Bolus Injection of Liraglutide Raises Plasma Glucose in Normal Rats by Activating Glucagon-like Peptide 1 Receptor in the Brain
Abstract
1. Introduction
2. Results
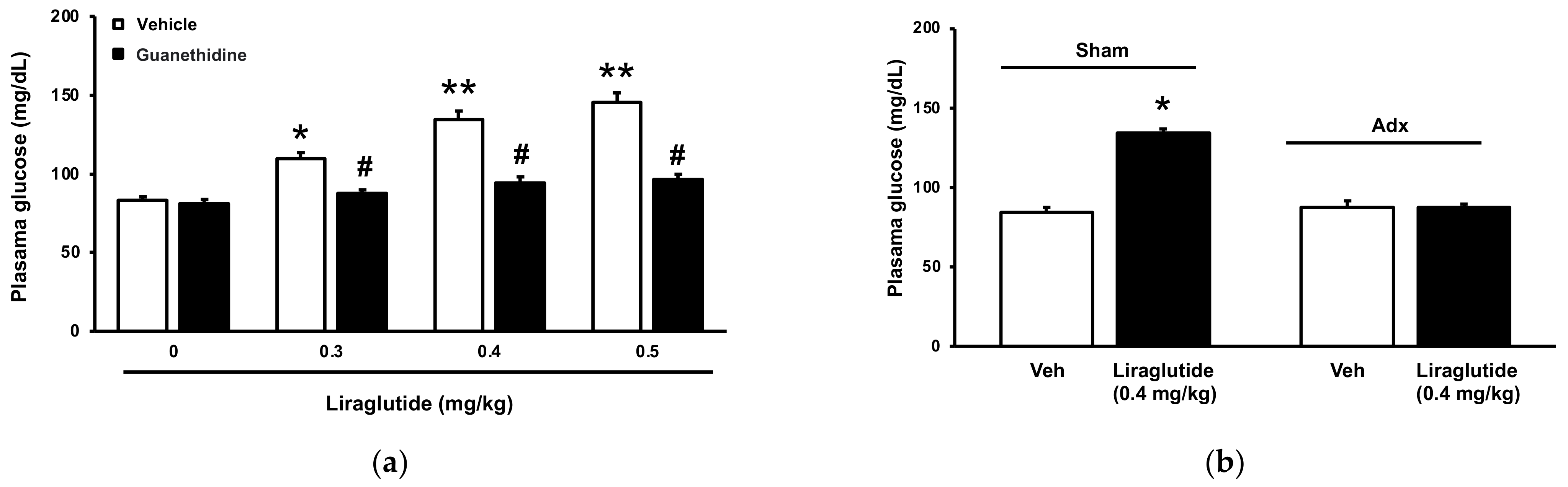
2.1. Acute Effects of Liraglutide on Plasma Glucose in Normal Rats
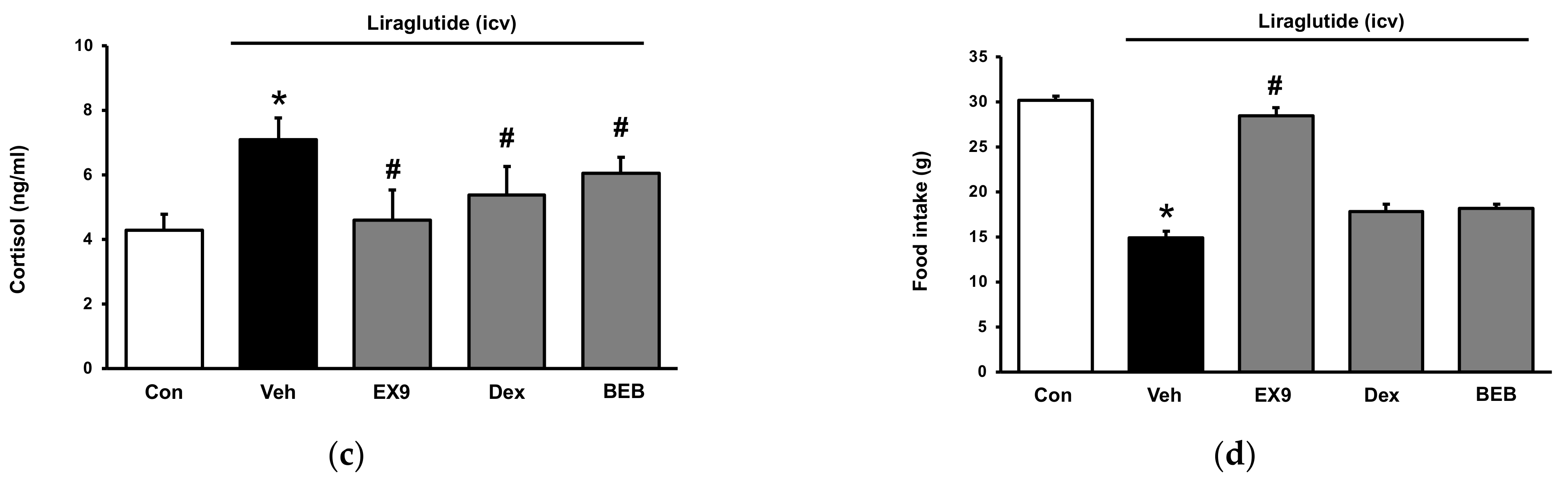
2.2. Acute Hyperglycemia in Rats Received Injection of Liraglutide into Brain
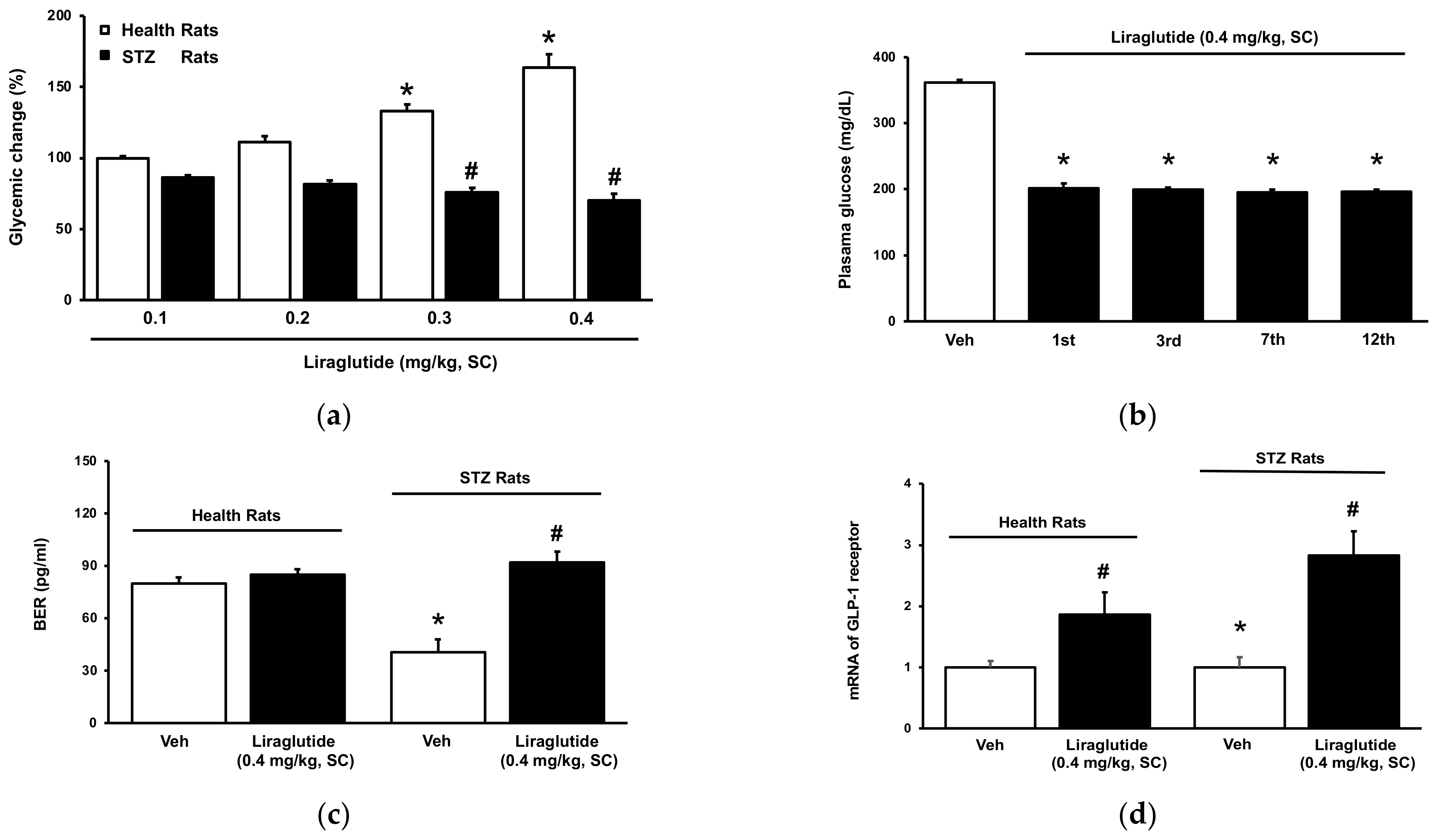
2.3. Tolerance of Hyperglycemia-Induced by Liraglutide in Normal Rats
2.4. Role of Receptor Sensitivity in the Dual Effects of Liraglutide In Vivo
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Model
4.3. Laboratory Determinations
4.4. Adrenalectomy in Rats
4.5. Incubation of Isolated Adrenal Gland
4.6. Intracerebroventricular (ICV) Injection
4.7. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. S1), 5–21. [Google Scholar] [CrossRef]
- Schwasinger-Schmidt, T.; Robbins, D.C.; Williams, S.J.; Novikova, L.; Stehno-Bittel, L. Long-term liraglutide treatment is associated with increased insulin content and secretion in beta-cells, and a loss of alpha-cells in ZDF rats. Pharmacol. Res. 2013, 76, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Montanya, E.; Sesti, G. A review of efficacy and safety data regarding the use of liraglutide, a once-daily human glucagon-like peptide 1 analogue, in the treatment of type 2 diabetes mellitus. Clin. Ther. 2009, 31, 2472–2488. [Google Scholar] [CrossRef] [PubMed]
- Cervera, A.; Wajcberg, E.; Sriwijitkamol, A.; Fernandez, M.; Zuo, P.; Triplitt, C.; Musi, N.; DeFronzo, R.A.; Cersosimo, E. Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E846–E852. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Management of endocrine disease: Are all GLP-1 agonists equal in the treatment of type 2 diabetes? Eur. J. Endocrinol. 2019, 181, R211–R234. [Google Scholar] [CrossRef]
- Gil-Lozano, M.; Romani-Perez, M.; Outeirino-Iglesias, V.; Vigo, E.; Gonzalez-Matias, L.C.; Brubaker, P.L.; Mallo, F. Corticotropin-releasing hormone and the sympathoadrenal system are major mediators in the effects of peripherally administered Exendin-4 on the hypothalamic-pituitary-adrenal axis of male rats. Endocrinology 2014, 155, 2511–2523. [Google Scholar] [CrossRef]
- Gil-Lozano, M.; Perez-Tilve, D.; Alvarez-Crespo, M.; Martis, A.; Fernandez, A.M.; Catalina, P.A.; Gonzalez-Matias, L.C.; Mallo, F. GLP-1(7-36)-amide and Exendin-4 stimulate the HPA axis in rodents and humans. Endocrinology 2010, 151, 2629–2640. [Google Scholar] [CrossRef]
- Winzeler, B.; da Conceicao, I.; Refardt, J.; Sailer, C.O.; Dutilh, G.; Christ-Crain, M. Effects of Glucagon-Like Peptide-1 Receptor Agonists on Hypothalamic-Pituitary-Adrenal Axis in Healthy Volunteers. J. Clin. Endocrinol. Metab. 2019, 104, 202–208. [Google Scholar] [CrossRef]
- Perez-Tilve, D.; Gonzalez-Matias, L.; Aulinger, B.A.; Alvarez-Crespo, M.; Gil-Lozano, M.; Alvarez, E.; Andrade-Olivie, A.M.; Tschop, M.H.; D’Alessio, D.A.; Mallo, F. Exendin-4 increases blood glucose levels acutely in rats by activation of the sympathetic nervous system. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1088–E1096. [Google Scholar] [CrossRef]
- Cheng, K.C.; Li, Y.X.; Shieh, P.C.; Cheng, J.T.; Hsu, C.C. Liraglutide Activates Glucagon-Like Peptide 1 Receptor to Attenuate Hyperglycemia through Endogenous Beta-Endorphin in Diabetic Rats. Pharmaceuticals 2020, 13, 407. [Google Scholar] [CrossRef]
- Nakade, Y.; Kitano, R.; Yamauchi, T.; Kimoto, S.; Sakamoto, K.; Inoue, T.; Kobayashi, Y.; Ohashi, T.; Sumida, Y.; Ito, K.; et al. Effect of Central Corticotropin-Releasing Factor on Hepatic Lipid Metabolism and Inflammation-Related Gene Expression in Rats. Int. J. Mol. Sci. 2021, 22, 3940. [Google Scholar] [CrossRef]
- Mi, J.; He, W.; Lv, J.; Zhuang, K.; Huang, H.; Quan, S. Effect of berberine on the HPA-axis pathway and skeletal muscle GLUT4 in type 2 diabetes mellitus rats. Diabetes Metab. Syndr. Obes. 2019, 12, 1717–1725. [Google Scholar] [CrossRef]
- Andrews, M.H.; Wood, S.A.; Windle, R.J.; Lightman, S.L.; Ingram, C.D. Acute glucocorticoid administration rapidly suppresses basal and stress-induced hypothalamo-pituitary-adrenal axis activity. Endocrinology 2012, 153, 200–211. [Google Scholar] [CrossRef]
- Lee, S.J.; Diener, K.; Kaufman, S.; Krieger, J.P.; Pettersen, K.G.; Jejelava, N.; Arnold, M.; Watts, A.G.; Langhans, W. Limiting glucocorticoid secretion increases the anorexigenic property of Exendin-4. Mol. Metab. 2016, 5, 552–565. [Google Scholar] [CrossRef]
- Gil-Lozano, M.; Romani-Perez, M.; Outeirino-Iglesias, V.; Vigo, E.; Brubaker, P.L.; Gonzalez-Matias, L.C.; Mallo, F. Effects of prolonged Exendin-4 administration on hypothalamic-pituitary-adrenal axis activity and water balance. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1105–E1117. [Google Scholar] [CrossRef]
- Yamamoto, H.; Lee, C.E.; Marcus, J.N.; Williams, T.D.; Overton, J.M.; Lopez, M.E.; Hollenberg, A.N.; Baggio, L.; Saper, C.B.; Drucker, D.J.; et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J. Clin. Investig. 2002, 110, 43–52. [Google Scholar] [CrossRef][Green Version]
- Diz-Chaves, Y.; Herrera-Perez, S.; Gonzalez-Matias, L.C.; Lamas, J.A.; Mallo, F. Glucagon-Like Peptide-1 (GLP-1) in the Integration of Neural and Endocrine Responses to Stress. Nutrients 2020, 12, 3304. [Google Scholar] [CrossRef]
- Mietlicki-Baase, E.G.; Ortinski, P.I.; Reiner, D.J.; Sinon, C.G.; McCutcheon, J.E.; Pierce, R.C.; Roitman, M.F.; Hayes, M.R. Glucagon-like peptide-1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic AMPA/kainate signaling. J. Neurosci. 2014, 34, 6985–6992. [Google Scholar] [CrossRef]
- Adams, J.M.; Pei, H.; Sandoval, D.A.; Seeley, R.J.; Chang, R.B.; Liberles, S.D.; Olson, D.P. Liraglutide Modulates Appetite and Body Weight Through Glucagon-Like Peptide 1 Receptor-Expressing Glutamatergic Neurons. Diabetes 2018, 67, 1538–1548. [Google Scholar] [CrossRef]
- Hsu, T.M.; Hahn, J.D.; Konanur, V.R.; Lam, A.; Kanoski, S.E. Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology 2015, 40, 327–337. [Google Scholar] [CrossRef]
- Gardiner, S.M.; March, J.E.; Kemp, P.A.; Bennett, T. Autonomic nervous system-dependent and -independent cardiovascular effects of Exendin-4 infusion in conscious rats. Br. J. Pharmacol. 2008, 154, 60–71. [Google Scholar] [CrossRef]
- Zelena, D.; Filaretova, L.; Mergl, Z.; Barna, I.; Toth, Z.E.; Makara, G.B. Hypothalamic paraventricular nucleus, but not vasopressin, participates in chronic hyperactivity of the HPA axis in diabetic rats. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E243–E250. [Google Scholar] [CrossRef]
- Scott, L.J. Dulaglutide: A Review in Type 2 Diabetes. Drugs 2020, 80, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Cukierman-Yaffe, T.; Gerstein, H.C.; Colhoun, H.M.; Diaz, R.; Garcia-Perez, L.E.; Lakshmanan, M.; Bethel, A.; Xavier, D.; Probstfield, J.; Riddle, M.C.; et al. Effect of dulaglutide on cognitive impairment in type 2 diabetes: An exploratory analysis of the REWIND trial. Lancet Neurol. 2020, 19, 582–590. [Google Scholar] [CrossRef]
- Vallof, D.; Kalafateli, A.L.; Jerlhag, E. Long-term treatment with a glucagon-like peptide-1 receptor agonist reduces ethanol intake in male and female rats. Transl. Psychiatry 2020, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Hunter, K.; Holscher, C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012, 13, 33. [Google Scholar] [CrossRef]
- Hwang, S.L.; Liu, I.M.; Tzeng, T.F.; Cheng, J.T. Activation of imidazoline receptors in adrenal gland to lower plasma glucose in streptozotocin-induced diabetic rats. Diabetologia 2005, 48, 767–775. [Google Scholar] [CrossRef]
- Chen, H.Y.; Wu, J.S.; Chen, J.J.; Cheng, J.T. Impaired regulation function in cardiovascular neurons of nucleus tractus solitarii in streptozotocin-induced diabetic rats. Neurosci. Lett. 2008, 431, 161–166. [Google Scholar] [CrossRef]
- Bao, Y.; Jiang, L.; Chen, H.; Zou, J.; Liu, Z.; Shi, Y. The Neuroprotective Effect of Liraglutide is Mediated by Glucagon-Like Peptide 1 Receptor-Mediated Activation of cAMP/PKA/CREB Pathway. Cell. Physiol. Biochem. 2015, 36, 2366–2378. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-C.; Cheng, J.-T.; Hsu, P.H.; Li, Y.; Cheng, K.-C. Bolus Injection of Liraglutide Raises Plasma Glucose in Normal Rats by Activating Glucagon-like Peptide 1 Receptor in the Brain. Pharmaceuticals 2022, 15, 904. https://doi.org/10.3390/ph15070904
Hsu C-C, Cheng J-T, Hsu PH, Li Y, Cheng K-C. Bolus Injection of Liraglutide Raises Plasma Glucose in Normal Rats by Activating Glucagon-like Peptide 1 Receptor in the Brain. Pharmaceuticals. 2022; 15(7):904. https://doi.org/10.3390/ph15070904
Chicago/Turabian StyleHsu, Chia-Chen, Juei-Tang Cheng, Ping Hao Hsu, Yingxiao Li, and Kai-Chun Cheng. 2022. "Bolus Injection of Liraglutide Raises Plasma Glucose in Normal Rats by Activating Glucagon-like Peptide 1 Receptor in the Brain" Pharmaceuticals 15, no. 7: 904. https://doi.org/10.3390/ph15070904
APA StyleHsu, C.-C., Cheng, J.-T., Hsu, P. H., Li, Y., & Cheng, K.-C. (2022). Bolus Injection of Liraglutide Raises Plasma Glucose in Normal Rats by Activating Glucagon-like Peptide 1 Receptor in the Brain. Pharmaceuticals, 15(7), 904. https://doi.org/10.3390/ph15070904




