Abstract
Platinum-based chemotherapy regimens have been proven to be effective in various cancers; however, considerable toxicities may develop and can even lead to treatment discontinuation. Diverse factors may influence adverse treatment events, with pharmacogenetic variations being one prime example. Polymorphisms within the glutathione S-transferase pi 1 (GSTP1) gene may especially alter enzyme activity and, consequently, various toxicities in patients receiving platinum-based chemotherapy. Due to a lack of consistency in the degree of elevated complication risk, we performed a systematic literature review and meta-analysis to determine the level of platinum-associated toxicity in patients with the GSTP1 rs1695 polymorphism. We conducted a systematic search for eligible studies published before January 2022 from PubMed, Web of Science, and EMBASE based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to evaluate the strength of the association between the rs1695 polymorphism and various toxicities. Ten eligible studies met the inclusion criteria. The pooled ORs for hematological toxicity and neutropenia in the patients with the variant (G) allele were 1.7- and 2.6-times higher than those with the AA genotype (95% CI 1.06–2.73 and 1.07–6.35), respectively. In contrast, the rs1695 polymorphism resulted in a 44% reduced gastrointestinal toxicity compared to wild-type homozygotes. Our study found that the GSTP1 rs1695 polymorphism was significantly correlated with platinum-induced toxicities. The study also revealed that rs1695 expression exhibited tissue-specific patterns and thus yielded opposite effects in different tissues. A personalized chemotherapy treatment based on these polymorphisms may be considered for cancer patients in the future.
1. Introduction
For several decades, platinum-based cancer treatment has been a key component of chemotherapy regimens; it has been reported that nearly 50% of all cancer treatments involve platinum-based regimens [1]. Among platinum-based cancer drugs, cisplatin, oxaliplatin, and carboplatin are the top three most used platinum chemotherapy agents [2]. Cisplatin, the first FDA-approved platinum compound for chemotherapy, is the most commonly used drug in the treatment of several cancers, including testicular cancer, ovarian cancer, head and neck cancer, esophageal cancer, and lung cancer. Carboplatin is a second-generation platinum drug with activity equivalent to cisplatin, while oxaliplatin is a third-generation platinum drug with similar effectiveness and safety to cisplatin. Although platinum-based agents have been effective, a variety of adverse events have been consistently reported.
Glutathione S-transferase (GST) is a phase II enzyme that detoxifies substances, including platinum chemotherapy drugs [3]. GST Pi 1 (GSTP1) belongs to one of four major classes of GSTs and is associated with a sensitivity to platinum chemotherapy drugs [4]. It plays an important role in mediating the interaction between medications and glutathione [5]; hence, polymorphisms within the GSTP1 may result in changes in enzyme activity. Especially given that alterations in GST can result in decreased platinum detoxification, causing various toxicities [3]. Since polymorphisms in GSTP1 may cause GST abnormalities, genetic screening can be proposed in patients receiving platinum-based chemotherapy.
GSTP1 I105V (rs1695 A > G) is a single nucleotide polymorphism (SNP) located within the substrate-binding domain, resulting in an amino-acid substitution [6]. It is known that this polymorphism is associated with enzyme activity and clinical outcomes of platinum-based regimens [7,8]. GSTP1 rs1695 was widely studied in candidate gene association investigations, but results were inconsistent [9,10]; in one study, patients with rs1695 variants had decreased neurotoxicity [11], whereas, in another, opposite results were presented [3]. In this context, identifying the association between polymorphisms and platinum-related toxicities in a larger number of subjects would be beneficial for individualized chemotherapy. Hence, this study aims to investigate the potential link between the rs1695 polymorphism and platinum-induced toxicity by using a systematic literature review and meta-analysis.
2. Methods
2.1. Literature Search Strategy
A systematic search was carried out by two investigators independently using PubMed, Web of Science, and EMBASE to find studies published before 26 January 2022. The following search terms were included: (cisplatin OR oxaliplatin OR carboplatin OR platinum compounds) AND (glutathione transferase* OR GST OR GSTM* OR GSTT* OR GSTP* OR GSTP1 OR rs1695 OR Ile105Val) AND (polymorph* OR genotyp* OR null OR deletion OR variant* OR mutation*) AND (toxicity OR adverse OR side-effects OR adverse effects). An initial screening of the titles and abstracts was conducted following the removal of duplicates. The studies that were selected for inclusion in this study were then reviewed in the full text following the eligibility criteria. This study is registered at the INPLASY (approval No. INPLASY202230025).
2.2. Inclusion and Exclusion Criteria
Studies were included if they (1) were randomized controlled trials (RCT) or cohort studies; (2) included adult patients receiving platinum-based regimens; (3) evaluated the association of rs1695 SNP with toxicity; (4) included applicable data on genotype in both cases and controls; or (5) published in English. Studies were excluded if they were (1) not involving toxicity outcomes; (2) not involving rs1695; (3) pharmacokinetic studies; (4) reviews, meeting abstracts, case reports, or case series, comments, letters, updates, news, editorials, or conferences; or (5) unable to provide appropriate data.
2.3. Data Extraction and Quality Assessment
For each study, the following data were collected: the last name of the first author, the year of publication, the number of patients, the country, the mean and range of the participants’ ages, the percentage of females, the type of cancer, the treatment regimen, and the definitions of toxicity outcomes. Selected studies were evaluated based on the Newcastle–Ottawa Scale (NOS). Among the components of the NOS are subject selection, comparability of study groups, and the exposure or outcome; every study can attain a total score of 9.
2.4. Statistical Analysis
To examine the association between the outcomes and polymorphism, odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. The heterogeneity of the selected studies was evaluated using I2. If I2 was greater than 50%, this indicated a high degree of heterogeneity; we applied the random-effects model. A fixed-effects model was applied when I2 was less or equal to 50%. We considered p-values lower than 0.05 to be significant. We performed statistical analyses using Review Manager (RevMan) version 5.4 (The Cochrane Collaboration, Copenhagen, Denmark).
Egger’s and Begg’s regression tests of the funnel plot were conducted to identify publication bias. The analysis of publication bias was conducted using the RStudio software (version 4.0.0; RStudio: Integrated Development for R, Boston, MA, USA). This meta-analysis was written based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Statement which comprises a checklist of 27 items.
3. Results and Discussion
Figure 1 shows a flow diagram of the literature search and selection process. There were a total of 632 records initially searched from PubMed (n = 139), Web of Science (n = 141), and EMBASE (n = 352). After removing duplicates (n = 243) and irrelevant studies from the title and abstract (n = 340), 49 records were selected for full-text review. Thirty-nine records were excluded due to the following reasons: not involving toxicity outcome (n = 8), not involving rs1695 (n = 7), pharmacokinetic studies (n = 5), abstracts, or case reports (n = 7), or unable to extract data (n = 12). Therefore, this meta-analysis included ten studies [3,7,8,11,12,13,14,15,16,17].
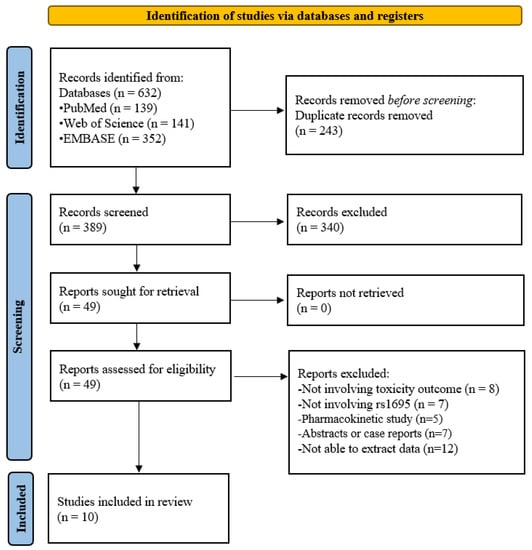
Figure 1.
Flow diagram of the study selection process.
Table 1 shows the baseline characteristics of the included studies. The studies were published between 2006 and 2021. Six studies were performed in Asia, two in Europe, and two in South America. This study included patients with different types of cancer, including esophageal cancer, lung cancer, and colon cancer. The studies with platinum-based regimens, including cisplatin, oxaliplatin, or carboplatin, were evaluated. Using NOS, each study received six or higher than six points out of nine.

Table 1.
Characteristics of studies included.
Figure 2 presents the OR and 95% CIs relating to the rs1695 polymorphism in the GSTP1 gene with the risks of various platinum-associated toxicities, such as gastrointestinal toxicity, hematological toxicity, neutropenia, and neurotoxicity. Gastrointestinal toxicity involved four studies and suggested that the rs1695 polymorphism was associated with an approximately 44% decrease in the odds of toxicity compared to the wild-type homozygotes. There were five hematological toxicity and three neutropenia studies in which the rs1695 polymorphism was associated with elevated toxicities by about 1.7- and 2.6-times, respectively (95% CI 1.06–2.73 and 1.07–6.35, respectively).
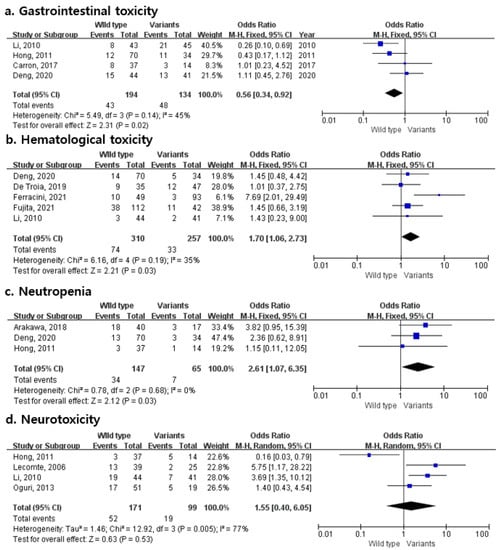
Figure 2.
Forest plots of the association between platinum-induced toxicities and GSTP1 polymorphism (a) gastrointestinal toxicity; (b) hematological toxicity; (c) neutropenia; (d) neurotoxicity.
Since there was no heterogeneity among the included studies for gastrointestinal toxicity, hematological toxicity, and neutropenia (I2 = 45%, 35%, and 0%, respectively), the effect size was calculated using a fixed-effects model. A random-effects model was applied to neurotoxicity (I2 = 77%). Egger’s and Begg’s tests did not show evidence for publication bias in this meta-analysis (gastrointestinal toxicity: Egger’s test, p = 0.776; Begg’s test, p = 0.500; hematological toxicity: Egger’s test, p = 0.520; Begg’s test, p = 0.142; neutropenia: Egger’s test, p = 0.430; Begg’s test, p = 0.602; neurotoxicity: Egger’s test, p = 0.536; Begg’s test, p = 0.174, respectively).
The principal finding of this study is that patients with the G allele of rs1695 have an approximately 1.7% and 2.61% increased odds of platinum-induced hematological toxicity and neutropenia, respectively (95% CI 1.06–2.73 and 1.07–6.35). On the other hand, the rs1695 polymorphism resulted in a 44% decrease in the odds of gastrointestinal toxicity compared to the wild-type homozygotes. The Begg’s and Egger’s tests showed no significant publication bias for toxicities.
Platinum-containing chemotherapy is the largest class of drugs used for cancer treatment and exhibits excellent drug responses. Identical DNA adducts formed by these anti-cancer medications [18] induce various cellular responses, such as transcriptional inhibition, cell cycle arrest, and DNA repair [19,20]. Together, platinum-based anticancer drugs inhibit tumor cell transcription and cause cell cycle arrest via the created DNA adducts. Moreover, platinum-based chemotherapy is advantageous because the platinum complex structure is designed to optimize DNA interaction. The enhanced permeability and retention effect has been applied to the development of the medications [21]. Yet, despite their clinical efficacy, platinum-based anti-cancer medications also exhibit various adverse events.
By catalyzing mercapturic acid synthesis, GSTs act as the first step in eliminating toxic compounds [22]. GSTP1, belonging to a class of GSTs, is known as one of the most vital detoxification enzymes in the body. It is responsible for initiating a pathway to lower the intracellular concentration of platinum medications and eliminate toxic compounds [23]. As GSTP1 polymorphisms can cause GST abnormalities, genetic testing can be suggested for patients receiving platinum-based chemotherapy. While several genetic differences in the GSTP1 gene are hypothesized to affect GST function, research is still ongoing to identify specific variants associated with platinum-induced toxicity.
Several studies have revealed that the rs1695 of GSTP1 mutation results from an A to G change at codon 105, causing a transition from isoleucine to valine, which is associated with decreased enzymatic activity and greatly increased platinum-related toxicities [24,25,26]. Previous studies have investigated the association between the GSTP1 rs1695 gene polymorphism and platinum-induced toxicities [24,27,28]; however, the conclusions were not consistent. Moreover, most meta-analyses were focused on one specific outcome, which created a need to employ a meta-analysis to amalgamate the various outcomes with a larger number of subjects and updated records.
This study showed that patients receiving platinum-based treatment with the G allele of rs1695 had about 1.7- and 2.6-fold higher hematological adverse events and neutropenia compared to those with the AA genotype, respectively. Hematological toxicity and neutropenia are serious adverse events that lead to treatment discontinuation. In this context, results from this study indicated that GSTP1 might serve as a potential marker and influence treatment regimens substantially.
Interestingly, unlike hematological adverse events and neutropenia, contrary results were found regarding gastrointestinal toxicities; the G allele carriers had an approximately 44% decrease in the odds of gastrointestinal toxicities compared to patients with the AA genotype. This may be attributable to the tissue-specific expression of rs1695. An expression quantitative trait loci (eQTL) analysis showed that the minor allele of rs1695 was associated with the decreased expression of GSTP1 in the colon and esophagus (gastroesophageal junction); therefore, lower GSTP1 expression could be related to decreased gastrointestinal toxicities. Although gastrointestinal adverse events are the most common toxicities from platinum-based regimens, previous studies failed to show significant associations or consistent results. This study provided a precise estimate of the effects by combining studies to increase the sample size and power. Thus, determining an individualized treatment strategy based on the polymorphism and estimating the patient’s risk of such toxicities would be important aspects in real clinical settings.
This meta-analysis has several limitations. First, the occurrence of platinum-induced toxicities can be affected by various factors, including other co-medications. Due to the inability to collect raw data, this study could not adjust for confounding factors when pooling odds ratios. Second, due to limited data availability, other critical toxicities including nephrotoxicity or cardiotoxicity could not be analyzed. Third, despite the lack of evidence of publication bias, the possibility cannot be ruled out because of the small sample size.
4. Conclusions
This meta-analysis identified an association between the selected SNP and platinum-induced complications by improving the estimation of its effect size. In particular, this study also demonstrated that rs1695 expression was tissue-specific and had opposite effects in different tissues. Based on this systematic review and meta-analysis on the rs1695 polymorphism and platinum-related toxicity, the future clinical decision regarding platinum-based regimens can be made.
Author Contributions
Conceptualization, D.-C.K. and K.-E.L.; Methodology, W.K., Y.-A.C. and D.-C.K.; Formal analysis, W.K.; Writing—original draft preparation, W.K. and K.-E.L.; Writing—review and editing, D.-C.K. and K.-E.L.; Funding acquisition, D.-C.K. and K.-E.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Medical Research Center Program (2017R1A5A2015541) of the National Research Foundation funded by the Korean government (Ministry of Science, ICT & Future Planning). This work was also supported by the Development Fund Foundation, Gyeongsang National University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Johnstone, T.C.; Park, G.Y.; Lippard, S.J. Understanding and improving platinum anticancer drugs—Phenanthriplatin. Anticancer Res. 2014, 34, 471–476. [Google Scholar] [PubMed]
- Market Study Report. Platinum Based Cancer Drugs Market Size to Reach US$1.8 Billion by 2026. Available online: https://www.globenewswire.com/news-release/2020/03/25/2006050/0/en/Platinum-based-cancer-drugs-market-size-to-reach-US-1-8-billion-by-2026.html (accessed on 18 February 2022).
- Lecomte, T.; Landi, B.; Beaune, P.; Laurent-Puig, P.; Loriot, M.-A. Glutathione S-Transferase P1 Polymorphism (Ile105Val) Predicts Cumulative Neuropathy in Patients Receiving Oxaliplatin-Based Chemotherapy. Clin. Cancer Res. 2006, 12, 3050–3056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoehlmacher, J.; Park, D.J.; Zhang, W.; Groshen, S.; Tsao-Wei, D.D.; Yu, M.C.; Lenz, H.-J. Association Between Glutathione S-Transferase P1, T1, and M1 Genetic Polymorphism and Survival of Patients With Metastatic Colorectal Cancer. JNCI J. Natl. Cancer Inst. 2002, 94, 936–942. [Google Scholar] [CrossRef] [Green Version]
- Oki, E.; Kakeji, Y.; Ohgaki, K.; Saeki, K.; Morita, M.; Emi, Y.; Maehara, Y. Impact of single nucleotide polymorphisms in glutathione S transferase gene GSTP1 in the treatment with ox-aliplatin based chemotherapy. Gan To Kagaku Ryoho 2008, 35, 1094–1096. [Google Scholar] [PubMed]
- Yadav, P.; Banerjee, A.; Boruah, N.; Singh, C.S.; Chatterjee, P.; Mukherjee, S.; Dakhar, H.; Nongrum, H.B.; Bhattacharjee, A.; Chatterjee, A. Glutathione S-transferasesP1 AA (105Ile) allele increases oral cancer risk, interacts strongly with c-Jun Kinase and weakly detoxifies areca-nut metabolites. Sci. Rep. 2020, 10, 6032. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.; Carron, J.; Lopes-Aguiar, L.; Costa, E.; Nogueira, G.; Lima, T.; Pincinato, E.; Visacri, M.; Quintanilha, J.; Moriel, P.; et al. GSTP1 c.313A>G, XPD c.934G>A, XPF c.2505T>C and CASP9 c.-1339A>G polymorphisms and severity of vomiting in head and neck cancer patients treated with cisplatin chemoradiation. Ann. Oncol. 2017, 28, v388. [Google Scholar] [CrossRef]
- Arakawa, Y.; Shirai, Y.; Hayashi, K.; Tanaka, Y.; Matsumoto, A.; Nishikawa, K.; Yano, S. Effects of gene polymorphisms on the risk of severe hyponatremia during DCF chemotherapy for patients with esophageal squamous cell carcinoma. Oncol. Lett. 2018, 16, 5455–5462. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Xu, B.; Yuan, P.; Ott, J.; Guan, Y.; Liu, Y.; Liu, Z.; Shen, Y.; Yu, D.; Lin, D. Genome-wide examination of genetic variants associated with response to platinum-based chemotherapy in patients with small-cell lung cancer. Pharm. Genom. 2010, 20, 389–395. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Zou, T.; Cui, J.; Yin, J.; Zheng, W.; Jiang, W.; Zhou, H.; Liu, Z. Pharmacogenomics of platinum-based chemotherapy response in NSCLC: A genotyping study and a pooled analysis. Oncotarget 2016, 7, 55741–55756. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Han, S.-W.; Ham, H.S.; Kim, T.-Y.; Choi, I.S.; Kim, B.-S.; Oh, D.-Y.; Im, S.-A.; Kang, G.H.; Bang, Y.-J. Phase II study of biweekly S-1 and oxaliplatin combination chemotherapy in metastatic colorectal cancer and pharmacogenetic analysis. Cancer Chemother. Pharmacol. 2011, 67, 1323–1331. [Google Scholar] [CrossRef]
- De Troia, B.; Dalu, D.; Filipazzi, V.; Isabella, L.; Tosca, N.; Ferrario, S.; Gambaro, A.R.; Somma, L.; Fasola, C.; Cheli, S.; et al. ABCB1 c.3435C>T polymorphism is associated with platinum toxicity: A preliminary study. Cancer Chemother. Pharmacol. 2019, 83, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Hou, J.; Deng, Q.; Zhong, Z. Predictive value of clinical toxicities of chemotherapy with fluoropyrimidines and oxaliplatin in colorectal cancer by DPYD and GSTP1 gene polymorphisms. World J. Surg. Oncol. 2020, 18, 321. [Google Scholar] [CrossRef] [PubMed]
- Ferracini, A.C.; Lopes-Aguiar, L.; Lourenço, G.J.; Yoshida, A.; Lima, C.S.P.; Sarian, L.O.; Derchain, S.; Kroetz, D.L.; Mazzola, P.G. GSTP1 and ABCB1 Polymorphisms Predicting Toxicities and Clinical Management on Carboplatin and Paclitaxel-Based Chemotherapy in Ovarian Cancer. Clin. Transl. Sci. 2021, 14, 720–728. [Google Scholar] [CrossRef]
- Fujita, K.; Motoyama, S.; Sato, Y.; Wakita, A.; Nagaki, Y.; Minamiya, Y.; Miura, M. Association between ABCC2 polymorphism and hematological toxicity in patients with esophageal cancer receiving platinum plus 5-fluorouracil therapy. Esophagus 2021, 19, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-F.; Yao, R.-Y.; Liu, K.-W.; Lv, H.-Y.; Jiang, T.; Liang, J. Genetic Polymorphism of GSTP1: Prediction of Clinical Outcome to Oxaliplatin/5-FU-based Chemotherapy in Advanced Gastric Cancer. J. Korean Med. Sci. 2010, 25, 846–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oguri, T.; Mitsuma, A.; Inada-Inoue, M.; Morita, S.; Shibata, T.; Shimokata, T.; Sugishita, M.; Nakayama, G.; Uehara, K.; Hasegawa, Y.; et al. Genetic polymorphisms associated with oxaliplatin-induced peripheral neurotoxicity in Japanese patients with colorectal cancer. Int. J. Clin. Pharmacol. Ther. 2013, 51, 475–481. [Google Scholar] [CrossRef]
- Chaney, S.G.; Campbell, S.; Bassett, E.; Wu, Y. Recognition and processing of cisplatin- and oxaliplatin-DNA adducts. Crit. Rev. Oncol. 2005, 53, 3–11. [Google Scholar] [CrossRef]
- Sedletska, Y.; Giraud-Panis, M.J.; Malinge, J.M. Cisplatin is a DNA-damaging antitumour compound triggering multifac-torial biochemical responses in cancer cells: Importance of apoptotic pathways. Curr. Med. Chem. Anticancer Agents 2005, 5, 251–265. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Apps, M.G.; Choi, E.; Wheate, N.J. The state-of-play and future of platinum drugs. Endocr.-Relat. Cancer 2015, 22, R219–R233. [Google Scholar] [CrossRef] [Green Version]
- Townsend, D.M.; Tew, K.D. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 2003, 22, 7369–7375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, M.A.; Stewart, R.K.; Smith, G.B.; Massey, T.E.; Bell, D. Human glutathione S-transferase P1 polymorphisms: Relationship to lung tissue enzyme activity and population frequency distribution. Carcinogenesis 1998, 19, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Santric, V.; Djokic, M.; Suvakov, S.; Pljesa-Ercegovac, M.; Nikitovic, M.; Radic, T.; Acimovic, M.; Stankovic, V.; Bumbasirevic, U.; Milojevic, B.; et al. GSTP1 rs1138272 Polymorphism Affects Prostate Cancer Risk. Medicina 2020, 56, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sophonnithiprasert, T.; Saelee, P.; Pongtheerat, T. Glutathione S-Transferase P1 Polymorphism on Exon 6 and Risk of Hepatocellular Carcinoma in Thai Male Patients. Oncology 2020, 98, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Mir, O.; Alexandre, J.; Tran, A.; Durand, J.-P.; Pons, G.; Treluyer, J.-M.; Goldwasser, F. Relationship between GSTP1 Ile105Val polymorphism and docetaxel-induced peripheral neuropathy: Clinical evidence of a role of oxidative stress in taxane toxicity. Ann. Oncol. 2009, 20, 736–740. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Q.; Gao, J.; Ji, Z.; Yuan, J.; Tian, Y.; Shen, L. Association between GSTP1 Ile105Val polymorphism and oxaliplatin-induced neuropathy: A systematic review and meta-analysis. Cancer Chemother. Pharmacol. 2013, 72, 305–314. [Google Scholar] [CrossRef]
- Lv, F.; Ma, Y.; Zhang, Y.; Li, Z. Relationship between GSTP1 rs1695 gene polymorphism and myelosuppression induced by platinum-based drugs: A meta-analysis. Int. J. Biol. Markers 2018, 33, 364–371. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).