TLC–Densitometry for Determination of Omeprazole in Simple and Combined Pharmaceutical Preparations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Validation of TLC–Densitometry Method
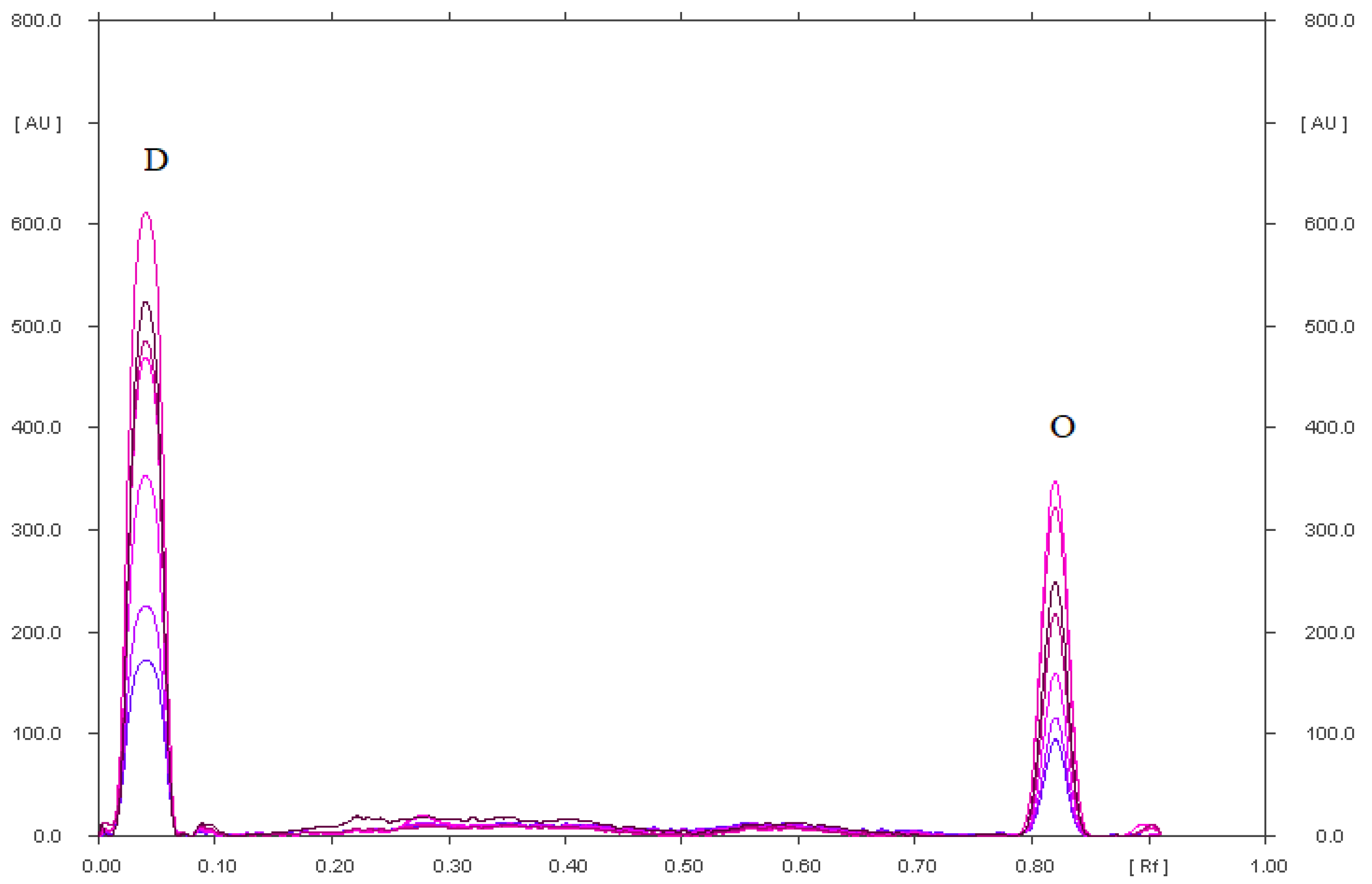
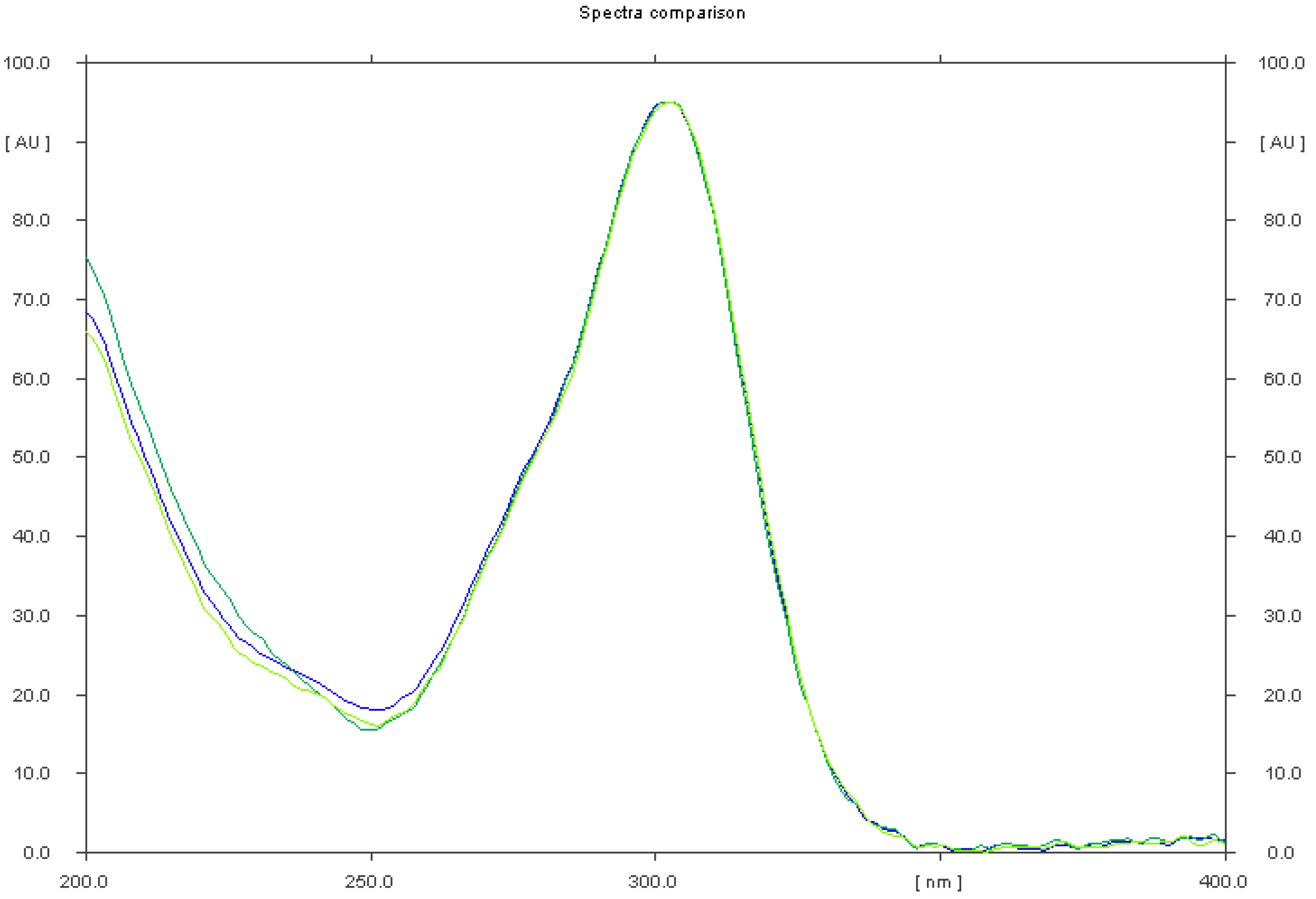
2.1.1. Selectivity of TLC–Densitometry Method
- chloroform–methanol–ammonia (36:4:0.60, v/v/v) (mobile phase IX).
- cyclohexane–chloroform–methanol–glacial acetic acid (6:3:0.5:0.5, v/v/v/v) (mobile phase X).
- in an acidic environment: 5-methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl) methyl]sulfonyl}-1H-benzimidazole (omeprazole-related compound A), 5-methoxy-2-{[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfonyl}-1H-benzimidazole, 4-dihydro-1-(5-methoxy-1H-benzo[d]imidazol-2-yl) -3,5-dimethyl-4oxopyridine-2-carboxylic acid;
- in an alkaline environment: 5-methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl) methyl]sulfonyl}-1H-benzimidazole (omeprazole-related compound A), 5-methoxy-2-{[(4-methoxy-dimethylpyridin-2-yl)methyl]sulfonyl}-1H-benzimidazole, 1,4-dihydro-1-(5-methoxy-1H-benzo[d]imidazol-2-yl)-3,5-dimethyl-4oxopyridine-2-carboxylic acid; and 5-methoxy-1H-benzimidozole-2-thiol;
- in solution with the addition of hydrogen peroxide (oxidation): 5-methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfonyl}-1H-benzimidazole (omeprazole-related compound), 4-dihydro-1-(5-methoxy-1H-benzo[d]imidazole-2-yl)-3,5-dimethyl-4-oxopyridine-2-carboxylic acid.
2.1.2. Linearity
2.1.3. Precision
2.1.4. Accuracy
2.1.5. Limit of Detection (LOD) and Limit of Quantification (LOQ)
2.1.6. Robustness
2.1.7. Quantitative Determination of Omeprazole in Simple and Combined Capsules
2.1.8. Comparison of the Limit of Detection of Omeprazole and Diclofenac Sodium Obtained in This Work with Literature Methods
3. Material and Methods
3.1. Pharmaceutical Reference Standards and Chemicals
3.2. Pharmaceutical Preparations
3.3. Preparation of Samples
3.4. Preparation of Standard Solutions
3.5. Thin-Layer Chromatography
3.6. Densitometric and Spectrodensitometric Study
3.7. Validation of the Thin-Layer Chromatography Method
3.7.1. Specificity
- chloroform–methanol 9:1 (v/v)
- chloroform–methanol 9:0.7 (v/v)
- chloroform–methanol 9:1.2 (v/v)
- chloroform–methanol 9:1.5 (v/v)
- chloroform–methanol–acetone 8.5:1:0.5 (v/v/v)
- chloroform–2-propanol–25% ammonia–acetonitrile 10.8:1.2:0.3:4 (v/v/v/v)
- chloroform–methanol–25% ammonia–acetonitrile 9:1:0.15:1 (v/v/v/v)
- chloroform–methanol–25% ammonia 9:0.7:0.10 (v/v/v)
- chloroform–methanol–25% ammonia 9:1:0.15 (v/v/v)
- cyclohexane–chloroform–methanol–glacial acetic acid 6:3:0.5:0.5 (v/v/v/v)
3.7.2. Linearity and Range
3.7.3. Accuracy
3.7.4. Precision
3.7.5. Limit of Detection (LOD) and Limit of Quantification (LOQ) Based on the Calibration Curves
3.7.6. Robustness Study
- The kind of chromatographic plates (1.05554 and 1.05570): these aluminum plates were precoated with silica gel 60F254. The plates 1.05570 had a dimension 10 cm × 20 cm. The plates 1.05554 had a dimension 20 cm × 20 cm and before were cut to size 10 cm × 20 cm
- Mobile phase volume (50 mL ± 5%): 50 mL of mobile phase was used as standard. However, in the study of the robustness of the method, a mobile phase with a volume of 50 mL ± 5% was used, i.e., 47.5 mL and 52.5 mL
- Temperature of the activation of the plates at 120 (±5) °C
- Development distance (±5 mm)
- Time of saturation of chromatographic chamber (±5 min)
3.7.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Strand, D.S.; Kim, D.; Peura, D.A. 25 years of proton pump inhibitors: A comprehensive review. Gut Liver 2017, 11, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P. Reliving 25 years of experience with omeprazole in acid-peptic diseases. J. Assoc. Physicians India 2018, 66, 72–78. [Google Scholar] [PubMed]
- Polish Pharmaceutical Society. Polish Pharmacopoeia, 11th ed.; Polish Pharmaceutical Society: Warsaw, Poland, 2017; pp. 2610–2611, 3676–3680. [Google Scholar]
- Sachs, G.; Shin, J.M.; Howden, C.W. Review article: The clinical pharmacology of proton pump inhibitors. Aliment. Pharmacol. Ther. 2006, 23, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Viazis, N.; Argyriou, K.; Kotzampassi, K.; Christodoulou, D.K.; Apostolopoulos, P.; Georgopoulos, S.D.; Liatsos, C.; Giouleme, O.; Koustenis, K.; Veretanos, C.; et al. A four-probiotics regimen combined with a standard helicobacter pylori-eradication treatment reduces side effects and increases eradication rates. Nutrients 2022, 14, 632. [Google Scholar] [CrossRef]
- Ruiz-Jarabo, I.; Paullada-Salmerón, J.A.; Jerez-Cepa, I.; Gonçalves Neto, J.B.; Bystriansky, J.S.; Mancera, J.M. Acute stress in lesser-spotted catshark (Scyliorhinus canicula Linnaeus, 1758) promotes amino acid catabolism and osmoregulatory imbalances. Animals 2022, 12, 1192. [Google Scholar] [CrossRef]
- Zatorski, H.; Salaga, M.; Zielińska, M.; Majchrzak, K.; Binienda, A.; Kordek, R.; Małecka-Panas, E.; Fichna, J. AdipoRon, an orally active, synthetic agonist of AdipoR1 and AdipoR2 receptors fas gastroprotective effect in experimentally induced gastric ulcers in mice. Molecules 2021, 26, 2946. [Google Scholar] [CrossRef]
- Todd, P.; Sorkin, E. Diclofenac sodium: A reappraisal of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs 1988, 35, 244–285. [Google Scholar] [CrossRef]
- DrugBank. Available online: https://go.drugbank.com/drugs (accessed on 22 July 2022).
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.A.; Radchenko, E.V.; Zefirov, N.S.; Makarenko, A.S.; et al. Virtual computational chemistry laboratory-design and description. J. Comput. Aided Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef]
- VCCLAB (Virtual Computational Chemistry Laboratory). 2005. Available online: http://www.vcclab.org (accessed on 22 July 2022).
- Sen, D.J.; Patel, J.G. Logaruthmic partition coefficient comparison study and molecular weight of synthesized prodrugs of ibuprofen + paracetamol, diclofenac sodium + paracetamol and ibuprofen + diclofenac sodium. Am. J. Adv. Drug Deliv. 2016, 4, 64–68. [Google Scholar]
- Gul, W.; Sajid, S.; Hamid, F.; Bhatti, S. Effect of acidic pH, and heat on the degradation of omeprazole and esomeprazole. Pharma Innov. J. 2015, 4, 19–21. [Google Scholar]
- Salem, H.; Riad, S.M.; Reda, M.; Ahmed, K. Simultaneous determination of omeprazole, tinidazole and clarithromycin in bulk powder and helicure tablets by TLC densitometric technique. J. Pharm. Educ. Res. 2013, 4, 34–40. [Google Scholar]
- Bhatt, N.M.; Chavadaa, V.D.; Sanyalb, M.; Shrivastava, P.S. Combining simplicity with cost-effectiveness: Investigation of poten of proton pump inhibitors through simulated formulations using a mono-TLC protocol. J. Chromatogr. A 2016, 1473, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Mangal, A.; Bhadoriya, S.S.; Verma, A.; Mishra, K.K. Novel application hydrotropic solubilization phenomenon in the thin layer chromatrography analysis of omeprazole. Int. J. Curr. Pharm. Res. 2011, 8, 15–16. [Google Scholar]
- Lobhe, G.A.; Banerjee, S.K.; Shirkhedkar, A.A.; Surana, S.J. Simultaneous determination of ondansetron hydrochloride and omeprazole in tablets by planar chromatography. Int. J. Res. Pharm. Chem. 2011, 1, 475–480. [Google Scholar]
- Agbaba, D.; Novovic, D.; Karljiković-Rajić, K.; Marinković, V. Densitometric determination of omeprazole, pantoprazole, and their impurities in pharmaceuticals. J. Planar Chromatogr. Modern TLC 2004, 17, 169–172. [Google Scholar] [CrossRef]
- El-Kimary, E.I.; Ragab, M.A.A. A validated high-performance thin-layer chromatographic method for the assay of two binary mixtures containing omeprazole or its isomer esomeprazole in pharmaceutical dosage forms. J. Planar Chromatogr. Modern TLC 2015, 28, 74–82. [Google Scholar] [CrossRef]
- Nassara, M.W.I.; Attiaa, K.A.M.; Mohamed, A.A.; Shahin, M. Application of TLC densitometric method for simultaneous determination of aspirin and omeprazole in pharmaceutical preparation. Innorig. Int. J. Sci. 2018, 5, 47–50. [Google Scholar]
- Kashid, A.M.; Kolhe, O.H. Simultaneous densitometric determination of aspirin and omeprazole by high-performance thin-layer chromatography. J. Planar Chromatogr. Modern TLC 2019, 32, 501–504. [Google Scholar] [CrossRef]
- Tambe, V.; Vichare, V.; Kolte, R. Development and validation of an HPTLC method for simultaneous estimation of omeprazole and ketoprofen in a developed tablet formulation. Int. J. PharmTech Res. 2014, 6, 634–640. [Google Scholar]
- Abdallah, N.A. HPLC and densitometric TLC methods for simultaneous determination of gemifloxacin with some co-administered drugs in human plasma. J. Chromatogr. Sep. Tech. 2014, 5, 1000220. [Google Scholar] [CrossRef]
- Abdelaleem, E.A.; Abou El Ella, D.A.; Mahmoud, A.M.; Abdelhamid, N.S. Green analysis of newly approved binary omeprazole/aspirin mixture in presence of aspirin impurity using ultra-high-performance liquid chromatography and thin-layer chromatography methods. Biomed. Chromatogr. 2021, 35, e4986. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Parveen, R.; Khan, S.A.; Alam, O.; Ahmed, S. Stability-indicating high-performance thin-layer chromatographic method for quantitative determination of omeprazole in capsule dosage form. J. AOAC Int. 2010, 93, 787–791. [Google Scholar] [CrossRef]
- Salem, H.; Riad, S.M.; Rezk, M.R.; Ahmed, K. Simultaneous determination of omeprazole, tinidazole and clarithromycin in bulk powder and Helicure® tablets by HPLC. J. Chromatogr. Sep. Tech. 2014, 5, 221. [Google Scholar] [CrossRef]
- Raval, P.B.; Puranik, M.; Wadher, S.J.; Yeole, P.G. A validated HPTLC method for determination of ondansetron in combination with omeprazole or rabeprazole in solid dosage form. Indian J. Pharm. Sci. 2008, 70, 386–390. [Google Scholar] [PubMed]
- Murakami, F.S.; Cruz, A.P.; Pereira, R.N.; Valente, B.R.; Silva, M.A.S. Development and validation of a RP-HPLC method to quantify omeprazole in delayed release tablets. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 113–121. [Google Scholar] [CrossRef]
- Flor, S.; Tripodi, V.; Scioscia, S.; Revello, L.; Lucangioli, B.S. Fast and sensitive new HPLC-UV method for determination of omeprazole and major related substances in pharmaceutical formulation. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 1666–1678. [Google Scholar] [CrossRef]
- Patel, B.; Patel, M.; Patel, J.; Suhagia, B. Simultaneous determination of omeprazole and domperidone in capsules by RP-HPLC and densitometric HPTLC. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 1749–1762. [Google Scholar] [CrossRef]
- Koppala, S.; Ranga, V.; Anireddy, J.S. Development and validation of a novel stability indicating RP-HPLC method for the simultaneous determination of related substances of ketoprofen and omeprazole in combined capsule dosage form. J. Chromatogr. Sci. 2016, 54, 765–775. [Google Scholar] [CrossRef]
- Maslarska, V.; Bozhanov, S.; Tsvetkova, B.; Peikova, L. High pressure liquid chromatographic method for simultaneous determination of ketoprofen and omeprazole. Pharmacia 2018, 65, 3–8. [Google Scholar]
- Kamal, A.H.; Marie, A.A.; Hammad, S.F. Stability indicating RP-HPLC method for simultaneous determination of omeprazole and aspirin in the presence of salicylicacidas degradation product. Microchem. J. 2020, 152, 104350. [Google Scholar] [CrossRef]
- Patel, R.K.; Patel, H.R.; Patel, V.A.; Ganure, A.L.; Patel, L.J. Development and validation of RP-HPLC method for simultaneous determination of omeprazole and diclofenac sodium in capsule dosage form. J. Pharm. Res. 2012, 5, 1640–1642. [Google Scholar]
- Kang, W.; Kim, D.; Kwon, K. Advanced method for determination of omeprazole in plasma by HPLC. Arch. Pharm. Res. 1999, 22, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Noubarani, M.; Keyhanfar, F.; Motevalian, M.; Mahmoudian, M. Improved HPLC method for determination of four PPIs, omeprazole, pantoprazole, lansoprazole and rabeprazole in human plasma. J. Pharm. Pharm. Sci. 2010, 13, 1–10. [Google Scholar] [CrossRef]
- Kalakonda, S.N.; Mohammad, B.D.; Kalyani, P.; Dussa, K.K. Development and validation of RP-HPLC method for the estimation of omeprazole in bulk and capsule dosage forms. Int. Curr. Pharm. J. 2012, 1, 366–369. [Google Scholar]
- Patel, J.; Patel, R.K.; Mochi, N.S. Development and validation of RP-HPLC method for simultaneous estimation of nimesulide and diclofenac sodium in tablet dosage form. Int. J. Res. Manag. Pharm. 2017, 6, 67–76. [Google Scholar]
- El-Sherif, Z.A.; Mohamed, A.O.; El-Bardicy, M.G.; El-Tarras, M.F. Reversed-phase high performance liquid chromatographic method for the determination of lansoprazole, omeprazole and pantoprazole sodium sesquihydrate in presence of their acid-induced degradation products. Chem. Pharm. Bull. 2006, 54, 814–818. [Google Scholar] [CrossRef]
- Hussain, A.; Naughton, D.P.; Barker, J. Potential effects of ibuprofen, remdesivir and omeprazole on dexamethasone metabolism in control sprague dawley male rat liver microsomes (drugs often used together alongside COVID-19 treatment). Molecules 2022, 27, 2238. [Google Scholar] [CrossRef]
- Chaitanya, K.S.; Unnisa, A. Novel UV-spectrophotometric methods for simultaneous estimation of omeprazole and diclofenac in bulk and pharmaceutical formulations. J. Pharm. Sci. Innov. 2014, 3, 428–433. [Google Scholar] [CrossRef]
- Wankhede, S.B.; Borole, V.N.; Chitlange, S.S. Simultaneous spectrophotometric estimation of diclofenac sodium and omeprazole in capsules. Asian J. Res. Chem. 2011, 4, 1172–1175. [Google Scholar]
- Wahbi, A.A.M.; Abdel-Razak, O.; Gazy, A.A.; Mahgoub, H.; Moneeb, M.S. Spectrophotometric determination of omeprazole, lansoprazole and pantoprazole in pharmaceutical formulations. J. Pharm. Biomed. Anal. 2002, 30, 1133–1142. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Botero-Coy, A.M.; Rosero-Moreano, M.; Lee, J.; Hernández, F.; Torres-Palma, R.A. An initial approach to the presence of pharmaceuticals in wastewater from hospitals in Colombia and their environmental risk. Water 2022, 14, 950. [Google Scholar] [CrossRef]
- Kwon, M.; Jeon, J.H.; Choi, M.K.; Song, I.S. The development and validation of a novel “Dual Cocktail” probe for cytochrome P450s and transporter functions to evaluate pharmacokinetic drug-drug and herb-drug interactions. Pharmaceutics 2020, 12, 938. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Ciltas, U. Determination of diclofenac in pharmaceutical preparations by voltammetry and gas chromatography methods. J. Pharm. Anal. 2015, 5, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ozaltin, N.; Temizer, A. Different pulse polarographic determination of omeprazole in pharmaceutical preparations. Electroanalysis 1994, 6, 799–803. [Google Scholar] [CrossRef]
- Parys, W.; Pyka-Pająk, A.; Dołowy, M. Application of thin-layer chromatography in combination with densitometry for the determination of diclofenac in enteric coated tablets. Pharmaceuticals 2019, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- ICH. ICH Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology, Q2(R1); ICH: Geneva, Switzerland, 2005; Available online: http://www.ich.org/products/guidelines/efficacy/article/efficacy-guidelines.htmL (accessed on 28 March 2022).
- Ferenczi Fodor, K.; Renger, B.; Végh, Z. The frustrated reviewer–Recurrent failures in manuscripts describing validation of quantitative TLC/HPTLC procedures for analysis of pharmaceuticals. J. Planar Chromatogr. Mod. TLC 2010, 23, 173–179. [Google Scholar] [CrossRef]
- DellaGreca, M.; Iesce, M.R.; Previtera, L.; Rubino, M. Degradation of lansoprazole and omeprazole in the aquatic environment. Chemosphere 2006, 63, 1087–1093. [Google Scholar] [CrossRef]
- Krzek, J.; Starek, M. Densitometric deetrmination of diclofenac, 1-(2,6-dichlorophenyl)indolin-2-one and indolin-2-one in pharmaceutical preparations and model solutions. J. Pharm. Biomed. Anal. 2002, 28, 227–243. [Google Scholar] [CrossRef]
- Elzayat, E.M.; Ibrahim, M.F.; Abdel-Rahman, A.A.; Alanazi, F.K.; Habib, W.A. A validated-stability-indicating UPLC method for determination of diclofenac sodium in its pure form and matrix formulations. Arab. J. Chem. 2017, 10 (Suppl. S2), S3245–S3254. [Google Scholar] [CrossRef]
- Bagal, M.V.; Gogate, P.R. Degradation of diclofenac sodium using combined processes based on hydrodynamic cavitation and heterogeneous photocatalysis. Ultrason. Sonochem. 2014, 21, 1035–1043. [Google Scholar] [CrossRef]
- Epold, I.; Dulova, N.; Trapido, M. Degradation of diclofenac in aqueous solution by homogeneous and heterogeneous photolysis. J. Environ. Eng. Ecol. Sci. 2012, 1, 3. [Google Scholar] [CrossRef]
- Jiménez-Salcedo, M.; Monge, M.; Tena, M.T. The photocatalytic degradation of sodium diclofenac in different water matrices using g-C3N4 nanosheets: A study of the intermediate by-products and mechanism. J. Environ. Chem. Eng. 2021, 9, 105827. [Google Scholar] [CrossRef]
- Hu, C.Y.; Liu, Y.J.; Kuan, W.H. pH-dependent degradation of diclofenac by a tunnel-structured manganese oxide. Water 2022, 12, 2203. [Google Scholar] [CrossRef]
- Belal, T.S.; Bedair, M.M.; Gazy, A.A.; Guirguis, K.M. Validated selective HPLC-DAD method for the simultaneous determination of diclofenac sodium and lidocaine hydrochloride in presence of four of their related substances and potential impurities. Acta Chromatogr. 2015, 27, 477–493. [Google Scholar] [CrossRef]
- United States Pharmacopeial Convention. The United States Pharmacopoeia, 34th ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 2011. [Google Scholar]
- Thongchai, W.; Liawruangrath, B.; Thongpoon, C.; Machan, T. High performance thin layer chromatographic method for the determination of diclofenac sodium in pharmaceutical formulations. Chiang Mai J. Sci. 2006, 33, 123–128. [Google Scholar]
- Konieczka, P.; Namiesnik, J. Validation of analytical procedures. In Evaluation and Quality Control of Analytical Measurement Results; Konieczka, P., Namiesnik, J., Eds.; WNT: Warsaw, Poland, 2007. (In Polish) [Google Scholar]
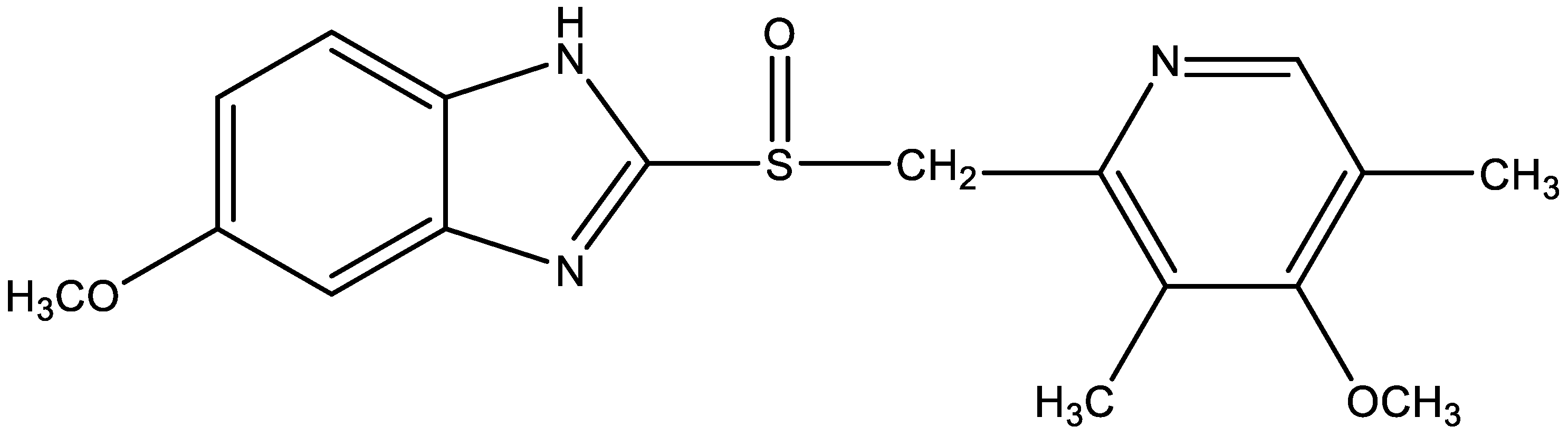
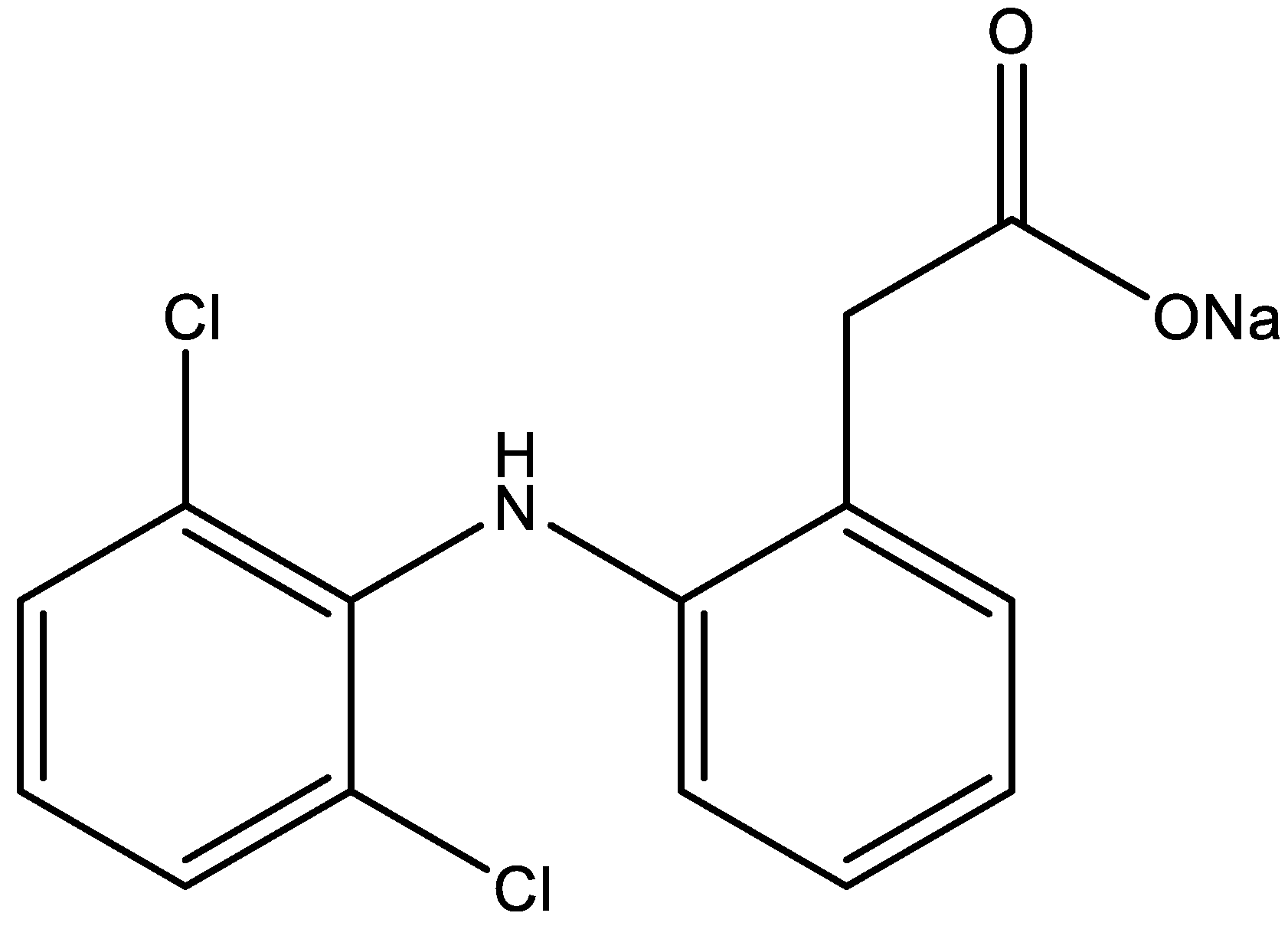

| Physicochemical Property | Omeprazole | Diclofenac Sodium |
|---|---|---|
| Empirical formula | C17H19N3O3S | C14H10Cl2NNaO2 |
| Molecular mass | 345.4 g/mol | 318.13 g/mol |
| Melting point | 155 °C | 283–285 °C |
| Solubility in water | 0.359 mg/mL | 21.3 g/L in 25 °C |
| pKa | 9.29 | 4.15 |
| logPexp | 2.33 | - |
| AlogPs | 1.56 | 4.75 |
| AClogP | 2.48 | 4.62 |
| xlogP2 | 0.60 | 3.85 |
| xlogP3 | 2.23 | 4.71 |
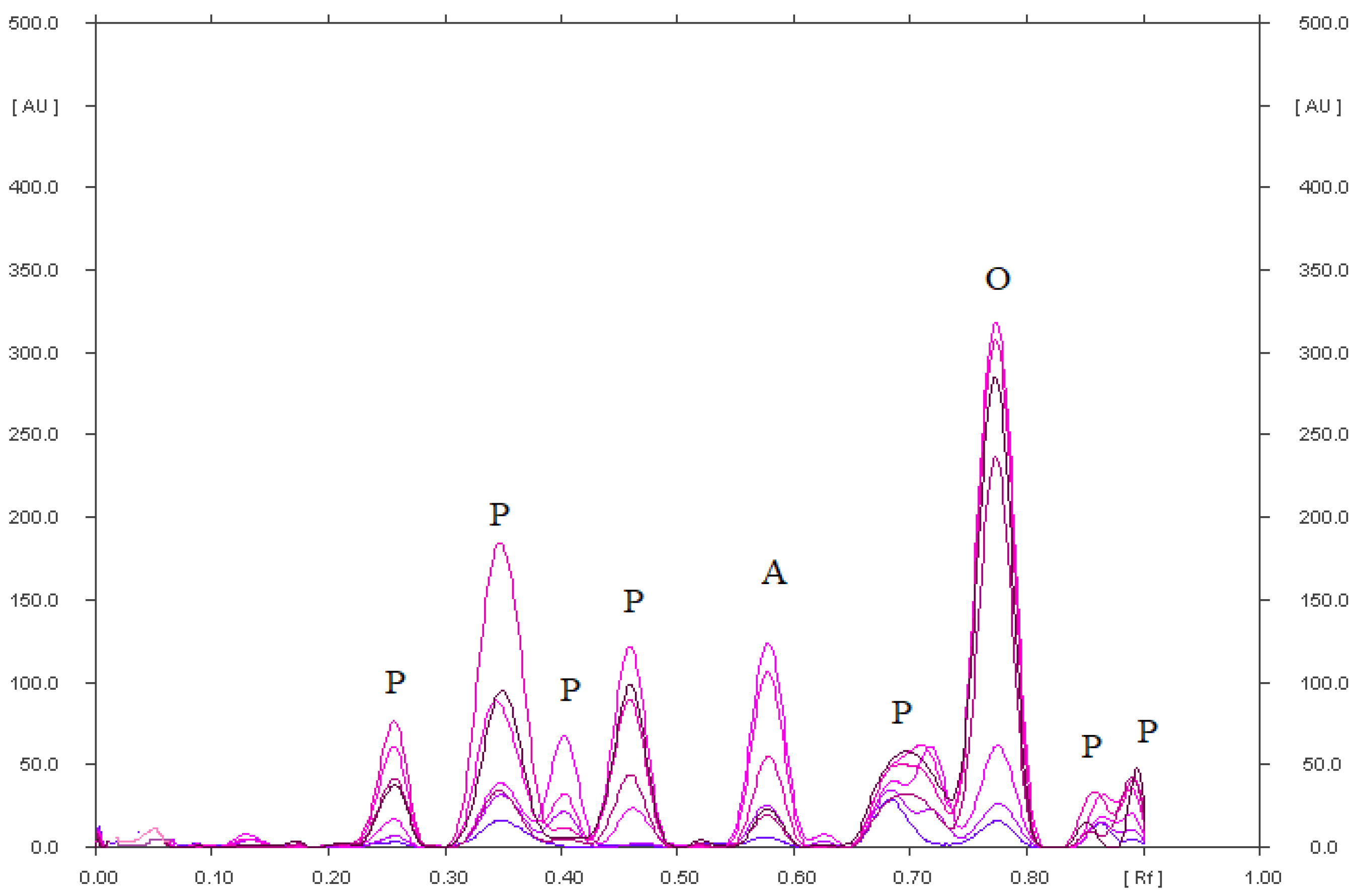
| Stress Conditions | RF of Omeprazole Degradation Products (P) and RF of Omeprazole (O) |
|---|---|
| Omeprazole in an acidic environment heated at 80 °C for 90 min. | 0.25; 0.35; 0.40; 0.45; 0.58 (b); 0.70; 0.85; 0.90 for P |
| 0.79 for O | |
| Omeprazole with the addition of saline, heated at 80 °C for 90 min. | 0.78; 0.89 for P |
| 0.82 for O | |
| Omeprazole in an alkaline environment, heated at 80 °C for 90 min. | 0.25; 0.34; 0.42; 0.57; 0.62 (b); 0.68; 0.72 for P |
| 0.79 for O | |
| Omeprazole with the addition of hydrogen peroxide, heated at 80 °C for 90 min. | 0.17; 0.40; 0.46; 0.51; 0.61 (b); 0.70; 0.79; 0.88 for P |
| 0.79 for O | |
| Omeprazole in a methanolic solution irradiated with UV at λ = 254 nm for 90 min. | 0.49; 0.70 for P |
| 0.81 for O | |
| Reference solution | 0.81 for O |
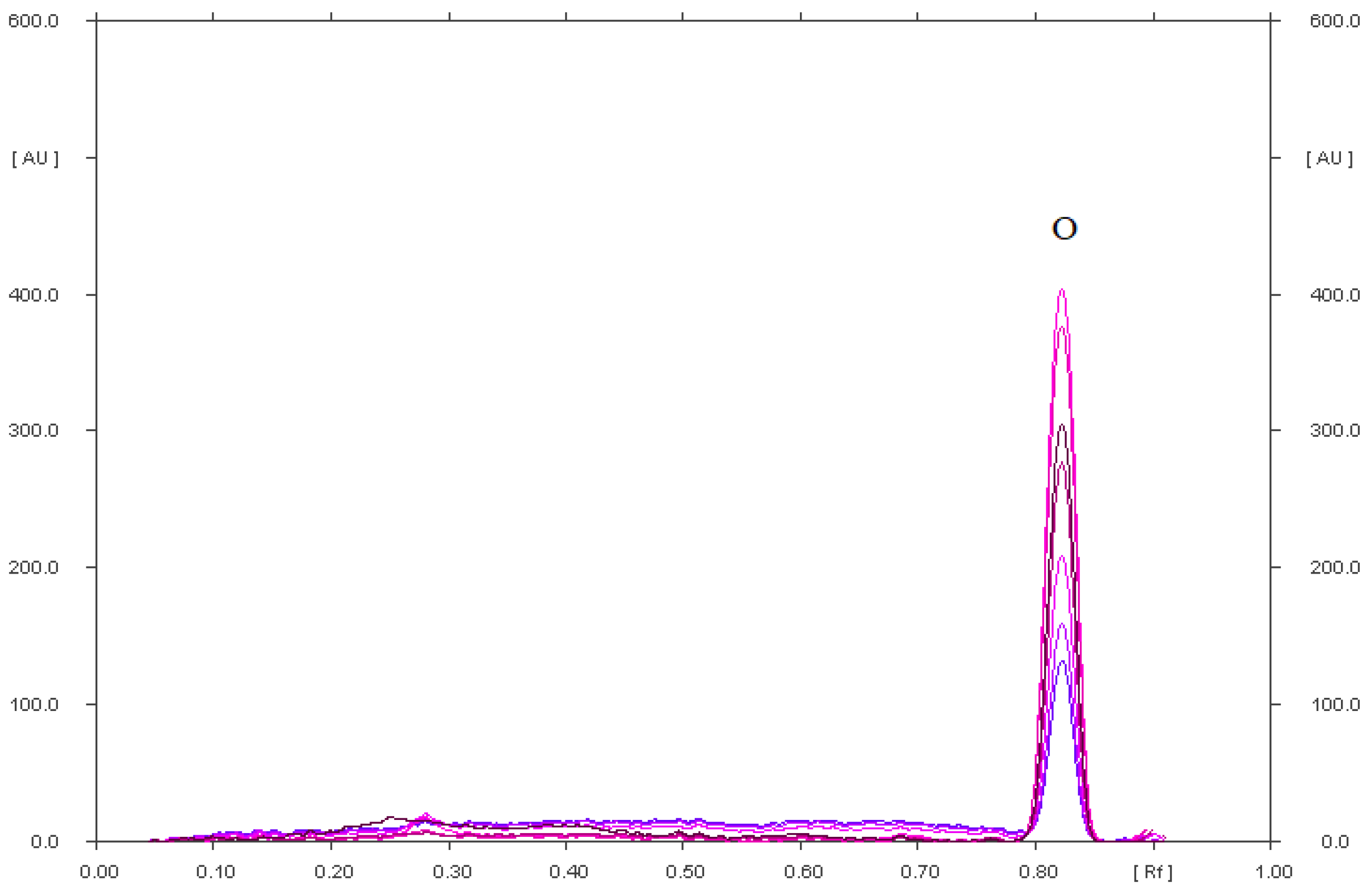
| Stress Conditions | RF of Degradation Products of Diclofenac Sodium (P) and RF of Diclofenac Sodium (D) |
|---|---|
| Diclofenac sodium in an acidic environment heated at 90 °C for 90 min (1.5 h) | 0.11, 0.38 0.63, 0.73, 0.80 (a) for P |
| 0.47 for D | |
| Diclofenac sodium in an acidic environment, heated at 90 °C for 5 h | 0.07, 0.11, 0.16, 0.73, 0.82 (a) for P |
| 0.49 for D | |
| Diclofenac sodium in a methanolic solution irradiated with UV at λ = 254 nm for 5 h. | 0.18, 0.37, 0.42, 0.65, 0.91 for P |
| 0.48 for D | |
| Diclofenac sodium, which was exposed to UV radiation (λ = 254 nm) on silica gel for 5 h. | 0.03, 0.21, 0.33, 0.41, 0.75, 0.91 for P |
| or O |
| Method Characteristic | Results | |||
|---|---|---|---|---|
| Specificity | Specific | |||
| Range [μg/spot] | 0.04–1.00 | |||
| Linearity | A = 1907.3 (±47.4) + 13,491.4 (±86.7) · x n = 13; r = 0.999; s = 98.8; F = 24222; p < 0.0001 | |||
| Limit of detection (LOD) [μg/spot] Limit of quantification (LOQ) [μg/spot] | 0.009 0.028 | |||
| For tablets Accuracy and precision | ||||
| DicloDuo Combi | Omeprazole Genoptim SPH | Bioprazol Bio Max | ||
| Accuracy, n = 6 | ||||
| for 50% standard added | R = 101.3%; CV = 2.21% | R = 99.6%; CV = 1.36% | R = 102.1%; CV = 2.01% | |
| for 100% standard added | R = 98.4%; CV = 1.89% | R = 100.8%; CV = 2.78% | R = 98.8%; CV = 1.98% | |
| for 150% standard added | R = 99.2%; CV = 2.46% | R = 101.6%; CV = 2.24% | R = 99.1%; CV = 1.78% | |
| Quantity of Precision (CV, [%]) n = 3 | ||||
| Interday | 0.10 µg/spot | 1.87 | 2.13 | 1.64 |
| 0.50 µg/spot | 2.13 | 0.89 | 1.89 | |
| 0.90 µg/spot | 2.56 | 0.99 | 1.05 | |
| Intraday | 0.10 µg/spot | 1.45 | 1.78 | 1.56 |
| 0.50 µg/spot | 2.45 | 2.13 | 1.91 | |
| 0.90 µg/spot | 2.81 | 1.89 | 1.08 | |
| Robustness | Robust | Robust | Robust | |
| Method Characteristic | Results | |
|---|---|---|
| Specificity | Specific | |
| Range [μg/spot] | 5.00–15.00 | |
| Linearity | A = 6880.4 (±51.8) + 278.4 (±4.9) ∙ x n = 11; r = 0.998; s = 51.8; F = 3181.9; p < 0.0001 | |
| Limit of detection (LOD) [μg/spot] | 0.61 | |
| Limit of quantification (LOQ) [μg/spot] | 1.84 | |
| For tablets Accuracy and precision | ||
| Accuracy (n = 6) | ||
| for 50% standard added (n = 6) | R = 103.1%; CV = 2.78% | |
| for 100% standard added (n = 6) | R = 98.5%; CV = 2.54% | |
| for 150% standard added (n = 6) | R = 98.4%; CV = 2.12% | |
| Quantity of Precision (CV, [%]) n = 3 | ||
| 6.00 μg/spot | 1.13 | |
| Interday | 10.00 μg/spot | 2.42 |
| 14.00 μg/spot | 1.88 | |
| 6.00 μg/spot | 2.45 | |
| Intraday | 10.00 μg/spot | 2.78 |
| 14.00 μg/spot | 2.38 | |
| Robustness | Robust | |
| Parameter | % RSD of Peak Area of | |||
|---|---|---|---|---|
| Diclofenac in DicloDuo Combi | Omeprazole in | |||
| DicloDuo Combi | Omeprazole Genoptim SPH | Bioprazol Bio Max | ||
| Chromatographic plates 1.05570 and 1.05554 | 1.11% | 0.95% | 1.13% | 1.29% |
| Mobile phase volume (50 mL ± 5%) | 0.89% | 0.85% | 0.88% | 0.96% |
| Temperature of the activation of the plates at 120 (±5) °C | 1.12% | 1.13% | 1.04% | 1.38% |
| Development distance (±5 mm) | 1.89% | 0.97% | 1.46% | 1.29% |
| Time od saturation (±5 min) | 1.28% | 1.33% | 1.12% | 0.99% |
| Number of Analysis | Pharmaceutical Preparation | |||
|---|---|---|---|---|
| DicloDuo Combi | Omeprazole Genoptim SPH | Bioprazol Bio Max | ||
| Diclofenac Sodium | Omeprazole | Omeprazole | Omeprazole | |
| 1 | 73.9 | 19.1 | 20.8 | 18.6 |
| 2 | 74.2 | 18.8 | 21.1 | 20.5 |
| 3 | 72.9 | 19.6 | 20.5 | 20.7 |
| 4 | 74.5 | 19.2 | 19.7 | 20.1 |
| 5 | 73.4 | 19.0 | 20.7 | 20.9 |
| 6 | 74.9 | 18.9 | 21.2 | 20.4 |
| Average amount [mg/capsule] | 74.0 | 19.1 | 20.7 | 20.2 |
| The label claim [mg/capsule] | 75 | 20 | 20 | 20 |
| Standard deviation (SD) | 0.73 | 0.28 | 0.54 | 0.83 |
| Coefficient of variation [CV%] | 0.53 | 0.08 | 0.29 | 0.69 |
| Confidence interval of arithmetic mean with confidence level equal 95% | μ = 74.0 ± 0.7 | μ = 19.1 ± 0.3 | μ = 20.7 ± 0.5 | μ = 20.2 ± 0.8 |
| Amount (%) in relations to the label claim | 98.7 | 95.5 | 103.5 | 101.0 |
| Analytical Method | LOD | Ref. |
|---|---|---|
| Omeprazole | ||
| TLC | 12.7 ng/spot | [15] |
| TLC | 0.961 ng/spot | [20] |
| TLC | 0.020 µg/spot | [24] |
| TLC | 0.009 µg/spot (9 ng/spot) 0.18 µg/mL | in this work |
| HPTLC | 0.005 µg/spot | [18] |
| HPTLC | 0.099 µg/spot | [27] |
| HPTLC | 0.074 µg/spot | [17] |
| HPTLC | 0.010 µg/spot | [19] |
| HPTLC | 2.64 ng/spot | [21] |
| HPTLC | 4.68 ng/spot | [22] |
| HPTLC | 40.83 ng/spot | [30] |
| HP-TLC | 7.9 ng/spot | [25] |
| HPLC-UV | 0.4 µg/mL | [29] |
| RP-HPLC | 131.27 ng/mL | [30] |
| RP-HPLC | 0.076 µg/mL | [33] |
| RP-HPLC | 0.0712 μg/mL | [34] |
| UHPLC | 1.48 µg/mL | [24] |
| RP-HPLC | 0.54 µg/mL | [39] |
| RP-HPLC | 0.06 µg/mL | [31] |
| UV-Spectrophotometric | 0.105 µg/mL | [41] |
| Spectrophotometric | 0.033 µg/mL | [43] |
| Diclofenac sodium | ||
| TLC | 0.0107 µg/spot | [15] |
| TLC | 0.28 µg/spot | [48] |
| TLC | 1 µg/mL | [60] |
| TLC | 0.61 µg/spot (122 µg/mL | in this work |
| RP-HPLC | 0.239 μg/mL | [34] |
| RP-HPLC | 0.011 µg/mL | [38] |
| GC–MS | 0.15 μg/mL | [46] |
| linear sweep voltammetry (LSV) | 4.8 μg/mL | [46] |
| UV-Spectrophotometric | 0.048 µg/mL | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parys, W.; Pyka-Pająk, A. TLC–Densitometry for Determination of Omeprazole in Simple and Combined Pharmaceutical Preparations. Pharmaceuticals 2022, 15, 1016. https://doi.org/10.3390/ph15081016
Parys W, Pyka-Pająk A. TLC–Densitometry for Determination of Omeprazole in Simple and Combined Pharmaceutical Preparations. Pharmaceuticals. 2022; 15(8):1016. https://doi.org/10.3390/ph15081016
Chicago/Turabian StyleParys, Wioletta, and Alina Pyka-Pająk. 2022. "TLC–Densitometry for Determination of Omeprazole in Simple and Combined Pharmaceutical Preparations" Pharmaceuticals 15, no. 8: 1016. https://doi.org/10.3390/ph15081016
APA StyleParys, W., & Pyka-Pająk, A. (2022). TLC–Densitometry for Determination of Omeprazole in Simple and Combined Pharmaceutical Preparations. Pharmaceuticals, 15(8), 1016. https://doi.org/10.3390/ph15081016







