Abstract
The rise in multiple-drug-resistant (MDR) phenotypes in Gram-negative pathogens is a major public health crisis. Pseudomonas aeruginosa is one of the leading causes of nosocomial infections in clinics. Treatment options for P. aeruginosa have become increasingly difficult due tdo its remarkable capacity to resist multiple antibiotics. The presence of intrinsic resistance factors and the ability to quickly adapt to antibiotic monotherapy warrant us to look for alternative strategies like combinatorial antibiotic therapy. Here, we report the frequency of P. aeruginosa multidrug-resistant and extensively drug-resistance (XDR) phenotypes in a super-specialty tertiary care hospital in north India. Approximately 60 percent of all isolated P. aeruginosa strains displayed the MDR phenotype. We found highest antibiotic resistance frequency in the emergency department (EMR), as 20 percent of isolates were resistant to 15 antipseudomonal antibiotics. Presence of plasmids with quinolone-resistance determinants were major drivers for resistance against fluoroquinolone. Additionally, we explored the possible combinatorial therapeutic options with four antipseudomonal antibiotics—colistin, ciprofloxacin, tobramycin, and meropenem. We uncovered an association between different antibiotic interactions. Our data show that the combination of colistin and ciprofloxacin could be an effective combinatorial regimen to treat infections caused by MDR and XDR P. aeruginosa.
1. Introduction
The expanding frequency of multidrug-0resistant pathogens and the virtually dry pipeline of new antibiotics have created a formidable challenge for our public health settings [1,2]. Infections due to Gram-negative pathogens, especially Pseudomonas aeruginosa, are responsible for high mortality around the globe [3,4,5]. P. aeruginosa is responsible for blood infections, respiratory tract infections, skin infections, urinary tract infections, and surgical site infections [6,7,8]. The presence of high-level intrinsic and acquired resistance determinants in this pathogen often results in poor clinical outcomes. Frequent reports of multidrug-resistant (MDR) and extensively drug-resistant (XDR) P. aeruginosa isolates from hospitals in low- and middle-income countries make the situation even more problematic for public health departments [9,10,11,12].
Treatment options available for P. aeruginosa infections are limited and shrinking rapidly [13]. Four antibiotic classes, i.e., fluoroquinolones, β-lactams, aminoglycosides, and polymyxins, are routinely prescribed against P. aeruginosa infections [7,13]. Fluoroquinolones are among the most active antibiotics against P. aeruginosa. However, frequent chromosomal mutations and horizontally acquired resistance elements enable this bacterium a swift escape against most of these antibiotics. Different resistance determinants such as target site (DNA gyrase and DNA topoisomerase IV) modifications, alteration in membrane permeability, and active efflux are responsible for fluoroquinolones resistance [14,15]. Both chromosomal-mediated and plasmid-mediated resistance determinants have been reported in P. aeruginosa [16]. Fluoroquinolone resistance may arise due to spontaneous mutations in chromosomal DNA gyrase and topoisomerase IV genes, and these genomic regions are called quinolone-resistance-determining regions (QRDR) [17]. However, in the recent past, plasmid-mediated quinolone resistance (PMQR) has been increasingly reported in Gram-negative bacteria all over the world [18,19]. Multiple qnr determinants (qnrA, qnrB, qnrS, and qnrD) have been identified in Gram-negative pathogens that are responsible for quinolones resistance [20,21]. Additionally, some plasmid-based novel determinants such as a modified acetyltransferase gene (aac(6′)-Ib-cr) and the efflux pump gene (qepA) have also been characterized in the recent past [22,23].
The lack of discovery of new antibiotics, especially antibiotics targeting Gram-negative bacteria, further contributes to the difficulty and often necessitates using existing antibiotics in a more innovative way such as combinatorial therapy [24]. Combinatorial therapy is an attractive approach against MDR and XDR isolates of P. aeruginosa [25,26,27]. Combinatorial therapy can provide multifaceted benefits in clinics as it can increase the empirical coverage against other pathogens, suppress the emergence of resistant phenotypes, and decrease toxicity [25,28]. P. aeruginosa has an additional worrisome feature, as it can rapidly adapt against antimicrobial monotherapy, and thus combinatorial therapy can also alleviate this inherent problem [29,30,31].
Many antibiotic interactions of fluoroquinolones have been reported against different pathogens, including P. aeruginosa, in the past [32]. However, it is still unclear how these complex antibiotic interactions depend on the level of antibiotic resistance. To better understand the interactions among routinely prescribed antibiotics against P. aeruginosa, we carried out extensive screening of antibiotic interactions against XDR P. aeruginosa. Here, we describe the co-existence of QRDR and PMQR, complex interconnection with the type of PMQR, and antibiotic interaction. We also show the interspecies transferability and stability of PMQR. This study aims to identify the optimal combinatorial therapy approaches effective against XDR P. aeruginosa.
2. Results
2.1. A High Level of Antibiotic Resistance Is Observed among P. aeruginosa
We collected 243 clinical strains of P. aeruginosa from various departments in a large tertiary care hospital located in northern India (All India Institute of Medical Sciences Rishikesh, Uttarakhand, India). About 60% of P. aeruginosa strains showed multiple drug-resistant phenotypes (resistant to three or more drug classes). We observed the highest rates of resistance for the fluoroquinolones, with resistance to ciprofloxacin, ranging from 50 to 100% in IPD (inpatients) and EMR (emergency) departments, respectively (Figure 1A,B). Surprisingly, we also found a high level of resistance against the last resort of antibiotics, i.e., carbapenems, with an average resistance of >50% in all departments. P. aeruginosa isolates from the EMR department showed higher resistance rates for fluoroquinolones, β-lactams, aminoglycosides, co-trimoxazole, and colistin than general trends for hospitalized patients. Overall, we observed the lowest resistance rates against ofloxacin, amikacin, and meropenem among fluoroquinolones, aminoglycosides, and carbapenems classes, respectively. Similarly, the resistance rate for colistin was lowest among all tested antipseudomonal antibiotics. About 10 and 20% of the P. aeruginosa isolates from the EMR department were resistant to 10 and 15 antibiotics, respectively (Figure 1C).
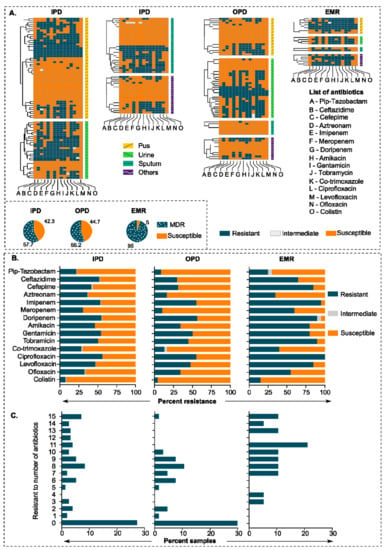
Figure 1.
Antibiotic resistance pattern in clinical strains of P. aeruginosa. (A) Heatmap showing an antibiotic resistance pattern of 243 clinical strains of P. aeruginosa against 15 antipseudomonal antibiotics. IPD, OPD, and EMR represent Inpatient Department, Outpatient Department, and Emergency Department, respectively. The source of isolation is indicated on the left side of the heatmaps. (B) Percent resistance of all samples (clinical isolates) in different departments against 15 antipseudomonal antibiotics. (C) Bar graphs showing a frequency of the number of antibiotics against department-wise P. aeruginosa resistance percentage.
2.2. Antibiotic–Antibiotic Interaction Screening Results Showed Colistin–Ciprofloxacin Is the Most Effective Antibiotic Combination against MDR P. aeruginosa
To identify the most effective antibiotic combinations, we selected five antipseudomonal antibiotic pairs, i.e., colistin–ciprofloxacin, colistin–meropenem, colistin–tobramycin, ciprofloxacin–tobramycin, and ciprofloxacin–meropenem based on the previously reported regimens [33,34,35,36]. We tested them against 17 XDR P. aeruginosa strains. The combination of tobramycin with either colistin or ciprofloxacin showed the lowest rate of synergistic effect (only in three and two strains, respectively) (Figure 2A,C,E). Meropenem–colistin and meropenem–ciprofloxacin combinations showed synergy in five and six strains, respectively (Figure 2D,F). However, the ciprofloxacin–colistin combination showed synergy in 11 strains out of 17 strains. Additionally, in 9 out of 11 strains, the concentration of ciprofloxacin and colistin decreased below the respective breakpoint defined by CLSI guidelines (Figure 2B). The CLSI MIC breakpoint for P. aeruginosa resistance phenotype against ciprofloxacin, colistin, meropenem, and tobramycin is ≥4, ≥4, ≥8, and ≥16 mg/L, respectively [37].
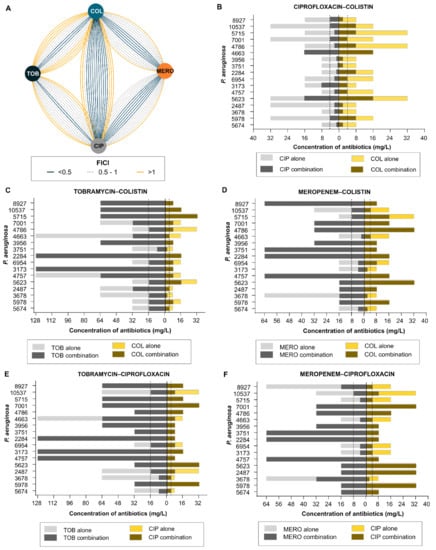
Figure 2.
(A) Antibiotic–antibiotic interaction network of four antipseudomonal antibiotics (colistin, meropenem, ciprofloxacin, and tobramycin) in 17 extensive drug-resistant clinical strains of P. aeruginosa. Nodes represent different antibiotics, edges represent Fractional Inhibitory Concentration Index (FICI), i.e., synergy (FICI < 0.5; dark blue), partial synergy (FICI = 0.5–1; dashed gray), or no interaction (FICI > 1; yellow). Interaction network was created with Cytoscape version 3.8.0. (B) Minimum inhibitory concentration of ciprofloxacin–colistin; (C) tobramycin–colistin; (D) meropenem–colistin; (E) tobramycin–ciprofloxacin; (F) Meropenem–ciprofloxacin; alone (light gray and light yellow, respectively) or in combination (dark gray and dijon yellow, respectively) against 17 extensively drug-resistant clinical strains of P. aeruginosa. The dashed line represents the MIC breakpoint of respective antibiotics as defined by CLSI guidelines. The CLSI MIC breakpoint for P. aeruginosa resistance phenotype against ciprofloxacin, colistin, meropenem, and tobramycin is ≥4, ≥4, ≥8, and ≥16 mg/L, respectively [37].
2.3. Prevalence of Inter-Species Plasmid Transfer
P. aeruginosa exploits multiple ways to tackle quinolone toxicity, as both plasmid-mediated and chromosomal-mediated resistance determinants play an important role. We tested twelve quinolone-resistant determinants for the presence of qnrA, qnrB, qnrD, qnrS, qepA, aqxA, aqxB, aac(6′)-Ib-cr, gyrA, gyrB, parC, and parE in 132 quinolone-resistant isolates of P. aeruginosa. The results are summarized in Supplementary Figure S1. Next, we selected six isolates based on the antibiotic resistance profile to check if plasmid-mediated quinolone-resistant (PMQR) determinants could be mobilized to other species through conjugation. We selected E. coli J53 Azr as a recipient host strain. Our results show that quinolone resistance could be transferred by conjugation to all six PMQR-positive donors (Figure 3A). The frequencies of transconjugation varied between 10−4 to 10−6 cells per recipient cells for different donor strains. Four donor strains (1916, 1826, 5978, and 4286) transferred plasmid at a frequency of 10−4 cells per recipient cells and two remaining strains (4663 and 5830) at 10−6 (Figure 3F). Out of nine PMQR determinants, four genes (qnrA, qnrB, qnrS, and aac(6′)-Ib-cr) were successfully transferred to E. coli J53 (Figure 3B). Plasmids that had quinolone-resistance genes can provide a selective advantage to host under an environment with quinolone pressure; however, maintaining plasmids without any selective advantage (selection pressure) could be costly to the host cells. Hence, we checked the stability of these transferred plasmids without any selective advantage and found that five out of six transconjugants (1916, 1826, 5978, 4286, and 4663) retained the plasmid for five days, while two transconjugants (4663 and 5978) retained plasmid even for ten days without any selection pressure (Figure 3C). Next, we checked whether the type of antibiotic–antibiotic interactions (Figure 3D) have any correlation for a given strain. We calculated the Pearson correlation coefficient for these variables (i.e., FICIs against different antibiotic interactions) and found some intriguing associations; for example, the colistin–meropenem combination was positively correlated with the colistin–tobramycin combination (r = 0.8, p < 0.05), while colistin–ciprofloxacin and colistin–tobramycin were negatively correlated (r = −0.8, p < 0.05) (Figure 3E). Although statistically insignificant, meropenem–colistin was positively associated with meropenem–ciprofloxacin and tobramycin–ciprofloxacin combinations (r = 0.7) (Figure 3E). Comprehensive correlation analysis of antibiotic interactions for any bacterial pathogen could help prioritize an appropriate treatment regimen.
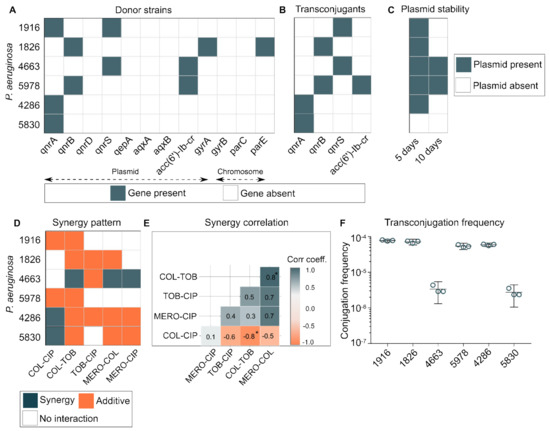
Figure 3.
(A) Presence of quinolone resistance determinants in six P. aeruginosa strains. Twelve quinolone-resistant determinants were tested for their presence (qnrA, qnrB, qnrD, qnrS, qepA, aqxA, aqxB, aac(6′)-Ib-cr, gyrA, gyrB, parC, parE). (B) Four plasmid-mediated quinolone-resistant determinants genes (qnrA, qnrB, qnrS, and aac(6′)-Ib-cr) from clinical strains of P. aeruginosa were transferred to E. coli J53. (C) Inter-species plasmid stability in E. coli J53 after 5 and 10 days of transconjugation. (D) Antibiotic–antibiotic interaction pattern in six P. aeruginosa strains. (E) Antibiotic interaction correlation between five antibiotic interaction types displayed against six P. aeruginosa strains. The correlation matrix was computed using library (ggcorrplot version 0.1.3) in R. (F) Transconjugation frequency of six P. aeruginosa plasmids to E. coli J53. *: correlation significance value p < 0.05.
2.4. Transferred Plasmids Provide a High Level of Resistance
Plasmids are one of the major drivers of antibiotic resistance against multiple antibiotic classes. To test whether plasmid-mediated quinolone resistance determinants present in P. aeruginosa could be transferred to other closely related species, we performed a transconjugation experiment with E. coli J53 (an azide resistant recipient). We found that plasmids present in clinical strains of P. aeruginosa could be easily mobilized to E. coli and with a high frequency. Moreover, transferred plasmids could provide a high level of resistance to the recipient cells. Transconjugants displayed a 256- to 2048-fold increase in MIC against ciprofloxacin, 8- to 16-fold increase against nalidixic acid and 256- to 512-fold increase against levofloxacin (Figure 4A–C). This increase in MIC is much higher than the resistance breakpoints defined by CLSI guidelines for respective antibiotics [37]. Harboring external genetic material could result in reduced fitness for the host cells [38]. However, when we tested the growth pattern of transconjugants with the parental host strain, we did not find any significant reduction in the growth rate of transconjugants as compared to the parental strain (Figure 4D–I).
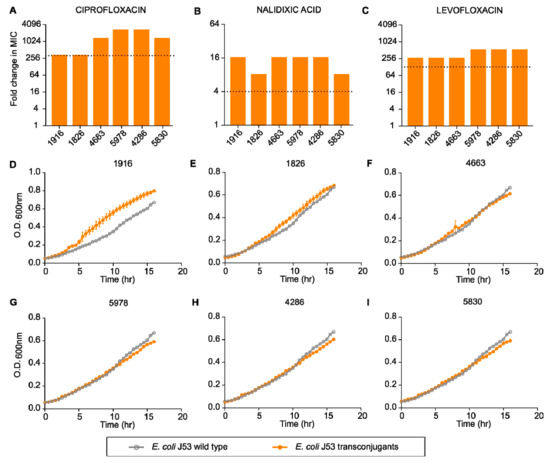
Figure 4.
Fold change in minimum inhibitory concentration of different transconjugants compared to E. coli J53 against quinolones: (A) ciprofloxacin, (B) nalidixic acid, (C) levofloxacin. Relative growth of different transconjugants ((D) 1916, (E) 1826, (F) 4663, (G) 5978, (H) 4286, (I) 5830) compared to E. coli J53.
2.5. Quinolone Resistant Strains Show Reduced Drug Accumulation
Bacterial efflux pumps play a central role in antibiotic resistance. Our results indicated that quinolone-resistant P. aeruginosa has higher efflux activity than the susceptible counterpart. Ethidium bromide is a common substrate for many efflux pumps in Gram-negative bacteria, and its reduced uptake inside these bacterial cells is indirectly proportional to the efflux activity [39,40]. We found that quinolone-resistant P. aeruginosa (5978, 5830, and 4663) show reduced EtBr accumulation kinetics (i.e., higher efflux) as compared to quinolone-susceptible P. aeruginosa (4985, 2189, and 6668) (Figure 5A). More specifically, the resistant strains showed 1.5 to 3 times decreased EtBr accumulation (p < 0.05) than the susceptible P. aeruginosa (Figure 5B).
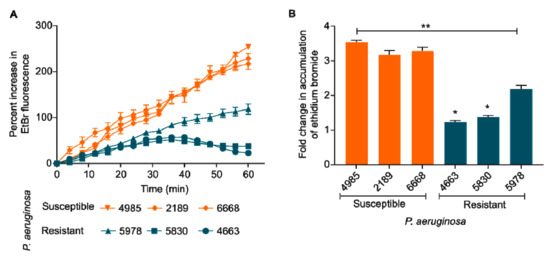
Figure 5.
(A) Ethidium bromide (EtBr) accumulation kinetics. Quinolone-resistant P. aeruginosa (5978, 5830, and 4663) show reduced EtBr accumulation kinetics (i.e., higher efflux) as compared to quinolone-susceptible P. aeruginosa (4985, 2189, and 6668). (B) Fold change in the accumulation of EtBr after 60 min. One way ANOVA followed by Mann-Whitney test. *: p < 0.05. **: p < 0.01.
3. Discussion
P. aeruginosa is currently one of the critical priority pathogens as defined by the World Health Organization [41]. They are also responsible for acute infections among patients admitted to ICUs. P. aeruginosa has been linked to more infection incidents and higher mortality rates in ICUs compared to other hospital wards. However, other clinical conditions of patients, such as pre-existing diseases, surgery, and immunosuppressive medications, play an important role in the mortality rate [6]. P. aeruginosa included in our study were mostly isolated from urine, followed by pus and sputum. Our data show a different trend as compared to previously reported clinical sources of isolated strains [42,43]. Our antibiotic resistance data show a high prevalence of MDR and XDR phenotypes in ICU patients as compared to IPD and OPD, which is also different from previously reported resistance frequencies among ICUs patients. We found a high prevalence of resistance against most of the antipseudomonal antibiotics except colistin and co-trimoxazole, which is in agreement with previously published surveys from different geographical locations [9,10]. However, we did not find any correlation between antibiotic resistance and sources of bacterial isolates, i.e., body site. For the treatment of P. aeruginosa infections at OPD departments in the All India Institute of Medical Sciences Rishikesh, clinicians routinely prescribe aztreonam and piperacillin–tazobactam; however, they also use other antibiotics such as meropenem, cefotaxime, colistin, and levofloxacin in the IPD and EMR department. Future studies can look into the evolution and correlation of antibiotic resistance versus prescription in different departments.
The antibiotic–antibiotic correlation matrix against P. aeruginosa indicates an intriguing pattern of interaction among antipseudomonal antibiotics. The colistin–ciprofloxacin combination showed better efficacy (9 out of 17 isolates) than that of colistin–meropenem (4 out of 17 isolates) or colistin–tobramycin (1 out of 17 isolates) in reducing the inhibitory concentration of individual antibiotics below the defined CLSI breakpoint (MIC breakpoint for ciprofloxacin, colistin, meropenem, and tobramycin is ≥4, ≥4, ≥8, and ≥16, respectively) [37]. Furthermore, the ciprofloxacin–meropenem (4 out of 17 isolates) and ciprofloxacin–tobramycin (2 out of 17 isolates) combinations were less effective than the colistin–ciprofloxacin combination. In light of these findings, a close look at pharmacokinetics and pharmacodynamics (PK/PD) of colistin and ciprofloxacin reveals some common characteristics. Both colistin and ciprofloxacin demonstrate concentration-dependent bactericidal activity. Additionally, the therapeutic efficacy of both colistin and ciprofloxacin are dependent on Cmax:MIC ratio [44]. The average plasma concentrations of these two antibiotics were ~5 mg/L within 3 h of administration. Previous reports of antibiotic combinations against P. aeruginosa have mixed outcomes. Several studies indicate better efficacy of the colistin–meropenem combination [45,46], while others advocate the colistin–ciprofloxacin combination [47,48]. Our results show that the type of antibiotic interaction is not uniform within the same species (i.e., not all strains of any given species show a specific pattern of antibiotic interaction). Perhaps such a mixed outcome of antibiotic interaction is expected. Antibiotic interaction depends on several factors such as genetic circuits and level of antibiotic resistance, which vary significantly for any given bacterial species [25,49]. However, whether antibiotic interactions show body site-specific or country-specific patterns remains currently unanswered, and it would be interesting to look into it in future studies.
Plasmids play a crucial role in spreading quinolone resistance across clinics and the environment. PMQR offers a selective advantage of hosting cells by providing a basal-level protection against quinolone toxicity and thus enables a favorable evolutionary window for escape. Our data show that plasmids containing quinolone-resistance determinants can be transferred to another bacterial host (here E. coli J53) and with a high frequency. Additionally, these plasmids remained stable for an extended period, even without any selection pressure. Indeed, plasmid stability can significantly vary from a few days to several months and even years without any selection pressure [50,51,52]. Plasmid maintenance can be challenging for host cells due to associated fitness costs [53]. However, without a direct selection pressure of antibiotics, plasmid maintenance greatly depends on nutrient availability. Additionally, if the rate of conjugation is sufficient to offset the fitness cost of carriage or loss from segregation, then plasmids may be stable or capable of increasing their frequency in bacterial populations even without any selection pressure [54,55]. Additional molecular mechanisms of such plasmid stability remain to be explored. In this study, we sought to explore the correlation between different types of antibiotic interaction for a given strain. We uncovered two statistically significant correlations; we found a negative correlation between the colistin–ciprofloxacin and the colistin–meropenem combination; similarly, strains showing colistin–meropenem synergy were always positively associated with colistin–tobramycin synergy. Modulation of intracellular concentration of partner antibiotics may partially explain this phenomenon, as it has been observed for many membrane-active compounds [56,57]. However, sometimes a membrane-active compound can also interfere with antibiotic import and thus decrease the intracellular concentration of the partner antibiotic; this has been seen with benzalkonium chloride (a membrane acting agent) interfering with the import of ciprofloxacin and gentamicin in E. coli [25].
The intrinsic resistance of P. aeruginosa against multiple biocides is largely contributed by efflux pumps [58]. Efflux pumps do not only play a pivotal role in providing the MDR phenotype but are also required for transporting virulence factors and signaling molecules [39]. Corroborating previous studies, our results show the basal level difference in activity of efflux pumps between resistant and susceptible isolates (Figure 5) [40,59]. Notably, due to heavy dependence on efflux pumps, targeting resistant isolates with membrane-active agents like colistin may show a proportionally higher effect than susceptible isolates.
Our study provided some new insights into synergistic interactions among antipseudomonal antibiotics. However, the current study has some limitations. First, the clinical isolates used in the study only represents a specific geographical location, and future studies may include isolates from different locations around the world. Second, we did not compare long-term evolutionary aspects of the combinations with respect to the generation of resistant mutants, and this would be interesting to look into it. Despite their growing biomedical significance, central questions about drug interactions remain unanswered; specifically, little is known about the underlying mechanisms of most drug interactions [60]. Overall, our data suggest that antibiotic interactions show a complex pattern. Several factors such as type of antibiotic resistance, presence, and level of expression of efflux pumps, plasmids, and nutritional status might contribute to it. Systemic identification of antibiotic interaction could provide us valuable insights and guide us in selecting appropriate antibiotic regimens for the emerging threat of MDR and XDR P. aeruginosa infections.
4. Materials and Methods
4.1. Chemicals and Biological Materials
All the bacteriological media were purchased from HIMEDIA, India. All antibiotics powder used in this study was obtained from Sigma Aldrich, St. Louis, MO, USA, while antibiotic discs were obtained from HIMEDIA, India. All clinical strains of P. aeruginosa were isolated from any of the following sources from patients with bacterial infections who were admitted to All India Institute of Medical Sciences Rishikesh, India between April 2018 to March 2019 (Supplementary Table S1): blood, urine, pus, pleural fluids, sputum, endotracheal aspirate, bronchoalveolar lavage, or infected tissue. Bacterial species other than P. aeruginosa were also isolated from infected samples; however, they are not part of this study. Clinical strains were routinely cultured on 5% sheep blood agar. Species identification of all causative microorganisms was performed using either Bruker’s MALDI Biotyper® Microbial Identification system (Bruker, Billerica, MA, USA) or MicroScan WalkAway 96 Plus ID/AST System (Bechman Coulter, Brea, CA, USA) as per manufacturer’s recommendation. E. coli J53 was obtained from the Department of Bacteriology, Postgraduate Institute of Medical Education and Research, Chandigarh.
4.2. Antibiotics Susceptibility Assay
Initial antibiotic susceptibility assay of all clinical strains was performed using an automated antibiotic susceptibility testing system (MicroScan WalkAway 96 Plus ID/AST System, Bechman Coulter, USA). Additionally, the susceptibility of 243 identified P. aeruginosa strains against 15 antipseudomonal antibiotics was also determined using the disk diffusion method according to CLSI recommendation [37]. For 17 extensively drug-resistance P. aeruginosa strains (Supplementary Table S1), minimum inhibitory concentration (MIC) values of the studied antimicrobials were determined using 2-fold broth microdilution method in a 96-well polystyrene plate with an initial inoculum of 106 CFU/mL in Cation adjusted Mueller Hinton broth (CAMHB). Antibiotic susceptibility results were interpreted according to the guidelines recommended by the Clinical and Laboratory Standards Institute (CLSI), USA [37]. Seventeen P. aeruginosa strains were selected based on the presence of PMQR (please refer to Section 4.4) and XDR phenotype.
4.3. Screening of Antibiotic-Antibiotic Combinations
In order to determine the best suitable antibiotic pairs for the treatment of MDR P. aeruginosa strains, four major antipseudomonal antibiotics, i.e., polymyxin B, tobramycin, meropenem, and ciprofloxacin (representing different antibiotic classes—polymyxins, aminoglycosides, carbapenems, and fluoroquinolones) were probed to each other using a two-dimensional checkerboard assay. Two-dimensional checkerboard assay was performed according to the previously described method [49]. The fractional inhibitory concentration index (FICI) of antibiotic pairs was determined to evaluate the type of drug interaction. Synergy was defined if the drug pair had an FICI value of ≤0.5, and additivity was defined as an FICI value of ≥0.5 to <4, whereas antagonism was defined as an FICI value of ≥4 [61].
4.4. Identification of Quinolone Resistance Determinants
Chromosomal and plasmid-mediated resistance determinants have been reported for quinolone resistance in Gram-negative bacteria. Hence, presence of chromosomal and plasmid-mediated quinolones resistance determinants (qnrA, qnrB, qnrD, qnrS, qepA, aqxA, aqxB, aac(6′)-Ib-cr, gyrA, gyrB, parC, parE) were checked using PCR. PCR conditions included an initial denaturation at 95 °C for 1 min followed by 30 cycles of 95 °C for 30 s and annealing for 30 s at 54 °C for 12 genes with an extension at 72 °C for 30 s. Cycling was followed by a final extension at 72 °C for 5 min. A complete list of primers used for PCR amplification along with the product sizes is provided in Table 1.

Table 1.
Table describing target genes and primers used for detecting PMQR (plasmid mediated quinolone resistance genes) and QRDR (chromosomal quinolone resistance determinants regions).
4.5. Bacterial Conjugation
Conjugation experiments were performed with six MDR P. aeruginosa strains as donors and E. coli J53 (azide-resistant) as the recipient in LB broth [69]. Briefly, donor and recipient cells were grown in LB broth to O.D.600nm~0.5. Conjugation was performed by mixing donor and recipient cells in a 1:1 ratio in LB broth followed by incubation at 37 °C for 12 h without shaking. Transconjugants were selected on LB agar plates co-supplemented with sodium azide (100 mg/L; Sigma-Aldrich, USA) and ciprofloxacin (4 mg/L) for counterselection of plasmid-encoded quinolone-resistant determinants. Transconjugation frequencies were calculated by dividing the number of transconjugants by the number of donor cells. The conjugation experiments were performed with three biological replicates.
4.6. Measurement of Plasmid Stability and Bacterial Fitness
To check the stability of inter-species plasmid transfer, transconjugants (E. coli J53 having plasmids) were serially passaged daily on antibiotic-free LB agar plates for ten days. For positive control, transconjugants were serially passaged on LB agar supplemented with ciprofloxacin. The presence of respective plasmid-mediated resistance determinants was assessed using PCR on the 5th and 10th days. For analyzing bacterial fitness, the growth of transconjugants and E. coli J53 (wild type) in antibiotic-free medium (LB medium) was compared by measuring optical density at 600 nm. Briefly, transconjugant and E. coli J53 cells grown overnight were incubated in fresh LB medium each at a starting O.D.600nm~0.05. Growth was measured using a spectrophotometer at an interval of 30 min for 16 h.
4.7. Ethidium Bromide Accumulation Assay
Ethidium bromide is a heterocyclic compound and a common substrate for major efflux pumps in Gram-negative bacteria. Ethidium bromide accumulation assay was performed as previously described [70,71]. P. aeruginosa cells were grown to an O.D.600nm~0.6, followed by washing with PBS, and finally resuspended in PBS to an O.D.600nm~0.2. Cells were loaded with 20 mg/L of ethidium bromide and 0.4% glucose (wt./vol) and were immediately aliquoted into a black 96-well plate (Fluorescence BRANDplates®, Essex, CT, USA). Fluorescence was measured using a Tecan Infinite® 200 PRO fluorescence spectrophotometer (Tecan, Männedorf, Switzerland) at an excitation wavelength of 520 nm and an emission wavelength of 590 nm for 60 min every 4 min.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph15020243/s1, Figure S1: Heatmap showing QRDR & PMQR; Table S1: Strains used in the study.
Author Contributions
Conceptualization, A.G. and A.K. (Ashish Kothari); methodology, A.K. (Ashish Kothari); formal analysis, A.G., A.K. (Ashish Kothari), N.J., S.K.K., A.K. (Ankur Kumar), K.K., S.K., A.P. and B.J.O.; writing—original draft preparation, A.G. and A.K. (Ashish Kothari); supervision, B.J.O.; funding acquisition, A.K. (Ashish Kothari). All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by intramural fund from All India Institute of Medical Sciences, Rishikesh, provided to A.K. (Ashish Kothari) as Ph.D. fellowship (319/IEC/Ph.D./2018).
Institutional Review Board Statement
This study was approved by the Institutional Review Board of the All India Institute of Medical Sciences Rishikesh, India (approval number ECR/736/Inst/UK/2015/RR-18).
Informed Consent Statement
Informed consent was obtained from all subjects (parents or guardians) involved in the study.
Data Availability Statement
Data is contained within the article and supplementary material.
Acknowledgments
The authors would like to thank staff members at the Department of Microbiology, All India Institute of Medical Sciences Rishikesh for technical support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Lewis, K. The Science of Antibiotic Discovery. Cell 2020, 181, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Babich, T.; Naucler, P.; Valik, J.K.; Giske, C.G.; Benito, N.; Cardona, R.; Rivera, A.; Pulcini, C.; Fattah, M.A.; Haquin, J.; et al. Risk factors for mortality among patients with Pseudomonas aeruginosa bacteraemia: A retrospective multicentre study. Int. J. Antimicrob. Agents 2020, 55, 105847. [Google Scholar] [CrossRef] [PubMed]
- Eklöf, J.; Sørensen, R.; Ingebrigtsen, T.S.; Sivapalan, P.; Achir, I.; Boel, J.B.; Bangsborg, J.; Ostergaard, C.; Dessau, R.B.; Jensen, U.S.; et al. Pseudomonas aeruginosa and risk of death and exacerbations in patients with chronic obstructive pulmonary disease: An observational cohort study of 22 053 patients. Clin. Microbiol. Infect. 2020, 26, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Jernigan, J.A.; Hatfield, K.M.; Wolford, H.; Nelson, R.E.; Olubajo, B.; Reddy, S.C.; McCarthy, N.; Paul, P.; McDonald, L.C.; Kallen, A.; et al. Multidrug-Resistant Bacterial Infections in U.S. Hospitalized Patients, 2012-2017. N. Engl. J. Med. 2020, 382, 1309–1319. [Google Scholar] [CrossRef]
- Breidenstein, E.B.; de la Fuente-Nunez, C.; Hancock, R.E. Pseudomonas aeruginosa: All roads lead to resistance. Trends Microbiol. 2011, 19, 419–426. [Google Scholar] [CrossRef]
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorli, L.; Luque, S.; Gomez-Zorrilla, S.; Benito, N.; Grau, S. Epidemiology and Treatment of Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Infections. Clin. Microbiol. Rev. 2019, 32, e00031-19. [Google Scholar] [CrossRef]
- Fujitani, S.; Sun, H.Y.; Yu, V.L.; Weingarten, J.A. Pneumonia Due to Pseudomonas aeruginosa Part I: Epidemiology, Clinical Diagnosis, and Source. Chest 2011, 139, 909–919. [Google Scholar] [CrossRef]
- Pérez, A.; Gato, E.; Pérez-Llarena, J.; Fernández-Cuenca, F.; Gude, M.J.; Oviaño, M.; Pachón, M.E.; Garnacho, J.; González, V.; Pascual, Á.; et al. High incidence of MDR and XDR Pseudomonas aeruginosa isolates obtained from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the MagicBullet clinical trial. J. Antimicrob. Chemother. 2019, 74, 1244–1252. [Google Scholar] [CrossRef]
- Zilberberg, M.D.; Shorr, A.F. Prevalence of multidrug-resistant Pseudomonas aeruginosa and carbapenem-resistant Enterobacteriaceae among specimens from hospitalized patients with pneumonia and bloodstream infections in the United States from 2000 to 2009. J. Hosp. Med. 2013, 8, 559–563. [Google Scholar] [CrossRef]
- Micek, S.T.; Wunderink, R.G.; Kollef, M.H.; Chen, C.; Rello, J.; Chastre, J.; Antonelli, M.; Welte, T.; Clair, B.; Ostermann, H.; et al. An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: Impact of multidrug resistance. Crit. Care 2015, 19, 219. [Google Scholar] [CrossRef] [PubMed]
- del Barrio-Tofiño, E.; López-Causapé, C.; Oliver, A. Pseudomonas aeruginosa epidemic high-risk clones and their association with horizontally-acquired β-lactamases: 2020 update. Int. J. Antimicrob. Agents 2020, 56, 106196. [Google Scholar] [CrossRef]
- O’Donnell, J.N.; Bidell, M.R.; Lodise, T.P. Approach to the Treatment of Patients with Serious Multidrug-Resistant Pseudomonas aeruginosa Infections. Pharmacotherapy 2020, 40, 952–969. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.G.; Fluit, A.C.; Milatovic, D.; Verhoef, J.; Schmitz, F.J. Mutations in GyrA, ParC, MexR and NfxB in clinical isolates of Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2003, 21, 409–413. [Google Scholar] [CrossRef]
- Drlica, K.; Malik, M. Fluoroquinolones: Action and resistance. Curr. Top. Med. Chem. 2003, 3, 249–282. [Google Scholar] [CrossRef] [PubMed]
- Gasink, L.B.; Fishman, N.O.; Weiner, M.G.; Nachamkin, I.; Bilker, W.B.; Lautenbach, E. Fluoroquinolone-resistant Pseudomonas aeruginosa: Assessment of risk factors and clinical impact. Am. J. Med. 2006, 119, 526.e519–526.e525. [Google Scholar] [CrossRef]
- Jacoby, G.A. Mechanisms of Resistance to Quinolones. Clin. Infect. Dis. 2005, 41, S120–S126. [Google Scholar] [CrossRef]
- Jacoby, G.A.; Strahilevitz, J.; Hooper, D.C. Plasmid-mediated quinolone resistance. Plasmids Biol. Impact Biotechnol. Discov. 2015, 475–503. [Google Scholar]
- Piekarska, K.; Wołkowicz, T.; Zacharczuk, K.; Rzeczkowska, M.; Chróst, A.; Bareja, E.; Olak, M.; Gierczyński, R. Co-existence of plasmid-mediated quinolone resistance determinants and mutations in gyrA and parC among fluoroquinolone-resistant clinical Enterobacteriaceae isolated in a tertiary hospital in Warsaw, Poland. Int. J. Antimicrob. Agents 2015, 45, 238–243. [Google Scholar] [CrossRef]
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J. Fluoroquinolone resistance: Mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H.; Chan, E.W.; Liu, L.Z.; Chen, S. PMQR genes oqxAB and aac(6′)Ib-cr accelerate the development of fluoroquinolone resistance in Salmonella typhimurium. Front. Microbiol. 2014, 5, 521. [Google Scholar] [CrossRef] [PubMed]
- Yamane, K.; Wachino, J.-I.; Suzuki, S.; Kimura, K.; Shibata, N.; Kato, H.; Shibayama, K.; Konda, T.; Arakawa, Y. New plasmid-mediated fluoroquinolone efflux pump, QepA, found in an Escherichia coli clinical isolate. Antimicrob. Agents Chemother. 2007, 51, 3354–3360. [Google Scholar] [CrossRef] [PubMed]
- Torella, J.P.; Chait, R.; Kishony, R. Optimal Drug Synergy in Antimicrobial Treatments. PLOS Comput. Biol. 2010, 6, e1000796. [Google Scholar] [CrossRef]
- Brochado, A.R.; Telzerow, A.; Bobonis, J.; Banzhaf, M.; Mateus, A.; Selkrig, J.; Huth, E.; Bassler, S.; Zamarreno Beas, J.; Zietek, M.; et al. Species-specific activity of antibacterial drug combinations. Nature 2018, 559, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Safdar, N.; Kethireddy, S.; Chateau, D. A survival benefit of combination antibiotic therapy for serious infections associated with sepsis and septic shock is contingent only on the risk of death: A meta-analytic/meta-regression study. Crit. Care Med. 2010, 38, 1651–1664. [Google Scholar] [CrossRef]
- Coates, A.R.M.; Hu, Y.; Holt, J.; Yeh, P. Antibiotic combination therapy against resistant bacterial infections: Synergy, rejuvenation and resistance reduction. Expert. Rev. Anti-Infect. Ther. 2020, 18, 5–15. [Google Scholar] [CrossRef]
- Chait, R.; Craney, A.; Kishony, R. Antibiotic interactions that select against resistance. Nature 2007, 446, 668. [Google Scholar] [CrossRef]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Petrosillo, N.; Ioannidou, E.; Falagas, M. Colistin monotherapy vs. combination therapy: Evidence from microbiological, animal and clinical studies. Clin. Microbiol. Infect. 2008, 14, 816–827. [Google Scholar] [CrossRef]
- Khawcharoenporn, T.; Chuncharunee, A.; Maluangnon, C.; Taweesakulvashra, T.; Tiamsak, P. Active monotherapy and combination therapy for extensively drug-resistant Pseudomonas aeruginosa pneumonia. Int. J. Antimicrob. Agents 2018, 52, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Cosgrove, S.E.; Maragakis, L.L. Combination Therapy for Treatment of Infections with Gram-Negative Bacteria. Clin. Microbiol. Rev. 2012, 25, 450–470. [Google Scholar] [CrossRef] [PubMed]
- Traugott, K.A.; Echevarria, K.; Maxwell, P.; Green, K.; Lewis, J.S., 2nd. Monotherapy or combination therapy? The Pseudomonas aeruginosa conundrum. Pharmacotherapy 2011, 31, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, L.; Li, W.; Xu, H.; He, P.; Yan, X.; Dai, H. Combination antibiotic therapy versus monotherapy for Pseudomonas aeruginosa bacteraemia: A meta-analysis of retrospective and prospective studies. Int. J. Antimicrob. Agents 2013, 42, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Righi, E.; Viscoli, C. Pseudomonas aeruginosa serious infections: Mono or combination antimicrobial therapy? Curr. Med. Chem. 2008, 15, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Maraolo, A.E.; Cascella, M.; Corcione, S.; Cuomo, A.; Nappa, S.; Borgia, G.; De Rosa, F.G.; Gentile, I. Management of multidrug-resistant Pseudomonas aeruginosa in the intensive care unit: State of the art. Expert. Rev. Anti-Infect. Ther. 2017, 15, 861–871. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 13th ed.; CLSI Standard M02; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- San Millan, A.; MacLean, R.C. Fitness Costs of Plasmids: A Limit to Plasmid Transmission. Microbiol. Spectr. 2017, 5, 5. [Google Scholar] [CrossRef]
- Alcalde-Rico, M.; Hernando-Amado, S.; Blanco, P.; Martínez, J.L. Multidrug Efflux Pumps at the Crossroad between Antibiotic Resistance and Bacterial Virulence. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Beinlich, K.L.; Chuanchuen, R.; Schweizer, H.P. Contribution of multidrug efflux pumps to multiple antibiotic resistance in veterinary clinical isolates of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2001, 198, 129–134. [Google Scholar] [CrossRef]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Pokharel, K.; Dawadi, B.R.; Bhatt, C.P.; Gupte, S. Prevalence of Pseudomonas Aeruginosa and its Antibiotic Sensitivity Pattern. J. Nepal. Health Res. Counc. 2019, 17, 109–113. [Google Scholar] [CrossRef]
- Merradi, M.; Kassah-Laouar, A.; Ayachi, A.; Heleili, N.; Menasria, T.; Hocquet, D.; Cholley, P.; Sauget, M. Occurrence of VIM-4 metallo-β-lactamase-producing Pseudomonas aeruginosa in an Algerian hospital. J. Infect. Dev. Ctries 2019, 13, 284–290. [Google Scholar] [CrossRef]
- Varley, A.J.; Sule, J.; Absalom, A.R. Principles of antibiotic therapy. Contin. Educ. Anaesth. Crit. Care Pain 2009, 9, 184–188. [Google Scholar] [CrossRef]
- Pankuch, G.A.; Lin, G.; Seifert, H.; Appelbaum, P.C. Activity of meropenem with and without ciprofloxacin and colistin against Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob. Agents Chemother. 2008, 52, 333–336. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramadan, R.A.; Gebriel, M.G.; Kadry, H.M.; Mosallem, A. Carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa: Characterization of carbapenemase genes and E-test evaluation of colistin-based combinations. Infect. Drug Resist. 2018, 11, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, B.B.; Padmaraj, S.R.; Rekha, P.D.; Tellis, R.C.; Prabhu, S.; Pothen, P. In vitro synergistic activity of colistin and ceftazidime or ciprofloxacin against multidrug-resistant clinical strains of Pseudomonas aeruginosa. Microb. Drug Resist. 2014, 20, 550–554. [Google Scholar] [CrossRef]
- Prentice, H.G.; Hann, I.M.; Nazareth, B.; Paterson, P.; Bhamra, A.; Kibbler, C.C. Oral ciprofloxacin plus colistin: Prophylaxis against bacterial infection in neutropenic patients. A strategy for the prevention of emergence of antimicrobial resistance. Br. J. Haematol. 2001, 115, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, A.; Gupta, V.; Shrivastava, S.K.; Pathania, R. Mechanistic insights into synergy between nalidixic acid and tetracycline against clinical isolates of Acinetobacter baumannii and Escherichia coli. Commun. Biol. 2021, 4, 542. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Fu, J.; Xie, N.; Ma, S.; Lei, L.; Zhai, W.; Shen, Y.; Sun, C.; Wang, S.; Shen, Z.; et al. Fitness Cost of blaNDM-5-Carrying p3R-IncX3 Plasmids in Wild-Type NDM-Free Enterobacteriaceae. Microorganisms 2020, 8, 377. [Google Scholar] [CrossRef]
- Wein, T.; Hülter, N.F.; Mizrahi, I.; Dagan, T. Emergence of plasmid stability under non-selective conditions maintains antibiotic resistance. Nat. Commun. 2019, 10, 2595. [Google Scholar] [CrossRef]
- Leinweber, H.; Alotaibi, S.M.I.; Overballe-Petersen, S.; Hansen, F.; Hasman, H.; Bortolaia, V.; Hammerum, A.M.; Ingmer, H. Vancomycin resistance in Enterococcus faecium isolated from Danish chicken meat is located on a pVEF4-like plasmid persisting in poultry for 18 years. Int. J. Antimicrob. Agents 2018, 52, 283–286. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Z.; Lu, X.; Peng, K.; Chen, S.; He, S.; Li, R. Structural Diversity, Fitness Cost, and Stability of a BlaNDM-1-Bearing Cointegrate Plasmid in Klebsiella pneumoniae and Escherichia coli. Microorganisms 2021, 9, 2435. [Google Scholar] [CrossRef] [PubMed]
- Enne, V.I.; Delsol, A.A.; Davis, G.R.; Hayward, S.L.; Roe, J.M.; Bennett, P.M. Assessment of the fitness impacts on Escherichia coli of acquisition of antibiotic resistance genes encoded by different types of genetic element. J. Antimicrob. Chemother. 2005, 56, 544–551. [Google Scholar] [CrossRef]
- Dimitriu, T.; Medaney, F.; Amanatidou, E.; Forsyth, J.; Ellis, R.J.; Raymond, B. Negative frequency dependent selection on plasmid carriage and low fitness costs maintain extended spectrum β-lactamases in Escherichia coli. Sci. Rep. 2019, 9, 17211. [Google Scholar] [CrossRef]
- Stokes, J.M.; MacNair, C.R.; Ilyas, B.; French, S.; Côté, J.P.; Bouwman, C.; Farha, M.A.; Sieron, A.O.; Whitfield, C.; Coombes, B.K.; et al. Pentamidine sensitizes Gram-negative pathogens to antibiotics and overcomes acquired colistin resistance. Nat. Microbiol. 2017, 2, 17028. [Google Scholar] [CrossRef] [PubMed]
- Farha, M.A.; Brown, E.D. Chemical probes of Escherichia coli uncovered through chemical-chemical interaction profiling with compounds of known biological activity. Chem. Biol. 2010, 17, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J. Mol. Microbiol. Biotechnol. 2001, 3, 255–264. [Google Scholar] [PubMed]
- Sobel, M.L.; Hocquet, D.; Cao, L.; Plesiat, P.; Poole, K. Mutations in PA3574 nalD Lead to Increased MexAB-OprM Expression and Multidrug Resistance in Laboratory and Clinical Isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2005, 49, 1782–1786. [Google Scholar] [CrossRef]
- Bollenbach, T. Antimicrobial interactions: Mechanisms and implications for drug discovery and resistance evolution. Curr. Opin. Microbiol. 2015, 27, 1–9. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Cattoir, V.; Poirel, L.; Rotimi, V.; Soussy, C.J.; Nordmann, P. Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBL-producing enterobacterial isolates. J. Antimicrob. Chemother. 2007, 60, 394–397. [Google Scholar] [CrossRef]
- Pribul, B.R.; Festivo, M.L.; Souza, M.M.S.d.; Rodrigues, D.d.P. Characterization of quinolone resistance in Salmonella spp. isolates from food products and human samples in Brazil. Braz. J. Microbiol. 2016, 47, 196–201. [Google Scholar] [CrossRef][Green Version]
- Chen, X.; Zhang, W.; Pan, W.; Yin, J.; Pan, Z.; Gao, S.; Jiao, X. Prevalence of qnr, aac(6’)-Ib-cr, qepA, and oqxAB in Escherichia coli isolates from humans, animals, and the environment. Antimicrob. Agents Chemother. 2012, 56, 3423–3427. [Google Scholar] [CrossRef]
- Kim, H.B.; Wang, M.; Park, C.H.; Kim, E.C.; Jacoby, G.A.; Hooper, D.C. oqxAB encoding a multidrug efflux pump in human clinical isolates of Enterobacteriaceae. Antimicrob. Agents Chemother. 2009, 53, 3582–3584. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Park, C.H.; Kim, C.J.; Kim, E.C.; Jacoby, G.A.; Hooper, D.C. Prevalence of plasmid-mediated quinolone resistance determinants over a 9-year period. Antimicrob. Agents Chemother. 2009, 53, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Jalal, S.; Ciofu, O.; Hoiby, N.; Gotoh, N.; Wretlind, B. Molecular mechanisms of fluoroquinolone resistance in Pseudomonas aeruginosa isolates from cystic fibrosis patients. Antimicrob. Agents Chemother. 2000, 44, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, Z.; Li, X.; Song, Y.; Kang, J.; Yin, D.; Gao, Y.; Shi, N.; Duan, J. Mutations in gyrB play an important role in ciprofloxacin-resistant Pseudomonas aeruginosa. Infect. Drug Resist. 2019, 12, 261–272. [Google Scholar] [CrossRef]
- Martínez-Martínez, L.; Pascual, A.; Jacoby, G.A. Quinolone resistance from a transferable plasmid. Lancet 1998, 351, 797–799. [Google Scholar] [CrossRef]
- Blair, J.M.; Piddock, L.J. How to measure export via bacterial multidrug resistance efflux pumps. Mbio 2016, 7, e00840-00816. [Google Scholar] [CrossRef] [PubMed]
- Wang-Kan, X.; Blair, J.M.A.; Chirullo, B.; Betts, J.; La Ragione, R.M.; Ivens, A.; Ricci, V.; Opperman, T.J.; Piddock, L.J.V. Lack of AcrB Efflux Function Confers Loss of Virulence on Salmonella enterica Serovar Typhimurium. Mbio 2017, 8, e00968-00917. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).