Potential Pharmaceutical Applications of Quercetin in Cardiovascular Diseases
Abstract
:1. Introduction
2. Literature Search Strategy
3. Results and Discussion
3.1. Chemical Structure of Quercetin and Its Main Derivatives
3.2. Quercetin’s Metabolic Pathways
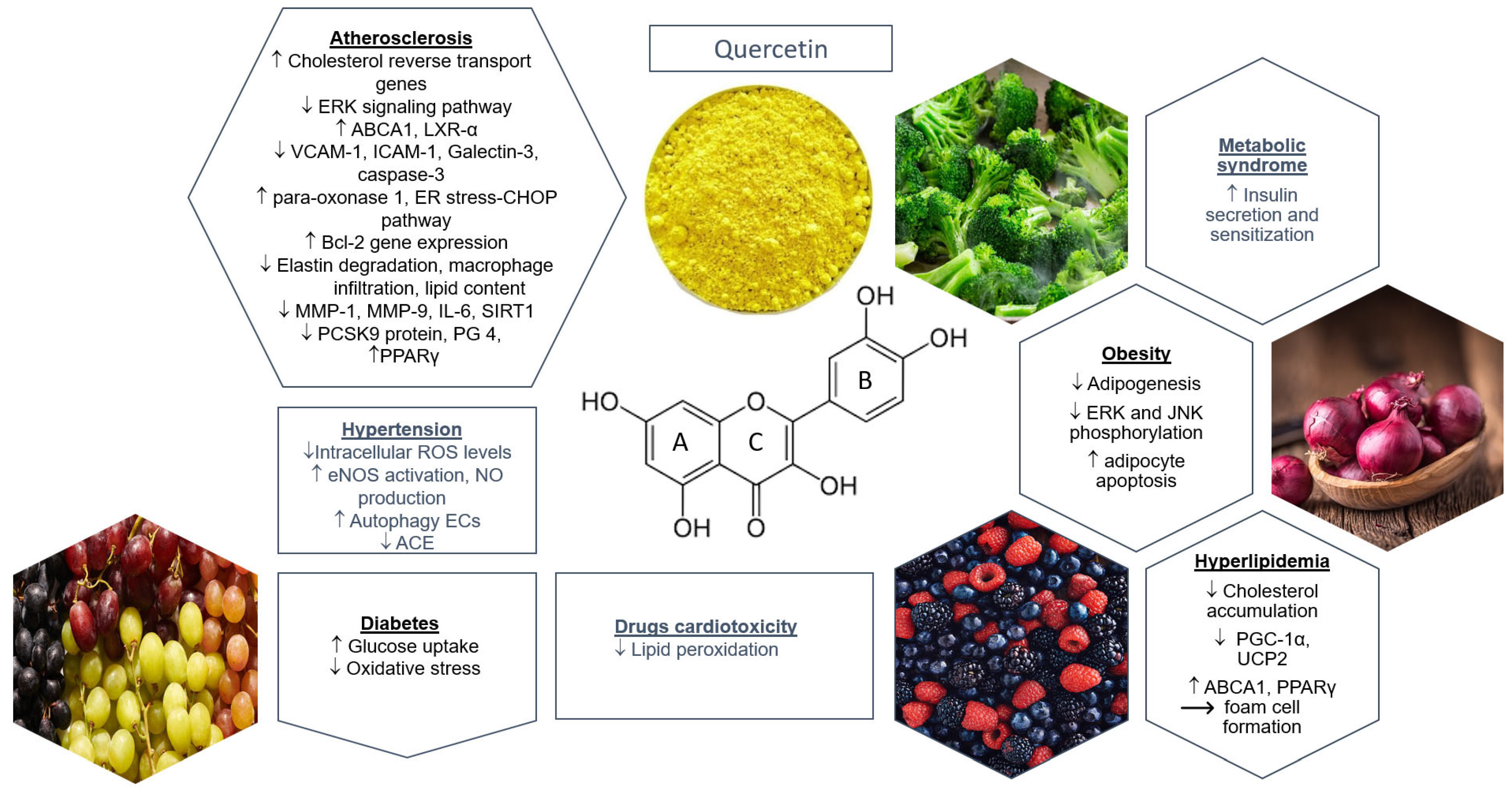
3.3. Quercetin and Cardiovascular Prevention Based on Preclinical Studies
3.3.1. Hypertension
3.3.2. Diabetes
3.3.3. Hyperlipidemia
3.3.4. Quercetin and Cardiac Protection: Cardiomyopathies, Cardiotoxicity, Myocardial Perfusion Injury, Myocardial Infarction
Cardiac Injury
Drug-Induced Cardiotoxicity
Metabolic Syndrome (MS)
Atherosclerosis
Obesity
3.4. Quercetin and Cardiovascular Prevention Based on Clinical Studies
3.4.1. Metabolic Syndrome (MS)
3.4.2. Obesity
3.4.3. Hypertension
3.4.4. Dyslipidemia
3.4.5. Hyperglycemia
3.4.6. Quercetin and CVDs
| Reference | Studies and Cohorts | Study Design | Outcomes |
|---|---|---|---|
| Huang et al., 2019 [130] | Nine RCTs, 525 pts; obese, HTN, PCOS, healthy individuals | Quercetin daily; dose: 100–1000 mg; duration: 2–12 weeks | ↔ body weight, BMI, waist circumference, waist-to-hip ratio |
| Huang et al., 2020 [125] | 17 RCTs, 896 pts; MS, T2DM, PCOS, obesity | Quercetin daily; dose: 30–1000 mg; duration: 2–12 weeks | ↓ SBP and DBP ↔ lipid and glucose profile Subgroup analysis (> 8 weeks after intervention): ↑ HDL, ↓ TG |
| Menezes et al., 2017 [126] | 18 RCTs, 530 pts; healthy individuals, MS, HTN | Flavonol daily; dose: 16–1200 mg; duration: 2–12 weeks | ↓ BP, FPG, LDL, Tchol, triacylglycerol ↑ HDL Subgroup analysis: favorable in dyslipidemic pts and pts of Asian origin |
| Ostadmohammadi et al., 2019 [150] | Nine RCTs, 781 pts; MS | Quercetin daily; dose: 150–1000 mg; duration 4–12 weeks | ↔ FPG, HbA1c, insulin resistance |
| Sahebkar et al., 2017 [144] | Five RCTs, 442 pts; central obesity, hypertriglyceridemia, T2DM, HTN | Quercetin daily; dose: 30–730 mg; duration: 2–10 weeks | ↓ TG (at doses > 400 mg/day) ↔ Tchol, LDL, HDL |
| Serban et al., 2016 [133] | Seven RCTs, 587 pts; HTN | Quercetin daily; dose: 100–1000 mg; duration: 4–10 weeks | ↓ SBP and DBP |
| Tabrizi et al., 2020 [147] | 16 RCTs, 1575 pts; MS | Quercetin daily; dose: 3.12–3000 mg; duration: 2 h postprandially for 12 weeks | ↓ Tchol, LDL, CRP ↔ TG, HDL, IL-6, TNF-α |
| Reference | Study Cohort/and Condition | Study Design | Outcomes |
|---|---|---|---|
| Biesinger et al., 2016 [138] | 18 pts; MS | Crossover RCT; quercetin dehydrate daily vs. placebo; dose: 25 mg; duration: 28 days; washout period: 2 weeks | ↔ HTN incidence |
| Brull et al., 2015 [141] | 68 pts; MS, overweight or obese, prehypertension and stage I HTN | RCT, crossover study; quercetin from onion skin extract daily vs. placebo; dose: 162 mg; duration: 6 wks; washout period: 6 weeks | ↔ HTN incidence, endothelin-1, ADMA, ACE activity, CRP, endothelin-1, sE-selectin, sVCAM-1, sICAM-1, RHI, AI |
| Burak et al., 2019 [143] | 67 healthy nonobese volunteers aged 19–35 years | Randomized double-blind placebo-controlled crossover study; 3.6 g/day ALA plus 190 mg/day quercetin vs. placebo; duration: 8 wks; washout period: 8 weeks | ↔ office systolic BP, mean 24-hour ambulatory BP, mean ambulatory BP, HDL, apolipoprotein A1, glucose, uric acid, oxidized LDL, CRP |
| Chekalina et al., 2018 [155] | 85 pts; CAD | RCT; intervention group (30 pts): quercetin, 120 mg daily; control group (55 pts): placebo; duration: 2 mo | ↓ IL-1b, TNF-α, expression of the IkBa gene in blood mononuclear cells |
| Conquer et al., 1998 [9] | 27 pts; healthy | RCT; intervention group (13 pts): quercetin, 1000 mg daily | ↔ HTN incidence, Tchol, LDL, HDL, TG, platelet aggregation, platelet thromboxane B2 production, resting heart rate |
| Edwards et al., 2007 [139] | 41 pts; prehypertension and stage I HTN | Crossover RCT; quercetin aglycone twice/day vs. placebo; dose: 365 mg; duration: 4 weeks; washout period: 2 weeks | ↔ HTN incidence, weight, BMI, indices of oxidative stress, TG, LDL, VLDL, HDL, Tchol, FPG Prehypertensive subjects: ↓ SBP, DBP, MAP |
| Egert et al., 2009 [137] | 93 pts; overweight or obese pts, MS | Double-blind placebo-controlled crossover study; quercetin daily vs. placebo; dose: 150 mg; duration: 6 weeks; washout period: 5 weeks | ↓ HDL, oxidized LDL ↔ incidence of HTN, Tchol, TG, LDL:HDL cholesterol, TG:HDL cholesterol ratios, TNF-α, CRP |
| Egert et al., 2010 [132] | 93 overweight/obese pts; MS, APOE3/3, 3/4, 4/4, 2/3, 2/4 | Double-blind placebo-controlled crossover study; quercetin daily vs. placebo; dose: 150 mg; duration: 6 weeks; washout period: 5 weeks | ↓ oxidized LDL, TNF-α ↔ CRP, body weight, waist circumference, fat mass, fat-free mass APOE3/3 group: ↓ SBP APOE4 group: ↓ HDL |
| Jin et al., 2021 [127] | 6417 subjects | Observational prospective cohort study; dietary intake flavonoids (quercetin + magnesium) | ↓ incidence of MS |
| Knekt et al., 1996 [156] | 5133 healthy adults aged 30–69 years | Cohort study; dietary intake of flavonoids | ↓ incidence of CAD |
| Larson et al., 2012 [142] | 17 men; normotensive and stage I HTN | Double-blind placebo-controlled crossover study; quercetin aglycone (dose: 1095 mg) vs. placebo; duration: acute single dose | Normotensive: ↔ BP, ACE activity Stage I HTN: ↓ SBP, DBP, mean BP ↔ ACE activity, NO metabolites, ET-1, ET-1:NO ratio metabolites |
| Lu et al., 2015 [148] | 24 healthy subjects; mild hypercholesterolemia | Pilot RCT; intervention group (12 pts): 100 ml quercetin-rich onion juice daily; control group (12 pts): placebo; duration: 8 weeks | ↓ waist circumference, Tchol, LDL |
| Mazza et al., 2021 [146] | 96 pts; dyslipidemia, HTN, statin intolerance | RCT; intervention group (48 pts): ezetimibe/quercetin, 10/100 mg daily; control group (48 pts): ezetimibe monotherapy; duration: 3 mo | ↓ TG, LDL |
| Nishimura et al., 2020 [149] | 70 healthy subjects | RCT; intervention group (35 pts): 9 g quercetin-rich onion powder daily; control group (35 pts): placebo; duration: 12 weeks | ↓ ALT ↔ visceral fat area, BP |
| Pfeuffer et al., 2013 [131] | 49 healthy men; APOE3/3, 3/4, 4/4 | Double-blind crossover study; quercetin daily vs. placebo; dose: 150 mg; washout period: 3 weeks | ↔ endothelial function APOE3/3 group: ↓ BMI, body weight, waist circumference |
| Sales et al., 2014 [152] | 15 pts; T2DM | Pilot study; intervention: capsules containing 200 mg dried leaves of E. punicifolia; duration: 3 mo | ↓ HbA1c, basal insulin, thyroid-stimulating hormone, CRP, SBP, DBP |
| Shatylo et al., 2021 [128] | 110 pts aged >60 years; MS | RCT; intervention group (55 pts): 240 mg quercetin daily; control group (55 pts): placebo; duration: 3 mo | ↓ SBP and DBP, body weight, BMI, Tchol, LDL, insulin, 2-hour glucose level ↔ HDL, TG, oxidative stress (CAT, SOD, AGEs) |
| Song et al., 2005 [151] | 38,018 healthy women aged >45 years | Cross-sectional study; dietary intake of flavonoids | ↔ incidence of T2DM |
| Yao et al., 2021 [136] | 15,662 subjects | Prospective cohort study; dietary intake of quercetin daily | ↔ HTN incidence |
| Zahedi et al., 2013 [140] | 62 women aged 35–55 years; T2DM | RCT; intervention group (34 pts): quercetin, 500 mg daily; control group (28 pts): placebo; duration: 10 wks | ↓ SBP, HDL ↔ HTN incidence, DBP, Tchol, LDL, TG, ratio of TG/HDL and LDL/HDL, TNF-α, IL-6, hs-CRP |
4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Hao, K.; Yu, F.; Shen, L.; Wang, F.; Yang, J.; Su, C. Field application of nanoliposomes delivered quercetin by inhibiting specific hsp70 gene expression against plant virus disease. J. Nanobiotechnol. 2022, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. Quercetin derivatives: Drug design, development, and biological activities, a review. Eur. J. Med. Chem. 2022, 229, 114068. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 162750. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef]
- Rusznyák, S.; Szent-Györgyi, A. Vitamin P: Flavonols as Vitamins. Nature 1936, 138, 27. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Chen, K.T.J.; Anantha, M.; Leung, A.W.Y.; Kulkarni, J.A.; Militao, G.G.C.; Wehbe, M.; Sutherland, B.; Cullis, P.R.; Bally, M.B. Characterization of a liposomal copper (II)-quercetin formulation suitable for parenteral use. Drug Deliv. Transl. Res. 2020, 10, 202–215. [Google Scholar] [CrossRef]
- Conquer, J.A.; Maiani, G.; Azzini, E.; Raguzzini, A.; Holub, B.J. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J. Nutr. 1998, 128, 593–597. [Google Scholar] [CrossRef]
- Janssen, K.; Mensink, R.P.; Cox, F.J.; Harryvan, J.L.; Hovenier, R.; Hollman, P.C.; Katan, M.B. Effects of the flavonoids quercetin and apigenin on hemostasis in healthy volunteers: Results from an in vitro and a dietary supplement study. Am. J. Clin. Nutr. 1998, 67, 255–262. [Google Scholar] [CrossRef]
- Nam, J.S.; Sharma, A.R.; Nguyen, L.T.; Chakraborty, C.; Sharma, G.; Lee, S.S. Application of Bioactive Quercetin in Oncotherapy: From Nutrition to Nanomedicine. Molecules 2016, 21, 108. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 29, e47. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Li, X.X.; Fang, Y.; Chen, X.; Xue, J. Therapeutic Potential of Quercetin as an Antiatherosclerotic Agent in Atherosclerotic Cardiovascular Disease: A Review. Evid Based Complement Altern. Med. 2020, 5926381. [Google Scholar] [CrossRef]
- Hai, Y.; Zhang, Y.; Liang, Y.; Ma, X.; Qi, X.; Xiao, J.; Xue, W.; Luo, Y.; Yue, T. Advance on the absorption, metabolism, and efficacy exertion of quercetin and its important derivatives. Food Front. 2020, 1, 420–434. [Google Scholar] [CrossRef]
- Khan, F.; Niaz, K.; Maqbool, F.; Ismail Hassan, F.; Abdollahi, M.; Nagulapalli Venkata, K.C.; Nabavi, S.M.; Bishayee, A. Molecular Targets Underlying the Anticancer Effects of Quercetin: An Update. Nutrients 2016, 8, 529. [Google Scholar] [CrossRef]
- Chen, X.; Yin, O.Q.P.; Zuo, Z.; Chow, M.S.S. Pharmacokinetics and Modeling of Quercetin and Metabolites. Pharm. Res. 2005, 22, 892–901. [Google Scholar] [CrossRef]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef]
- Van der Woude, H.; Boersma, M.G.; Vervoort, J.; Rietjens, I.M. Identification of 14 quercetin phase II mono- and mixed conjugates and their formation by rat and human phase II in vitro model systems. Chem. Res. Toxicol. 2004, 17, 1520–1530. [Google Scholar] [CrossRef]
- Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. [Google Scholar] [CrossRef]
- Morand, C.; Crespy, V.; Manach, C.; Besson, C.; Demigné, C.; Rémésy, C. Plasma metabolites of quercetin and their antioxidant properties. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1998, 275, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.L.; Xiao, N.; Li, X.W.; Fan, Y.; Alolga, R.N.; Sun, X.Y.; Wang, S.L.; Li, P.; Qi, L.W. Pharmacokinetic comparison between quercetin and quercetin 3-O-β-glucuronide in rats by UHPLC-MS/MS. Sci. Rep. 2016, 6, 35460. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, S.J.; Cho, J.; Gharbi, A.; Han, H.D.; Kang, T.H.; Lee, Y.; Park, W.-S.; Jung, I.-D.; Park, Y.-M. Tamarixetin Exhibits Anti-inflammatory Activity and Prevents Bacterial Sepsis by Increasing IL-10 Production. J. Nat. Prod. 2018, 81, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.; Sesink, A.L.; Faassen-Peters, M.; Hollman, P.C. The type of sugar moiety is a major determinant of the small intestinal uptake and subsequent biliary excretion of dietary quercetin glycosides. Br. J. Nutr. 2004, 91, 841–847. [Google Scholar] [CrossRef]
- Wong, C.C.; Akiyama, Y.; Abe, T.; Lippiat, J.D.; Orfila, C.; Williamson, G. Carrier-mediated transport of quercetin conjugates: Involvement of organic anion transporters and organic anion transporting polypeptides. Biochem. Pharmacol. 2012, 84, 564–570. [Google Scholar] [CrossRef]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Sudano, I.; Salvetti, A. Endothelial Dysfunction in Hypertension. J. Cardiovasc. Pharmacol. 2001, 38, S11–S14. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Ling, W.; Murugan, D.; Lau, Y.; Vanhoutte, P.M.; Mustafa, M.R. Sodium nitrite exerts an antihypertensive effect and improves endothelial function through activation of eNOS in the SHR. Sci. Rep. 2016, 6, 33048. [Google Scholar] [CrossRef]
- Lin, X.; Han, T.; Fan, Y.; Wu, S.; Wang, F.; Wang, C. Quercetin improves vascular endothelial function through promotion of autophagy in hypertensive rats. Life Sci. 2020, 258, 118106. [Google Scholar] [CrossRef]
- Shen, Y.; Croft, K.D.; Hodgson, J.M.; Kyle, R.; Lee, I.L.; Wang, Y.; Stocker, R.; Ward, N.C. Quercetin and its metabolites improve vessel function by inducing eNOS activity via phosphorylation of AMPK. Biochem. Pharmacol. 2012, 84, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.C.; Parente, J.M.; Belo, V.A.; Mendes, A.S.; Gonzaga, N.A.; do Vale, G.T.; Ceron, C.S.; Tanus-Santos, J.E.; Tirapelli, C.R.; Castro, M.M. Quercetin decreases the activity of matrix metalloproteinase-2 and ameliorates vascular remodeling in renovascular hypertension. Atherosclerosis 2018, 270, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Hasima, N.; Ozpolat, B. Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cancer. Cell Death Dis. 2014, 5, e1509. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, R.; Li, J.; Mao, J.; Lei, Y.; Wu, J.; Zeng, J.; Zhang, T.; Wu, H.; Chen, L.; et al. Quercetin induces protective autophagy in gastric cancer cells: Involvement of Akt-mTOR- and hypoxia-induced factor 1α-mediated signaling. Autophagy 2011, 7, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, W.; Yang, Z.Y.; Zhou, X.S.; Gong, C.; Zhang, T.R.; Wei, X.; Ma, D.; Ye, F.; Gao, Q.L. Quercetin induces protective autophagy and apoptosis through ER stress via the p-STAT3/Bcl-2 axis in ovarian cancer. Apoptosis 2017, 22, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Jofré, I.; Pezoa, C.; Cuevas, M.; Scheuermann, E.; Freires, I.A.; Rosalen, P.L.; de Alencar, S.M.; Romero, F. Antioxidant and Vasodilator Activity of Ugni molinae Turcz. (Murtilla) and Its Modulatory Mechanism in Hypotensive Response. Oxid. Med. Cell. Longev. 2016, 6513416. [Google Scholar] [CrossRef]
- Carullo, G.; Ahmed, A.; Trezza, A.; Spiga, O.; Brizzi, A.; Saponara, S.; Fusi, F.; Aiello, F. Design, synthesis and pharmacological evaluation of ester-based quercetin derivatives as selective vascular KCa1.1 channel stimulators. Bioorgan. Chem. 2020, 105, 104404. [Google Scholar] [CrossRef]
- Elbarbry, F.; Abdelkawy, K.; Moshirian, N.; Abdel-Megied, A.M. The Antihypertensive Effect of Quercetin in Young Spontaneously Hypertensive Rats; Role of Arachidonic Acid Metabolism. Int. J. Mol. Sci. 2020, 21, 6554. [Google Scholar] [CrossRef]
- Duarte, J.; Perez-Palencia, R.; Vargas, F.; Ocete, M.A.; Perez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br. J. Pharmacol. 2001, 133, 117–124. [Google Scholar] [CrossRef]
- Carlstrom, J.; Symons, J.D.; Wu, T.C.; Bruno, R.S.; Litwin, S.E.; Jalili, T. A quercetin supplemented diet does not prevent cardiovascular complications in spontaneously hypertensive rats. J. Nutr. 2007, 137, 628–633. [Google Scholar] [CrossRef]
- Häckl, L.P.; Cuttle, G.; Dovichi, S.S.; Lima-Landman, M.T.; Nicolau, M. Inhibition of angiotesin-converting enzyme by quercetin alters the vascular response to brandykinin and angiotensin I. Pharmacology 2002, 65, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Said, A.; Tundis, R.; Rashed, K.; Statti, G.A.; Hufner, A.; Menichini, F. Inhibition of angiotensin converting enzyme (ACE) by flavonoids isolated from Ailanthus excelsa (Roxb) (Simaroubaceae). Phytother. Res. 2007, 21, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Raghuvanshi, R.; Ceylan, F.D.; Bolling, B.W. Quercetin and Its Metabolites Inhibit Recombinant Human Angiotensin-Converting Enzyme 2 (ACE2) Activity. J. Agric. Food Chem. 2020, 68, 13982–13989. [Google Scholar] [CrossRef] [PubMed]
- Abdelkarem, H.M.; Fadda, L.H. Flaxseed and quercetin improve anti-inflammatory cytokine level and insulin sensitivity in animal model of metabolic syndrome, the fructose-fed rats. Arab. J. Chem. 2017, 10, 3015–3020. [Google Scholar] [CrossRef]
- Alam, M.M.; Meerza, D.; Naseem, I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014, 109, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.B.S.; Porto, M.L.; Santos, M.C.L.F.S.; Campagnaro, B.P.; Gava, A.L.; Meyrelles, S.S.; Pereira, T.M.C.; Vasquez, E.C. Theprotective effects of oral low-dose quercetin on diabetic nephropathy in hypercholesterolemic mice. Front. Physiol. 2015, 6, 247. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Nachar, A.; Thong, F.; Sweeney, G.; Haddad, P.S. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn. Mag. 2015, 11, 74–81. [Google Scholar]
- Dhanya, R.; Arya, A.D.; Nisha, P.; Jayamurthy, P. Quercetin, a Lead Compound against Type 2 Diabetes Ameliorates Glucose Uptake via AMPK Pathway in Skeletal Muscle Cell Line. Front. Pharmacol. 2017, 8, 336. [Google Scholar] [CrossRef]
- Dhanya, R.; Arun, K.B.; Syama, H.P.; Nisha, P.; Sundaresan, A.; Santhosh Kumar, T.R.; Jayamurthy, P. Rutin and quercetin enhance glucose uptake in L6 myotubes under oxidative stress induced by tertiary butyl hydrogen peroxide. Food Chem. 2014, 158, 546–554. [Google Scholar] [CrossRef]
- Eid, H.M.; Martineau, L.C.; Saleem, A.; Muhammad, A.; Vallerand, D.; Benhaddou-Andaloussi, A.; Nistor, L.; Afshar, A.; Arnason, J.T.; Haddad, P.S. Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant Vaccinium vitis-idaea. Mol. Nutr. Food Res. 2010, 54, 991–1003. [Google Scholar] [CrossRef]
- Jiang, H.; Yamashita, Y.; Nakamura, A.; Croft, K.; Ashida, H. Quercetin and its metabolite isorhamnetin promote glucose uptake through different signalling pathways in myotubes. Sci. Rep. 2019, 9, 2690. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.-J.; Li, Y.; Cao, Q.-H.; Wu, H.-X.; Tang, X.-Y.; Gao, X.-H.; Yub, J.Q.; Chen, Z.; Yang, Y. In vitro and in vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomed. Pharmacother. 2019, 109, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Juźwiak, S.; Wójcicki, J.; Mokrzycki, K.; Marchlewicz, M.; Białecka, M.; Wenda-Rózewicka, L.; Gawrońska-Szklarz, B.; Droździk, M. Effect of quercetin on experimental hyperlipidemia and atherosclerosis in rabbits. Pharmacol. Rep. 2005, 57, 604–609. [Google Scholar] [PubMed]
- Castillo, R.L.; Herrera, E.A.; Gonzalez-Candia, A.; Reyes-Farias, M.; de la Jara, N.; Peña, J.P.; Carrasco-Pozo, C. Quercetin Prevents Diastolic Dysfunction Induced by a High-Cholesterol Diet: Role of Oxidative Stress and Bioenergetics in Hyperglycemic Rats. Oxid. Med. Cell. Longev. 2018, 1–14, 7239123. [Google Scholar] [CrossRef]
- Sun, L.; Li, E.; Wang, F.; Wang, T.; Qin, Z.; Niu, S.; Qiu, C. Quercetin increases macrophage cholesterol efflux to inhibit foam cell formation through activating PPARγ-ABCA1 pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 10854–10860. [Google Scholar]
- Cui, Y.; Hou, P.; Li, F.; Liu, Q.; Qin, S.; Zhou, G.; Xu, X.; Si, Y.; Guo, S. Quercetin improves macrophage reverse cholesterol transport in apolipoprotein E-deficient mice fed a high-fat diet. Lipids Health Dis. 2017, 16, 1–7. [Google Scholar] [CrossRef]
- Huwait, E.A.; Saddeek, S.Y.; Al-Massabi, R.F.; Almowallad, S.J.; Pushparaj, P.N.; Kalamegam, G. Antiatherogenic Effects of Quercetin in the THP-1 Macrophage Model In Vitro, With Insights into Its Signaling Mechanisms Using In Silico Analysis. Front. Pharmacol. 2021, 12, 698138. [Google Scholar] [CrossRef]
- Liang, N.; Li, Y.M.; He, Z.; Hao, W.; Zhao, Y.; Liu, J.; Zhu, H.; Kwek, E.; Ma, K.Y.; He, W.S.; et al. Rutin and Quercetin Decrease Cholesterol in HepG2 Cells but Not Plasma Cholesterol in Hamsters by Oral Administration. Molecules 2021, 26, 3766. [Google Scholar] [CrossRef]
- Ferenczyova, K.; Kalocayova, B.; Bartekova, M. Potential Implications of Quercetin and its Derivatives in Cardioprotection. Int. J. Mol. Sci. 2020, 21, 1585. [Google Scholar] [CrossRef]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef]
- Jing, Z.; Wang, Z.; Li, X.; Li, X.; Cao, T.; Bi, Y.; Zhou, J.; Chen, X.; Yu, D.; Zhu, L.; et al. Protective Effect of Quercetin on Posttraumatic Cardiac Injury. Sci. Rep. 2016, 6, 30812. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.S.; Oliveira, E.D.; Santos-Miranda, A.; Cruz, J.S.; Gondim, A.N.S.; Menezes-Filho, J.E.R.; Souza, D.S.; Pinho-da-Silva, L.; Jesus, I.C.G.; Roman-Campos, D.; et al. Dissection of the Effects of Quercetin on Mouse Myocardium. Basic Clin. Pharmacol. Toxicol. 2017, 120, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, M.; Zeng, H.; Liu, P.; Zhu, X.; Zhou, F.; Liu, J.; Zhang, J.; Dong, Z.; Tang, Y.; et al. Quercetin Attenuates Ethanol-Induced Iron Uptake and Myocardial Injury by Regulating the Angiotensin II-L-Type Calcium Channel. Mol. Nutr. Food Res. 2018, 62, 1700772. [Google Scholar]
- Bali, E.B.; Ergin, V.; Rackova, L.; Bayraktar, O.; Küçükboyaci, N.; Karasu, Ç. Olive leaf extracts protect cardiomyocytes against 4-hydroxynonenal-induced toxicity in vitro: Comparison with oleuropein, hydroxytyrosol, and quercetin. Planta Med. 2014, 80, 984–992. [Google Scholar] [CrossRef]
- Li, C.; Wang, T.; Zhang, C.; Xuan, J.; Su, C.; Wang, Y. Quercetin attenuates cardiomyocyte apoptosis via inhibition of JNK and p38 mitogen-activated protein kinase signaling pathways. Gene 2016, 577, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Lozano, O.; Lázaro-Alfaro, A.; Silva-Platas, C.; Oropeza-Almazán, Y.; Torres-Quintanilla, A.; Bernal-Ramírez, J.; Alves-Figueiredo, H.; García-Rivas, G. Nanoencapsulated Quercetin Improves Cardioprotection during Hypoxia-Reoxygenation Injury through Preservation of Mitochondrial Function. Oxid. Med. Cell. Longev. 2019, 7683051. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhang, T.; Meng, Q.; Yan, P.; Wang, X.; Luo, D.; Zhou, X.; Ji, R. Quercetin Improves Cardiomyocyte Vulnerability to Hypoxia by Regulating SIRT1/TMBIM6-Related Mitophagy and Endoplasmic Reticulum Stress. Oxid. Med. Cell. Longev. 2021, 5529913. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.Y.; Chen, F.; Xu, M.; Yao, L.P.; Zhang, Y.J.; Zhuang, Y. Quercetin attenuates myocardial ischemia-reperfusion injury via downregulation of the HMGB1-TLR4-NF-κB signaling pathway. Am. J. Transl. Res. 2018, 10, 1273–1283. [Google Scholar]
- Jin, H.B.; Yang, Y.B.; Song, Y.L.; Zhang, Y.C.; Li, Y.R. Protective roles of quercetin in acute myocardial ischemia and reperfusion injury in rats. Mol. Biol. Rep. 2012, 39, 11005–11009. [Google Scholar] [CrossRef]
- Albadrani, G.M.; BinMowyna, M.N.; Bin-Jumah, M.N.; El-Akabawy, G.; Aldera, H.; AL-Farga, A.M. Quercetin prevents myocardial infarction adverse remodeling in rats by attenuating TGF-β1/Smad3 signaling: Different mechanisms of action. Saudi J. Biol. Sci. 2021, 28, 2772–2782. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.Z.; Wu, Y.; Ke, J.J.; He, X.H.; Wang, Y.L. Quercetin postconditioning attenuates myocardial ischemia/reperfusion injury in rats through the PI3K/Akt pathway. Braz. J. Med. Biol. 2013, 46, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Mamoshina, P.; Rodriguez, B.; Bueno-Orovio, A. Toward a broader view of mechanisms of drug cardiotoxicity. Cell Rep. Med. 2021, 2, 100216. [Google Scholar] [CrossRef] [PubMed]
- Manta, K.; Papakyriakopoulou, P.; Chountoulesi, M.; Diamantis, D.A.; Spaneas, D.; Vakali, V.; Naziris, N.; Chatziathanasiadou, M.V.; Andreadelis, I.; Moschovou, K.; et al. Preparation and Biophysical Characterization of Quercetin Inclusion Complexes with β-Cyclodextrin Derivatives to be Formulated as Possible Nose-to-Brain Quercetin Delivery Systems. Mol. Pharm. 2020, 17, 4241–4255. [Google Scholar] [CrossRef] [PubMed]
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic. Biol. Med. 1994, 16, 845–850. [Google Scholar] [CrossRef]
- Matouk, A.I.; Taye, A.; Heeba, G.H.; El-Moselhy, M.A. Quercetin augments the protective effect of losartan against chronic doxorubicin cardiotoxicity in rats. Environ. Toxicol. Pharmacol. 2013, 36, 443–450. [Google Scholar] [CrossRef]
- Nazmi, A.S.; Ahmad, S.J.; Pillai, K.K.; Akhtar, M.; Ahmad, A.; Najmi, A.K. Protective effects of Bombyx mori, quercetin and benazepril against doxorubicin induced cardiotoxicity and nephrotoxicity. J. Saudi Chem. Soc. 2016, 20, 573–578. [Google Scholar] [CrossRef]
- Salvatorelli, E.; Guarnieri, S.; Menna, P.; Liberi, G.; Calafiore, A.M.; Mariggiò, M.A.; Mordente, A.; Gianni, L.; Minotti, G. Defective one- or two-electron reduction of the anticancer anthracycline epirubicin in human heart. Relative importance of vesicular sequestration and impaired efficiency of electron addition. J. Biol. Chem. 2006, 281, 10990–11001. [Google Scholar] [CrossRef]
- Prasun, P. Mitochondrial dysfunction in metabolic syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165838. [Google Scholar] [CrossRef]
- Iwara, I.; Mboso, E.O.; Eteng, O.; Elot, K.N.; Igile, G.O.; Ebong, P.E. Peristrophe Bicalyculata Extract and Quercetin Ameliorate High Fat Diet- Streptozotocin- Induced Type II Diabetes in Wistar Rats. SSRN 2022, 2, 100060. [Google Scholar] [CrossRef]
- Zhou, J.-F.; Wang, W.-J.; Yin, Z.-P.; Zheng, G.-D.; Chen, J.-G.; Li, J.-E.; Chen, L.L.; Zhang, Q.-F. Quercetin is a promising pancreatic lipase inhibitor in reducing fat absorption in vivo. Food Biosci. 2021, 43, 101248. [Google Scholar] [CrossRef]
- Boydens, C.; Pauwels, B.; Vanden Daele, L.; Van de Voorde, J. Protective effect of resveratrol and quercetin on in vitro-induced diabetic mouse corpus cavernosum. Cardiovasc. Diabetol. 2016, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Song, W.; Liang, X.; Zhang, Q.; Shi, Y.; Liu, W.; Shi, X. Protective effect of quercetin on streptozotocin-induced diabetic peripheral neuropathy rats through modulating gut microbiota and reactive oxygen species level. Biomed. Pharmacother. 2020, 127, 110147. [Google Scholar] [CrossRef] [PubMed]
- Mariee, A.D.; Abd-Allah, G.M.; El-Beshbishy, H.A. Protective effect of dietary flavonoid quercetin against lipemic-oxidative hepatic injury in hypercholesterolemic rats. Pharm. Biol. 2012, 50, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.; Chang, W.T.; Shiau, D.K.; Chou, P.H.; Wu, M.F.; Hsu, C.L. Antiobesity Efficacy of Quercetin-Rich Supplement on Diet-Induced Obese Rats: Effects on Body Composition, Serum Lipid Profile, and Gene Expression. J. Agric. Food Chem. 2018, 66, 70–80. [Google Scholar] [CrossRef]
- Kuipers, E.N.; Dam, A.; Held, N.M.; Mol, I.M.; Houtkooper, R.H.; Rensen, P.; Boon, M.R. Quercetin Lowers Plasma Triglycerides Accompanied by White Adipose Tissue Browning in Diet-Induced Obese Mice. Int. J. Mol. Sci. 2018, 19, 1786. [Google Scholar] [CrossRef]
- Wang, M.; Xiao, F.L.; Mao, Y.J.; Ying, L.L.; Zhou, B.; Li, Y. Quercetin decreases the triglyceride content through the PPAR signalling pathway in primary hepatocytes of broiler chickens. Biotechnol. Biotechnol. Equip. 2019, 33, 1000–1010. [Google Scholar] [CrossRef]
- Muselin, F.; Cristina, R.T.; Dumitrescu, E.; Doma, A.O.; Radulov, I.; Berbecea, A.A.; Horablaga, A.; Morariu, F.E.; Manea, D.N.; Horablaga, N.M. Quercetin Beneficial Role in the Homeostatic Variation of Certain Trace Elements in Dyslipidemic Mice. Evid.-Based Complement. Altern. Med. 2022, 3299505. [Google Scholar] [CrossRef]
- Jia, Q.; Cao, H.; Shen, D.; Li, S.; Yan, L.; Chen, C.; Xing, S.; Dou, F. Quercetin protects against atherosclerosis by regulating the expression of PCSK9, CD36, PPARγ, LXRα and ABCA1. Int. J. Mol. Med. 2019, 44, 893–902. [Google Scholar] [CrossRef]
- Gao, X.R.; Chen, Z.; Fang, K.; Xu, J.X.; Ge, J.F. Protective effect of quercetin against the metabolic dysfunction of glucose and lipids and its associated learning and memory impairments in NAFLD rats. Lipids Health Dis. 2021, 20, 164. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, L.; Guo, X.; Zhang, S.; Wang, J.; Zhou, F.; Liu, L.; Tang, Y.; Yao, P. Quercetin attenuates high fat diet-induced atherosclerosis in apolipoprotein E knockout mice: A critical role of NADPH oxidase. Food Chem. Toxicol. 2017, 105, 22–33. [Google Scholar] [CrossRef]
- Garelnabi, M.; Mahini, H.; Wilson, T. Quercetin intake with exercise modulates lipoprotein metabolism and reduces atherosclerosis plaque formation. J. Int. Soc. Sports Nutr. 2014, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Hayek, T.; Fuhrman, B.; Vaya, J.; Rosenblat, M.; Belinky, P.; Coleman, R.; Elis, A.; Aviram, M. Reduced progression of atherosclerosis in apolipoprotein E-deficient mice following consumption of red wine, or its polyphenols quercetin or catechin, is associated with reduced susceptibility of LDL to oxidation and aggregation. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2744–2752. [Google Scholar] [CrossRef] [PubMed]
- Zhi, K.; Li, M.; Bai, J.; Wu, Y.; Zhou, S.; Zhang, X.; Qu, L. Quercitrin treatment protects endothelial progenitor cells from oxidative damage via inducing autophagy through extracellular signal-regulated kinase. Angiogenesis 2016, 19, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Jia, Q.; Shen, D.; Yan, L.; Chen, C.; Xing, S. Quercetin has a protective effect on atherosclerosis via enhancement of autophagy in ApoE-/- mice. Exp. Ther. Med. 2019, 18, 2451–2458. [Google Scholar] [CrossRef]
- Xue, F.; Nie, X.; Shi, J.; Liu, Q.; Wang, Z.; Li, X.; Zhou, J.; Su, J.; Xue, M.; Chen, W.D.; et al. Quercetin Inhibits LPS-Induced Inflammation and ox-LDL-Induced Lipid Deposition. Front. Pharmacol. 2017, 8, 40. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, L.; Zhao, G.; Du, X. Quercetin reduces atherosclerotic lesions by altering the gut microbiota and reducing atherogenic lipid metabolites. J. Appl. Microbiol. 2019, 127, 1824–1834. [Google Scholar] [CrossRef]
- Li, S.; Cao, H.; Shen, D.; Chen, C.; Xing, S.; Dou, F.; Jia, Q. Effect of Quercetin on Atherosclerosis Based on Expressions of ABCA1, LXR-α and PCSK9 in ApoE-/- Mice. Chin. J. Integr. Med. 2020, 26, 114–121. [Google Scholar] [CrossRef]
- Bhaskar, S.; Sudhakaran, P.; Helen, A. Quercetin attenuates atherosclerotic inflammation and adhesion molecule expression by modulating TLR-NF-κB signaling pathway. Cell. Immunol. 2016, 310, 131–140. [Google Scholar] [CrossRef]
- Lin, W.; Wang, W.; Wang, D.; Ling, W. Quercetin protects against atherosclerosis by inhibiting dendritic cell activation. Mol. Nutr. Food Res. 2017, 61, 1700031. [Google Scholar] [CrossRef]
- Bian, Y.; Liu, P.; Zhong, J.; Hu, Y.; Zhuang, S.; Fan, K.; Liu, Z. Quercetin Attenuates Adhesion Molecule Expression in Intestinal Microvascular Endothelial Cells by Modulating Multiple Pathways. Dig. Dis. Sci. 2018, 63, 3297–3304. [Google Scholar] [CrossRef]
- Atrahimovich, D.; Samson, A.; Khattib, A.; Vaya, J.; Khatib, S. Punicalagin Decreases Serum Glucose Levels and Increases PON1 Activity and HDL Anti-Inflammatory Values in Balb/c Mice Fed a High-Fat Diet. Oxid. Med. Cell. Longev. 2018, 2673076. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiao, L.; He, H.; Zeng, H.; Liu, J.; Jiang, C.; Mei, G.; Yu, J.; Chen, H.; Yao, P.; et al. Quercetin Attenuates Atherosclerotic Inflammation by Inhibiting Galectin-3-NLRP3 Signaling Pathway. Mol. Nutr. Food Res. 2021, 65, e2000746. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, C.; Yao, X.; Zhang, H. Quercetin attenuates high fructose feeding-induced atherosclerosis by suppressing inflammation and apoptosis via ROS-regulated PI3K/AKT signaling pathway. Biomed. Pharmacother. 2017, 85, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Garige, M.; Varatharajalu, R.; Marmillot, P.; Gottipatti, C.; Leckey, L.; Lakshman, R.M. Quercetin up-regulates paraoxonase 1 gene expression with concomitant protection against LDL oxidation. Biochem. Biophys. Res. Commun. 2009, 379, 1001–1004. [Google Scholar] [CrossRef]
- Yao, S.; Sang, H.; Song, G.; Yang, N.; Liu, Q.; Zhang, Y.; Jiao, P.; Zong, C.; Qin, S. Quercetin protects macrophages from oxidized low-density lipoprotein-induced apoptosis by inhibiting the endoplasmic reticulum stress-C/EBP homologous protein pathway. Exp. Biol. Med. 2012, 237, 822–831. [Google Scholar] [CrossRef]
- Kondo, M.; Izawa-Ishizawa, Y.; Goda, M.; Hosooka, M.; Kagimoto, Y.; Saito, N.; Matsuoka, R.; Zamami, Y.; Chuma, M.; Yagi, K.; et al. Preventive Effects of Quercetin against the Onset of Atherosclerosis-Related Acute Aortic Syndromes in Mice. Int. J. Mol. Sci. 2020, 21, 7226. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, L.; Wang, Y.; Ma, D.; Li, X. Quercetin Attenuates Atherosclerosis via Modulating Oxidized LDL-Induced Endothelial Cellular Senescence. Front. Pharmacol. 2020, 11, 512. [Google Scholar]
- Song, S.; Jang, S.; Kang, H.; Wei, B.; Jeoun, U.; Yoon, G.; Hwang, E.S. Modulation of Mitochondrial Membrane Potential and ROS Generation by Nicotinamide in a Manner Independent of SIRT1 and Mitophagy. Mol. Cells 2017, 40, 503–514. [Google Scholar]
- Song, L.; Xu, M.; Lopes-Virella, M.; Huang, Y. Quercetin inhibits matrix metalloproteinase-1 expression in human vascular endothelial cells through extracellular signal-regulated kinase. Arch. Biochem. Biophys. 2001, 391, 72–78. [Google Scholar] [CrossRef]
- Saragusti, A.; Ortega, M.; Cabrera, J.; Estrin, D.; Marti, M.; Chiabrando, G. Inhibitory effect of quercetin on matrix metalloproteinase 9 activity molecular mechanism and structure-activity relationship of the flavonoid-enzyme interaction. Eur. J. Pharmacol. 2010, 644, 138–145. [Google Scholar] [CrossRef]
- Jung, C.H.; Cho, I.; Ahn, J.; Jeon, T.-I.; Ha, T.-Y. Quercetin Reduces High-Fat Diet-Induced Fat Accumulation in the Liver by Regulating Lipid Metabolism Genes. Phyt. Res. 2012, 27, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, M.; Sato, Y.; Kondo, S.; Adachi, S.; Yoshizawa, F.; Sato, Y. Quercetin enhances fatty acid β-oxidation by inducing lipophagy in AML12 hepatocytes. Heliyon 2021, 7, e07324. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, R.A.; Elshikh, M.S.; Mohamed, M.O.; Darweesh, M.F.; Hussein, D.S.; Almutairi, S.M.; Embaby, A.S. Quercetin mitigates the adverse effects of high fat diet on pancreatic and renal tissues in adult male albino rats. J. King Saud Univ. Sci. 2022, 34, 101946. [Google Scholar] [CrossRef]
- Puteri, M.U.; Azmi, N.U.; Kato, M.; Saputri, F.C. PCSK9 Promotes Cardiovascular Diseases: Recent Evidence about Its Association with Platelet Activation-Induced Myocardial Infarction. Life 2022, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Kapelouzou, A.; Tsanikidis, H.; Vitta, I.; Liapis, C.D.; Sailer, N. Effects of rosiglitazone/metformin fixed-dose combination therapy and metformin monotherapy on serum vaspin, adiponectin and IL-6 levels in drug-naïve patients with type 2 diabetes. Exp. Clin. Endocrinol. Diabetes 2011, 119, 63–68. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Rushchak, V.; Voronina, A.; Shayakhmetova, A.; Chashchyn, M. Quercetin effectiveness in the prevention of pathological processes at the metabolic syndrome. Toxicol. Lett. 2013, 221, 171. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Kim, S.; Park, J.; Ha, T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem. Biophys. Res. Commun. 2008, 373, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.A.; Muñoz, V.R.; Kuga, G.K.; Gaspar, R.C.; Nakandakari, S.; Crisol, B.M.; Botezelli, J.D.; Pauli, L.; da Silva, A.; de Moura, L.P.; et al. Obesity Increases Mitogen-Activated Protein Kinase Phosphatase-3 Levels in the Hypothalamus of Mice. Front. Cell. Neurosci. 2017, 11, 313. [Google Scholar] [CrossRef]
- Hemmati, M.; Mostafavi, S.E.; Zarban, A.; Hoshyar, R. Protective Effects of Quercetin on Hyperglycemia and Stress Proteins Expression in Rats with Streptozocin-Induced Diabetes. Brieflands 2018, 15, e64964. [Google Scholar] [CrossRef]
- Le, N.H.; Kim, C.S.; Park, T.; Park, J.H.; Sung, M.K.; Lee, D.G.; Hong, S.M.; Choe, S.Y.; Goto, T.; Kawada, T.; et al. Quercetin protects against obesity-induced skeletal muscle inflammation and atrophy. Mediat. Inflamm. 2014, 834294. [Google Scholar] [CrossRef]
- Feng, J.; Ge, C.; Li, W.; Li, R. 3-(3-Hydroxyphenyl)propionic acid, a microbial metabolite of quercetin, inhibits monocyte binding to endothelial cells via modulating E-selectin expression. Fitoterapia 2022, 156, 105071. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.E.; Velidakis, N.; Khattab, E.; Kassimis, G.; Patsourakos, N. The interplay between statins and adipokines. Is this another explanation of statins’ ‘pleiotropic’ effects? Cytokine 2021, 148, 155698. [Google Scholar] [CrossRef] [PubMed]
- Mirsafaei, L.; Reiner, Ž.; Shafabakhsh, R.; Asemi, Z. Molecular and Biological Functions of Quercetin as a Natural Solution for Cardiovascular Disease Prevention and Treatment. Plant Foods Hum. Nutr. 2020, 75, 307–315. [Google Scholar] [CrossRef]
- Huang, H.; Liao, D.; Dong, Y.; Pu, R. Effect of quercetin supplementation on plasma lipid profiles, blood pressure, and glucose levels: A systematic review and meta-analysis. Nutr. Rev. 2020, 78, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Menezes, R.; Rodriguez-Mateos, A.; Kaltsatou, A.; González-Sarrías, A.; Greyling, A.; Giannaki, C.; Andres-Lacueva, C.; Milenkovic, D.; Gibney, E.R.; Dumont, J.; et al. Impact of Flavonols on Cardiometabolic Biomarkers: A Meta-Analysis of Randomized Controlled Human Trials to Explore the Role of Inter-Individual Variability. Nutrients 2017, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Liu, J.; Jia, Y.; Han, T.; Zhao, X.; Sun, C.; Na, L. The association of dietary flavonoids, magnesium and their interactions with the metabolic syndrome in Chinese adults: A prospective cohort study. Br. J. Nutr. 2021, 126, 892–902. [Google Scholar] [CrossRef]
- Shatylo, V.; Antoniuk-Shcheglova, I.; Naskalova, S.; Bondarenko, O.; Havalko, A.; Krasnienkov, D.; Zabuga, O.; Kukharskyy, V.; Guryanov, V.; Vaiserman, A. Cardio-metabolic benefits of quercetin in elderly patients with metabolic syndrome. PharmaNutrition 2021, 15, 100250. [Google Scholar] [CrossRef]
- Cano-Martínez, A.; Bautista-Pérez, R.; Castrejón-Téllez, V.; Carreón-Torres, E.; Pérez-Torres, I.; Díaz-Díaz, E.; Flores-Estrada, J.; Guarner-Lans, V.; Rubio-Ruíz, M.E. Resveratrol and Quercetin as Regulators of Inflammatory and Purinergic Receptors to Attenuate Liver Damage Associated to Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 8939. [Google Scholar] [CrossRef]
- Pfeuffer, M.; Auinger, A.; Bley, U.; Kraus-Stojanowic, I.; Laue, C.; Winkler, P.; Rüfer, C.E.; Frank, J.; Bösch-Saadatmandi, C.; Rimbach, G.; et al. Effect of quercetin on traits of the metabolic syndrome, endothelial function and inflammation in men with different APOE isoforms. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 403–409. [Google Scholar] [CrossRef]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. Camb. 2009, 102, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Serban, M.C.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, e002713. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Tamtaji, O.R.; Milajerdi, A.; Dadgostar, E.; Kolahdooz, F.; Chamani, M.; Amirani, E.; Mirzaei, H.; Asemi, Z. The Effects of Quercetin Supplementation on Blood Pressures and Endothelial Function Among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Curr. Pharm. Des. 2019, 25, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Dai, K.; Meng, G.; Zhang, Q.; Liu, L.; Wu, H.; Gu, Y.; Sun, S.; Wang, X.; Jia, Q.; et al. Low dietary quercetin intake by food frequency questionnaire analysis is not associated with hypertension occurrence. Clin. Nutr. 2021, 40, 3748–3753. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, S.; Michaels, H.A.; Quadros, A.S.; Qian, Y.; Rabovsky, A.B.; Badger, R.S.; Jalili, T. A combination of isolated phytochemicals and botanical extracts lowers diastolic blood pressure in a randomized controlled trial of hypertensive subjects. Eur. J. Clin. Nutr. 2016, 70, 10–16. [Google Scholar] [CrossRef]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin reduces blood pressure in hypertensive subjects. J. Nutr. 2007, 137, 2405–2411. [Google Scholar] [CrossRef]
- Zahedi, M.; Ghiasvand, R.; Feizi, A.; Asgari, G.; Darvish, L. Does Quercetin Improve Cardiovascular Risk factors and Inflammatory Biomarkers in Women with Type 2 Diabetes: A Double-blind Randomized Controlled Clinical Trial. Int. J. Prev. Med. 2013, 4, 777–785. [Google Scholar]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: A randomised double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef]
- Larson, A.; Witman, M.A.; Guo, Y.; Ives, S.; Richardson, R.S.; Bruno, R.S.; Jalili, T.; Symons, J.D. Acute, quercetin-induced reductions in blood pressure in hypertensive individuals are not secondary to lower plasma angiotensin-converting enzyme activity or endothelin-1: Nitric oxide. Nutr. Res. 2012, 32, 557–564. [Google Scholar] [CrossRef]
- Burak, C.; Wolffram, S.; Zur, B.; Langguth, P.; Fimmers, R.; Alteheld, B.; Stehle, P.; Egert, S. Effect of alpha-linolenic acid in combination with the flavonol quercetin on markers of cardiovascular disease risk in healthy, non-obese adults: A randomized, double-blinded placebo-controlled crossover trial. Nutrition 2019, 58, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A. Effects of quercetin supplementation on lipid profile: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2017, 57, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Gong, X.; Li, M. Quercetin Actions on Lipid Profiles in Overweight and Obese Individuals: A Systematic Review and Meta-Analysis. Curr. Pharm. Des. 2019, 25, 3087–3095. [Google Scholar] [CrossRef] [PubMed]
- Mazza, A.; Nicoletti, M.; Lenti, S.; Torin, G.; Rigatelli, G.; Fratter, A. Effectiveness and safety of nutraceutical compounds added to ezetimibe treatment in hypertensive and hyper-cholesterolemic subjects with statinintolerance. J. Hypertens. 2021, 39 (Suppl. S1), e371. [Google Scholar] [CrossRef]
- Tabrizi, R.; Tamtaji, O.R.; Mirhosseini, N.; Lankarani, K.B.; Akbari, M.; Heydari, S.T.; Dadgostar, E.; Asemi, Z. The effects of quercetin supplementation on lipid profiles and inflammatory markers among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1855–1868. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.M.; Chiu, H.F.; Shen, Y.C.; Chung, C.C.; Venkatakrishnan, K.; Wang, C.K. Hypocholesterolemic Efficacy of Quercetin Rich Onion Juice in Healthy Mild Hypercholesterolemic Adults: A Pilot Study. Plant Foods Hum. Nutr. 2015, 70, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Muro, T.; Kobori, M.; Nishihira, J. Effect of Daily Ingestion of Quercetin-Rich Onion Powder for 12 Weeks on Visceral Fat: A Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Study. Nutrients 2019, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Ostadmohammadi, V.; Milajerdi, A.; Ayati, E.; Kolahdooz, F.; Asemi, Z. Effects of quercetin supplementation on glycemic control among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 1330–1340. [Google Scholar] [CrossRef]
- Song, Y.; Manson, J.E.; Buring, J.E.; Sesso, H.D.; Liu, S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: A prospective study and cross-sectional analysis. J. Am. Coll. Nutr. 2005, 24, 376–384. [Google Scholar] [CrossRef]
- Sales, D.S.; Carmona, F.; de Azevedo, B.C.; Taleb-Contini, S.H.; Bartolomeu, A.C.; Honorato, F.B.; Martinez, E.Z.; Pereira, A.M. Eugenia punicifolia (Kunth) DC. as an adjuvant treatment for type-2 diabetes mellitus: A non-controlled, pilot study. Phytother. Res. 2014, 28, 1816–1821. [Google Scholar] [CrossRef]
- Tabasco, R.; Sánchez-Patán, F.; Monagas, M.; Bartolomé, B.; Victoria Moreno-Arribas, M.; Peláez, C.; Requena, T. Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: Resistance and metabolism. Food Microbiol. 2011, 28, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gao, B.; Siqin, B.; He, Q.; Zhang, R.; Meng, X.; Zhang, N.; Zhang, N.; Li, M. Gut Microbiota: A Novel Regulator of Cardiovascular Disease and Key Factor in the Therapeutic Effects of Flavonoids. Front. Pharmacol. 2021, 12, 651926. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhong, Y.; Feng, N.; Guo, Z.; Wang, S.; Xing, D. New horizons in the roles and associations of COX-2 and novel natural inhibitors in cardiovascular diseases. Mol. Med. 2021, 27, 123. [Google Scholar] [CrossRef] [PubMed]
- Dagher, O.; Mury, P.; Thorin-Trescases, N.; Noly, P.; Thorin, E.; Carrier, M. Therapeutic Potential of Quercetin to Alleviate Endothelial Dysfunction in Age-Related Cardiovascular Diseases. Front. Cardiovasc. Med. 2021, 8, 658400. [Google Scholar] [CrossRef]
- Chekalina, N.; Burmak, Y.; Petrov, Y.; Borisova, Z.; Manusha, Y.; Kazakov, Y.; Kaidashev, I. Quercetin reduces the transcriptional activity of NF-kB in stable coronary artery disease. Indian Heart J. 2018, 70, 593–597. [Google Scholar] [CrossRef]
- Knekt, P.; Jarvinen, R.; Reunanen, A.; Maatela, J. Flavonoid intake and coronary mortality in Finland: A cohort study. BMJ 1996, 312, 478–481. [Google Scholar] [CrossRef]

| Reference | Animal Model | Study Design | Outcomes |
|---|---|---|---|
| Alam et al., 2014 [45] | Swiss albino mice | 20 mg/kg/day quercetin orally for 3 weeks | ↓ FBG ↑ GLUT-4 |
| Albadrani et al., 2020 [70] | Wistar albino rats | 50 mg/kg/day quercetin orally for 4 weeks | ↓ TGF-b1/Smad3 signaling |
| Abdelkarem and Fadda, 2017 [44] | Sprague–Dawley rats | 50 mg/kg/day quercetin orally for 4 weeks | ↓ serum glucose, TC, LDL-C, TG, leptin, adiponectin |
| Carlstrom et al., 2007 [40] | Spontaneously hypertensive rats (SHR) and Wistar Kyoto rats (WKY) | 1.5 g quercetin/kg/day quercetin orally (gavage) for 5 or 11 weeks (SHR) | ↔ BP, cardiac hypertrophy, vascular dysfunction, vascular remodeling, and indices of oxidative stress in SHR |
| Castillo et al., 2018 [54] | Wistar albino rats | HC diet supplemented with 0.5% w/w quercetin for 4 weeks | ↓ TG, TC, glucose, oxidative stress suppression, LDL-C and VLDL-C increase, HDL-C decrease |
| Cui et al., 2017 [56] | apoE−/− mice | 12.5 mg/kg/day quercetin via gavage | ↑ RCT |
| Duarte et al., 2009 [39] | Spontaneously hypertensive rats (SHR) and normotensive Wistar Kyoto rats (WKY) | 10 mg/kg/day quercetin orally (gavage) for 5 weeks | ↓ SBP, DBP, and MAP in SHR |
| Elbarbry et al., 2020 [38] | Spontaneously hypertensive rats (SHR) | 10, 30, and 60 mg/L quercetin in drinking water for 7 weeks | ↓ MAP at a high dose of quercetin |
| Gao et al., 2021 [89] | Nonalcoholic fatty liver disease (NAFLD) rat model | 80, 40, or 20 mg/kg/day quercetin via gavage for 4 weeks | ↓ TC, TG, blood glucose levels |
| Garelnabi et al., 2022 [91] | C57BL6 LDLr−/− mice | 100 μg/day quercetin orally for 4 weeks | ↓ AP, MCP-1 |
| Gomes et al., 2015 [46] | apoE−/− mice | 10 mg/kg/day quercetin orally for 4 weeks | ↓ plasma glucose, TG, TC, tendency to reduce proteinuria and glomerular injury |
| Häckl et al., 2002 [41] | Wistar albino rats | Oral or IV preadministration of 88.7 μmol/kg and 14.7 μmol/kg quercetin, respectively, for 45 and 5 min before a bradykinin IV injection | ↑ hypotensive effect of bradykinin ↓ MAP |
| Hemmati et al., 2018 [120] | Wistar albino rats | 15 mg/kg/day quercetin (i.p. injection) for 3 weeks | ↓ MDA, mRNA levels of HSP27, HSP70, HSF-1, and glucose-6-phosphatase ↑ glucokinase expression |
| Iwara et al., 2022 [79] | Albino rats | 10 mg/kg/day quercetin orally for 3 weeks | ↓ FBG ↑ BW, AST, ALP, ALT, albumin |
| Jia et al., 2019 [88] | apoE−/− mice | 12.5 mg/kg/day quercetin orally for 12 weeks | ↓ TC, LDL-C, oxLDL, TNF-α, IL-6, plaques ↑ IL-10, PPAR-γ, LXRα ABCA1 |
| Jin et al., 2012 [96] | Sprague–Dawley rats | 1 mg/kg quercetin IV | ↓ TNF-α, IL-10 |
| Jung et al., 2012 [111] | C57BL/6J mice | HC diet supplemented with 0.025% w/w quercetin for 9 weeks | ↓ BW, size of the epididymal adipose tissue and liver tissue, TBARS, fat, altered expression of the lipid metabolism-related genes |
| Juźwiak et al., 2005 [53] | Mongrel rabbits | 0.05 mg/kg/day quercetin orally for 4 and 12 weeks | ↓ TG, TC, plaque formation, thickening of the tunica intima of the aorta |
| Kuipers et al., 2018 [85] | C57Bl/6J mice | HC diet supplemented with 0.1% w/w quercetin for 12 weeks | ↓ TG, white adipose tissue browning |
| Le et al., 2014 [121] | C57BL/6J mice | HC diet supplemented with 0.05% and 0.1% w/w quercetin for 9 weeks | ↓ TNF-α, MCP-1 skeletal muscle atrophy |
| Liang et al., 2021 [58] | Hypercholesterolemia hamsters | 2.5 g/kg/day quercetin orally for 8 weeks | ↔ TC |
| Lin et al., 2020 [30] | Spontaneously hypertensive rats (SHR) | 10 mg/kg/day quercetin orally (gavage) for 6 weeks | ↓ SBP, DBP ↑ autophagy |
| Mariee et al., 2012 [83] | Sprague–Dawley rats | 15 mg/kg/day quercetin orally for 2 weeks | ↓ TG, TC, LDL-C, ALT, AST, γ-GT, liver TBARS ↑ HDL-C, GSH |
| Matouk et al., 2013 [75] | Wistar albino rats | 10 mg/kg/day quercetin orally for 4 weeks | ↓ TNF-α, LDH, CK-MB, MDA, NO ↓ CAT, ↓SOD |
| Muselin et al., 2022 [87] | BALB/c mice | 500 mg/L quercetin in drinking water (duration of the study not reported) | ↓ TC, LDL-C, TG |
| Nazmi et al., 2016 [76] | Wistar albino rats | 2 mg/kg/day quercetin orally for 1 week | ↑ AST, LDH, BUN, creatinine, GSH |
| Pereira et al., 2018 [32] | 2K1C hypertensive Wistar albino rats | 10 mg/kg/day quercetin via gavage for 3 weeks | ↓ SBP, BW, ROS, MLP |
| Rasheed et al., 2022 [113] | Albino rats | 50 mg/kg/day of quercetin orally for 12 weeks | Improvement of the histopathological degenerative and inflammatory changes ↓ mean area % of collagen fibers |
| Ting et al., 2018 [84] | Wistar albino rats | 13 mg/kg/day quercetin orally for 8 weeks | ↓ BW, ALT, TG, TC, size of perirenal adipocytes ↑ adiponectin expression, AST |
| Wang et al., 2013 [71] | Sprague–Dawley rats | 10 mg/kg quercetin (i.p. injection) 5 min before reperfusion | ↓ infarct size, serum levels of creatine kinase and lactate dehydrogenase, caspase-3 immunoreactivity, and Bax expression ↑ Akt phosphorylation and Bcl-2 expression |
| Zhou et al., 2021 [80] | Sprague–Dawley rats | Oral preadministration of 5 and 10 mg/kg quercetin | ↓ TG, fat absorption ↑ fat excretion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papakyriakopoulou, P.; Velidakis, N.; Khattab, E.; Valsami, G.; Korakianitis, I.; Kadoglou, N.P. Potential Pharmaceutical Applications of Quercetin in Cardiovascular Diseases. Pharmaceuticals 2022, 15, 1019. https://doi.org/10.3390/ph15081019
Papakyriakopoulou P, Velidakis N, Khattab E, Valsami G, Korakianitis I, Kadoglou NP. Potential Pharmaceutical Applications of Quercetin in Cardiovascular Diseases. Pharmaceuticals. 2022; 15(8):1019. https://doi.org/10.3390/ph15081019
Chicago/Turabian StylePapakyriakopoulou, Paraskevi, Nikolaos Velidakis, Elina Khattab, Georgia Valsami, Ioannis Korakianitis, and Nikolaos PE Kadoglou. 2022. "Potential Pharmaceutical Applications of Quercetin in Cardiovascular Diseases" Pharmaceuticals 15, no. 8: 1019. https://doi.org/10.3390/ph15081019
APA StylePapakyriakopoulou, P., Velidakis, N., Khattab, E., Valsami, G., Korakianitis, I., & Kadoglou, N. P. (2022). Potential Pharmaceutical Applications of Quercetin in Cardiovascular Diseases. Pharmaceuticals, 15(8), 1019. https://doi.org/10.3390/ph15081019







