleDash, Shreshtha, Jian Zuo, and Peter S. Steyger. 2022. "Local Delivery of Therapeutics to the Cochlea Using Nanoparticles and Other Biomaterials" Pharmaceuticals 15, no. 9: 1115. https://doi.org/10.3390/ph15091115
APA StyleDash, S., Zuo, J., & Steyger, P. S. (2022). Local Delivery of Therapeutics to the Cochlea Using Nanoparticles and Other Biomaterials. Pharmaceuticals, 15(9), 1115. https://doi.org/10.3390/ph15091115






