7-Acetoxyhorminone from Salvia multicaulis Vahl. as Promising Inhibitor of 3-Hydroxy-3-methylglutaryl Coenzyme A (HMG-CoA) Reductase
Abstract
:1. Introduction
2. Results
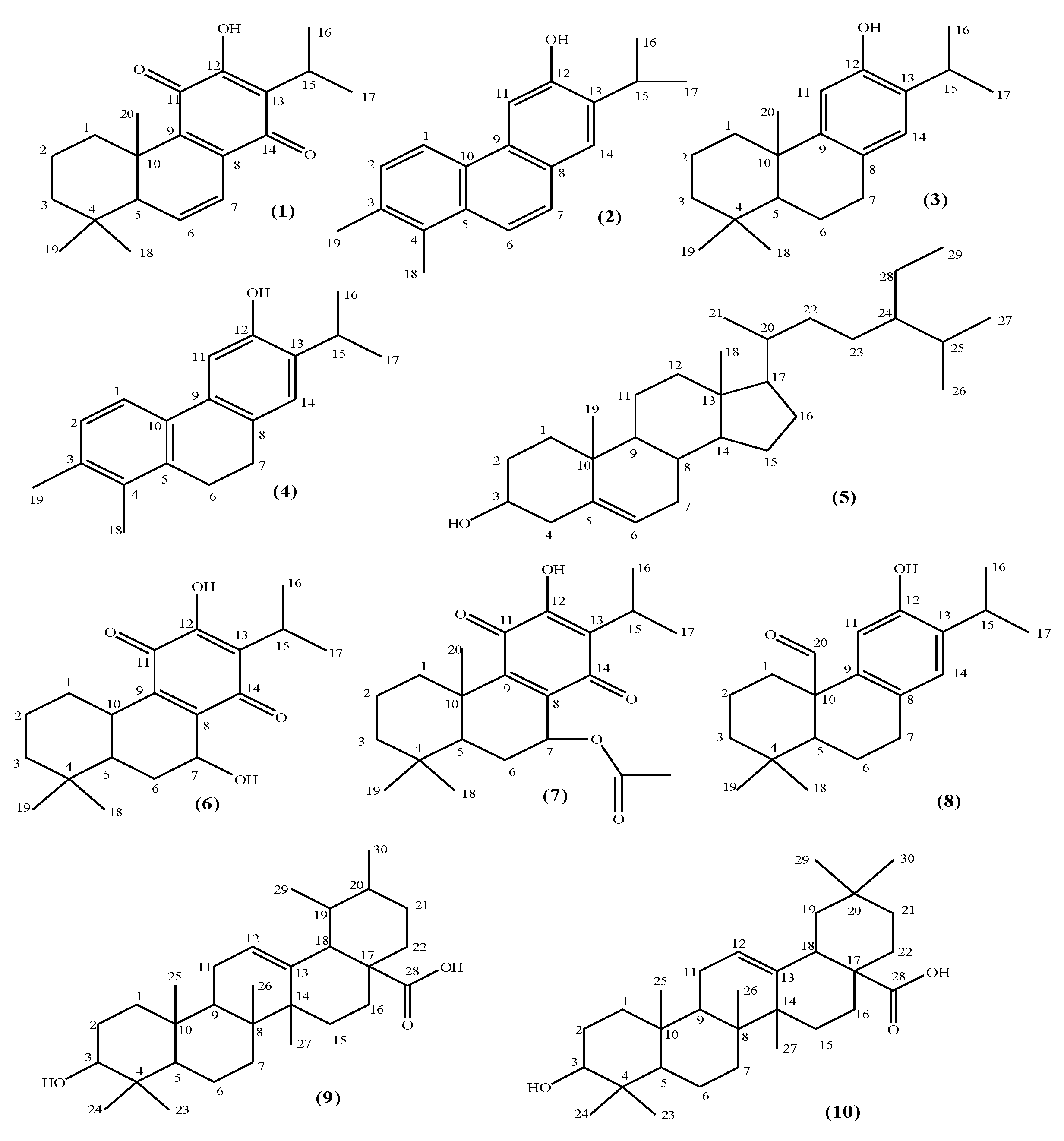
2.1. Isolation of the Compounds
2.2. Structure Elucidation
2.3. HMG-CoA Reductase Inhibitory Activity of the Isolated Compounds
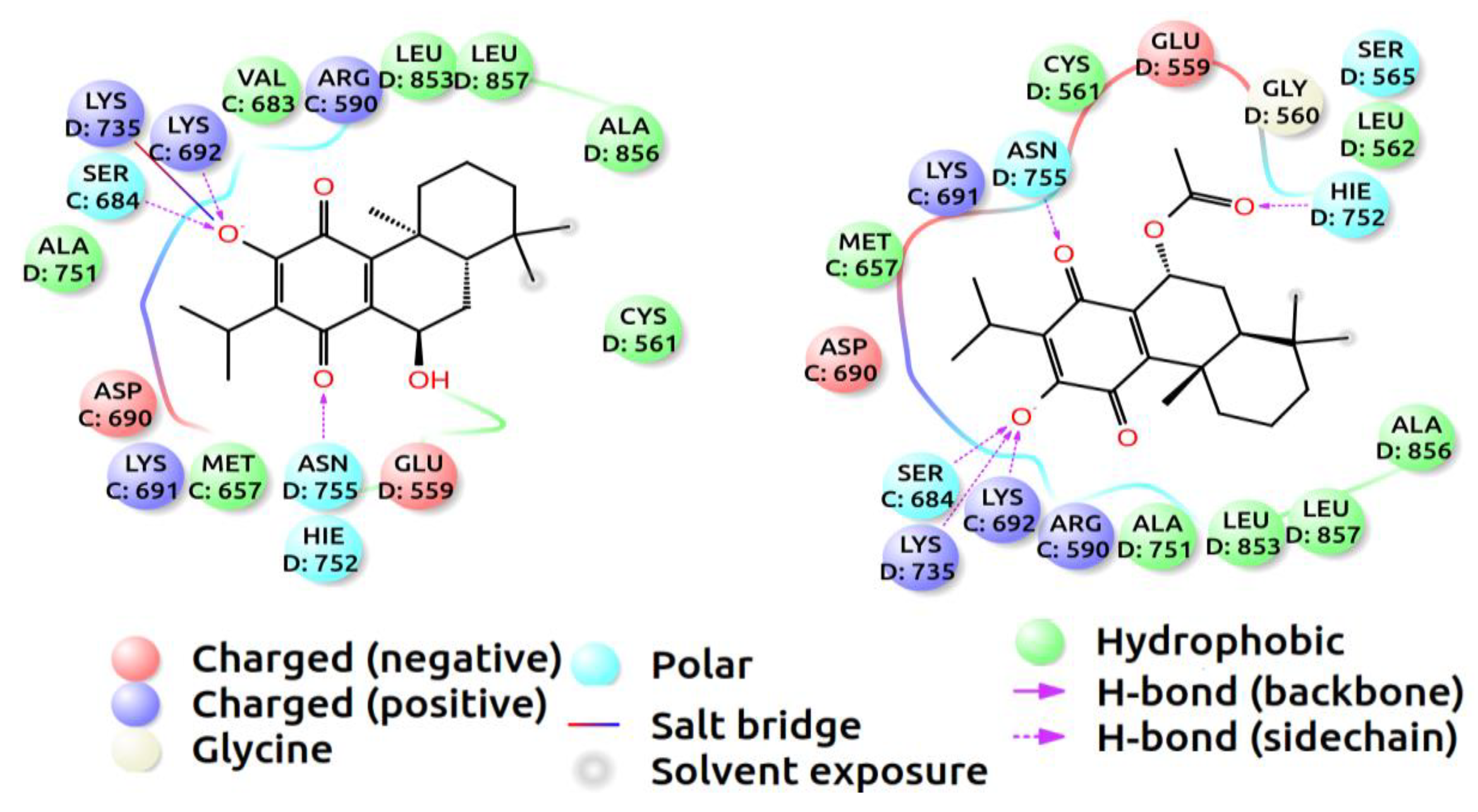
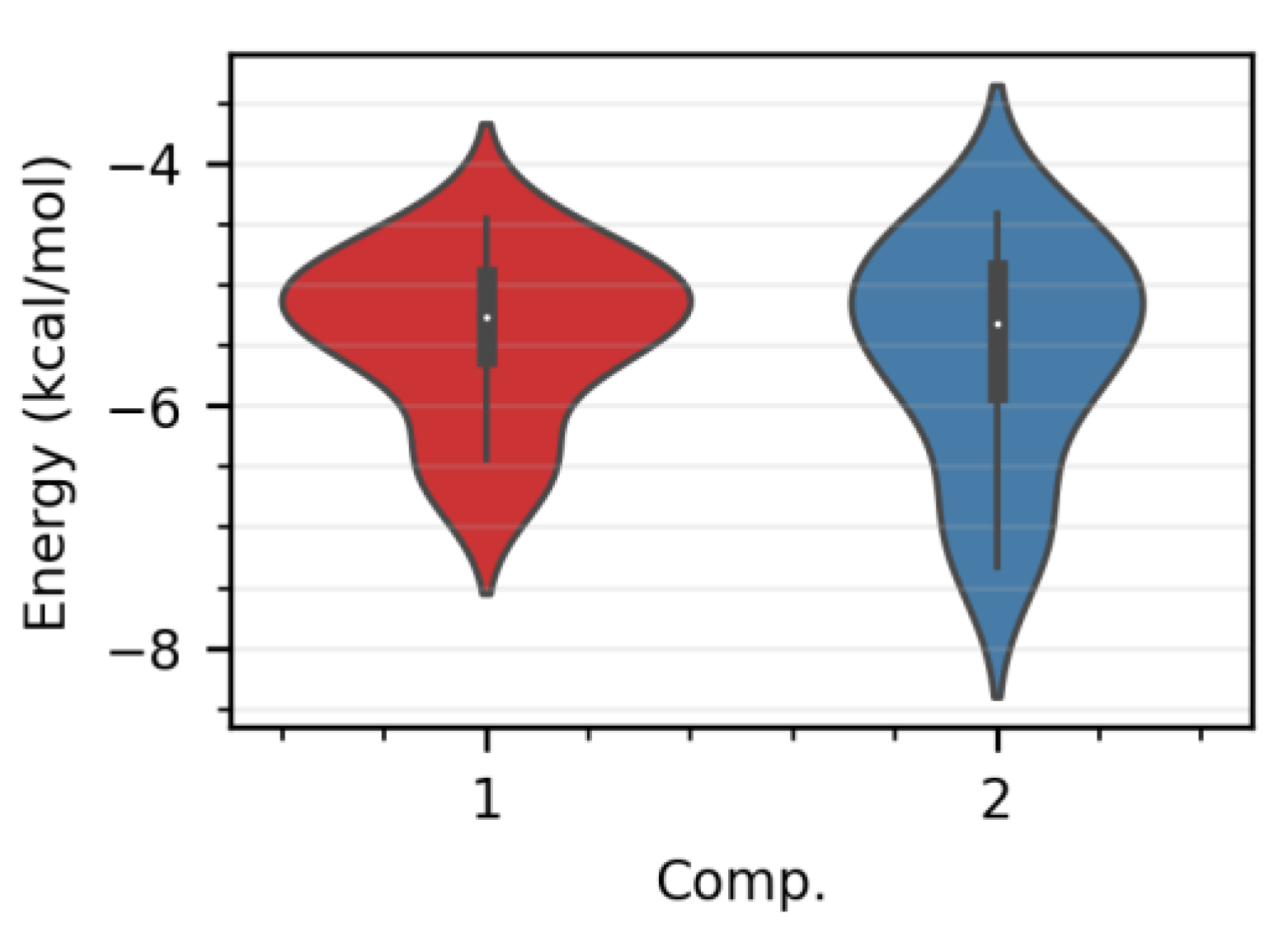
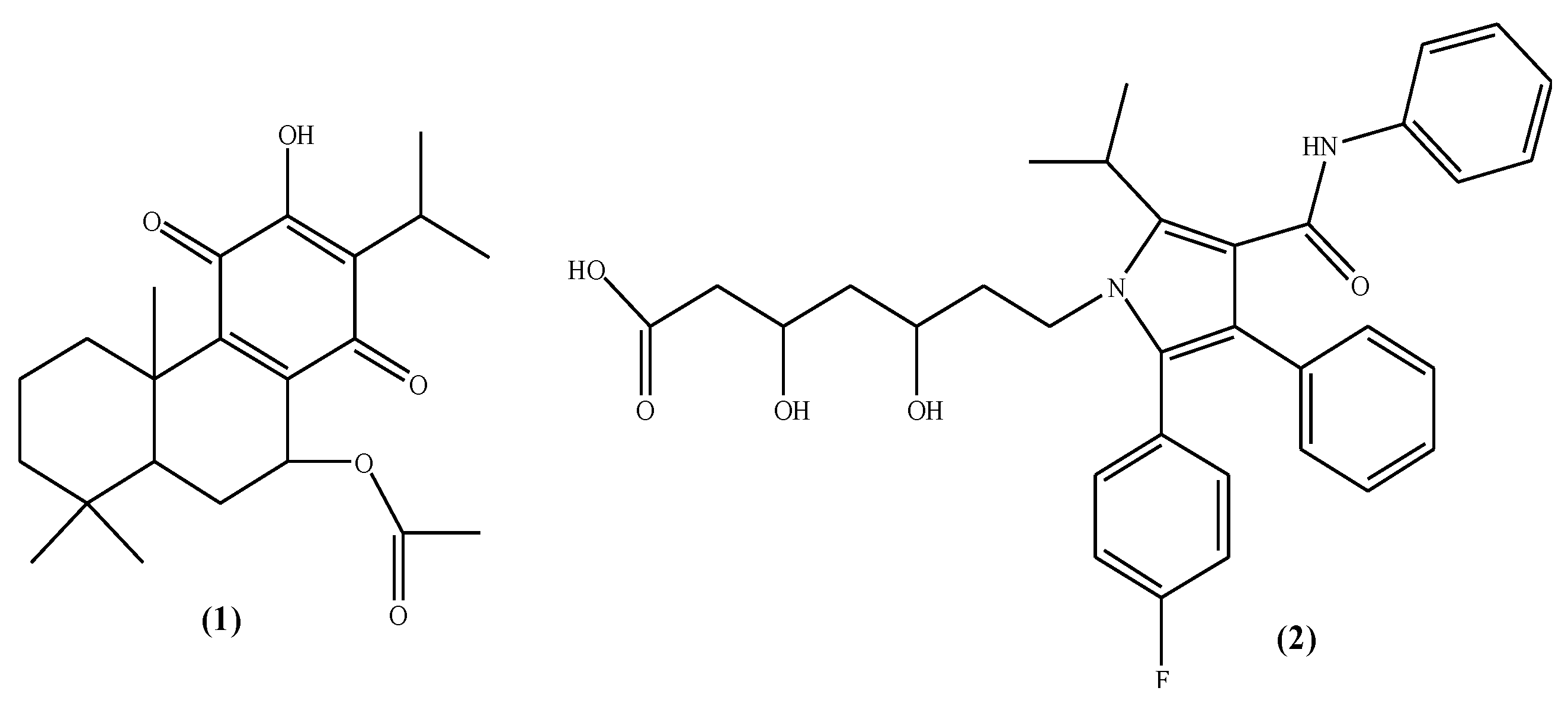
2.4. Molecular Docking Data
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Fractionation
4.4. Microtiter Assay for HMG-CoA Reductase Inhibition
4.5. Molecular Docking Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gholamhoseinian, A.; Shahouzehi, B.; Sharifi-Far, F. Inhibitory activity of some plant methanol extracts on 3-hydroxy-3-methylglutaryl coenzyme a reductase. Int. J. Pharmacol. 2010, 6, 705–711. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Nain, S. A mini-review on hyperlipidemia: Common clinical problem. J. Interv. Cardiol. 2018, 4, 10–11. [Google Scholar]
- Steinberg, D. Low density lipoprotein oxidation and its pathobiological significance. J. Biol. Chem. 1997, 272, 20963–20966. [Google Scholar] [CrossRef] [Green Version]
- Baskaran, G.; Shukor, M.Y.; Salvamani, S.; Ahmad, S.A.; Shaharuddin, N.A.; Pattiram, P.D. HMG-CoA reductase inhibitory activity and phytocomponent investigation of Basella alba leaf extract as a treatment for hypercholesterolemia. Drug Des. Devel. Ther. 2015, 9, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Gülcan, H.O.; Yiğitkan, S.; Orhan, I.E. The natural products as hydroxymethylglutaryl-CoA reductase inhibitors. Lett. Drug Des. Discov. 2019, 16, 1130–1137. [Google Scholar] [CrossRef]
- Yigitkan, S. Investigation of Some Plants Grown in Turkey for Their HMG (3-hydroxy-3-methyl-glutaryl) CoA Reductase Inhibitory Effect (Supervisor: Prof.Dr. Ilkay Erdogan Orhan). Ph.D. Thesis, Gazi University, Ankara, Turkey, 2020. [Google Scholar]
- Shen, C.; Huang, L.; Xiang, H.; Deng, M.; Gao, H.; Zhu, Z.; Lui, M.; Luo, G. Inhibitory effects on the HMG-CoA Reductase in the chemical constituents of the Cassia mimosoides Linn. Rev. Romana Medicina Lab. 2016, 24, 413–422. [Google Scholar] [CrossRef] [Green Version]
- Bağcı, E.; Koçak, A. Essential oil composition of the aerial parts of two Salvia L. (S. multicaulis Vahl. Enum. and S. tricochlada Bentham) species from East Anatolian region (Turkey). Int. J. Sci. Technol. Res. 2008, 3, 13–18. [Google Scholar]
- Firat, M. An addition to the flora of Turkey: Salvia reuterıana (Lamiaceae), with contributions to its taxonomy. Iran. J. Bot. 2020, 26, 137–140. [Google Scholar]
- Tabanca, N.; Demirci, B.; Başer, K.H.C.; Aytac, Z.; Ekici, M.; Khan, S.I.; Jacob, M.R.; Wedge, D.E. Chemical composition and antifungal activity of Salvia macrochlamys and Salvia recognita essential oils. J. Agric. Food Chem. 2006, 54, 6593–6597. [Google Scholar] [CrossRef]
- Tabanca, N.; Demirci, B.; Aytaç, Z.; Baser, K.H.C. The chemical composition of Salvia verticillata L. subsp. verticillata from Turkey. Nat. Volatiles Essent. Oils. 2017, 4, 18–28. [Google Scholar]
- Özek, G.; Demirci, F.; Özek, T.; Tabanca, N.; Wedge, D.E.; Khan, S.I.; Başer, K.H.C.; Duran, A.H. Gas chromatographic-mass spectrometric analysis of volatiles obtained by four different techniques from Salvia rosifolia Sm. and evaluation for biological activity. J. Chromatogr. A 2010, 1217, 741–748. [Google Scholar] [CrossRef]
- Aşkun, T.; Başer, K.H.C.; Tümen, G.; Kürkçüoğlu, M. Characterization of essential oils of some Salvia species and their antimycobacterial activities. Turk. J. Biol. 2010, 34, 89–95. [Google Scholar]
- Süntar, I.; Küpeli Akkol, E.; Keleş, H.; Öktem, A.; Başer, K.H.C.; Yeşilada, E. A novel wound healing ointment: A formulation of Hypericum perforatum oil and sage and oregano essential oils based on traditional Turkish knowledge. J. Ethnopharmacol. 2011, 134, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Yarıs, E.; Adsız, L.B.; Yener, I.; Tuncay, E.; Yilmaz, M.A.; Akdeniz, M.; Kaplaner, E.; Firat, M.; Ertas, A.; Kolak, U. Isolation of secondary metabolites of two endemic species: Salvia rosifolia Sm. and Salvia cerino-pruinosa Rech. f. var. elazigensis (Lamiaceae). J. Food Meas. Charact. 2021, 15, 4929–4938. [Google Scholar] [CrossRef]
- Barhoumi, L.M.; Al-Jaber, H.I.; Zarga, M.H.A. A new diterpene and other constituents Salvia multicaulis from Jordan. Nat. Prod. Res. 2021, 24, 1–8. [Google Scholar] [CrossRef]
- Hajar, R. Statins: Past and present. Heart Views. 2011, 12, 121–127. [Google Scholar] [CrossRef]
- Kültür, Ş. Medicinal plants used in Kırklareli Province (Turkey). J. Ethnopharmacol. 2007, 111, 341–364. [Google Scholar] [CrossRef]
- Güneş, F.; Özhatay, N. An ethnobotanical study from Kars (Eastern) Turkey. Biodivers. Conserv. 2011, 4, 30–41. [Google Scholar]
- Li, M.; Li, Q.; Zhang, C.; Zhang, N.; Cui, Z.; Huang, L.; Xiao, P. An ethnopharmacological investigation of medicinal Salvia plants (Lamiaceae) in China. Acta Pharm. Sin. B. 2013, 3, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Endo, A. A historical perspective on the discovery of statins. Proc. Jpn. Acad. 2010, 86, 484–493. [Google Scholar] [CrossRef] [Green Version]
- Endo, A. A gift from nature: The birth of the statins. Nat. Med. 2008, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, N.; Ashitani, T.; Hayasaka, Y.; Murayama, T.; Ogiyama, K.; Takahashi, K. Antitermitic activities of abietane-type diterpenes from Taxodium distichum cones. J. Chem. Ecol. 2009, 35, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Ulubelen, A.; Topçu, G.; Chen, S.; Cai, P.; Snyder, J.K. A new abietane diterpene from Salvia wiedemannii Boiss. J. Org. Chem. 1991, 56, 7354–7356. [Google Scholar] [CrossRef]
- Tezuka, Y.; Kasimu, R.; Li, J.X.; Basnet, P.; Tanaka, K.; Namba, T.; Kadota, S. Constituents of roots of Salvia deserta Schang. (Xinjiang-Danshen). Chem. Pharm. Bull. 1998, 46, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Öztekin, N.; Başkan, S.; Evrim Kepekçi, S.; Erim, F.B.; Topçu, G. Isolation and analysis of bioactive diterpenoids in Salvia species (Salvia chionantha and Salvia kronenburgii) by micellar electrokinetic capillary chromatography. J. Pharm. Biomed. 2010, 51, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, B. 1H and 13C-NMR spectral assignments of some natural abietane diterpenoids. Magn. Reson. Chem. 2003, 41, 741–746. [Google Scholar] [CrossRef]
- Takashi, M.; Yoshinori, E.; Masaru, O. The synthesis of (+)-pisiferol and (+)-pisiferal. Bull. Chem. Soc. Jpn. 1983, 56, 7–8. [Google Scholar]
- Ulubelen, A.; Topçu, G.; Johansson, C.B. Norditerpenoids and diterpenoids from Salvia multicaulis with antituberculous activity. J. Nat. Prod. 1997, 60, 1275–1280. [Google Scholar] [CrossRef]
- Topçu, G.; Ertaş, A.; Kolak, U.; Öztürk, M.; Ulubelen, A. Antioxidant activity tests on novel triterpenoids from Salvia Macrochlamys. Arkivoc 2007, 7, 195–208. [Google Scholar] [CrossRef] [Green Version]
- Ertaş, A.; Topçu, G.; Kolak, U.; Temel, H.; Yener, I.; Yılmaz, M.A. Screening of some Salvia (sage) species by HPLC-IT-TOF-MS, purification of their secondary metabolites, and determination of their anticancer effect. In The Scientific and Technological Research Council of Turkey (TÜBİTAK); Project No: 114Z801; Ankara, Turkey, 2018. [Google Scholar]
- Ertas, A.; Goren, A.C.; Haşimi, N.; Tolan, V.; Kolak, U. Chemical constituents of Mentha longifolia subsp. noeana with antioxidant, anticholinesterase and antimicrobial activities. Rec. Nat. Prod. 2015, 9, 105–115. [Google Scholar]
- Karr, S. Epidemiology and management of hyperlipidemia. Am. J. Manag. Care. 2017, 23 (Suppl. 9), 139. [Google Scholar]
- Schachter, M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: An update. Fundam. Clin. Pharmacol. 2005, 19, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Azemawah, V.; Movahed, M.R.; Centuori, P.; Penaflor, R.; Riel, P.L.; Situ, S.; Hashemzadeh, M. State of the art comprehensive review of individual statins, their differences, pharmacology, and clinical implications. Cardiovasc. Drugs Ther. 2019, 33, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, T.E. Role of statin therapy in primary prevention of cardiovascular disease in elderly patients. Curr. Atheroscler. Rep. 2019, 21, 28. [Google Scholar] [CrossRef] [Green Version]
- Abd, T.; Jacobson, T.A. Statin-induced myopathy: A review and update. Expert Opin. Drug Saf. 2011, 10, 373–387. [Google Scholar] [CrossRef]
- Robinson, J.G. Statins and diabetes risk: How real is it and what are the mechanisms? Curr. Opin. Lipidol. 2015, 26, 228–235. [Google Scholar] [CrossRef]
- Simic, I.; Reiner, Z. Adverse effects of statins—Myths and reality. Curr. Pharm. Des. 2015, 21, 1220–1226. [Google Scholar] [CrossRef]
- Auer, J.; Sinzinger, H.; Franklin, B.; Berent, R. Muscle-and skeletal-related side-effects of statins: Tip of the iceberg? Eur. J. Prev. Cardiol. 2016, 23, 88–110. [Google Scholar] [CrossRef]
- Bellosta, S.; Corsini, A. Statin drug interactions and related adverse reactions. Expert Opin. Drug Saf. 2012, 11, 933–946. [Google Scholar] [CrossRef]
- Yandrapalli, S.; Malik, A.; Guber, K.; Rochlani, Y.; Pemmasani, G.; Jasti, M.; Aronow, W.S. Statins and the potential for higher diabetes mellitus risk. Expert Rev. Clin. Pharmacol. 2019, 12, 825–830. [Google Scholar] [CrossRef]
- Pedro-Botet, J.; Climent, E.; Benaiges, D. Muscle and statins: From toxicity to the nocebo effect. Expert Opin. Drug Saf. 2019, 18, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, M.; Zhao, T.; Zhao, B.; Jia, L.; Zhu, Y.; Xiang, R. Danhong injection inhibits the development of atherosclerosis in both Apoe−/− and Ldlr−/− mice. J. Cardiovasc. Pharmacol. 2014, 63, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.S.; Soares-Freitas, R.A.M.; Arêas, J.A.G.; Gandra, E.A.; de las Mercedes Salas-Mellado, M. Peptides from chia present antibacterial activity and inhibit cholesterol synthesis. Plant Foods Hum. Nutr. 2018, 73, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Singh, S.P.; Srivastava, A.; Puri, A.; Chhonker, Y.S.; Bhatta, R.S.; Siddiqi, M.I. Discovery of a new class of HMG-CoA reductase inhibitor from Polyalthia longifolia as potential lipid lowering agent. Eur. J. Med. Chem. 2011, 46, 5206–5211. [Google Scholar] [CrossRef]
- Laksman, T.S.; Puttrevu, S.K.; Pradhan, R.; Mishra, A.; Verma, S.; Chhonker, Y.S.; Bhatta, R.S. Pharmacokinetics, metabolism, bioavailability, tissue distribution and excretion studies of 16α-hydroxycleroda-3, 13 (14) Z-dien-15, 16-olide—A novel HMG-CoA reductase inhibitor. Naunyn Schmiedebe. Arch. Pharmacol. 2018, 391, 965–973. [Google Scholar]
- Halvorsen, B.; Ranheim, T.; Nenseter, M.S.; Huggett, A.C.; Drevon, C.A. Effect of a coffee lipid (cafestol) on cholesterol metabolism in human skin fibroblasts. J. Lipid Res. 1998, 39, 901–912. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, I.; Kang, S.S. Cholesterol biosynthesis inhibitors of microbial origin. Nat. Prod. Chem. 2006, 33, 731. [Google Scholar]
- Manzoni, M.; Rollini, M. Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl. Microbiol. Biotechnol. 2002, 58, 555–564. [Google Scholar]
- Wang, K.; Bao, L.; Xiong, W.; Ma, K.; Han, J.; Wang, W.; Liu, H. Lanostane triterpenes from the Tibetan medicinal mushroom Ganoderma leucocontextum and their inhibitory effects on HMG-CoA reductase and α-glucosidase. J. Nat. Prod. 2015, 78, 1977–1989. [Google Scholar] [CrossRef]
- Pfefferkorn, J.A.; Song, Y.; Sun, K.L.; Miller, S.R.; Trivedi, B.K.; Choi, C.; Sorenson, R.J.; Bratton, L.D.; Unangst, P.C.; Larsen, S.D.; et al. Design and synthesis of hepatoselective, pyrrole-based HMG-CoA reductase inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 4538–4544. [Google Scholar] [CrossRef]
- Madhavi Sastry, G.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 3. [Google Scholar] [CrossRef]
- Farid, R.; Day, T.; Friesner, R.A.; Pearlstein, R.A. New insights about HERG blockade obtained from protein modeling, potential energy mapping, and docking studies. Bioorg. Med. Chem. 2006, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel procedure for modeling ligand/receptor induced fit effects. J. Med. Chem. 2006, 49, 2. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.; Beard, H.S.; Farid, R. Use of an induced fit receptor structure in virtual screening. Chem. Biol. Drug. Des. 2006, 67, 83–84. [Google Scholar] [CrossRef] [PubMed]
| Extracts and Fractions | HMG-CoA Reductase Inhibition (Inhibition % ± SD a at 100 µg/mL) |
|---|---|
| Aerial part-petroleum ether | NA b |
| Root-petroleum ether | NA |
| Aerial part-ethanol | 57.16 ± 0.24 |
| Root-ethanol | 60.26 ± 0.19 |
| Aerial part-dichloromethane–acetone (1:1) | 55.21 ± 0.48 |
| Root-dichloromethane–acetone (1:1) | 71.97 ± 0.36 |
| M-1: Fr. 1–4 | NA |
| M-2: Fr. 5–6 | 16.30 ± 0.33 |
| M-3: Fr. 7–9 | 50.17 ± 1.24 c |
| M-4: Fr. 10–13 | 3.50 ± 2.61 |
| M-5: Fr. 14 | 15.22 ± 0.08 |
| M-6: Fr. 15–17 | 54.81 ± 0.80 |
| M-7: Fr. 18–20 | 52.83 ± 0.31 |
| M-8: Fr. 21–22 | 57.39 ± 0.06 |
| M-9: Fr. 23–24 | 32.12 ± 0.11 |
| M-10: Fr. 25–26 | 56.45 ± 0.14 |
| M-11: Fr. 27–32 | 29.66 ± 2.57 |
| M-12: Fr. 33–35 | 28.20 ± 0.20 |
| M-13: Fr. 36–40 | NA |
| M-14: Fr. 41–48 | NA |
| M-15: Fr. 49–52 | NA |
| Atorvastatin | 91.06 ± 0.46 |
| No | Compounds | HMG-CoA Reductase Inhibition (Inhibition % ± SD a at 100 µg/mL) |
|---|---|---|
| 1 | 6,7-Dehydroyleanone | 28.50 ± 0.13 |
| 2 | 12-Demethylmulticaulin | 35.29 ± 0.05 |
| 3 | Ferruginol | 18.11 ± 0.10 |
| 4 | 12-Hydroxy abieta-1, 3, 5(10), 8, 11, 13-hexaene | 2.26 ± 0.21 |
| 5 | β-Sitosterol | 12.52 ± 0.14 |
| 6 | Horminone–7-acetoxyhorminone | 76.26 ± 0.14 c (IC50 = 52.3 ± 0.78 µg/mL) |
| 7 | 7-Acetoxyhorminone | 84.15 ± 0.10 (IC50 = 63.6 ± 1.21 µg/mL) |
| 8 | Pisiferal | NA b |
| 9 | Ursolic acid | 43.32 ± 0.11 |
| 10 | Oleanolic acid | 25.00 ± 0.14 |
| Atorvastatin | 97.16 ± 0.01 (IC50 = 3.0 ± 0.36 µg/mL) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yigitkan, S.; Ertas, A.; Salmas, R.E.; Firat, M.; Orhan, I.E. 7-Acetoxyhorminone from Salvia multicaulis Vahl. as Promising Inhibitor of 3-Hydroxy-3-methylglutaryl Coenzyme A (HMG-CoA) Reductase. Pharmaceuticals 2022, 15, 198. https://doi.org/10.3390/ph15020198
Yigitkan S, Ertas A, Salmas RE, Firat M, Orhan IE. 7-Acetoxyhorminone from Salvia multicaulis Vahl. as Promising Inhibitor of 3-Hydroxy-3-methylglutaryl Coenzyme A (HMG-CoA) Reductase. Pharmaceuticals. 2022; 15(2):198. https://doi.org/10.3390/ph15020198
Chicago/Turabian StyleYigitkan, Serkan, Abdulselam Ertas, Ramin Ekhteiari Salmas, Mehmet Firat, and Ilkay Erdogan Orhan. 2022. "7-Acetoxyhorminone from Salvia multicaulis Vahl. as Promising Inhibitor of 3-Hydroxy-3-methylglutaryl Coenzyme A (HMG-CoA) Reductase" Pharmaceuticals 15, no. 2: 198. https://doi.org/10.3390/ph15020198
APA StyleYigitkan, S., Ertas, A., Salmas, R. E., Firat, M., & Orhan, I. E. (2022). 7-Acetoxyhorminone from Salvia multicaulis Vahl. as Promising Inhibitor of 3-Hydroxy-3-methylglutaryl Coenzyme A (HMG-CoA) Reductase. Pharmaceuticals, 15(2), 198. https://doi.org/10.3390/ph15020198








