Systematic Review of Gossypol/AT-101 in Cancer Clinical Trials
Abstract
1. Introduction
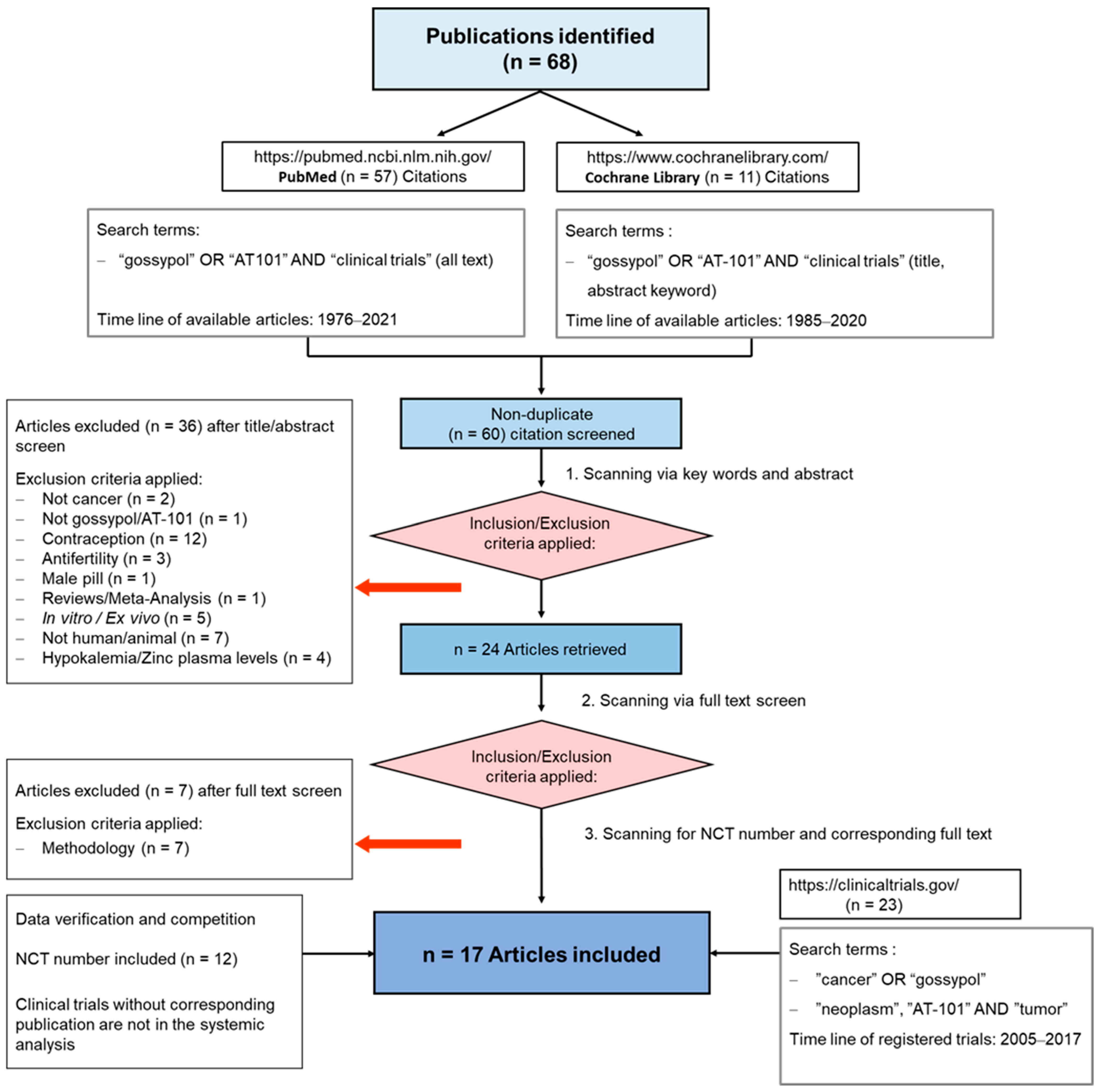
2. Materials and Methods
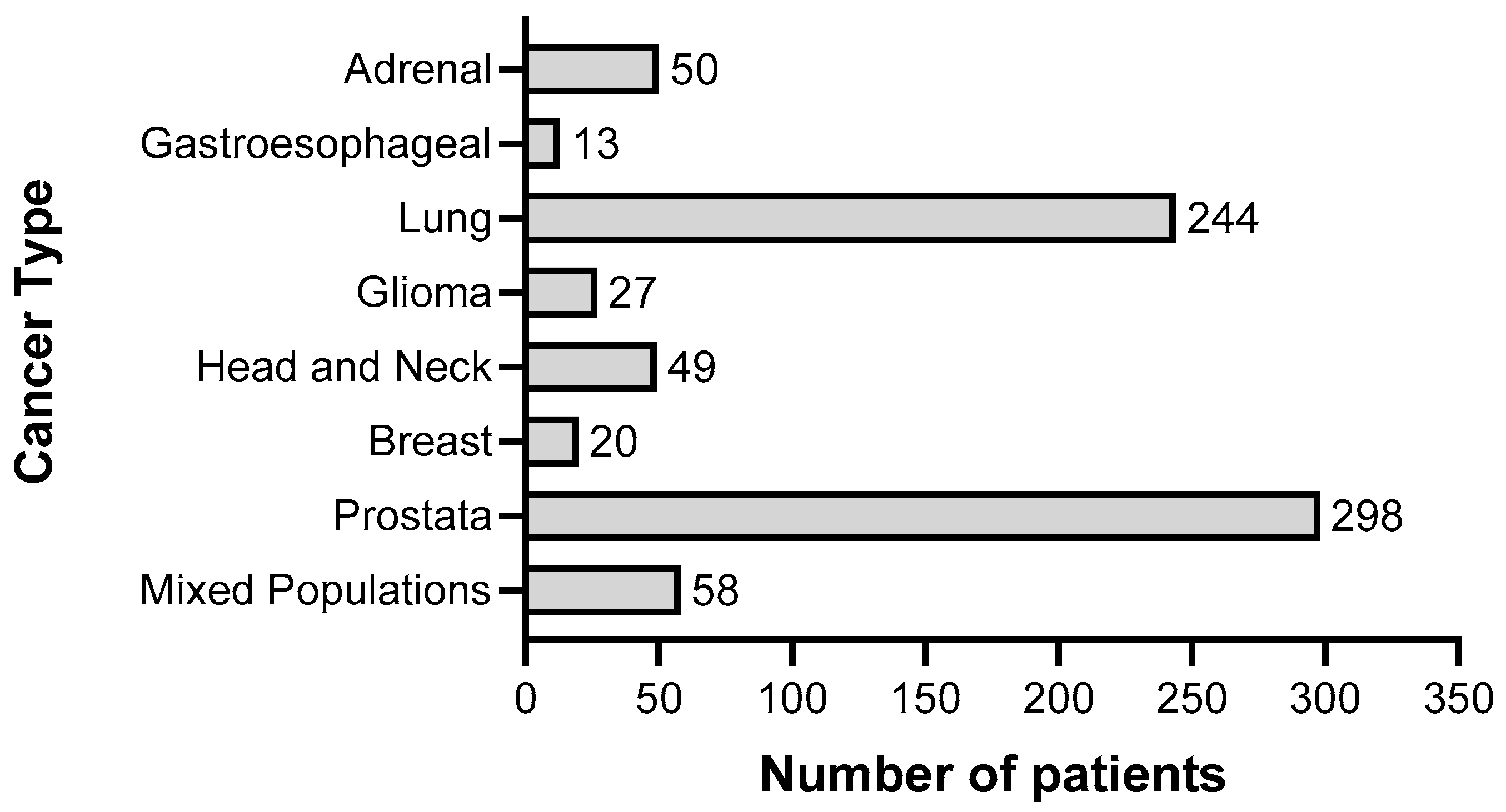
3. Results
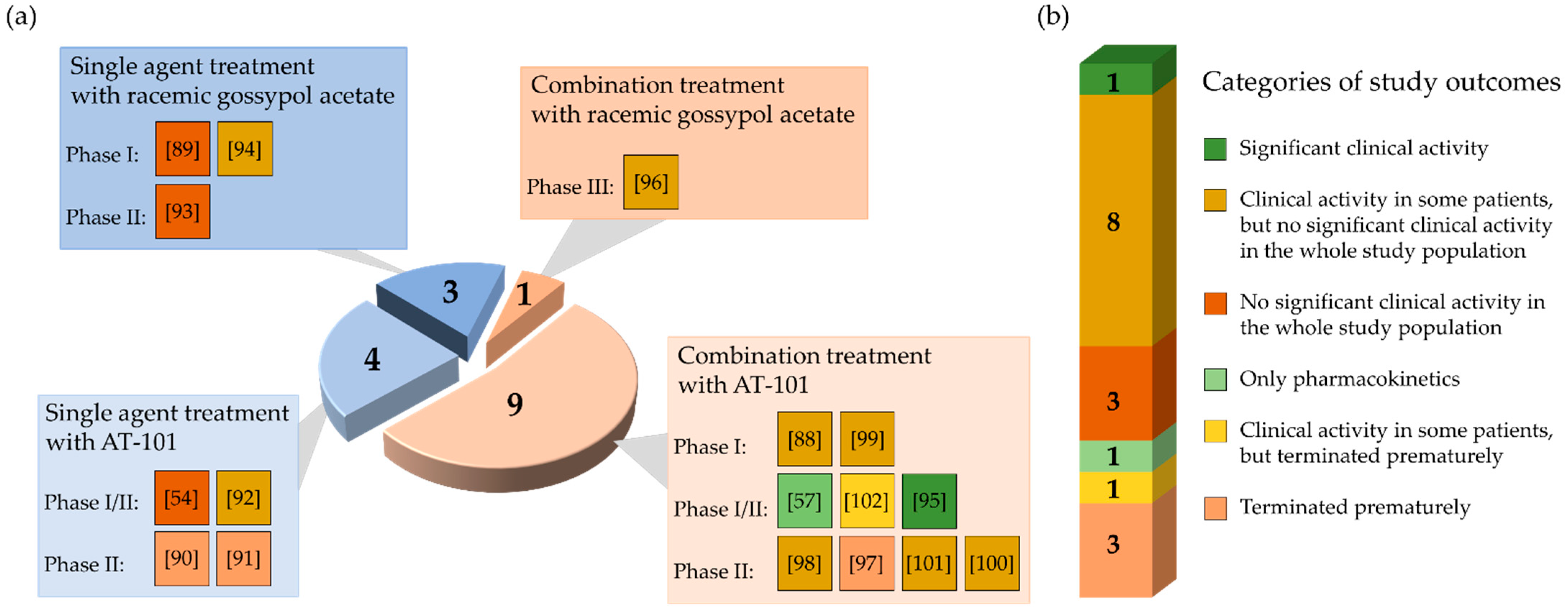
3.1. Trials Determining Therapeutic Effect and Toxicity of Single-Agent Gossypol/AT-101
3.2. Trials Evaluating Efficacy and Safety of Gossypol/AT-101 in Combination with Standard Chemo- and Radiation Therapies in Cancer Patients
3.3. Investigations Not Published as an Original Manuscript/Work on PubMed, MEDLINE, and Cochrane Databases but Containing Results of Clinical Investigations
4. Summary
4.1. Bioavailability, Digestion, Transporters, and Liver Toxicity
4.2. Potential Benefit and Current Limitations
4.3. Dose-Limiting Toxicities
4.4. Association of Gossypol/AT-101 with Cancer Parameters
4.5. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADT | androgen deprivation therapy |
| AE | adverse events |
| AIF | apoptosis-inducing factor |
| ALT | alanine aminotransferase |
| APE1/Ref-1 | apurinic/apyrimidinic endonuclease 1/redox enhancing factor-1 |
| AST | aspartate aminotransferase |
| AUC | area under the concentration time curve |
| BID | latin: bis in die; twice a day |
| Bcl-2 | B-cell lymphoma 2 |
| BH3 | Bcl-2 homology domain 3 |
| CEA | carcinoembryonic antigen |
| CHD1 | chromodomain helicase DNA-binding protein |
| co | control |
| cCR | clinical complete response |
| CR | complete response |
| CRPC | castrate-resistant prostate cancer |
| CTCAE | Common Terminology Criteria for Adverse Events |
| CXCL8 | chemokine C-X-C motif ligand 8 |
| DCR | disease control rate |
| DLT | dose-limiting toxicity |
| DNA | deoxyribonucleic acid |
| ECG | electrocardiogram |
| ECOG | Eastern Cooperative Oncology Group |
| eg | experimental group |
| EGFR | epidermal growth factor receptor |
| ES-SCLC | extensive stage—small cell lung cancer |
| GEC | gastroesophageal carcinoma |
| GI | gastrointestinal |
| HDAC | histone deacetylase |
| HDACi | histone deacetylase inhibitor |
| HNSCC | head and neck squamous cell carcinoma |
| IL-8 | interleukin-8 |
| IV | intravenously |
| LHRH | luteinizing hormone-releasing hormone |
| MOMP | mitochondrial outer membrane permeabilization |
| mOS | median overall survival |
| mPFS | median progression-free survival |
| MR | minor response |
| MTD | maximally tolerated dose |
| n. a. | not available |
| ORR | objective response rate |
| OR | objective response |
| OS | overall survival |
| P-gp | p-glycoprotein |
| PBMC | peripheral blood mononuclear cell |
| PCWG | Prostate Cancer Clinical Trials Working Group |
| PD | progressive disease |
| PFS | progression-free survival |
| PO BID | latin: per os bis in die; orally, twice a day |
| PO QD | latin: per os quaque die; orally, once a day |
| PR | partial response |
| PSA | prostate-specific antigen |
| R/M | recurrent/metastatic |
| Rb | retinoblastoma protein |
| RECIST | response evaluation criteria in solid tumors |
| ROS | reactive oxygen species |
| RR | response rate |
| SAE | serious adverse event |
| SCLC | small cell lung cancer |
| SD | stable disease |
| SOX9 | SRY-box transcription factor 9 |
| SWOG | Southwestern Oncology Group |
| VEGF | vascular endothelial growth factor |
| YAP1 | yes-associated protein 1 |
References
- Gadelha, I.C.N.; Fonseca, N.B.S.; Oloris, S.C.S.; Melo, M.M.; Soto-Blanco, B. Gossypol toxicity from cottonseed products. Sci. World J. 2014, 2014, 231635. [Google Scholar] [CrossRef] [PubMed]
- Kenar, J.A. Reaction chemistry of gossypol and its derivatives. J. Am. Oil Chem. Soc. 2006, 83, 269–302. [Google Scholar] [CrossRef]
- Abou-Donia, M.B. Physiological effects and metabolism of gossypol. In Residue Reviews; Springer: New York, NY, USA, 1976; pp. 125–160. [Google Scholar]
- Shelley, M.D.; Hartley, L.; Fish, R.G.; Groundwater, P.; Morgan, J.; Mort, D.; Mason, M.; Evans, A. Stereo-specific cytotoxic effects of gossypol enantiomers and gossypolone in tumour cell lines. Cancer Lett. 1999, 135, 171–180. [Google Scholar] [CrossRef]
- Sampath, D.S.; Balaram, P. Resolution of racemic gossypol and interaction of individual enantiomers with serum albumins and model peptides. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1986, 882, 183–186. [Google Scholar] [CrossRef]
- Liang, H.; Duo-Kai, Z.; Yi-Kang, S. Resolution of racemic gossypol. J. Ethnopharmacol. 1987, 20, 13–20. [Google Scholar] [CrossRef]
- Matlin, S.A.; Zhou, R. Resolution of gossypol: Analytical and preparative HPLC. J. High Resol. Chromatogr. 1984, 7, 629–631. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Zhang, Y.; Yasin, A.; Zhang, L. Investigating Stability and Tautomerization of Gossypol-A Spectroscopy Study. Molecules 2019, 24, 1286. [Google Scholar] [CrossRef]
- Hron, R.J.; Kim, H.L.; Calhoun, M.C.; Fisher, G.S. Determination of (+)-, (−)-, and total gossypol in cottonseed by high-performance liquid chromatography. J. Am. Oil Chem. Soc. 1999, 76, 1351–1355. [Google Scholar] [CrossRef]
- Jaroszewski, J.W.; Strøm-Hansen, T.; Hansen, S.H.; Thastrup, O.; Kofod, H. On the botanical distribution of chiral forms of gossypol. Planta Med. 1992, 58, 454–458. [Google Scholar] [CrossRef]
- Wu, D.F.; Yu, Y.W.; Tang, Z.M.; Wang, M.Z. Pharmacokinetics of (+/−)-, (+)-, and (−)-gossypol in humans and dogs. Clin. Pharm. 1986, 39, 613–618. [Google Scholar] [CrossRef]
- Stipanovic, R.D.; Puckhaber, L.S.; Bell, A.A.; Percival, A.E.; Jacobs, J. Occurrence of (+)- and (−)-gossypol in wild species of cotton and in Gossypium hirsutum Var. marie-galante (Watt) Hutchinson. J. Agric. Food Chem. 2005, 53, 6266–6271. [Google Scholar] [CrossRef] [PubMed]
- Hoshiai, H.; Uehara, S.; Mori, R.; Nagaike, F.; Tsuiki, A.; Suzuki, M. Gossypol as oral contraceptive for male: Trial case report. Tohoku J. Exp. Med. 1982, 138, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Lagerlöf, R.K.; Tone, J.N. The effect of gossypol acetic acid on female reproduction. Drug Chem. Toxicol. 1985, 8, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.Z.; Wang, Z.G. Gossypol: A potential antifertility agent for males. Annu. Rev. Pharmacol. Toxicol. 1984, 24, 329–360. [Google Scholar] [CrossRef] [PubMed]
- Dodou, K.; Anderson, R.J.; Lough, W.J.; Small, D.A.P.; Shelley, M.D.; Groundwater, P.W. Synthesis of gossypol atropisomers and derivatives and evaluation of their anti-proliferative and anti-oxidant activity. Bioorg. Med. Chem. 2005, 13, 4228–4237. [Google Scholar] [CrossRef]
- Tai-Shun, L.; Schinazi, R.F.; Zhu, J.; Birks, E.; Carbone, R.; Yikang, S.; Kemei, W.; Liang, H.; Prusoff, W.H. Anti-HIV-1 activity and cellular pharmacology of various analogs of gossypol. Biochem. Pharmacol. 1993, 46, 251–255. [Google Scholar] [CrossRef]
- Lin, T.S.; Schinazi, R.; Griffith, B.P.; August, E.M.; Eriksson, B.F.; Zheng, D.K.; Huang, L.A.; Prusoff, W.H. Selective inhibition of human immunodeficiency virus type 1 replication by the (−) but not the (+) enantiomer of gossypol. Antimicrob. Agents Chemother. 1989, 33, 2149–2151. [Google Scholar] [CrossRef]
- Radloff, R.J.; Deck, L.M.; Royer, R.E.; Vander Jagt, D.L. Antiviral activities of gossypol and its derivatives against herpes simplex virus type II. Pharmacol. Res. Commun. 1986, 18, 1063–1073. [Google Scholar] [CrossRef]
- Razakantoanina, V.; Nguyen Kim, P.P.; Jaureguiberry, G. Antimalarial activity of new gossypol derivatives. Parasitol. Res. 2000, 86, 665–668. [Google Scholar] [CrossRef]
- Royer, R.E.; Deck, L.M.; Campos, N.M.; Hunsaker, L.A.; Vander Jagt, D.L. Biologically active derivatives of gossypol: Synthesis and antimalarial activities of peri-acylated gossylic nitriles. J. Med. Chem. 1986, 29, 1799–1801. [Google Scholar] [CrossRef]
- Keshmiri-Neghab, H.; Goliaei, B. Therapeutic potential of gossypol: An overview. Pharm. Biol. 2014, 52, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Vadehra, D.V.; Kalla, N.R.; Saxena, M.; Hashia, R.; Kaur, P.; Gupta, L.K. Antimicrobial Activity of Gossypol Acetic-Acid. IRCS Med. Sci.-Biochem. 1985, 13, 10–11. [Google Scholar]
- Ye, W.; Chang, H.-L.; Wang, L.-S.; Huang, Y.-W.; Shu, S.; Sugimoto, Y.; Dowd, M.K.; Wan, P.J.; Lin, Y.C. Induction of apoptosis by (−)-gossypol-enriched cottonseed oil in human breast cancer cells. Int. J. Mol. Med. 2010, 26, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Piao, L.; Xu, P.; Ye, W.; Zhong, S.; Lin, S.-H.; Kulp, S.K.; Mao, Y.; Cho, Y.; Lee, L.J.; et al. Liposomes containing (−)-gossypol-enriched cottonseed oil suppress Bcl-2 and Bcl-xL expression in breast cancer cells. Pharm. Res. 2011, 28, 3256–3264. [Google Scholar] [CrossRef]
- Cao, H.; Sethumadhavan, K.; Cao, F.; Wang, T.T.Y. Gossypol decreased cell viability and down-regulated the expression of a number of genes in human colon cancer cells. Sci. Rep. 2021, 11, 5922. [Google Scholar] [CrossRef]
- Yuan, Y.; Tang, A.J.; Castoreno, A.B.; Kuo, S.-Y.; Wang, Q.; Kuballa, P.; Xavier, R.; Shamji, A.F.; Schreiber, S.L.; Wagner, B.K. Gossypol and an HMT G9a inhibitor act in synergy to induce cell death in pancreatic cancer cells. Cell Death Dis. 2013, 4, e690. [Google Scholar] [CrossRef]
- Pang, X.; Wu, Y.; Wu, Y.; Lu, B.; Chen, J.; Wang, J.; Yi, Z.; Qu, W.; Liu, M. (−)-Gossypol suppresses the growth of human prostate cancer xenografts via modulating VEGF signaling-mediated angiogenesis. Mol. Cancer 2011, 10, 795–805. [Google Scholar] [CrossRef]
- Le Blanc, M.; Russo, J.; Kudelka, A.P.; Smith, J.A. An in vitro study of inhibitory activity of gossypol, a cottonseed extract, in human carcinoma cell lines. Pharmacol. Res. 2002, 46, 551–555. [Google Scholar] [CrossRef]
- Cao, H.; Sethumadhavan, K.; Bland, J.M. Isolation of Cottonseed Extracts That Affect Human Cancer Cell Growth. Sci. Rep. 2018, 8, 10458. [Google Scholar] [CrossRef]
- Danial, N.N.; Korsmeyer, S.J. Cell Death. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Zeng, Y.; Ma, J.; Xu, L.; Wu, D. Natural Product Gossypol and its Derivatives in Precision Cancer Medicine. Curr. Med. Chem. 2019, 26, 1849–1873. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.-X.; Uto, T.; Tong, X.; Takeshita, T.; Tanigawa, S.; Imamura, I.; Ose, T.; Fujii, M. Involvement of reactive oxygen species-independent mitochondrial pathway in gossypol-induced apoptosis. Arch. Biochem. Biophys. 2004, 428, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, W.-T.; Tsai, M.-D.; Jow, G.-M.; Tien, L.-T.; Lee, Y.-J. Involvement of Smac, p53, and caspase pathways in induction of apoptosis by gossypol in human retinoblastoma cells. Mol. Vis. 2012, 18, 2033–2042. [Google Scholar] [PubMed]
- Meng, Y.; Tang, W.; Dai, Y.; Wu, X.; Liu, M.; Ji, Q.; Ji, M.; Pienta, K.; Lawrence, T.; Xu, L. Natural BH3 mimetic (−)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa. Mol. Cancer 2008, 7, 2192–2202. [Google Scholar] [CrossRef]
- Wolter, K.G.; Wang, S.J.; Henson, B.S.; Wang, S.; Griffith, K.A.; Kumar, B.; Chen, J.; Carey, T.E.; Bradford, C.R.; D’Silva, N.J. (−)-gossypol inhibits growth and promotes apoptosis of human head and neck squamous cell carcinoma in vivo. Neoplasia 2006, 8, 163–172. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007, 26, 1324–1337. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Wang, L.-S.; Dowd, M.K.; Wan, P.J.; Lin, Y.C. (−)-Gossypol reduces invasiveness in metastatic prostate cancer cells. Anticancer Res. 2009, 29, 2179–2188. [Google Scholar]
- Zhang, M.; Liu, H.; Guo, R.; Ling, Y.; Wu, X.; Li, B.; Roller, P.P.; Wang, S.; Yang, D. Molecular mechanism of gossypol-induced cell growth inhibition and cell death of HT-29 human colon carcinoma cells. Biochem. Pharmacol. 2003, 66, 93–103. [Google Scholar] [CrossRef]
- Macoska, J.A.; Adsule, S.; Tantivejkul, K.; Wang, S.; Pienta, K.J.; Lee, C.T. (−)Gossypol promotes the apoptosis of bladder cancer cells in vitro. Pharmacol. Res. 2008, 58, 323–331. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Huang, X.-F.; Mu, S.-J.; An, Q.-X.; Xia, A.-J.; Chen, R.; Wu, D.-C. Inhibition of proliferation of prostate cancer cell line, PC-3, in vitro and in vivo using (−)-gossypol. Asian J. 2010, 12, 390–399. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Wang, L.-S.; Chang, H.-L.; Ye, W.; Dowd, M.K.; Wan, P.J.; Lin, Y.C. Molecular mechanisms of (−)-gossypol-induced apoptosis in human prostate cancer cells. Anticancer Res. 2006, 26, 1925–1933. [Google Scholar] [PubMed]
- Mohammad, R.M.; Wang, S.; Aboukameel, A.; Chen, B.; Wu, X.; Chen, J.; Al-Katib, A. Preclinical studies of a nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-X(L) (−)-gossypol against diffuse large cell lymphoma. Mol. Cancer 2005, 4, 13–21. [Google Scholar]
- Oliver, C.L.; Miranda, M.B.; Shangary, S.; Land, S.; Wang, S.; Johnson, D.E. (−)-Gossypol acts directly on the mitochondria to overcome Bcl-2- and Bcl-X(L)-mediated apoptosis resistance. Mol. Cancer 2005, 4, 23–31. [Google Scholar]
- Balakrishnan, K.; Wierda, W.G.; Keating, M.J.; Gandhi, V. Gossypol, a BH3 mimetic, induces apoptosis in chronic lymphocytic leukemia cells. Blood 2008, 112, 1971–1980. [Google Scholar] [CrossRef]
- Lei, X.; Chen, Y.; Du, G.; Yu, W.; Wang, X.; Qu, H.; Xia, B.; He, H.; Mao, J.; Zong, W.; et al. Gossypol induces Bax/Bak-independent activation of apoptosis and cytochrome c release via a conformational change in Bcl-2. FASEB J. 2006, 20, 2147–2149. [Google Scholar] [CrossRef]
- Xiong, J.; Li, J.; Yang, Q.; Wang, J.; Su, T.; Zhou, S. Gossypol has anti-cancer effects by dual-targeting MDM2 and VEGF in human breast cancer. Breast Cancer Res. 2017, 19, 27. [Google Scholar] [CrossRef]
- Lian, J.; Wu, X.; He, F.; Karnak, D.; Tang, W.; Meng, Y.; Xiang, D.; Ji, M.; Lawrence, T.S.; Xu, L. A natural BH3 mimetic induces autophagy in apoptosis-resistant prostate cancer via modulating Bcl-2–Beclin1 interaction at endoplasmic reticulum. Cell Death Differ. 2011, 18, 60–71. [Google Scholar] [CrossRef]
- Gao, P.; Bauvy, C.; Souquère, S.; Tonelli, G.; Liu, L.; Zhu, Y.; Qiao, Z.; Bakula, D.; Proikas-Cezanne, T.; Pierron, G.; et al. The Bcl-2 homology domain 3 mimetic gossypol induces both Beclin 1-dependent and Beclin 1-independent cytoprotective autophagy in cancer cells. J. Biol. Chem. 2010, 285, 25570–25581. [Google Scholar] [CrossRef]
- Wang, B.; Chen, L.; Ni, Z.; Dai, X.; Qin, L.; Wu, Y.; Li, X.; Xu, L.; Lian, J.; He, F. Hsp90 inhibitor 17-AAG sensitizes Bcl-2 inhibitor (−)-gossypol by suppressing ERK-mediated protective autophagy and Mcl-1 accumulation in hepatocellular carcinoma cells. Exp. Cell Res. 2014, 328, 379–387. [Google Scholar] [CrossRef]
- Jang, G.-H.; Lee, M. BH3-mimetic gossypol-induced autophagic cell death in mutant BRAF melanoma cells with high expression of p21Cip¹. Life Sci. 2014, 102, 41–48. [Google Scholar] [CrossRef]
- Ni, Z.; Dai, X.; Wang, B.; Ding, W.; Cheng, P.; Xu, L.; Lian, J.; He, F. Natural Bcl-2 inhibitor (−)-gossypol induces protective autophagy via reactive oxygen species-high mobility group box 1 pathway in Burkitt lymphoma. Leuk. Lymphoma 2013, 54, 2263–2268. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-W.; Wang, L.-S.; Chang, H.-L.; Ye, W.; Sugimoto, Y.; Dowd, M.K.; Wan, P.J.; Lin, Y.C. Effects of serum on (−)-gossypol-suppressed growth in human prostate cancer cells. Anticancer Res. 2006, 26, 3613–3620. [Google Scholar] [PubMed]
- van Poznak, C.; Seidman, A.D.; Reidenberg, M.M.; Moasser, M.M.; Sklarin, N.; van Zee, K.; Borgen, P.; Gollub, M.; Bacotti, D.; Yao, T.J.; et al. Oral gossypol in the treatment of patients with refractory metastatic breast cancer: A phase I/II clinical trial. Breast Cancer Res. Treat. 2001, 66, 239–248. [Google Scholar] [CrossRef]
- Jiang, J.; Sugimoto, Y.; Liu, S.; CHANG, H.-L.; Park, K.-Y.; Kulp, S.K.; Lin, Y.C. The inhibitory effects of gossypol on human prostate cancer cells-PC3 are associated with transforming growth factor beta1 (TGFbeta1) signal transduction pathway. Anticancer Res. 2004, 24, 91–100. [Google Scholar]
- Moon, D.-O.; Kim, M.-O.; Lee, J.-D.; Kim, G.-Y. Gossypol suppresses NF-kappaB activity and NF-kappaB-related gene expression in human leukemia U937 cells. Cancer Lett. 2008, 264, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Zerp, S.F.; Stoter, T.R.; Hoebers, F.J.P.; van den Brekel, M.W.M.; Dubbelman, R.; Kuipers, G.K.; Lafleur, M.V.M.; Slotman, B.J.; Verheij, M. Targeting anti-apoptotic Bcl-2 by AT-101 to increase radiation efficacy: Data from in vitro and clinical pharmacokinetic studies in head and neck cancer. Radiat. Oncol. 2015, 10, 158. [Google Scholar] [CrossRef]
- Qian, C.; Li, M.; Sui, J.; Ren, T.; Li, Z.; Zhang, L.; Zhou, L.; Cheng, Y.; Wang, D. Identification of a novel potential antitumor activity of gossypol as an APE1/Ref-1 inhibitor. Drug Des. Devel. Ther. 2014, 8, 485–496. [Google Scholar] [CrossRef][Green Version]
- Xanthoudakis, S.; Curran, T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 1992, 11, 653–665. [Google Scholar] [CrossRef]
- Demple, B.; Herman, T.; Chen, D.S. Cloning and expression of APE, the cDNA encoding the major human apurinic endonuclease: Definition of a family of DNA repair enzymes. Proc. Natl. Acad. Sci. USA 1991, 88, 11450–11454. [Google Scholar] [CrossRef]
- Wang, D.; Xiang, D.-B.; Yang, X.; Chen, L.-S.; Li, M.-X.; Zhong, Z.-Y.; Zhang, Y.-S. APE1 overexpression is associated with cisplatin resistance in non-small cell lung cancer and targeted inhibition of APE1 enhances the activity of cisplatin in A549 cells. Lung Cancer 2009, 66, 298–304. [Google Scholar] [CrossRef]
- Xie, J.-Y.; Li, M.-X.; Xiang, D.-B.; Mou, J.-H.; Qing, Y.; Zeng, L.-L.; Yang, Z.-Z.; Guan, W.; Wang, D. Elevated expression of APE1/Ref-1 and its regulation on IL-6 and IL-8 in bone marrow stromal cells of multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2010, 10, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Di Maso, V.; Mediavilla, M.G.; Vascotto, C.; Lupo, F.; Baccarani, U.; Avellini, C.; Tell, G.; Tiribelli, C.; Crocè, L.S. Transcriptional Up-Regulation of APE1/Ref-1 in Hepatic Tumor: Role in Hepatocytes Resistance to Oxidative Stress and Apoptosis. PLoS ONE 2015, 10, e0143289. [Google Scholar] [CrossRef] [PubMed]
- Juhnke, M.; Heumann, A.; Chirico, V.; Höflmayer, D.; Menz, A.; Hinsch, A.; Hube-Magg, C.; Kluth, M.; Lang, D.S.; Möller-Koop, C.; et al. Apurinic/apyrimidinic endonuclease 1 (APE1/Ref-1) overexpression is an independent prognostic marker in prostate cancer without TMPRSS2:ERG fusion. Mol. Carcinog. 2017, 56, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Shan, J.; Li, M.; Qing, Y.; Qian, C.; Wang, G.; Li, Q.; Lu, G.; Li, C.; Peng, Y.; et al. Small-molecule BH3 mimetic and pan-Bcl-2 inhibitor AT-101 enhances the antitumor efficacy of cisplatin through inhibition of APE1 repair and redox activity in non-small-cell lung cancer. Drug Des. Devel. Ther. 2015, 9, 2887–2910. [Google Scholar] [CrossRef]
- Ren, T.; Shan, J.; Qing, Y.; Qian, C.; Li, Q.; Lu, G.; Li, M.; Li, C.; Peng, Y.; Luo, H.; et al. Sequential treatment with AT-101 enhances cisplatin chemosensitivity in human non-small cell lung cancer cells through inhibition of apurinic/apyrimidinic endonuclease 1-activated IL-6/STAT3 signaling pathway. Drug Des. Devel. Ther. 2014, 8, 2517–2529. [Google Scholar] [CrossRef]
- Zhao, R.; Zhou, S.; Xia, B.; Zhang, C.; Hai, P.; Zhe, H.; Wang, Y. AT-101 enhances gefitinib sensitivity in non-small cell lung cancer with EGFR T790M mutations. BMC Cancer 2016, 16, 491. [Google Scholar] [CrossRef]
- Johnstone, R.W. Histone-deacetylase inhibitors: Novel drugs for the treatment of cancer. Nat. Rev. Drug Discov. 2002, 1, 287–299. [Google Scholar] [CrossRef]
- Mai, A. The therapeutic uses of chromatin-modifying agents. Expert Opin. Ther. Targets 2007, 11, 835–851. [Google Scholar] [CrossRef]
- Mazzio, E.A.; Soliman, K.F.A. HTP Nutraceutical Screening for Histone Deacetylase Inhibitors and Effects of HDACis on Tumor-suppressing miRNAs by Trichostatin A and Grapeseed (Vitis vinifera) in HeLa cells. Cancer Genom. Proteom. 2017, 14, 17–33. [Google Scholar] [CrossRef][Green Version]
- Berger, A.; Venturelli, S.; Kallnischkies, M.; Böcker, A.; Busch, C.; Weiland, T.; Noor, S.; Leischner, C.; Weiss, T.S.; Lauer, U.M.; et al. Kaempferol, a new nutrition-derived pan-inhibitor of human histone deacetylases. J. Nutr. Biochem. 2013, 24, 977–985. [Google Scholar] [CrossRef]
- Venturelli, S.; Berger, A.; Böcker, A.; Busch, C.; Weiland, T.; Noor, S.; Leischner, C.; Schleicher, S.; Mayer, M.; Weiss, T.S.; et al. Resveratrol as a pan-HDAC inhibitor alters the acetylation status of histone corrected proteins in human-derived hepatoblastoma cells. PLoS ONE 2013, 8, e73097. [Google Scholar] [CrossRef]
- Alfarouk, K.O.; Stock, C.-M.; Taylor, S.; Walsh, M.; Muddathir, A.K.; Verduzco, D.; Bashir, A.H.H.; Mohammed, O.Y.; Elhassan, G.O.; Harguindey, S.; et al. Resistance to cancer chemotherapy: Failure in drug response from ADME to P-gp. Cancer Cell Int. 2015, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, D.; Rajamani, P.; Singh, R.P. Effects of phytochemicals on ionization radiation-mediated carcinogenesis and cancer therapy. Mutat. Res. 2011, 728, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Bostan, M.; Mihaila, M.; Petrica-Matei, G.G.; Radu, N.; Hainarosie, R.; Stefanescu, C.D.; Roman, V.; Diaconu, C.C. Resveratrol Modulation of Apoptosis and Cell Cycle Response to Cisplatin in Head and Neck Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 6322. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Hannan, M.A.; Dash, R.; Rahman, M.H.; Islam, R.; Uddin, M.J.; Sohag, A.A.M.; Rahman, M.H.; Rhim, H. Phytochemicals as a Complement to Cancer Chemotherapy: Pharmacological Modulation of the Autophagy-Apoptosis Pathway. Front. Pharmacol. 2021, 12, 639628. [Google Scholar] [CrossRef] [PubMed]
- Paoluzzi, L.; Gonen, M.; Gardner, J.R.; Mastrella, J.; Yang, D.; Holmlund, J.; Sorensen, M.; Leopold, L.; Manova, K.; Marcucci, G.; et al. Targeting Bcl-2 family members with the BH3 mimetic AT-101 markedly enhances the therapeutic effects of chemotherapeutic agents in in vitro and in vivo models of B-cell lymphoma. Blood 2008, 111, 5350–5358. [Google Scholar] [CrossRef]
- Wong, F.Y.; Liem, N.; Xie, C.; Yan, F.L.; Wong, W.C.; Wang, L.; Yong, W.-P. Combination therapy with gossypol reveals synergism against gemcitabine resistance in cancer cells with high BCL-2 expression. PLoS ONE 2012, 7, e50786. [Google Scholar] [CrossRef]
- Lian, J.; Ni, Z.; Dai, X.; Su, C.; Smith, A.R.; Xu, L.; He, F. Sorafenib sensitizes (−)-gossypol-induced growth suppression in androgen-independent prostate cancer cells via Mcl-1 inhibition and Bak activation. Mol. Cancer 2012, 11, 416–426. [Google Scholar] [CrossRef]
- Caylioglu, D.; Meyer, R.J.; Hellmold, D.; Kubelt, C.; Synowitz, M.; Held-Feindt, J. Effects of the Anti-Tumorigenic Agent AT101 on Human Glioblastoma Cells in the Microenvironmental Glioma Stem Cell Niche. Int. J. Mol. Sci. 2021, 22, 3606. [Google Scholar] [CrossRef]
- Xu, L.; Yang, D.; Wang, S.; Tang, W.; Liu, M.; Davis, M.; Chen, J.; Rae, J.M.; Lawrence, T.; Lippman, M.E. (−)-Gossypol enhances response to radiation therapy and results in tumor regression of human prostate cancer. Mol. Cancer 2005, 4, 197–205. [Google Scholar]
- Akagunduz, O.; Karaca, B.; Atmaca, H.; Uzunoglu, S.; Karabulut, B.; Sanli, U.A.; Haydaroglu, A.; Uslu, R. Radiosensitization of hormone-refractory prostate cancer cells by gossypol treatment. J. Balk. Union Oncol. 2010, 15, 763–767. [Google Scholar]
- Keshmiri-Neghab, H.; Goliaei, B.; Nikoofar, A. Gossypol enhances radiation induced autophagy in glioblastoma multiforme. Gen. Physiol. Biophys. 2014, 33, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Kasten-Pisula, U.; Windhorst, S.; Dahm-Daphi, J.; Mayr, G.; Dikomey, E. Radiosensitization of tumour cell lines by the polyphenol Gossypol results from depressed double-strand break repair and not from enhanced apoptosis. Radiother. Oncol. 2007, 83, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Moretti, L.; Li, B.; Kim, K.W.; Chen, H.; Lu, B. AT-101, a pan-Bcl-2 inhibitor, leads to radiosensitization of non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 680–687. [Google Scholar] [CrossRef]
- Zerp, S.F.; Stoter, R.; Kuipers, G.; Yang, D.; Lippman, M.E.; van Blitterswijk, W.J.; Bartelink, H.; Rooswinkel, R.; Lafleur, V.; Verheij, M. AT-101, a small molecule inhibitor of anti-apoptotic Bcl-2 family members, activates the SAPK/JNK pathway and enhances radiation-induced apoptosis. Radiat. Oncol. 2009, 4, 47. [Google Scholar] [CrossRef]
- Adamski, V.; Schmitt, C.; Ceynowa, F.; Adelung, R.; Lucius, R.; Synowitz, M.; Hattermann, K.; Held-Feindt, J. Effects of sequentially applied single and combined temozolomide, hydroxychloroquine and AT101 treatment in a long-term stimulation glioblastoma in vitro model. J. Cancer Res. Clin. Oncol. 2018, 144, 1475–1485. [Google Scholar] [CrossRef]
- Stein, M.N.; Goodin, S.; Gounder, M.; Gibbon, D.; Moss, R.; Portal, D.; Lindquist, D.; Zhao, Y.; Takebe, N.; Tan, A.; et al. A phase I study of AT-101, a BH3 mimetic, in combination with paclitaxel and carboplatin in solid tumors. Investig. New Drugs 2020, 38, 855–865. [Google Scholar] [CrossRef]
- Stein, R.C.; Joseph, A.E.; Matlin, S.A.; Cunningham, D.C.; Ford, H.T.; Coombes, R.C. A preliminary clinical study of gossypol in advanced human cancer. Cancer Chemother. Pharmacol. 1992, 30, 480–482. [Google Scholar] [CrossRef]
- Xie, H.; Yin, J.; Shah, M.H.; Menefee, M.E.; Bible, K.C.; Reidy-Lagunes, D.; Kane, M.A.; Quinn, D.I.; Gandara, D.R.; Erlichman, C.; et al. A phase II study of the orally administered negative enantiomer of gossypol (AT-101), a BH3 mimetic, in patients with advanced adrenal cortical carcinoma. Investig. New Drugs 2019, 37, 755–762. [Google Scholar] [CrossRef]
- Baggstrom, M.Q.; Qi, Y.; Koczywas, M.; Argiris, A.; Johnson, E.A.; Millward, M.J.; Murphy, S.C.; Erlichman, C.; Rudin, C.M.; Govindan, R. A phase II study of AT-101 (Gossypol) in chemotherapy-sensitive recurrent extensive-stage small cell lung cancer. J. Thorac. Oncol. 2011, 6, 1757–1760. [Google Scholar] [CrossRef]
- Liu, G.; Kelly, W.K.; Wilding, G.; Leopold, L.; Brill, K.; Somer, B. An open-label, multicenter, phase I/II study of single-agent AT-101 in men with castrate-resistant prostate cancer. Clin. Cancer Res. 2009, 15, 3172–3176. [Google Scholar] [CrossRef] [PubMed]
- Bushunow, P.; Reidenberg, M.M.; Wasenko, J.; Winfield, J.; Lorenzo, B.; Lemke, S.; Himpler, B.; Corona, R.; Coyle, T. Gossypol treatment of recurrent adult malignant gliomas. J. Neuro-Oncol. 1999, 43, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Flack, M.R.; Pyle, R.G.; Mullen, N.M.; Lorenzo, B.; Wu, Y.W.; Knazek, R.A.; Nisula, B.C.; Reidenberg, M.M. Oral gossypol in the treatment of metastatic adrenal cancer. J. Clin. Endocrinol. Metab. 1993, 76, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Chen, Q.; Li, Y.; Lei, G.; Scott, A.; Huo, L.; Li, C.Y.; Estrella, J.S.; Correa, A.; Pizzi, M.P.; et al. Targeting cancer stem cells with a pan-BCL-2 inhibitor in preclinical and clinical settings in patients with gastroesophageal carcinoma. Gut 2021, 70, gutjnl-2020-321175. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Zhang, L.; Li, M.; Dai, N.; Luo, H.; Shan, J.; Yang, X.; Xu, M.; Feng, Y.; et al. A randomized, double-blind, placebo-controlled study of B-cell lymphoma 2 homology 3 mimetic gossypol combined with docetaxel and cisplatin for advanced non-small cell lung cancer with high expression of apurinic/apyrimidinic endonuclease 1. Investig. New Drugs 2020, 38, 1862–1871. [Google Scholar] [CrossRef]
- Swiecicki, P.L.; Bellile, E.; Sacco, A.G.; Pearson, A.T.; Taylor, J.M.G.; Jackson, T.L.; Chepeha, D.B.; Spector, M.E.; Shuman, A.; Malloy, K.; et al. A phase II trial of the BCL-2 homolog domain 3 mimetic AT-101 in combination with docetaxel for recurrent, locally advanced, or metastatic head and neck cancer. Investig. New Drugs 2016, 34, 481–489. [Google Scholar] [CrossRef]
- Stein, M.N.; Hussain, M.; Stadler, W.M.; Liu, G.; Tereshchenko, I.V.; Goodin, S.; Jeyamohan, C.; Kaufman, H.L.; Mehnert, J.; DiPaola, R.S. A Phase II Study of AT-101 to Overcome Bcl-2--Mediated Resistance to Androgen Deprivation Therapy in Patients With Newly Diagnosed Castration-Sensitive Metastatic Prostate Cancer. Clin. Genitourin. Cancer 2016, 14, 22–27. [Google Scholar] [CrossRef]
- Schelman, W.R.; Mohammed, T.A.; Traynor, A.M.; Kolesar, J.M.; Marnocha, R.M.; Eickhoff, J.; Keppen, M.; Alberti, D.B.; Wilding, G.; Takebe, N.; et al. A phase I study of AT-101 with cisplatin and etoposide in patients with advanced solid tumors with an expanded cohort in extensive-stage small cell lung cancer. Investig. New Drugs 2014, 32, 295–302. [Google Scholar] [CrossRef]
- Sonpavde, G.; Matveev, V.; Burke, J.M.; Caton, J.R.; Fleming, M.T.; Hutson, T.E.; Galsky, M.D.; Berry, W.R.; Karlov, P.; Holmlund, J.T.; et al. Randomized phase II trial of docetaxel plus prednisone in combination with placebo or AT-101, an oral small molecule Bcl-2 family antagonist, as first-line therapy for metastatic castration-resistant prostate cancer. Ann. Oncol. 2012, 23, 1803–1808. [Google Scholar] [CrossRef]
- Ready, N.; Karaseva, N.A.; Orlov, S.V.; Luft, A.V.; Popovych, O.; Holmlund, J.T.; Wood, B.A.; Leopold, L. Double-blind, placebo-controlled, randomized phase 2 study of the proapoptotic agent AT-101 plus docetaxel, in second-line non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 781–785. [Google Scholar] [CrossRef]
- Heist, R.S.; Fain, J.; Chinnasami, B.; Khan, W.; Molina, J.R.; Sequist, L.V.; Temel, J.S.; Fidias, P.; Brainerd, V.; Leopold, L.; et al. Phase I/II study of AT-101 with topotecan in relapsed and refractory small cell lung cancer. J. Thorac. Oncol. 2010, 5, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Wu, D. An overview of the clinical pharmacology and therapeutic potential of gossypol as a male contraceptive agent and in gynaecological disease. Drugs 1989, 38, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Loberg, R.D.; McGregor, N.; Ying, C.; Sargent, E.; Pienta, K.J. In vivo evaluation of AT-101 (R-(−)-gossypol acetic acid) in androgen-independent growth of VCaP prostate cancer cells in combination with surgical castration. Neoplasia 2007, 9, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- James, D.F.; Prada, C.E.; Castro, J.E.; Kipps, T.J. AT 101, an Inhibitor of Bcl-2 Family Members Is Cytotoxic to a Heterogeneous Group of CLL Samples and Synergistic with Rituximab. Blood 2005, 106, 2979. [Google Scholar] [CrossRef]
- Castro, J.E.; Loria, O.J.; Aguillon, R.A.; James, D.; Llanos, C.A.; Rassenti, L.; Wood, B.A.; Homlund, J.T.; Kipps, T.J. A Phase II, Open Label Study of AT-101 in Combination with Rituximab in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia. Evaluation of Two Dose Regimens. Blood 2007, 110, 3119. [Google Scholar] [CrossRef]
- Sonpavde, G.; Pond, G.R.; Armstrong, A.J.; Galsky, M.D.; Leopold, L.; Wood, B.A.; Wang, S.-L.; Paolini, J.; Chen, I.; Chow-Maneval, E.; et al. Radiographic progression by Prostate Cancer Working Group (PCWG)-2 criteria as an intermediate endpoint for drug development in metastatic castration-resistant prostate cancer. BJU Int. 2014, 114, E25–E31. [Google Scholar] [CrossRef]
- Yang, S.T.; Ying, X.; Tang, H.; Wang, J.C.; Wu, B.B. Intestinal Absorption, Pharmacokinetics and Tissue Distribution of Gossypol Nanosuspensions. AMR 2014, 1015, 708–712. [Google Scholar] [CrossRef]
- Chadha, S.; Sanyal, S.N.; Kanwar, U. Reversibility of the effects of gossypol acetic acid, an antispermatogenic/antifertility agent on the intestinal structure and functions of male albino rats. Res. Exp. Med. 1989, 189, 205–219. [Google Scholar] [CrossRef]
- Chadha, S.; Sanyal, S.N.; Kanwar, U. Alterations and reversibility of the effects of gossypol acetic acid on the intestinal uptake of end product nutrients in normal and protein calorie-malnourished male rats. Ann. Nutr. Metab. 1991, 35, 53–60. [Google Scholar] [CrossRef]
- Trischitta, F.; Faggio, C. Gossypol affects ion transport in the isolated intestine of the seawater adapted eel, Anguilla anguilla. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 151, 139–143. [Google Scholar] [CrossRef]
- Jia, L.; Coward, L.C.; Kerstner-Wood, C.D.; Cork, R.L.; Gorman, G.S.; Noker, P.E.; Kitada, S.; Pellecchia, M.; Reed, J.C. Comparison of pharmacokinetic and metabolic profiling among gossypol, apogossypol and apogossypol hexaacetate. Cancer Chemother. Pharmacol. 2008, 61, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Cater, C.M.; Lyman, C.M. Reaction of gossypol with amino acids and other amino compounds. J. Am. Oil Chem. Soc. 1969, 46, 649–653. [Google Scholar] [CrossRef]
- Fu, Y.F.; Zhang, S.L.; Lu, Z.M.; Wang, W. Effects of gossypol on the activity of kidney (Na+ + K+)-ATPase and the functions of erythrocyte membrane. Contraception 1988, 37, 179–184. [Google Scholar] [CrossRef]
- Johansen, R.; Misra, H.P. Effects of gossypol on the hepatic drug metabolizing system in rats. Contraception 1990, 42, 683–690. [Google Scholar] [CrossRef]
- Ma, X.N.; Back, D.J. Inhibition of hepatic microsomal enzymes by gossypol in the rat. Contraception 1984, 30, 89–97. [Google Scholar] [CrossRef]
- Aneja, R.; Dass, S.K.; Prakash, S.; Chandra, R. Effect of gossypol in association with chromium protoporphyrin on heme metabolic enzymes. Artif. Cells Blood Substit. Biotechnol. 2004, 32, 159–172. [Google Scholar] [CrossRef]
- Ali, S.F.; El-Sewedy, S.M. Effect of gossypol on liver metabolic enzymes in male rats. Toxicol. Lett. 1984, 23, 299–306. [Google Scholar] [CrossRef]
- Liu, H.; Sun, H.; Lu, D.; Zhang, Y.; Zhang, X.; Ma, Z.; Wu, B. Identification of glucuronidation and biliary excretion as the main mechanisms for gossypol clearance: In vivo and in vitro evidence. Xenobiotica 2014, 44, 696–707. [Google Scholar] [CrossRef]
- Lin, Y.C.; Nuber, D.C.; Gu, Y.; Cutler, G.; Hinchcliff, K.W.; Haibel, G. Gossypol pharmacokinetics in mid-lactation Brown Swiss dairy cows. Vet. Res. Commun. 1991, 15, 379–385. [Google Scholar] [CrossRef]
- Kalla, N.R.; Sud, S. Distribution of gossypol. Acta Eur. Fertil. 1990, 21, 77–80. [Google Scholar]
- Abou-Donia, M.B.; Othman, M.A.; Obih, P. Interspecies comparison of pharmacokinetic profile and bioavailability of (±)-gossypol in male Fischer-344 rats and male B6C3F mice. Toxicology 1989, 55, 37–51. [Google Scholar] [CrossRef]
- Othman, M.A.; Abou-Donia, M.B. Pharmacokinetic profile of (+/−)-gossypol in male Sprague-Dawley rats following single intravenous and oral and subchronic oral administration. Proc. Soc. Exp. Biol. Med. 1988, 188, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, B.D.; Spalding, A.C.; Campos, M.S.; Ashimori, N.; Dong, Z.; Wang, S.; Lawrence, T.S.; Nör, J.E. Metronomic small molecule inhibitor of Bcl-2 (TW-37) is antiangiogenic and potentiates the antitumor effect of ionizing radiation. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Imai, A.; Zeitlin, B.D.; Visioli, F.; Dong, Z.; Zhang, Z.; Krishnamurthy, S.; Light, E.; Worden, F.; Wang, S.; Nör, J.E. Metronomic dosing of BH3 mimetic small molecule yields robust antiangiogenic and antitumor effects. Cancer Res. 2012, 72, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Li, Q.; Li, Y.; Duan, W.; Huang, C.; Zheng, X.; Sun, L.; Luo, J.; Wang, D.; Zhang, S.; et al. Prediction of survival prognosis of non-small cell lung cancer by APE1 through regulation of Epithelial-Mesenchymal Transition. Oncotarget 2016, 7, 28523–28539. [Google Scholar] [CrossRef]
- Hussain, M.; Tangen, C.M.; Higano, C.; Schelhammer, P.F.; Faulkner, J.; Crawford, E.D.; Wilding, G.; Akdas, A.; Small, E.J.; Donnelly, B.; et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: Data from Southwest Oncology Group Trial 9346 (INT-0162). J. Clin. Oncol. 2006, 24, 3984–3990. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, H.; Fan, X.; Luo, L.; Duan, F.; Jiang, Z.; Wang, Q.; Leung, E.L.H.; Liu, L.; Yao, X. Gossypol Inhibits Non-small Cell Lung Cancer Cells Proliferation by Targeting EGFRL858R/T790M. Front. Pharmacol. 2018, 9, 728. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, G.-Y.; Cao, D.; Pan, H.; Li, Y.-W. Gossypol overcomes EGFR-TKIs resistance in non-small cell lung cancer cells by targeting YAP/TAZ and EGFRL858R/T790M. Biomed. Pharmacother. 2019, 115, 108860. [Google Scholar] [CrossRef]
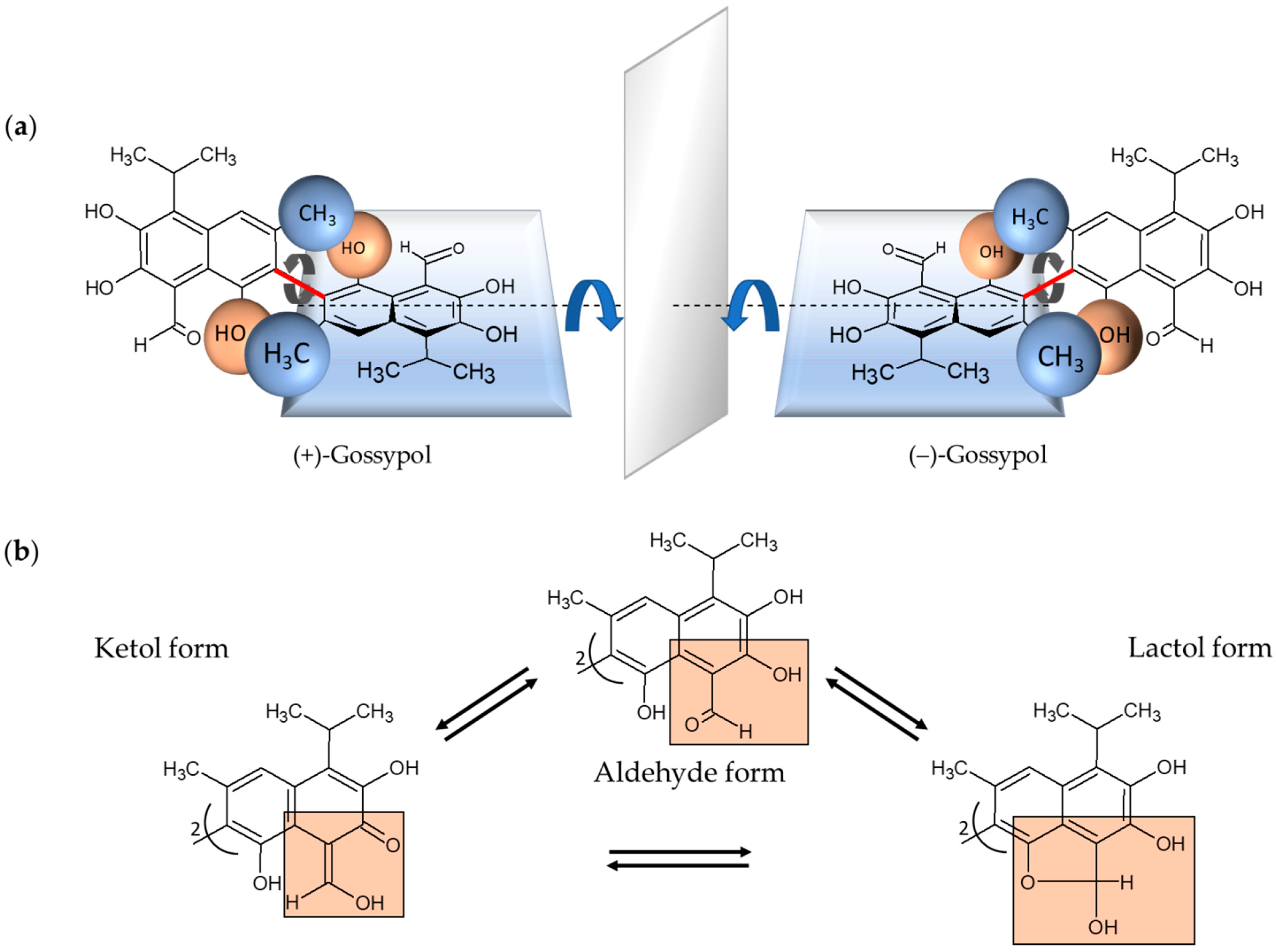
| NCT Number Publication Date Country Reference | Tumor Entity Patient Diagnosis n | Trial Design | Treatment Type and Frequency | Toxicity | Reported Outcomes/Conclusions |
|---|---|---|---|---|---|
| NCT00848016 2019 USA [90] | patients with histologically confirmed metastatic, recurrent, or primarily unresectable advanced adrenal cortical carcinoma n = 29 | nonrandomized, single-center phase II |
|
|
|
| NCT00773955 2011 USA [91] | recurrent chemosensitive ES-SCLC n = 14 | phase II |
|
|
|
| NCT00286806 2009 USA [92] | progressive CRPC n = 23 | open-label, multicenter, phase I/II |
|
|
|
| NCT n. a. 2001 USA [54] | refractory metastatic breast cancer n = 20 | phase I/II |
|
|
|
| NCT n. a. 1999 USA [93] | pathologically confirmed glial tumors which had recurred after radiation therapy n = 27 | phase II |
|
|
|
| NCT n. a. 1993 USA [94] | metastatic adrenal cancer n = 21 | phase I |
|
|
|
| NCT n. a. 1992 UK [89] | advanced human cancer n = 34 | phase I |
|
|
|
| NCT Number Publication Date Country Reference | Tumor Entity Patient Diagnosis Number (n) | Trial Design | Treatment Type and Frequency | Concurrent Treatment | Toxicity | Reported Outcomes/Conclusions |
|---|---|---|---|---|---|---|
| NCT00561197 2021 USA [95] | GEC n = 13 | open label, phase I/II |
|
|
|
|
| NCT01977209 2020 China [96] | advanced NSCLC n = 62 (co = 31, eg = 31) | double-blind, randomized, placebo-controlled, phase III |
|
|
|
|
| NCT00891072 2020 USA [88] | advanced solid tumors n = 24 | open label, dose escalating, nonrandomized, single-center, phase I |
|
|
|
|
| NCT01285635 2016 USA [97] | un-resectable, recurrent, or locally advanced or metastatic HNSCC, not amenable to curative radiation or surgery n = 35 | open label, randomized, phase II |
|
|
|
|
| NCT00666666 2016 USA [98] | newly diagnosed castration-sensitive metastatic prostate cancer n = 55 | open label, multicenter study, phase II |
|
|
|
|
| NCT n. a. 2015 Netherlands [57] | locally advanced inoperable head and neck cancer (HNSCC) n = 14 | phase I/II |
|
|
|
|
| NCT00544596 2014 USA [99] | patients with advanced solid tumors (1. cohort), and an expanded cohort of patients with ES-SCLC (2. cohort, n = 7) n = 27 | open label, dose escalating, phase I |
|
|
|
|
| NCT00571675/ NCT00286793 2012 USA/Russian Federation [100] | metastatic CRPC n = 220 | double-blind, placebo-controlled, two-arm trial with 1:1 randomization of phase II |
|
|
|
|
| NCT00544960 2011 USA/Russian Federation/Ukraine [101] | advanced or metastatic NSCLC n = 105 | double-blind, randomized (1:1), placebo-controlled phase II |
|
|
|
|
| NCT00397293 2010 USA [102] | relapsed and refractory SCLC, who had progressed on prior platinum-containing chemotherapy n = 36 | open-labeled, multicenter, phase I/II |
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renner, O.; Mayer, M.; Leischner, C.; Burkard, M.; Berger, A.; Lauer, U.M.; Venturelli, S.; Bischoff, S.C. Systematic Review of Gossypol/AT-101 in Cancer Clinical Trials. Pharmaceuticals 2022, 15, 144. https://doi.org/10.3390/ph15020144
Renner O, Mayer M, Leischner C, Burkard M, Berger A, Lauer UM, Venturelli S, Bischoff SC. Systematic Review of Gossypol/AT-101 in Cancer Clinical Trials. Pharmaceuticals. 2022; 15(2):144. https://doi.org/10.3390/ph15020144
Chicago/Turabian StyleRenner, Olga, Mascha Mayer, Christian Leischner, Markus Burkard, Alexander Berger, Ulrich M. Lauer, Sascha Venturelli, and Stephan C. Bischoff. 2022. "Systematic Review of Gossypol/AT-101 in Cancer Clinical Trials" Pharmaceuticals 15, no. 2: 144. https://doi.org/10.3390/ph15020144
APA StyleRenner, O., Mayer, M., Leischner, C., Burkard, M., Berger, A., Lauer, U. M., Venturelli, S., & Bischoff, S. C. (2022). Systematic Review of Gossypol/AT-101 in Cancer Clinical Trials. Pharmaceuticals, 15(2), 144. https://doi.org/10.3390/ph15020144








