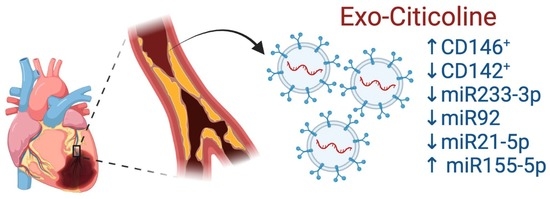
Citicoline Modifies the Expression of Specific miRNAs Related to Cardioprotection in Patients with ST-Segment Elevation Myocardial Infarction Subjected to Coronary Angioplasty
Abstract
1. Introduction
2. Results
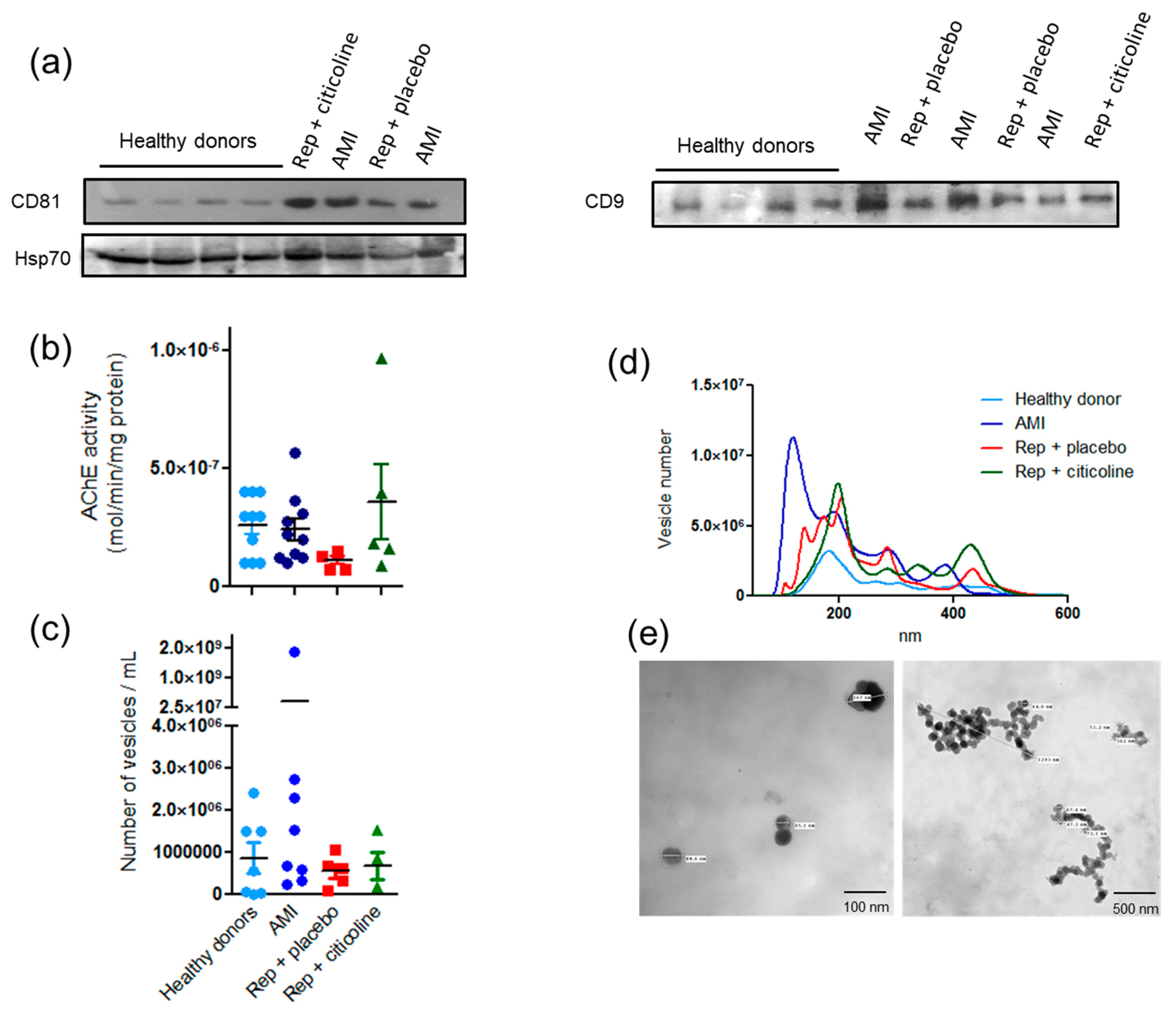
2.1. Characterization of Exosome-Enriched Fractions
2.2. Exosomes Secretion from Different Cellular Types
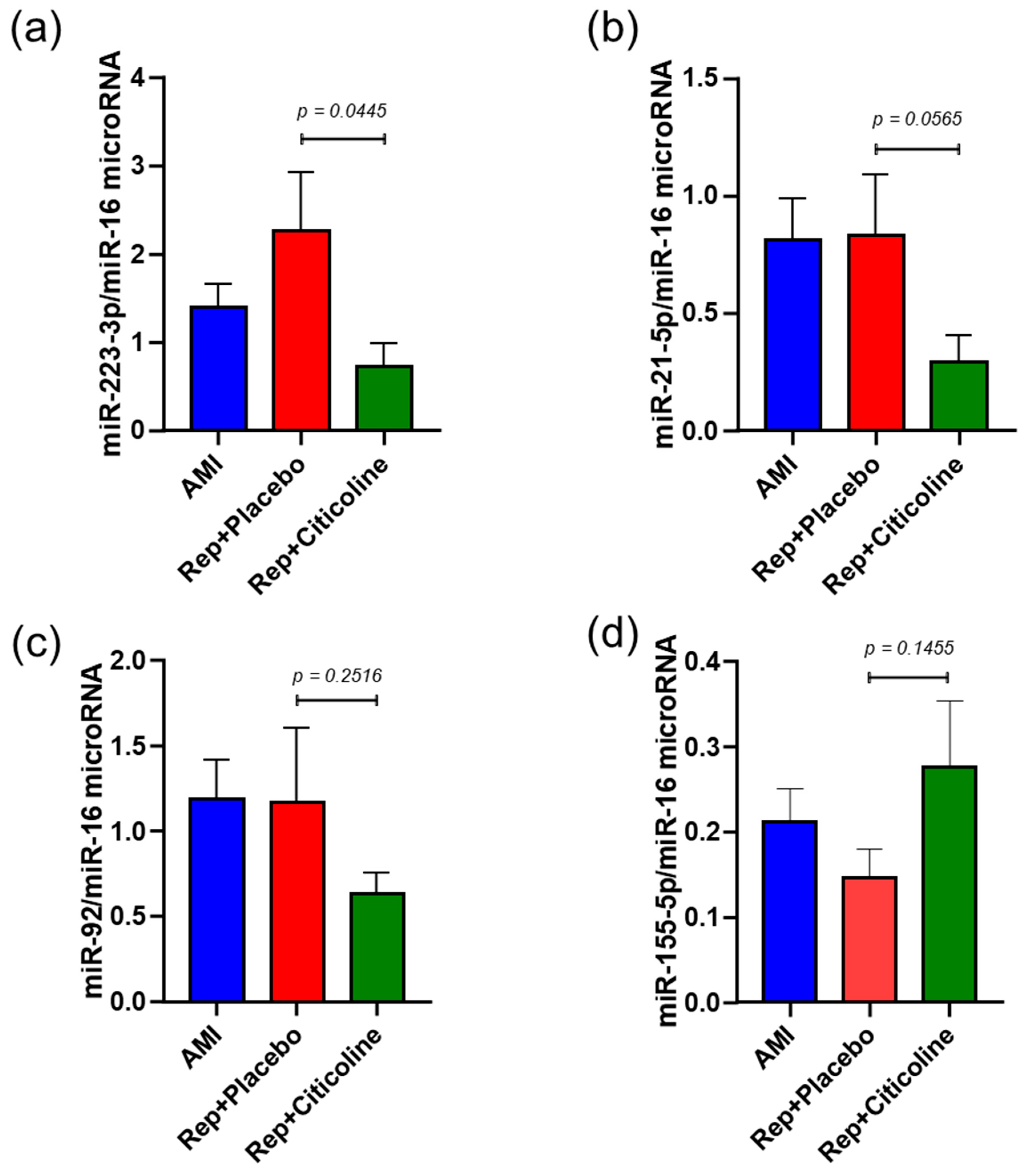
2.3. Citicoline Modifies miR-233-3p, miR21-5p, and miR-92 levels in Exosomes from Infarcted Patients Subjected to Angioplasty
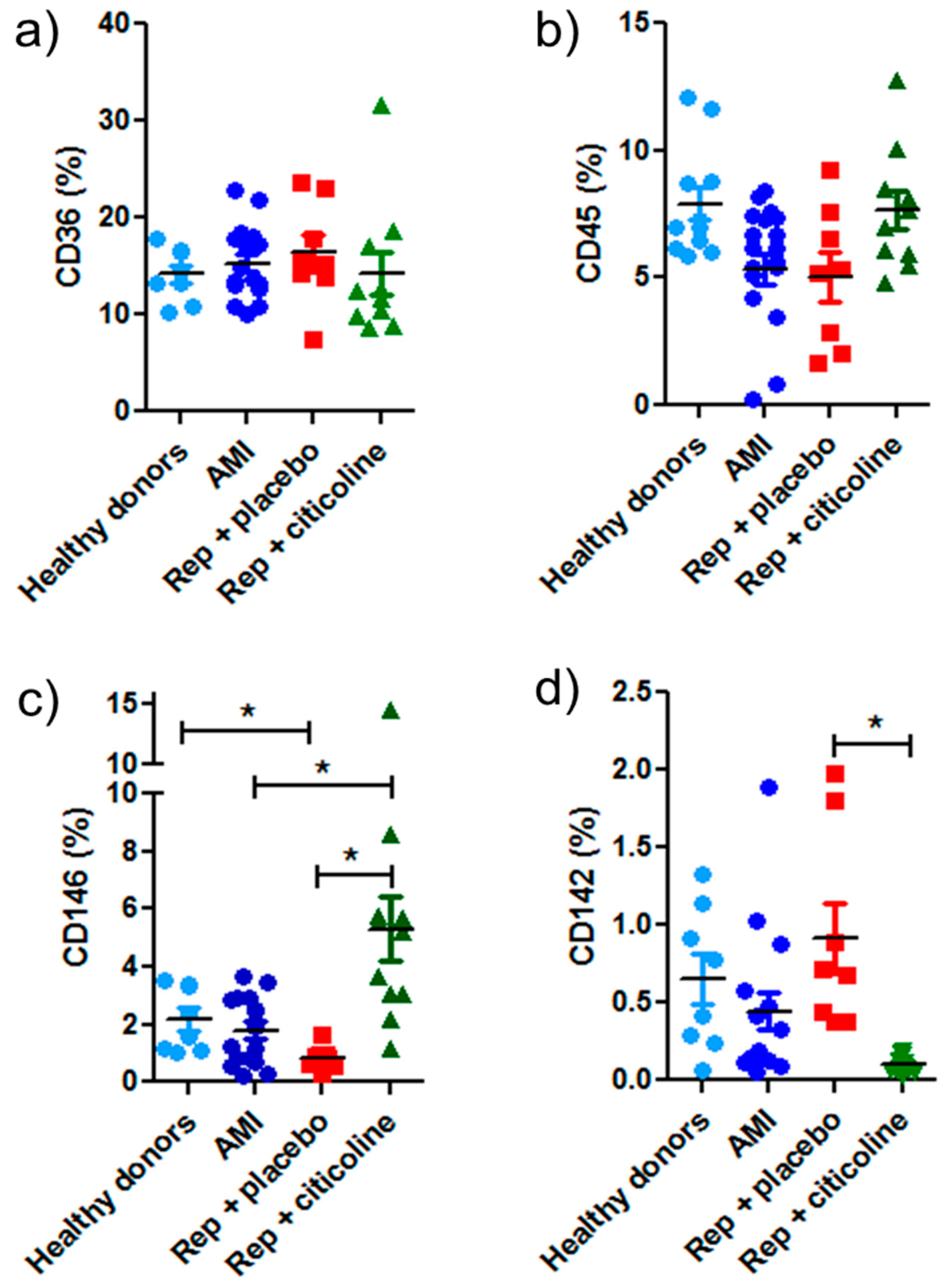
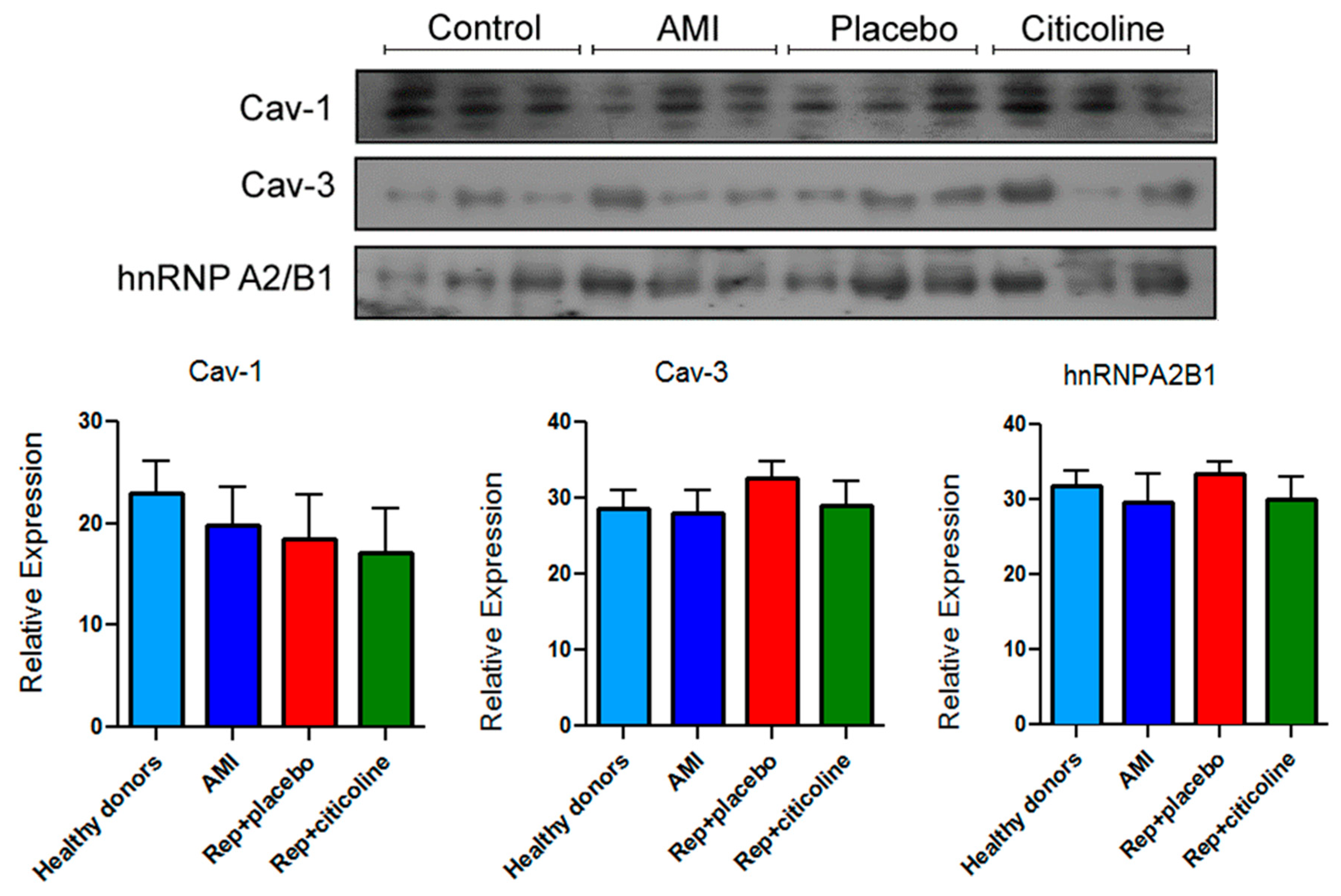
2.4. Caveolin and hnRNPA2B1 Content in Exosomes from Infarcted Patients Subjected to Angioplasty
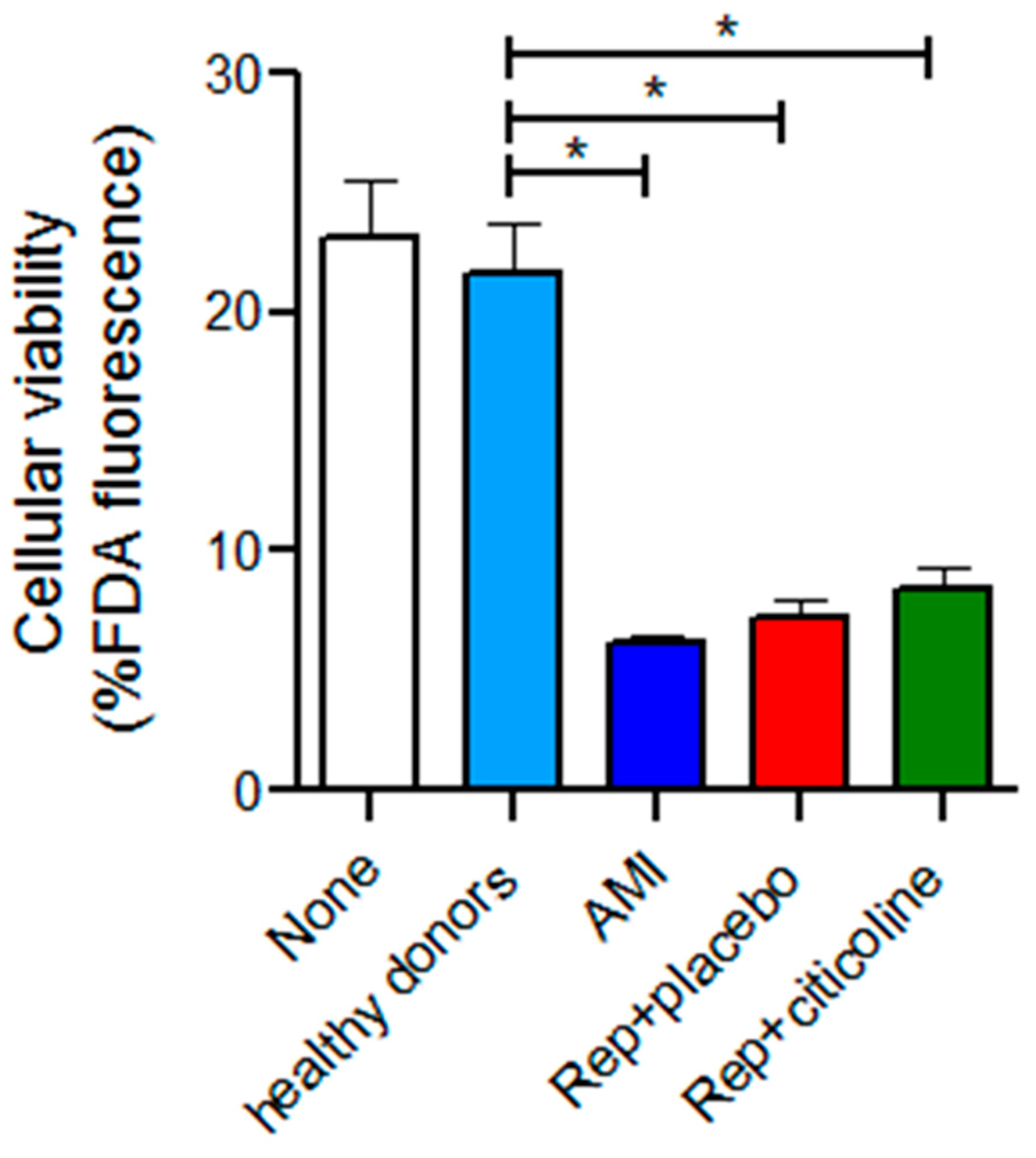
2.5. Effect of the Incubation of Exosomes from Reperfused Patients Treated with Citicoline in H9c2 Viability
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Group of Patients
4.3. Obtention of Exosomes from Healthy Donors and Patients Subjected to Angioplasty
4.4. Characterization of Exosome-Enriched Fractions
4.5. Flow Cytometry
4.6. Isolation of miRNAs from Plasma Exosomes
4.7. miRNAs Determination by RT-qPCR
4.8. Western-Blot Analysis
4.9. Cell Culture and Model of Hypoxia/Reoxygenation (H/R)
4.10. Cellular Viability
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Edgar, J.R. Q & A: What are exosomes, exactly? BMC Biol. 2016, 14, 1–7. [Google Scholar]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, Y.; Ono, K.; Horie, T.; Nishi, H.; Nagao, K.; Kinoshita, M.; Watanabe, S.; Baba, O.; Kojima, Y.; Shizuta, S.; et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ. Cardiovasc. Genet. 2011, 4, 446–454. [Google Scholar] [CrossRef]
- Cesselli, D.; Parisse, P.; Aleksova, A.; Veneziano, C.; Cervellin, C.; Zanello, A.; Beltrami, A.P. Extracellular vesicles: How drug and pathology interfere with their biogenesis and function. Front. Physiol. 2018, 9, 1394. [Google Scholar] [CrossRef]
- Boulanger, C.M.; Loyer, X.; Rautou, P.-E.; Amabile, N. Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 2017, 14, 259–272. [Google Scholar] [CrossRef]
- Yang, C.; Yang, J.; Fan, Z.; Yang, J. ECs-derived exosomes: A novel therapeutic target for myocardial ischemia-reperfusion injury. Int. J. Cardiol. 2021, 333, 51. [Google Scholar] [CrossRef]
- Bernal-Mizrachi, L.; Jy, W.; Jimenez, J.J.; Pastor, J.; Mauro, L.M.; Horstman, L.L.; de Marchena, E.; Ahn, Y.S. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am. Heart J. 2003, 145, 962–970. [Google Scholar] [CrossRef]
- Cheng, M.; Yang, J.; Zhao, X.; Zhang, E.; Zeng, Q.; Yu, Y.; Yang, L.; Wu, B.; Yi, G.; Mao, X.; et al. Circulating myocardial microRNAs from infarcted hearts are carried in exosomes and mobilize bone marrow progenitor cells. Nat. Commun. 2019, 10, 959. [Google Scholar] [CrossRef]
- De Hoog, V.C.; Timmers, L.; Schoneveld, A.H.; Wang, J.W.; van de Weg, S.M.; Sze, S.K.; van Keulen, J.K.; Hoes, A.W.; den Ruijter, H.M.; de Kleijn, D.P.V.; et al. Serum extracellular vesicle protein levels are associated with acute coronary syndrome. Eur. Heart J. Acute Cardiovasc. Care 2013, 2, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Coskun, C.; Avci, B.; Yalcin, M.; Yermezler, A.; Yilmaz, M.S.; Savci, V. Protective effect of CDP-choline on ischemia-reperfusion-induced myocardial tissue injury in rats. Ir. J. Med. Sci. 2014, 183, 539–548. [Google Scholar] [CrossRef] [PubMed]
- González-Pacheco, H.; Méndez-Domínguez, A.; Hernández, S.; López-Marure, R.; Vazquez-Mellado, M.J.; Aguilar, C.; Rocha-Zavaleta, L. Pre-conditioning with CDP-choline attenuates oxidative stress-induced cardiac myocyte death in a hypoxia/reperfusion model. Sci. World J. 2014, 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Esquivel, L.; Pavón, N.; Buelna-Chontal, M.; González-Pacheco, H.; Belmont, J.; Chávez, E. Citicoline (CDP-choline) protects myocardium from ischemia/reperfusion injury via inhibiting mitochondrial permeability transition. Life Sci. 2014, 96, 53–58. [Google Scholar] [CrossRef]
- Hernández-Esquivel, L.; Pavón, N.; Buelna-Chontal, M.; González-Pacheco, H.; Belmont, J.; Chávez, E. Cardioprotective properties of citicoline against hyperthyroidism-induced reperfusion damage in rat hearts. Biochem. Cell Biol. 2015, 93, 185–191. [Google Scholar] [CrossRef]
- Secades, J.J.; Alvarez-Sabin, J.; Castillo, J.; Díez-Tejedor, E.; Martinez-Vila, E.; Ríos, J.; Oudovenko, N. Citicoline for Acute Ischemic Stroke: A Systematic Review and Formal Meta-analysis of Randomized, Double-Blind, and Placebo-Controlled Trials. J. Stroke Cerebrovasc. Dis. 2016, 25, 1984–1996. [Google Scholar] [CrossRef]
- Cubells, J.M.; Hernando, C. Clinical trial on the use of cytidine diphosphate choline in Parkinson’s disease. C. Clin. Ther. 1988, 10, 664–671. [Google Scholar]
- Alvarez, X.A.; Mouzo, R.; Pichel, V.; Pérez, P.; Laredo, M.; Fernández-Novoa, L.; Corzo, L.; Zas, R.; Alcaraz, M.; Secades, J.J.; et al. Double-blind placebo-controlled study with citicoline in APOE genotyped Alzheimer’s disease patients. Effects on cognitive performance, brain bio-electrical activity and cerebral perfusion. Methods Find Exp. Clin. Pharmacol. 1999, 21, 633–644. [Google Scholar]
- Aminzadeh, A.; Salarinejad, A. Citicoline protects against lead-induced oxidative injury in neuronal PC12 cells. Biochem. Cell Biol. 2019, 97, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.M.; Wechsler, L.R.; Sabounjian, L.A.; Schwiderski, U.E. Citicoline Stroke Study Group. A phase III randomized efficacy trial of 2000 mg citicoline in acute ischemic stroke patients. Neurology 2001, 57, 1595–1602. [Google Scholar] [CrossRef]
- Dávalos, A.; Alvarez-Sabín, J.; Castillo, J.; Díez-Tejedor, E.; Ferro, J.; Martínez-Vila, E.; Serena, J.; Segura, T.; Cruz, V.T.; Masjuan, J.; et al. Citicoline in the treatment of acute ischaemic stroke: An international, randomised, multicentre, placebo-controlled study (ICTUS trial). Lancet 2012, 380, 349–357. [Google Scholar] [CrossRef]
- Tiwari, R.P.; Jain, A.; Khan, Z.; Kohli, V.; Bharmal, R.N.; Kartikeyan, S.; Bisen, P.S. Cardiac Troponins I and T: Molecular Markers for Early Diagnosis, Prognosis, and Accurate Triaging of Patients with Acute Myocardial Infarction. Mol. Diagn. Ther. 2012, 16, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Soria, C.A.M.; Alejo, G.C.; González, J.J.E.; Sánchez, J.A.; Sánchez, J.M. Usefulness of rapid bedside assay of cardiac troponin I and creatine phosphokinase-MB in acute ischemic coronary syndromes. Arch. Cardiol. Mex. 2006, 76, 37–46. [Google Scholar]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Katus, H.A.; Lindahl, B.; Morrow, D.A.; Clemmensen, P.M.; et al. Third Universal Definition of Myocardial Infarction. Glob. Heart. 2012, 7, 275–295. [Google Scholar] [CrossRef]
- Overgaard, K. The Effects of Citicoline on Acute Ischemic Stroke: A Review. J. Stroke Cerebrovasc. Dis. 2014, 23, 1764–1769. [Google Scholar] [CrossRef]
- Jeanneteau, J.; Hibert, P.; Martinez, M.C.; Tual-Chalot, S.; Tamareille, S.; Furber, A.; Andriantsitohaina, R.; Prunier, F. Microparticle release in remote ischemic conditioning mechanism. Am. J. Physiol. Heart. Circ. Physiol. 2012, 303, H871–H877. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steppich, B.A.; Braun, S.L.; Stein, A.; Demetz, G.; Groha, P.; Schömig, A.; von Beckerath, N.; Kastrati, A.; Ott, I. Plasma TF activity predicts cardiovascular mortality in patients with acute myocardial infarction. Thromb. J. 2009, 7, 11. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, G.-C. Role of microRNAs in the reperfused myocardium towards post-infarct remodelling. Cardiovasc. Res. 2012, 94, 284–292. [Google Scholar] [CrossRef]
- Qin, D.; Wang, X.; Li, Y.; Yang, L.; Wang, R.; Peng, J.; Essandoh, K.; Mu, X.; Peng, T.; Han, Q.; et al. MicroRNA-223-5p and -3p Cooperatively Suppress Necroptosis in Ischemic/Reperfused Hearts. J. Biol. Chem. 2016, 291, 20247–20259. [Google Scholar] [CrossRef]
- Zazueta, C.; Buelna-Chontal, M.; Macías-López, A.; Román-Anguiano, N.G.; González-Pacheco, H.; Pavón, N.; Springall, R.; Aranda-Frausto, A.; Bojalil, R.; Silva-Palacios, A.; et al. Cytidine-5′-Diphosphocholine Protects the Liver From Ischemia/Reperfusion Injury Preserving Mitochondrial Function and Reducing Oxidative Stress. Liver Transplant. 2018, 24, 1070–1083. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhu, P.; Yang, J.; Liu, X.; Dong, S.; Wang, X.; Chun, B.; Zhuang, J.; Zhang, C. Ischaemic preconditioning-regulated miR-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target PDCD4. Cardiovasc. Res. 2010, 87, 431–439. [Google Scholar] [CrossRef]
- Sayed, D.; He, M.; Hong, C.; Gao, S.; Rane, S.; Yang, Z.; Abdellatif, M. MicroRNA-21 is a downstream effector of AKT that medi-ates its antiapoptotic effects via suppression of Fas ligand. J. Biol. Chem. 2010, 285, 20281–20290. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, G.; Zhao, W.; Hu, Y. Inhibition of MiR-92a May Protect Endothelial Cells After Acute Myocardial Infarction in Rats: Role of KLF2/4. Med. Sci. Monit. 2016, 22, 2451–2462. [Google Scholar] [CrossRef] [PubMed]
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K.; et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 2009, 324, 1710–1713. [Google Scholar] [CrossRef]
- Hinkel, R.; Penzkofer, D.; Zühlke, S.; Fischer, A.; Husada, W.; Xu, Q.-F.; Baloch, E.; van Rooij, E.; Zeiher, A.M.; Kupatt, C.; et al. Inhibition of microRNA-92a protects against ischemia/reperfusion injury in a large-animal model. Circulation 2013, 128, 1066–1075. [Google Scholar] [CrossRef]
- Groot, M.; Lee, H. Sorting Mechanisms for MicroRNAs into Extracellular Vesicles and Their Associated Diseases. Cells 2020, 9, 1044. [Google Scholar] [CrossRef]
- Simón, L.; Campos, A.; Leyton, L.; Quest, A.F.G. Caveolin-1 function at the plasma membrane and in intracellular compartments in cancer. Cancer Metastasis Rev. 2020, 39, 435–453. [Google Scholar] [CrossRef]
- Logozzi, M.; De Milito, A.; Lugini, L.; Borghi, M.; Calabrò, L.; Spada, M.; Perdicchio, M.; Marino, M.L.; Federici, C.; Iessi, E.; et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS ONE 2009, 4, e5219. [Google Scholar] [CrossRef]
- Flick, M.; Albrecht, M.; Oei, G.T.M.L.; Steenstra, R.; Kerindongo, R.P.; Zuurbier, C.J.; Patel, H.H.; Hollmann, M.W.; Preckel, B.; Weber, N.C. Helium postconditioning regulates expression of caveolin-1 and -3 and induces RISK pathway activation after ischaemia/reperfusion in cardiac tissue of rats. Eur. J. Pharmacol. 2016, 791, 718–725. [Google Scholar] [CrossRef]
- Weber, N.C.; Schilling, J.M.; Warmbrunn, M.V.; Dhanani, M.; Kerindongo, R.; Siamwala, J.; Song, Y.; Zemljic-Harpf, A.E.; Fannon, M.J.; Hollmann, M.W.; et al. Helium-induced changes in circulating caveolin in mice suggest a novel mechanism of cardiac protection. Int. J. Mol. Sci. 2019, 20, 2640. [Google Scholar] [CrossRef] [PubMed]
- Villarroya-Beltri, C.; Gutierrez-Vazquez, C.; Sanchez-Cabo, F.; Pérez-Hernández, D.; Vázquez, J.; Martin-Cofreces, N.; Martinez-Herrera, D.J.; Pascual-Montano, A.; Mittelbrunn, M.; Sánchez-Madrid, F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013, 4, 2980. [Google Scholar] [CrossRef] [PubMed]
- Lange, T.; Stracke, S.; Rettig, R.; Lendeckel, U.; Kuhn, J.; Schlüter, R.; Rippe, V.; Endlich, K.; Endlich, N. Identification of miR-16 as an endogenous reference gene for the normalization of urinary exosomal miRNA expression data from CKD patients. PLoS ONE 2017, 12, e0183435. [Google Scholar] [CrossRef] [PubMed]
- Ragni, E.; Perucca Orfei, C.; De Luca, P.; Colombini, A.; Viganò, M.; Lugano, G.; Bollati, V.; De Girolamo, L. Identification of miRNA Reference Genes in Extracellular Vesicles from Adipose Derived Mesenchymal Stem Cells for Studying Osteoarthritis. Int. J. Mol. Sci. 2019, 20, 1108. [Google Scholar] [CrossRef] [PubMed]
- Marabita, F.; De Candia, P.; Torri, A.; Tegnér, J.; Abrignani, S.; Rossi, R.L. Normalization of circulating microRNA expression data obtained by quantitative real-time RT-PCR. Brief. Bioinform. 2016, 17, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-F.; Hsieh, C.-C.; Wang, S.-C.; Chang, C.-Y.; Hung, C.-H.; Kuo, P.-L.; Liu, Y.-R.; Li, C.-Y.; Liu, P.-L. Simvastatin attenuates cardiac fibrosis via regulation of cardiomyocyte-derived exosome secretion. J. Clin. Med. 2019, 8, 794. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Cai, Z.; Yang, F.; Yu, L.; Yu, Z.; Jiang, L.; Wang, Q.; Yang, Y.; Wang, L.; Cao, X.; Wang, J. Activated T cell exosomes promote tumor invasion via fas signaling pathway. J. Immunol. 2012, 188, 5954–5961. [Google Scholar] [CrossRef] [PubMed]
- Ballinas-Verdugo, M.A.; Jiménez-Ortega, R.F.; Martínez-Martínez, E.; Rivas, N.; Contreras-López, E.A.; Carbó, R.; Sánchez, F.; Bojalil, R.; Márquez-Velasco, R.; Sánchez-Muñoz, F.; et al. Circulating miR-146a as a possible candidate biomarker in the indeterminate phase of Chagas disease. Biol. Res. 2021, 54, 21. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://pubmed.ncbi.nlm.nih.gov/?term=miRNAs+and+ischemia+and+reperfusion+and+heart&sort=date (accessed on 1 April 2022).

| Rep + Placebo | Rep + Citicoline | |
|---|---|---|
| Age (year) | 56.38 ± 10.57 | 53.63 ± 8.11 |
| Corporal mass index | 27.75 ± 4.709 | 27.85 ± 3.594 |
| Left ascending artery | 20% | 25% |
| Right coronary artery | 31% | 24% |
| Hypertension | 10.3% | 25.9% |
| Obesity | 58% | 77% |
| Diabetes | 14% | 20.7% |
| Smokers | 27.5% | 37% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Palacios, A.; Arroyo-Campuzano, M.; Flores-García, M.; Patlán, M.; Hernández-Díazcouder, A.; Alcántara, D.; Ramírez-Camacho, I.; Arana-Hidalgo, D.; Soria-Castro, E.; Sánchez, F.; et al. Citicoline Modifies the Expression of Specific miRNAs Related to Cardioprotection in Patients with ST-Segment Elevation Myocardial Infarction Subjected to Coronary Angioplasty. Pharmaceuticals 2022, 15, 925. https://doi.org/10.3390/ph15080925
Silva-Palacios A, Arroyo-Campuzano M, Flores-García M, Patlán M, Hernández-Díazcouder A, Alcántara D, Ramírez-Camacho I, Arana-Hidalgo D, Soria-Castro E, Sánchez F, et al. Citicoline Modifies the Expression of Specific miRNAs Related to Cardioprotection in Patients with ST-Segment Elevation Myocardial Infarction Subjected to Coronary Angioplasty. Pharmaceuticals. 2022; 15(8):925. https://doi.org/10.3390/ph15080925
Chicago/Turabian StyleSilva-Palacios, Alejandro, Miguel Arroyo-Campuzano, Mirthala Flores-García, Mariana Patlán, Adrián Hernández-Díazcouder, Diego Alcántara, Ixchel Ramírez-Camacho, Dana Arana-Hidalgo, Elizabeth Soria-Castro, Fausto Sánchez, and et al. 2022. "Citicoline Modifies the Expression of Specific miRNAs Related to Cardioprotection in Patients with ST-Segment Elevation Myocardial Infarction Subjected to Coronary Angioplasty" Pharmaceuticals 15, no. 8: 925. https://doi.org/10.3390/ph15080925
APA StyleSilva-Palacios, A., Arroyo-Campuzano, M., Flores-García, M., Patlán, M., Hernández-Díazcouder, A., Alcántara, D., Ramírez-Camacho, I., Arana-Hidalgo, D., Soria-Castro, E., Sánchez, F., González-Pacheco, H., & Zazueta, C. (2022). Citicoline Modifies the Expression of Specific miRNAs Related to Cardioprotection in Patients with ST-Segment Elevation Myocardial Infarction Subjected to Coronary Angioplasty. Pharmaceuticals, 15(8), 925. https://doi.org/10.3390/ph15080925