Activation of SIRT-1 Pathway by Nanoceria Sheds Light on Its Ameliorative Effect on Doxorubicin-Induced Cognitive Impairment (Chemobrain): Restraining Its Neuroinflammation, Synaptic Dysplasticity and Apoptosis
Abstract
1. Introduction
2. Results
2.1. Effect of Nanoceria on Cognitive Function of the Hippocampus
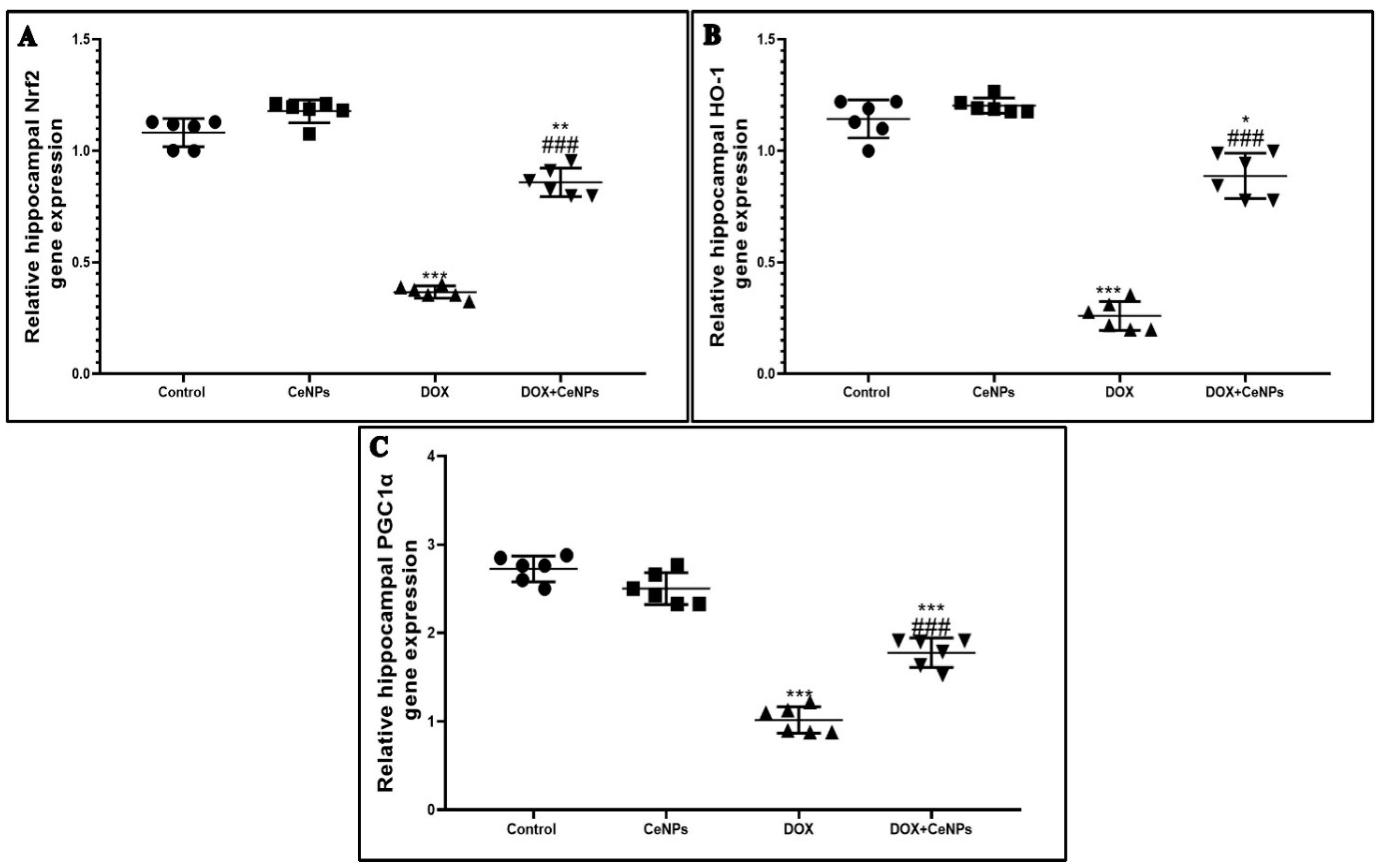
2.2. Effect of Nanoceria on DOX-Induced Hippocampal Oxidative Stress and Gene Expression of Nrf2, HO-1 and PGC1-α
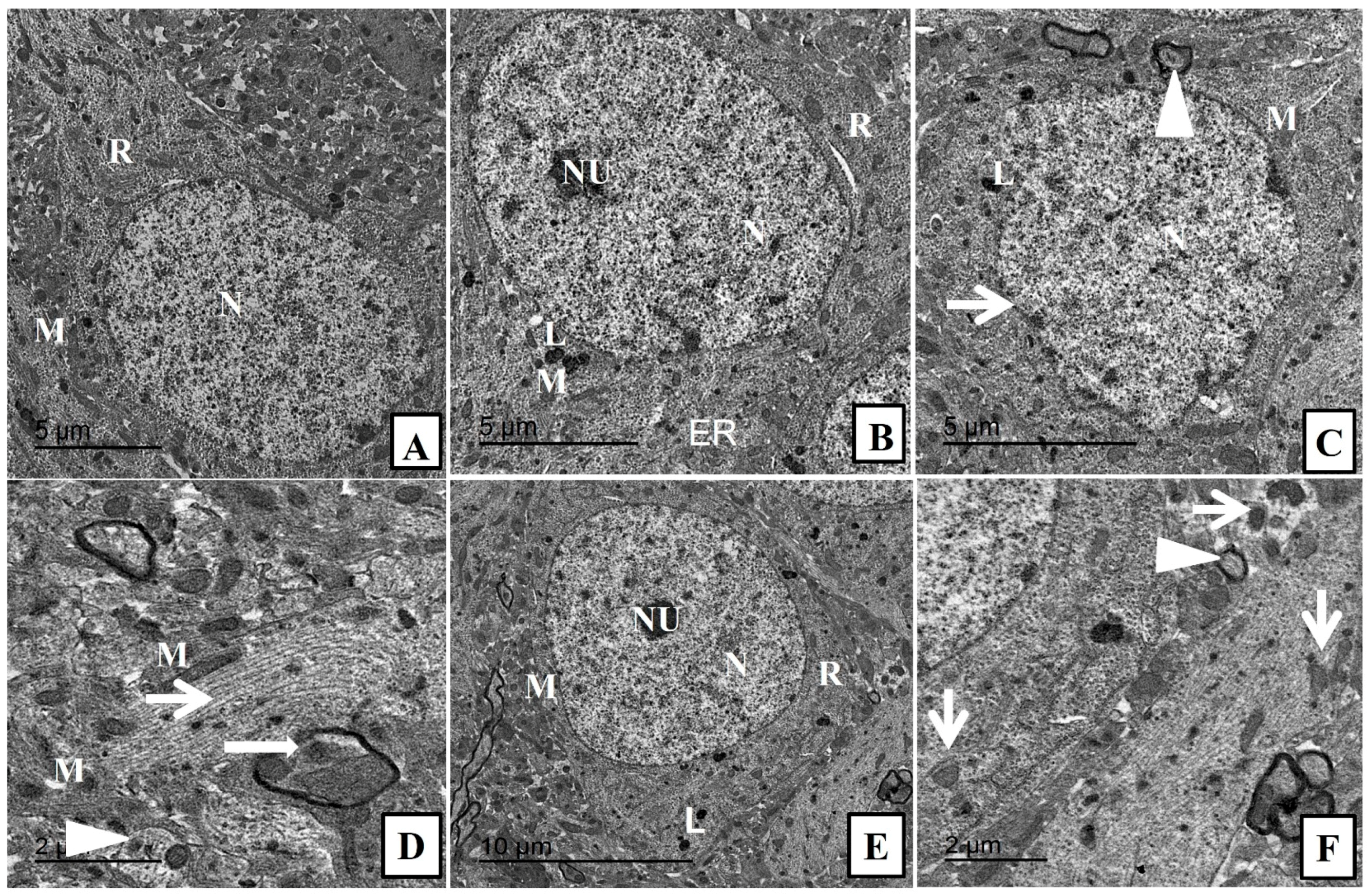
2.3. Effect of Nanoceria on the Histoarchitecture of Hippocampal Tissue
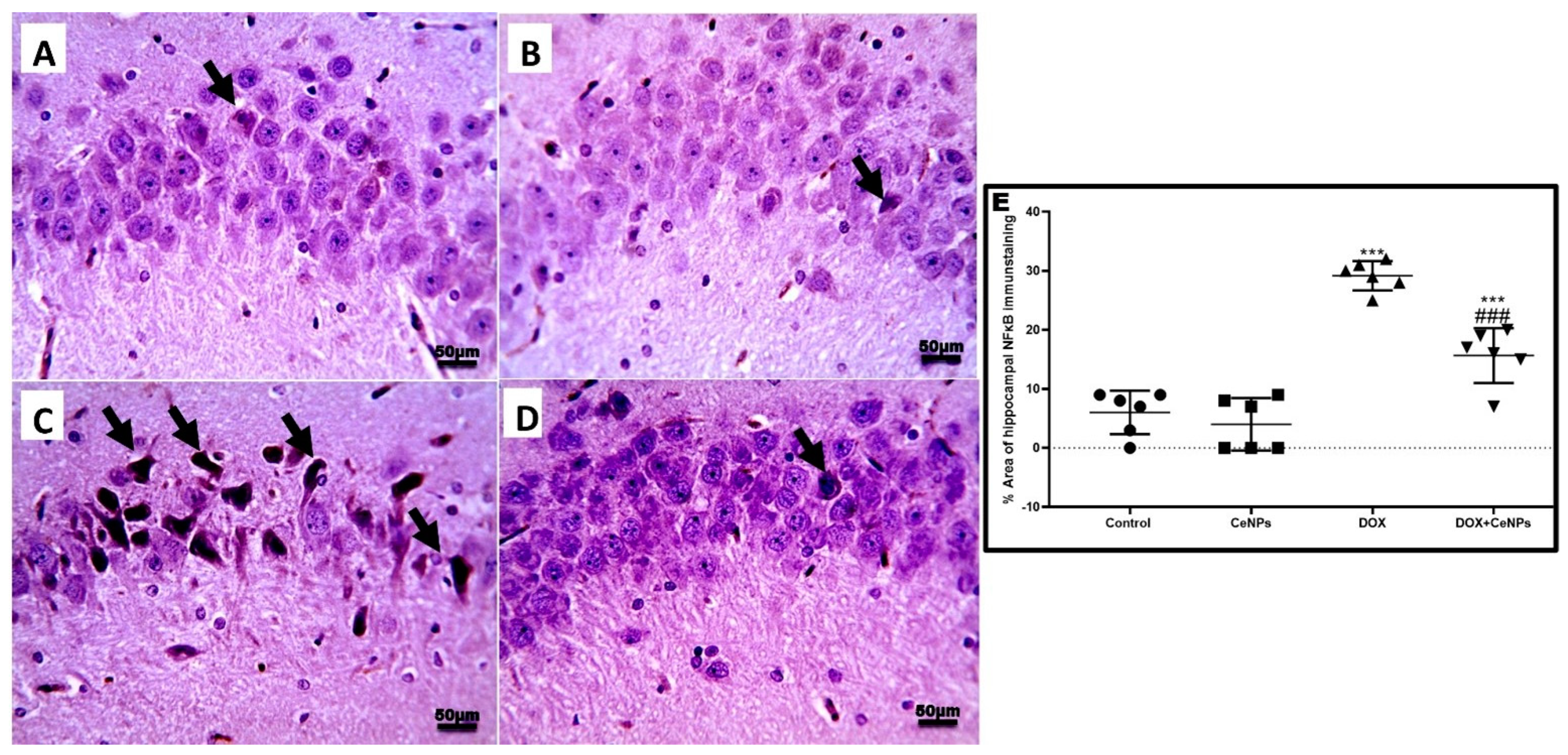
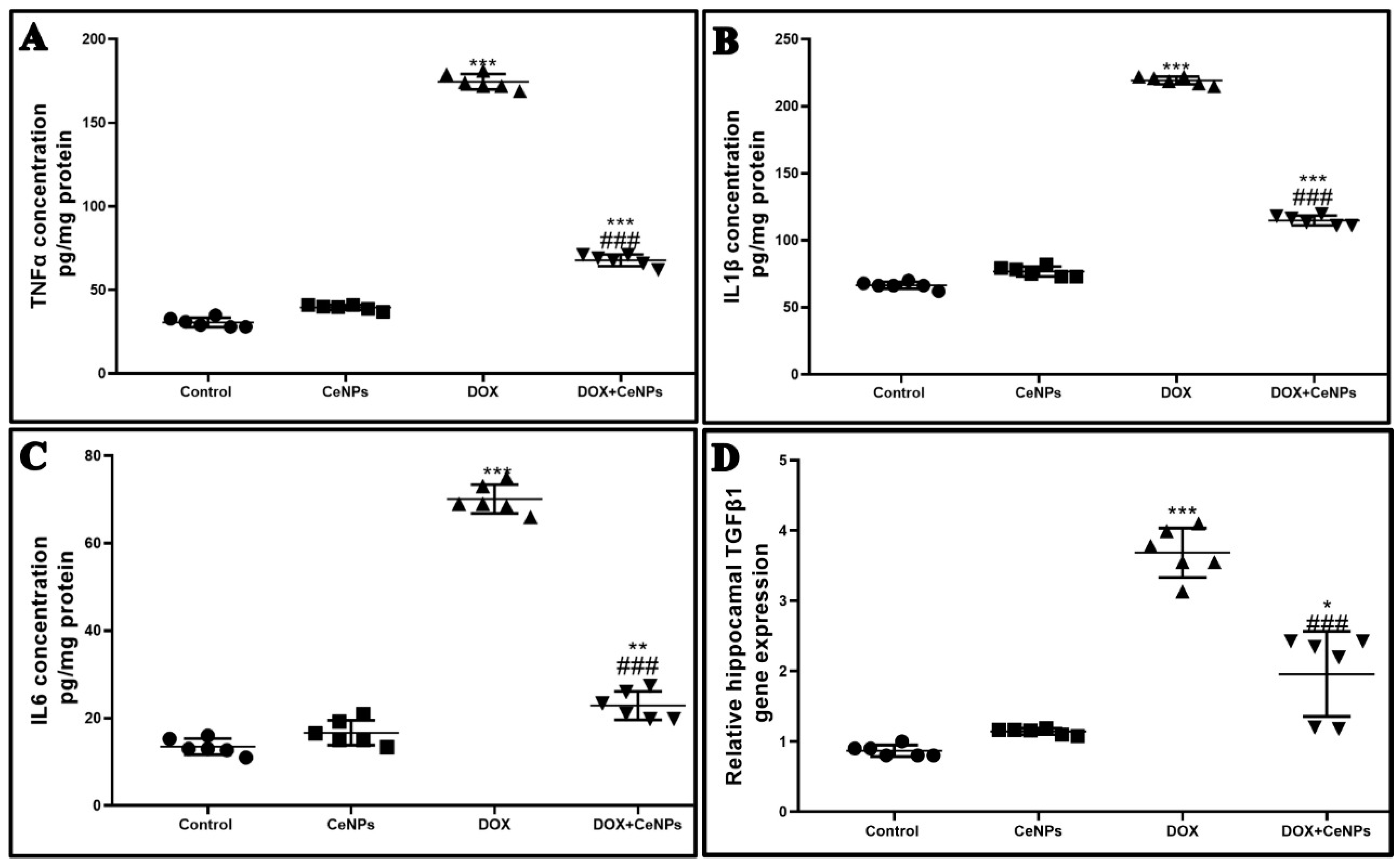
2.4. Effect of Nanoceria on DOX-Induced Hippocampal Neuroinflammation and Astrogliosis
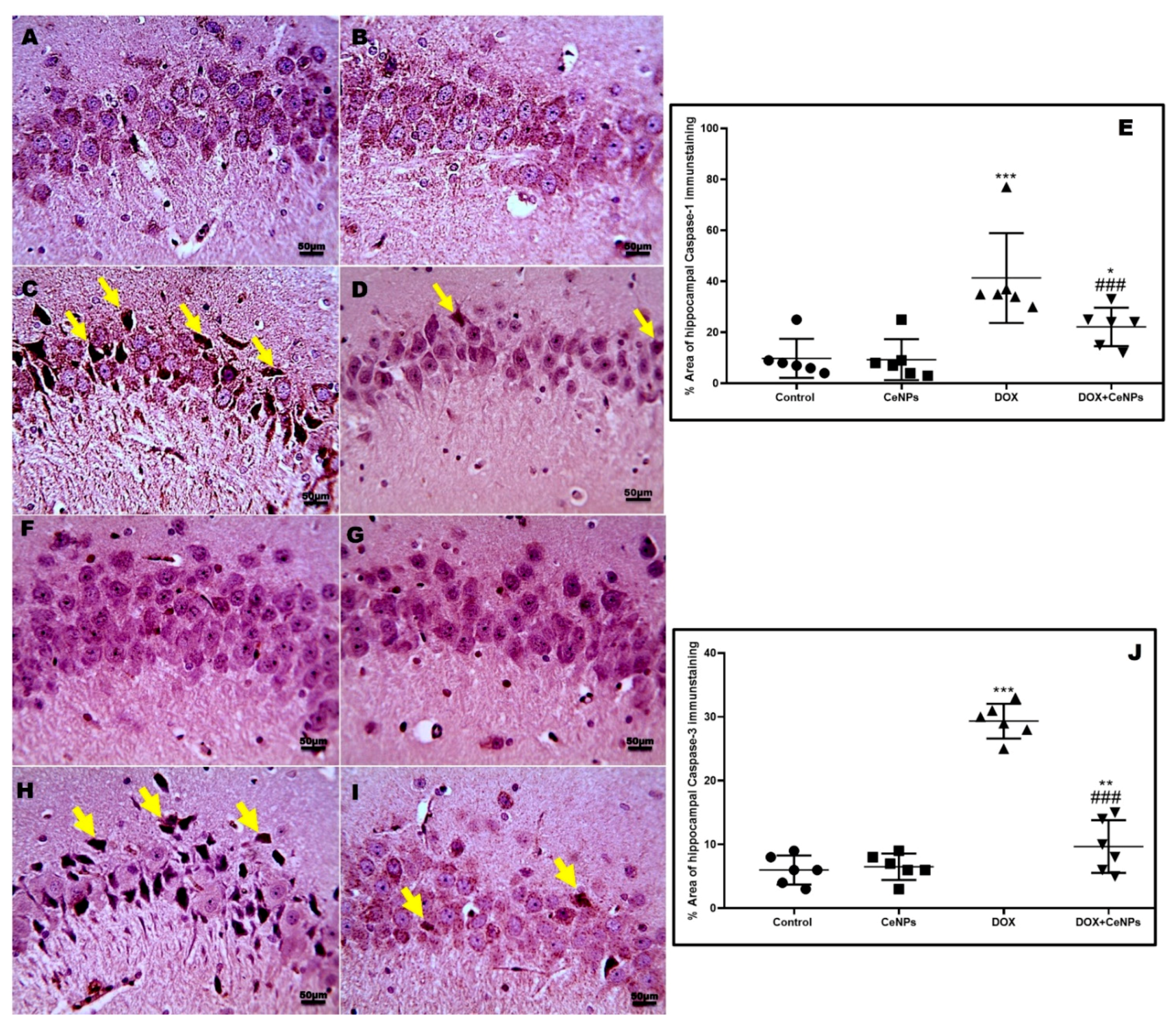
2.5. Effect of CeNPs on DOX-Induced Hippocampal Neuronal Cells Apoptosis and Pyroptosis
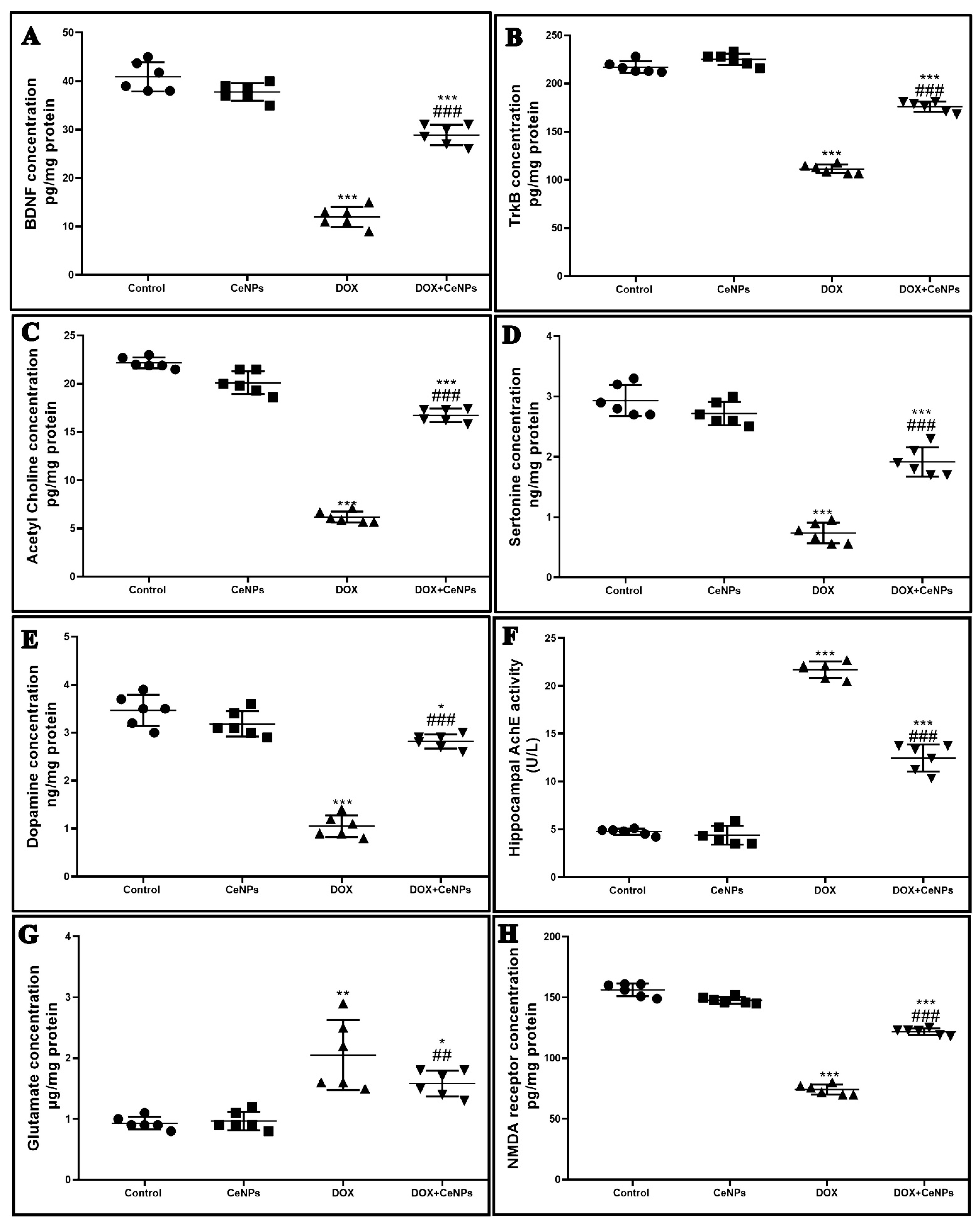
2.6. Effect of CeNPson Hippocampal Synaptic Plasticity
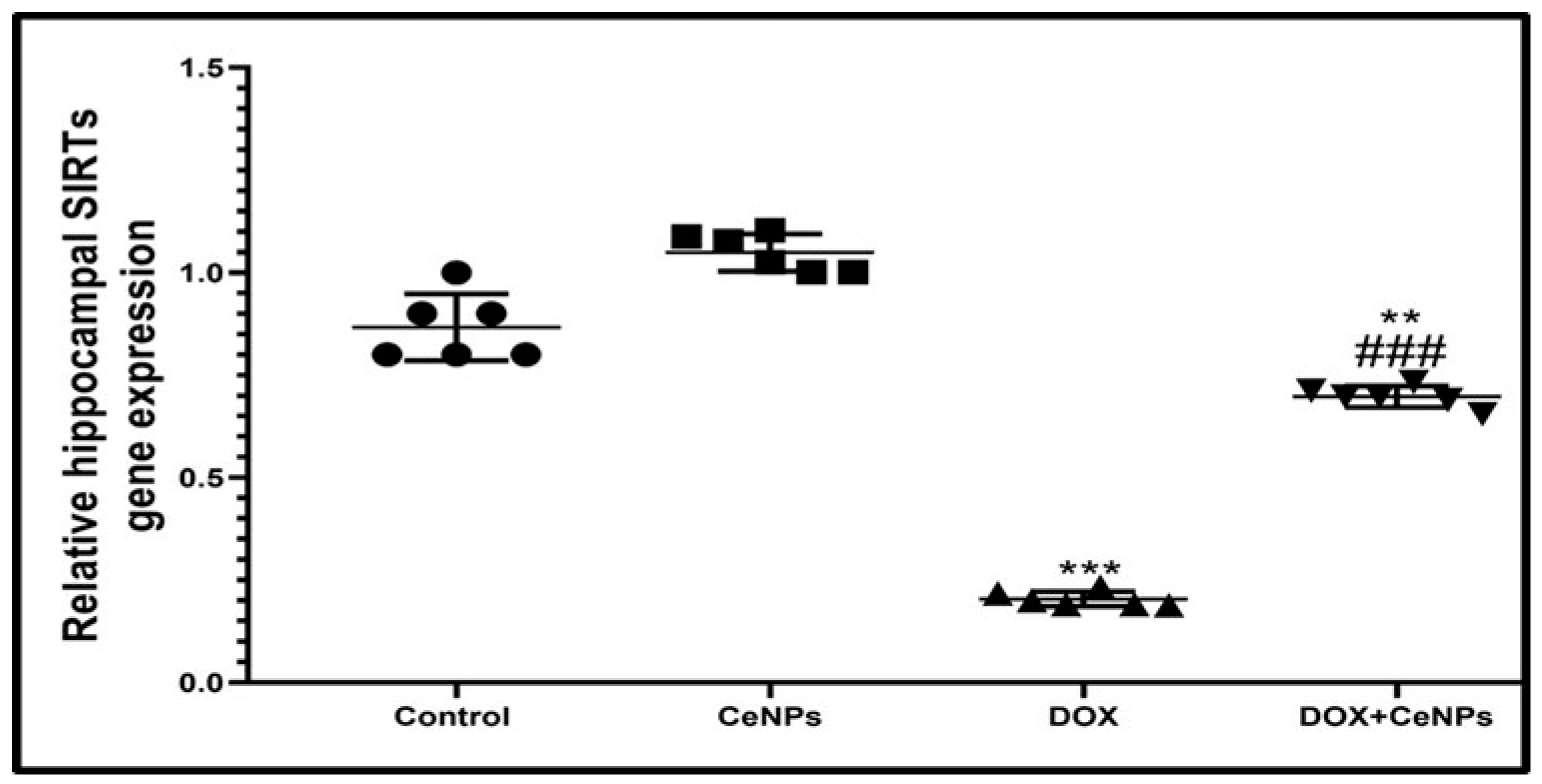
2.7. Effect of CeNPs on Hippocampal SIRT-1
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals
4.3. Experimental Design
4.4. Behavioral Tests
4.4.1. Passive Avoidance Test
4.4.2. Assessment of Locomotion
4.4.3. Morris Water Maze (MWM) Test
4.5. Samples Collection
4.6. Biochemical Analysis
4.7. Histopathological Examination
4.8. Immunohistochemical Assay of GFAP, NFκB, Caspase 3, Caspase 1 and Synaptophysin
4.9. Transmission Electron Microscopy
4.10. RT-PCR Assessment
4.11. Western Blotting for Cytochrome c
4.12. Enzyme-Linked Immunoassay (ELISA) for BDNF, Trkβ Receptor, Proinflammatory Cytokines (TNFα, IL-1β and IL6), Neurotransmitters (Acetylcholine, Acetylcholinesterase, Serotonin, Dopamine, GABA and Glutamate) and NMDA Receptor
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanaskie, M.L.; Loeb, S.J. The experience of cognitive change in women with breast cancer following chemotherapy. J. Cancer Surviv. Res. 2015, 9, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Kesler, S.R.; Ahles, T.A.; Morrow, G.R. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int. Rev. Psychiatry 2014, 26, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Correa, D.D.; Ahles, T.A. Neurocognitive changes in cancer survivors. Cancer J. 2008, 14, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Jim, H.S.; Phillips, K.M.; Chait, S.; Faul, L.A.; Popa, M.A.; Lee, Y.H.; Hussin, M.G.; Jacobsen, P.B.; Small, B.J. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J. Clin. Oncol. 2012, 30, 3578–3587. [Google Scholar] [CrossRef]
- Whittaker, A.L.; George, R.P.; O’Malley, L. Prevalence of cognitive impairment following chemotherapy treatment for breast cancer: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 2135. [Google Scholar] [CrossRef]
- Christie, L.A.; Acharya, M.M.; Parihar, V.K.; Nguyen, A.; Martirosian, V.; Limoli, C.L. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin. Cancer Res. 2012, 18, 1954–1965. [Google Scholar] [CrossRef]
- Volkova, M.; Russell, R. Anthracycline cardiotoxicity: Prevalence, pathogenesis and treatment. Curr. Cardiol. Rev. 2011, 7, 214–220. [Google Scholar] [CrossRef]
- Gutierrez, P.L. The role of NAD(P)H oxidoreductase (DT-Diaphorase) in the bioactivation of quinone-containing antitumor agents: A review. Free Radic. Biol. Med. 2000, 29, 263–275. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharm. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef]
- Nakagawa, H.; Fujita, T.; Kubo, S.; Tokiyoshi, K.; Yamada, M.; Kanayama, T.; Hagiwara, Y.; Nakanomyo, H.; Hiraoka, M. Difference in CDDP penetration into CSF between selective intraarterial chemotherapy in patients with malignant glioma and intravenous or intracarotid administration in patients with metastatic brain tumor. Cancer Chemother. Pharmacol. 1996, 37, 317–326. [Google Scholar] [CrossRef]
- Sardi, I.; La Marca, G.; Cardellicchio, S.; Giunti, L.; Malvagia, S.; Genitori, L.; Massimino, M.; de Martino, M.; Giovannini, M.G. Pharmacological modulation of blood-brain barrier increases permeability of doxorubicin into the rat brain. Am. J. Cancer Res. 2013, 3, 424–432. [Google Scholar] [PubMed]
- Tangpong, J.; Cole, M.P.; Sultana, R.; Joshi, G.; Estus, S.; Vore, M.; St Clair, W.; Ratanachaiyavong, S.; St Clair, D.K.; Butterfield, D.A. Adriamycin-induced, TNF-alpha-mediated central nervous system toxicity. Neurobiol. Dis. 2006, 23, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Ongnok, B.; Chattipakorn, N.; Chattipakorn, S.C. Doxorubicin and cisplatin-induced cognitive impairment: The possible mechanisms and interventions. Exp. Neurol. 2020, 324, 113118. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.M.; Artavanis-Tsakonas, S.; Louvi, A. The Notch pathway in CNS homeostasis and neurodegeneration. WIREs Dev. Biol. 2020, 9, e358. [Google Scholar] [CrossRef]
- Xu, J.; Jackson, C.W.; Khoury, N.; Escobar, I.; Perez-Pinzon, M.A. Brain SIRT1 Mediates Metabolic Homeostasis and Neuroprotection. Front. Endocrinol. 2018, 9, 702. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, W.; Pan, H.; Feldser, H.G.; Lainez, E.; Miller, C.; Leung, S.; Zhong, Z.; Zhao, H.; Sweitzer, S.; et al. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-κB activity. PLoS ONE 2012, 7, e46364. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Lee, H.Y.; Min, K.J. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019, 52, 24–34. [Google Scholar] [CrossRef]
- Shi, X.; Pi, L.; Zhou, S.; Li, X.; Min, F.; Wang, S.; Liu, Z.; Wu, J. Activation of Sirtuin 1 Attenuates High Glucose-Induced Neuronal Apoptosis by Deacetylating p53. Front. Endocrinol. 2018, 9, 274. [Google Scholar] [CrossRef]
- Michán, S.; Li, Y.; Chou, M.M.; Parrella, E.; Ge, H.; Long, J.M.; Allard, J.S.; Lewis, K.; Miller, M.; Xu, W.; et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J. Neurosci. 2010, 30, 9695–9707. [Google Scholar] [CrossRef]
- Gao, J.; Wang, W.Y.; Mao, Y.W.; Gräff, J.; Guan, J.S.; Pan, L.; Mak, G.; Kim, D.; Su, S.C.; Tsai, L.H. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 2010, 466, 1105–1109. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between oxidative stress and SIRT1: Impact on the aging process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef] [PubMed]
- Dahle, J.T.; Arai, Y. Environmental geochemistry of cerium: Applications and toxicology of cerium oxide nanoparticles. Int. J. Environ. Res. Public Health. 2015, 12, 1253–1278. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Qu, X. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia. Mater. 2014, 6, e90. [Google Scholar] [CrossRef]
- Xu, C.; Lin, Y.; Wang, J.; Wu, L.; Wei, W.; Ren, J.; Qu, X. Nanoceria-triggered synergetic drug release based on CeO2-capped mesoporous silica host–guest interactions and switchable enzymatic activity and cellular effects of CeO2. Adv. Healthc. Mater. 2013, 2, 1591–1599. [Google Scholar] [CrossRef]
- Li, M.; Shi, P.; Xu, C.; Ren, J.S.; Qu, X.G. Cerium oxide caged metal chelator: Anti-aggregation and anti-oxidation integrated H2O2-responsive controlled drug release for potential Alzheimer’s disease treatment. Chem. Sci. 2013, 4, 2536–2542. [Google Scholar] [CrossRef]
- Dowding, J.M.; Seal, S.; Self, W.T. Cerium oxide nanoparticles accelerate the decay of peroxynitrite (ONOO(-)). Drug Deliv. Transl. Res. 2013, 3, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Estevez, A.Y.; Pritchard, S.; Harper, K.; Aston, J.W.; Lynch, A.; Lucky, J.J.; Ludington, J.S.; Chatani, P.; Mosenthal, W.P.; Leiter, J.C.; et al. Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Radic. Biol. Med. 2011, 51, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Heckman, K.L.; DeCoteau, W.; Estevez, A.; Reed, K.J.; Costanzo, W.; Sanford, D.; Leiter, J.C.; Clauss, J.; Knapp, K.; Gomez, C. Custom cerium oxide nanoparticles protect against a free radical mediated autoimmune degenerative disease in the brain. ACS Nano 2013, 7, 10582–10596. [Google Scholar] [CrossRef]
- Sangomla, S.; Saifi, M.A.; Khurana, A.; Godugu, C. Nanoceria ameliorates doxorubicin induced cardiotoxicity: Possible mitigation via reduction of oxidative stress and inflammation. J. Trace Elem. Med. Biol. 2018, 47, 53–62. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Perez, J.M. Surface-chargedependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS Nano 2010, 4, 5321–5331. [Google Scholar] [CrossRef]
- Sack, M.; Alili, L.; Karaman, E.; Das, S.; Gupta, A.; Seal, S.; Brenneisen, P. Combination of conventional chemotherapeutics with redox-active cerium oxide nanoparticles—a novel aspect in cancer therapy. Mol. Cancer Ther. 2014, 13, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Tarnuzzer, R.W.; Colon, J.; Patil, S.; Seal, S. Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano Lett. 2005, 5, 2573–2577. [Google Scholar] [CrossRef] [PubMed]
- Hirst, S.M.; Karakoti, A.S.; Tyler, R.D.; Sriranganathan, N.; Seal, S.; Reilly, C.M. Anti-inflammatory properties of cerium oxide nanoparticles. Small 2009, 5, 2848–2856. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-X.; Zhu, Y.-F.; Chang, H.-F.; Liang, Y. Nanoceria restrains PM2. 5-induced metabolic disorder and hypothalamus inflammation by inhibition of astrocytes activation related NF-κB pathway in Nrf2 deficient mice. Free Radic. Biol. Med. 2016, 99, 259–272. [Google Scholar] [CrossRef]
- Hegazy, M.A.E.; Maklad, H.M.; Elmonsif, D.A.A.; Elnozhy, F.Y.; Alqubiea, M.A.; Alenezi, F.A. The possible role of cerium oxide (CeO2) nanoparticles in prevention of neurobehavioral and neurochemical changes in 6-hydroxydopamine induced parkinsonian disease. Alex. J. Med. 2017, 53, 351–360. [Google Scholar] [CrossRef]
- Saifi, M.A.; Sangomla, S.; Khurana, A.; Godugu, C. Protective effect of nanoceria on cisplatin-induced nephrotoxicity by amelioration of oxidative stress and pro-inflammatory mechanisms. Biol. Trace Elem. Res. 2019, 189, 145–156. [Google Scholar] [CrossRef]
- El-Agamy, S.E.; Abdel-Aziz, A.K.; Wahdan, S.; Esmat, A.; Azab, S.S. Astaxanthin ameliorates doxorubicin-induced cognitive impairment (chemobrain) in experimental rat model: Impact on oxidative, inflammatory, and apoptotic machineries. Mol. Neurobiol. 2018, 55, 5727–5740. [Google Scholar] [CrossRef]
- Kitamura, Y.; Hattori, S.; Yoneda, S.; Watanabe, S.; Kanemoto, E.; Sugimoto, M.; Kawai, T.; Machida, A.; Kanzaki, H.; Miyazaki, I. Doxorubicin and cyclophosphamide treatment produces anxiety like behavior and spatial cognition impairment in rats: Possible involvement of hippocampal neurogenesis via brain-derived neurotrophic factor and cyclin D1 regulation. Behav. Brain Res. 2015, 292, 184–193. [Google Scholar] [CrossRef]
- Jin, J.; Maren, S. Prefrontal-Hippocampal Interactions in Memory and Emotion. Front. Syst. Neurosci. 2015, 9, 170. [Google Scholar] [CrossRef]
- Squire, L.R.; Genzel, L.; Wixted, J.T.; Morris, R.G. Memory consolidation. Cold Spring Harb. Perspect. Biol. 2015, 7, a021766. [Google Scholar] [CrossRef]
- Danish, S.M.; Gupta, A.; Khan, U.A.; Hasan, N.; Ahmad, F.J.; Warsi, M.H.; Ali, A.M.A.; Zafar, A.; Jain, G.K. Intranasal Cerium Oxide Nanoparticles Ameliorate Cognitive Function in Rats with Alzheimer’s via Anti-Oxidative Pathway. Pharmaceutics 2022, 14, 756. [Google Scholar] [CrossRef] [PubMed]
- Aluise, C.D.; Sultana, R.; Tangpong, J.; Vore, M.; St Clair, D.; Moscow, J.A.; Butterfield, D.A. Chemo brain (chemo fog) as a potential side effect of doxorubicin administration: Role of cytokine-induced, oxidative/nitrosative stress in cognitive dysfunction. Adv. Exp. Med. Biol. 2010, 678, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.; Aluise, C.D.; Cole, M.P.; Sultana, R.; Pierce, W.M.; Vore, M.; St Clair, D.K.; Butterfield, D.A. Alterations in brain antioxidant enzymes and redox proteomic identification of oxidized brain proteins induced by the anti-cancer drug adriamycin: Implications for oxidative stress-mediated chemobrain. Neuroscience 2010, 166, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Dudka, J.; Gieroba, R.; Korga, A.; Burdan, F.; Matysiak, W.; Jodlowska- Jedrych, B.; Mandziuk, S.; Korobowicz, E.; Murias, M. Different effects of resveratrol on dose-related Doxorubicin-induced heart and liver toxicity. Evid Based Complement. Altern. Med. 2012, 2012, 606183. [Google Scholar] [CrossRef]
- Joshi, G.; Hardas, S.; Sultana, R.; St Clair, D.K.; Vore, M.; Butterfield, D.A. Glutathione elevation by gamma-glutamyl cysteine ethyl ester as a potential therapeutic strategy for preventing oxidative stress in brain mediated by in vivo administration of adriamycin: Implication for chemobrain. J. Neurosci. Res. 2007, 85, 497–503. [Google Scholar] [CrossRef]
- Valle, I.; Alvarez-Barrientos, A.; Arza, E.; Lamas, S.; Monsalve, M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 2005, 66, 562–573. [Google Scholar] [CrossRef]
- Chen, M.; Samuel, V.P.; Wu, Y.; Dang, M.; Lin, Y.; Sriramaneni, R.; Sah, S.K.; Chinnaboina, G.K.; Zhang, G. Nrf2/HO-1 Mediated Protective Activity of Genistein Against Doxorubicin-Induced Cardiac Toxicity. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 143–152. [Google Scholar] [CrossRef]
- Rzigalinski, B.A.; Carfagna, C.S.; Ehrich, M. Cerium oxide nanoparticles in neuroprotection and considerations for efficacy and safety. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1444. [Google Scholar] [CrossRef]
- Song, K.; Li, Y.; Zhang, H.; An, N.; Wei, Y.; Wang, L.; Tian, C.; Yuan, M.; Sun, Y.; Xiang, Y.; et al. Oxidative stress-mediated blood-brain barrier (BBB) disruption in neurological diseases. Oxid. Med. Cell. Longev. 2020, 2020, 4356386. [Google Scholar] [CrossRef]
- Karakoti, A.; Singh, S.; Dowding, J.M.; Seal, S.; Welf, W.T. Redox active radical scavenging nanomaterials. Chem. Soc. Rev. 2010, 39, 4422–4432. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, C.-H.; Raitano, J.M.; Hanson, J.C.; Caliebe, W.A.; Khalid, S.; Chan, S.-W. Phase stability in ceria-zirconia binary oxide nanoparticles: The effect of the Ce3+ concentration and the redox environment. J. Appl. Phys. 2006, 99, 084313. [Google Scholar] [CrossRef]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Fang, T.; Lu, L.Q.; Yi, L. Neuroprotective potential of cerium oxide nanoparticles for focal cerebral ischemic stroke. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Hasanvand, D.; Amiri, I.; Soleimani, A.S.; Saidijam, M.; Shabab, N.; Artimani, T. Effects of CeO2 nanoparticles on the HO-1, NQO1, and GCLC expression in the testes of diabetic rats. Can. J. Physiol. Pharmacol. 2018, 96, 963–969. [Google Scholar] [CrossRef]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-kappaB activation by reactive oxygen species: Fifteen years later. Biochem Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Nishioku, T.; Matsumoto, J.; Dohgu, S.; Sumi, N.; Miyao, K.; Takata, F.; Shuto, H.; Yamauchi, A.; Kataoka, Y. Tumor necrosis factor-alpha mediates the blood-brain barrier dysfunction induced by activated microglia in mouse brain microvascular endothelial cells. J. Pharm. Sci. 2010, 112, 251–254. [Google Scholar] [CrossRef]
- Kimelberg, H.K.; Nedergaard, M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics 2010, 7, 338–353. [Google Scholar] [CrossRef]
- Baune, B.T.; Camara, M.-L.; Eyre, H.; Jawahar, C.; Anscomb, H.; Körner, H. Tumour necrosis factor—Alpha mediated mechanisms of cognitive dysfunction. Transl. Neurosci. 2012, 3, 263–277. [Google Scholar] [CrossRef]
- Katsuno, M.; Adachi, H.; Banno, H.; Suzuki, K.; Tanaka, F.; Sobue, G. Transforming growth factor-β signaling in motor neuron diseases. Curr. Mol. Med. 2011, 11, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Preller, V.; Gerber, A.; Wrenger, S.; Togni, M.; Marguet, D.; Tadje, J.; Lendeckel, U.; Rocken, C.; Faust, J.; Neubert, K.; et al. TGF beta1-mediated control of central nervous system inflammation and autoimmunity through the inhibitory receptor CD26. J. Immunol. 2007, 178, 4632–4640. [Google Scholar] [CrossRef] [PubMed]
- Stipursky, J.; Francis, D.; Dezonne, R.S.; Bérgamo de Araújo, A.P.; Souza, L.; Moraes, C.A.; Alcantara Gomes, F.C. TGF-β1 promotes cerebral cortex radial glia-astrocyte differentiation in vivo. Front. Cell Neurosci. 2014, 8, 393, Erratum in: Front. Cell Neurosci. 2015, 9, 232.. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Y.; Sun, H.; Yin, Z.; Guo, X.; Yan, J. Anti-Inflammatory Effects of Cerium Dioxide Nanoparticles on Peritonitis in Rats Induced by Staphylococcus epidermidis Infection. Adv. Polym. Technol. 2020, 2020, 3591508. [Google Scholar] [CrossRef]
- Elshony, N.; Nassar, A.M.K.; El-Sayed, Y.S.; Samak, D.; Noreldin, A.; Wasef, L.; Saleh, H.; Elewa, Y.H.A.; Tawfeek, S.E.; Saati, A.A.; et al. Ameliorative Role of Cerium Oxide Nanoparticles Against Fipronil Impact on Brain Function, Oxidative Stress, and Apoptotic Cascades in Albino Rats. Front. Neurosci. 2021, 15, 651471. [Google Scholar] [CrossRef] [PubMed]
- Voet, S.; Srinivasan, S.; Lamkanfi, M.; van Loo, G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol. Med. 2019, 11, e10248. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2020, 140, 821–832. [Google Scholar] [CrossRef]
- Wei, S.; Ma, W.; Li, X.; Jiang, C.; Sun, T.; Li, Y.; Zhang, B.; Li, W. Involvement of ROS/NLRP3 Inflammasome Signaling Pathway in Doxorubicin-Induced Cardiotoxicity. Cardiovasc. Toxicol. 2020, 20, 507–519. [Google Scholar] [CrossRef]
- Javadov, S.; Kuznetsov, A. Mitochondrial permeability transition and cell death: The role of cyclophilin d. Front. Physiol. 2013, 4, 76. [Google Scholar] [CrossRef]
- Bogner, C.; Leber, B.; Andrews, D.W. Apoptosis: Embedded in membranes. Curr. Opin. Cell Biol. 2010, 22, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, C.J.; Kwak, H.B.; No, M.H.; Heo, J.W.; Kim, T.W. Physical exercise prevents cognitive impairment by enhancing hippocampal neuroplasticity and mitochondrial function in doxorubicin-induced chemobrain. Neuropharmacology 2018, 133, 451–461. [Google Scholar] [CrossRef]
- Solgi, T.; Amiri, I.; Asl, S.S.; Saidijam, M.; Seresht, B.M.; Artimani, T. Antiapoptotic and antioxidative effects of cerium oxide nanoparticles on the testicular tissues of streptozotocin-induced diabetic rats: An experimental study. Int. J. Reprod. Biomed. 2021, 19, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.; Afonso, P.M.; Salazar, I.L.; Duarte, C.B. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res. 2015, 1621, 82–101. [Google Scholar] [CrossRef] [PubMed]
- Alhowail, A.H.; Bloemer, J.; Majrashi, M.; Pinky, P.D.; Bhattacharya, S.; Yongli, Z.; Bhattacharya, D.; Eggert, M.; Woodie, L.; Buabeid, M.A.; et al. Doxorubicin-induced neurotoxicity is associated with acute alterations in synaptic plasticity, apoptosis, and lipid peroxidation. Toxicol. Mech. Methods. 2019, 29, 457–466. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, B.; Santucci, S.; Benedetti, E.; Di Loreto, S.; Phani, R.; Falone, S.; Amicarelli, F.; Ceru, M.; Cimini, A. Cerium Oxide Nanoparticles Trigger Neuronal Survival in a Human Alzheimer Disease Model By Modulating BDNF Pathway. Curr. Nanosci. 2009, 5, 167–176. [Google Scholar] [CrossRef]
- Du, J.; Zhang, A.; Li, J.; Liu, X.; Wu, S.; Wang, B.; Wang, Y.; Jia, H. Doxorubicin-Induced Cognitive Impairment: The Mechanistic Insights. Front. Oncol. 2021, 11, 673340. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef]
- Kwatra, M.; Jangra, A.; Mishra, M.; Sharma, Y.; Ahmed, S.; Ghosh, P.; Kumar, V.; Vohora, D.; Khanam, R. Naringin and Sertraline Ameliorate Doxorubicin-Induced Behavioral Deficits Through Modulation of Serotonin Level and Mitochondrial Complexes Protection Pathway in Rat Hippocampus. Neurochem. Res. 2016, 41, 2352–2366. [Google Scholar] [CrossRef]
- Fernandez, S.P.; Muzerelle, A.; Scotto-Lomassese, S.; Barik, J.; Gruart, A.; Delgado-García, J.M.; Gaspar, P. Constitutive and Acquired Serotonin Deficiency Alters Memory and Hippocampal Synaptic Plasticity. Neuropsychopharmacology 2017, 42, 512–523. [Google Scholar] [CrossRef]
- Bethus, I.; Tse, D.; Morris, R.G. Dopamine and Memory: Modulation of the Persistence of Memory for Novel Hippocampal NMDA Receptor-Dependent Paired Associates. J. Neurosci. 2010, 30, 1610–1618. [Google Scholar] [CrossRef]
- Thomas, T.C.; Beitchman, J.A.; Pomerleau, F.; Noel, T.; Jungsuwadee, P.; Butterfield, D.A.; Clair, D.K.S.; Vore, M.; Gerhardt, G.A. Acute Treatment With Doxorubicin Affects Glutamate Neurotransmission in the Mouse Frontal Cortex and Hippocampus. Brain Res. 2017, 1672, 10–17. [Google Scholar] [CrossRef]
- Habbas, S.; Santello, M.; Becker, D.; Stubbe, H.; Zappia, G.; Liaudet, N.; Klaus, F.R.; Kollias, G.; Fontana, A.; Pryce, C.R.; et al. Neuroinflammatory TNFα Impairs Memory via Astrocyte Signaling. Cell 2015, 163, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Miller, A.H.; Sanacora, G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology 2017, 42, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.X.; Xue, S.F.; Deng, J.; Zhang, M.; Shi, G.; Zhou, T. Polyacrylic acid-coated cerium oxide nanoparticles: An oxidase mimic applied for colorimetric assay to organophosphorus pesticides. Biosens. Bioelectron. 2016, 85, 457–463. [Google Scholar] [CrossRef]
- Disdier, C.; Chalansonnet, M.; Gagnaire, F.; Gaté, L.; Cosnier, F.; Devoy, J.; Saba, W.; Lund, A.K.; Brun, E.; Mabondzo, A. Brain inflammation, blood brain barrier dysfunction and neuronal synaptophysin decrease after inhalation exposure to titanium dioxide nanoaerosol in aging rats. Sci. Rep. 2017, 7, 12196. [Google Scholar] [CrossRef] [PubMed]
- El-Agamy, S.E.; Abdel-Aziz, A.K.; Esmat, A.; Azab, S.S. Chemotherapy and Cognition: Comprehensive Review on Doxorubicin-Induced Chemobrain. Cancer Chemother. Pharm. 2019, 84, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.Y.; Zhang, Y.; Coughlin, B.L.; Cleary, L.J.; Byrne, J.H. Doxorubicin Attenuates Serotonin-Induced Long-Term Synaptic Facilitation by Phosphorylation of p38 Mitogen-Activated Protein Kinase. J. Neurosci. 2014, 34, 13289–13300. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, C.; Xiong, L.; Xie, J.; Huang, C.; Pi, R.; Huang, Z.; Li, L. Icaritin Alleviates Glutamate- Induced Neuronal Damage by Inactivating Glun2b-Containing NMDARs Through the ERK/DAPK1 Pathway. Front. Neurosci. 2021, 15, 525615. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Nepal, N.; Rogers, S.; Manne, N.D.; Arvapalli, R.; Rice, K.M.; Asano, S.; Fankenhanel, E.; Ma, J.Y.; Shokuhfar, T.; et al. Lipopolysaccharide-induced MAP kinase activation in RAW 264.7 cells attenuated by cerium oxide nanoparticles. Data Brief. 2015, 4, 96–99. [Google Scholar] [CrossRef]
- Ruan, Y.; Dong, C.; Patel, J.; Duan, C.; Wang, X.; Wu, X.; Cao, Y.; Pu, L.; Lu, D.; Shen, T.; et al. SIRT1 suppresses doxorubicin-induced cardiotoxicity by regulating the oxidative stress and p38MAPK pathways. Cell Physiol. Biochem. 2015, 35, 1116–1124. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, H.; Zhang, B.; Zhang, E.; Wu, Y. Tumor Necrosis Factor-alpha (TNF-α) Enhances miR-155-Mediated Endothelial Senescence by Targeting Sirtuin1 (SIRT1). Med. Sci. Monit. 2019, 25, 8820–8835. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X.; Chen, W.; Bo, N.; Wang, X.; Chi, Z.; Wu, W. Sirtuin 1 activation enhances the PGC-1α/mitochondrial antioxidant system pathway in status epilepticus. Mol. Med. Rep. 2015, 11, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; He, H.; Zuo, Z.; Xu, Z.; Wei, Z.; Deng, J. The role of different SIRT1-mediated signaling pathways in toxic injury. Cell Mol. Biol. Lett. 2019, 24, 36. [Google Scholar] [CrossRef]
- Shen, J.; Xu, L.; Qu, C.; Sun, H.; Zhang, J. Resveratrol prevents cognitive deficits induced by chronic unpredictable mild stress: Sirt1/miR-134 signaling pathway regulates CREB/BDNF expression in hippocampus in vivo and in vitro. Behav. Brain Res. 2018, 349, 1–7. [Google Scholar] [CrossRef]
- Yang, X.; Si, P.; Qin, H.; Yin, L.; Yan, L.J.; Zhang, C. The Neuroprotective Effects of SIRT1 on NMDA-Induced Excitotoxicity. Oxidative Med. Cell Longev. 2017, 2017, 2823454. [Google Scholar] [CrossRef]
- Ibrahim, H.G.; Attia, N.; Fatma El Zahraa, A.H.; El Heneidy, M.A. Cerium oxide nanoparticles: In pursuit of liver protection against doxorubicin-induced injury in rats. Biomed. Pharmacother. 2018, 103, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Khaksar, M.R.; Rahimifard, M.; Baeeri, M.; Maqbool, F.; Navaei-Nigjeh, M.; Hassani, S.; Moeini-Nodeh, S.; Kebriaeezadeh, A.; Abdollahi, M. Protective effects of cerium oxide and yttrium oxide nanoparticles on reduction of oxidative stress induced by sub-acute exposure to diazinon in the rat pancreas. J. Trace Elem. Med. Biol. 2017, 41, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, A.K.; Mantawy, E.M.; Said, R.S.; Helwa, R. The tyrosine kinase inhibitor, sunitinib malate, induces cognitive impairment in vivo via dysregulating VEGFR signaling, apoptotic and autophagic machineries. Exp. Neurol. 2016, 283, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Kim, J.; Shim, J.; Kim, C.Y.; Jang, J.H.; Lee, K.W.; Lee, H.J. Decaffeinated coffee prevents scopolamine-induced memory impairment in rats. Behav. Brain Res. 2013, 245, 113–119. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Churchill, Livingstone/Elsevier: London, UK, 2008. [Google Scholar]
- Habib, R.; Wahdan, S.A.; Gad, A.M.; Azab, S.S. Infliximab abrogates cadmium-induced testicular damage and spermiotoxicity via enhancement of steroidogenesis and suppression of inflammation and apoptosis mediators. Ecotoxicol. Environ. Saf. 2019, 182, 109398. [Google Scholar] [CrossRef]
- Salem, M.; Altayeb, Z. Light and Electron Microscopic Study on the Possible Protective Effect of Nigella Sativa Oil on Cisplatin Hepatotoxicity in Albino Rats. Egypt J. Histol. 2017, 40, 68–79. [Google Scholar] [CrossRef]
- Gallaghar, S.R.; Smith, J.A. Current Protocols in Molecular Biology; Ausubel, M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., Struhl, K., Eds.; John Wiley & Sons, Inc.: Media, PA, USA, 1988; Volume 1–2, p. 146. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]






| Control Group | CeNPs Group | DOX Group | DOX + CeNPs Group | |
|---|---|---|---|---|
| GSH (μmol/g tissue) | 1.49 ± 0.07 | 1.67 ± 0.03 | 0.51 ± 0.04 *** | 1.29 ± 0.34 ### |
| SOD (U/g tissue) | 158 ± 9.20 | 183 ± 6.30 ** | 75 ± 5.50 *** | 124 ± 5.40 ***, ### |
| MDA (nmol/g tissue) | 7.4 ± 0.51 | 6.7 ± 0.42 | 21.4 ± 0.58 *** | 13.4 ± 0.46 ***, ### |
| CAT (U/g tissue) | 2.7 ± 0.27 | 3.3 ± 0.20 ** | 1.2 ± 0.15 *** | 1.9 ± 0.16 ***, ## |
| Forward Sequence | Reverse Sequence | Gene Accession Number | |
|---|---|---|---|
| NLRP3 | GTGGAGATCCTAGGTTTCTCTG | CAGGATCTCATTCTCTTGGATC | NM_001191642.1 |
| P38 MAPK | CACAGCACCTCAGCAATGAT | AGGCCTATCTTCCCAGGAAA | NM_053842.2 |
| ERK1 | TCAAGCCTTCCAACCTC | GCAGCCCACAGACCAAA | XM_046421134.1 |
| Nrf2 | AGGACATGGAGCAAGTTTGG | TTGCCCTAAGCTCATCTCGT | NM_031789.2 |
| HO-1 | TCAGGTGTCCAGAGAAGGCTTT | CTCTTCCAGGGCCGTGTAGA | NM_012580.2 |
| SIRT-1 | GACGACGAGGGCGAGGAG | ACAGGAGGTTGTCTCGGTAGC | XM_006223877.1 |
| TGF-β1 | GACTCTCCACCTGCAAGACC | GGACTGGCGAGCCTTAGTTT | NM_021578.2 |
| PGC1-α | ATCCTCTTCAAGATCCTGTTACT | CGTGCTCATTGGCTTCATAG | XM_032916070.1 |
| GAPDH | CCTTCTCCATGGTGGTGAAGA | CACCATCTTCCAGGAGCGAG | NM_001394060.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, M.; Elazab, S.T.; Badawy, A.M.; Saati, A.A.; Qusty, N.F.; Al-Kushi, A.G.; Sarhan, A.; Osman, A.; Farage, A.E. Activation of SIRT-1 Pathway by Nanoceria Sheds Light on Its Ameliorative Effect on Doxorubicin-Induced Cognitive Impairment (Chemobrain): Restraining Its Neuroinflammation, Synaptic Dysplasticity and Apoptosis. Pharmaceuticals 2022, 15, 918. https://doi.org/10.3390/ph15080918
Taha M, Elazab ST, Badawy AM, Saati AA, Qusty NF, Al-Kushi AG, Sarhan A, Osman A, Farage AE. Activation of SIRT-1 Pathway by Nanoceria Sheds Light on Its Ameliorative Effect on Doxorubicin-Induced Cognitive Impairment (Chemobrain): Restraining Its Neuroinflammation, Synaptic Dysplasticity and Apoptosis. Pharmaceuticals. 2022; 15(8):918. https://doi.org/10.3390/ph15080918
Chicago/Turabian StyleTaha, Medhat, Sara T. Elazab, Alaa. M. Badawy, Abdullah A. Saati, Naeem F. Qusty, Abdullah G. Al-Kushi, Anas Sarhan, Amira Osman, and Amira E. Farage. 2022. "Activation of SIRT-1 Pathway by Nanoceria Sheds Light on Its Ameliorative Effect on Doxorubicin-Induced Cognitive Impairment (Chemobrain): Restraining Its Neuroinflammation, Synaptic Dysplasticity and Apoptosis" Pharmaceuticals 15, no. 8: 918. https://doi.org/10.3390/ph15080918
APA StyleTaha, M., Elazab, S. T., Badawy, A. M., Saati, A. A., Qusty, N. F., Al-Kushi, A. G., Sarhan, A., Osman, A., & Farage, A. E. (2022). Activation of SIRT-1 Pathway by Nanoceria Sheds Light on Its Ameliorative Effect on Doxorubicin-Induced Cognitive Impairment (Chemobrain): Restraining Its Neuroinflammation, Synaptic Dysplasticity and Apoptosis. Pharmaceuticals, 15(8), 918. https://doi.org/10.3390/ph15080918








