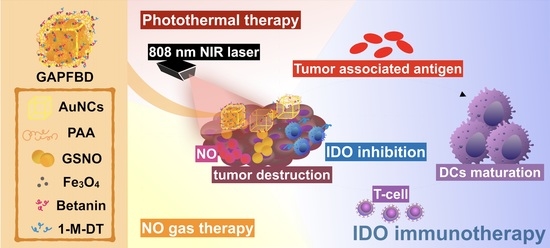
Dual-Sensitive Gold-Nanocubes Platform with Synergistic Immunotherapy for Inducing Immune Cycle Using NIR-Mediated PTT/NO/IDO
Abstract
:1. Introduction
2. Results and Discussion
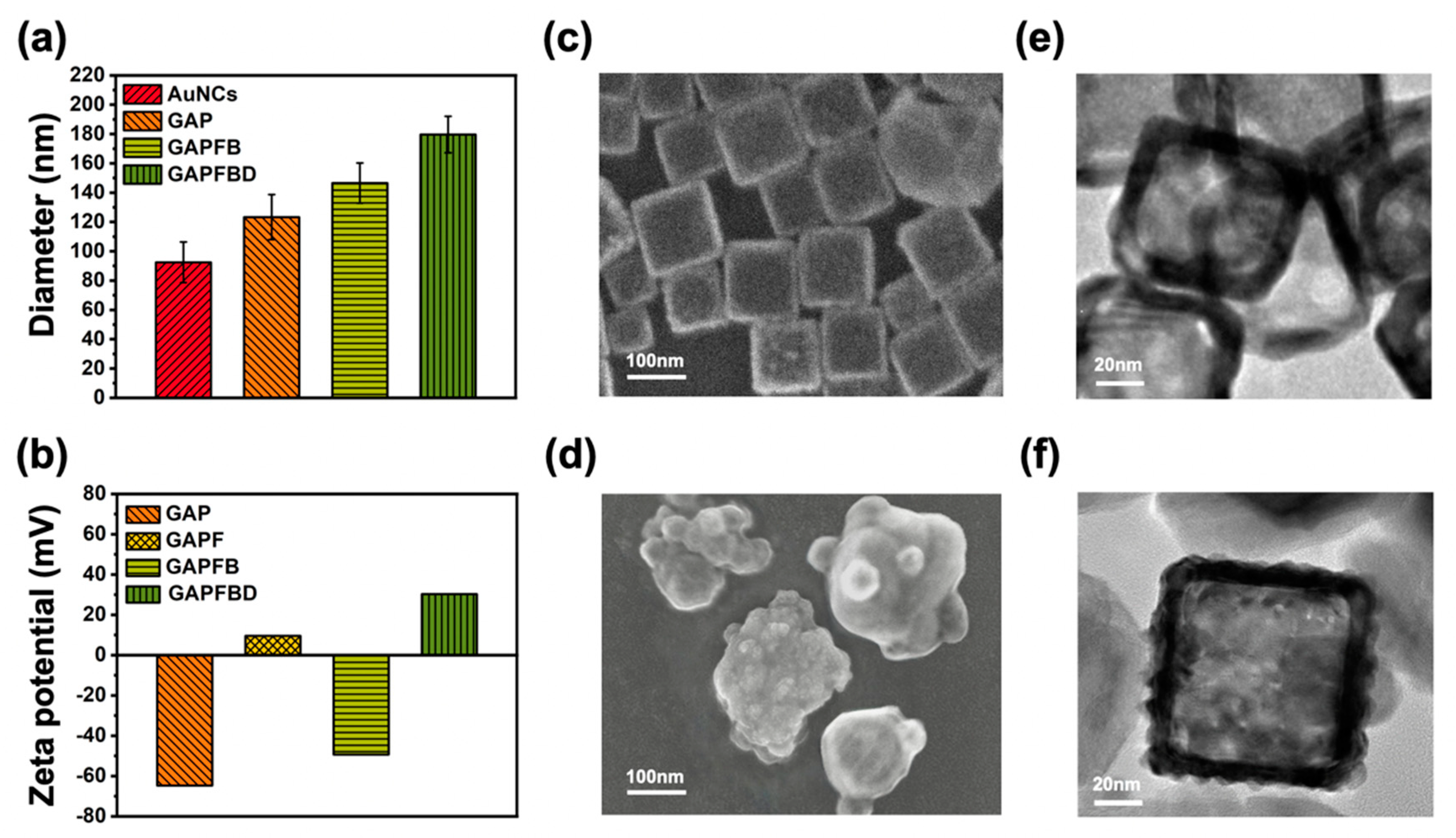
2.1. Characterization of GSNO-AuNCs-PAA-Fe3O4-Betanin-1-M-DT (GAPFBD)
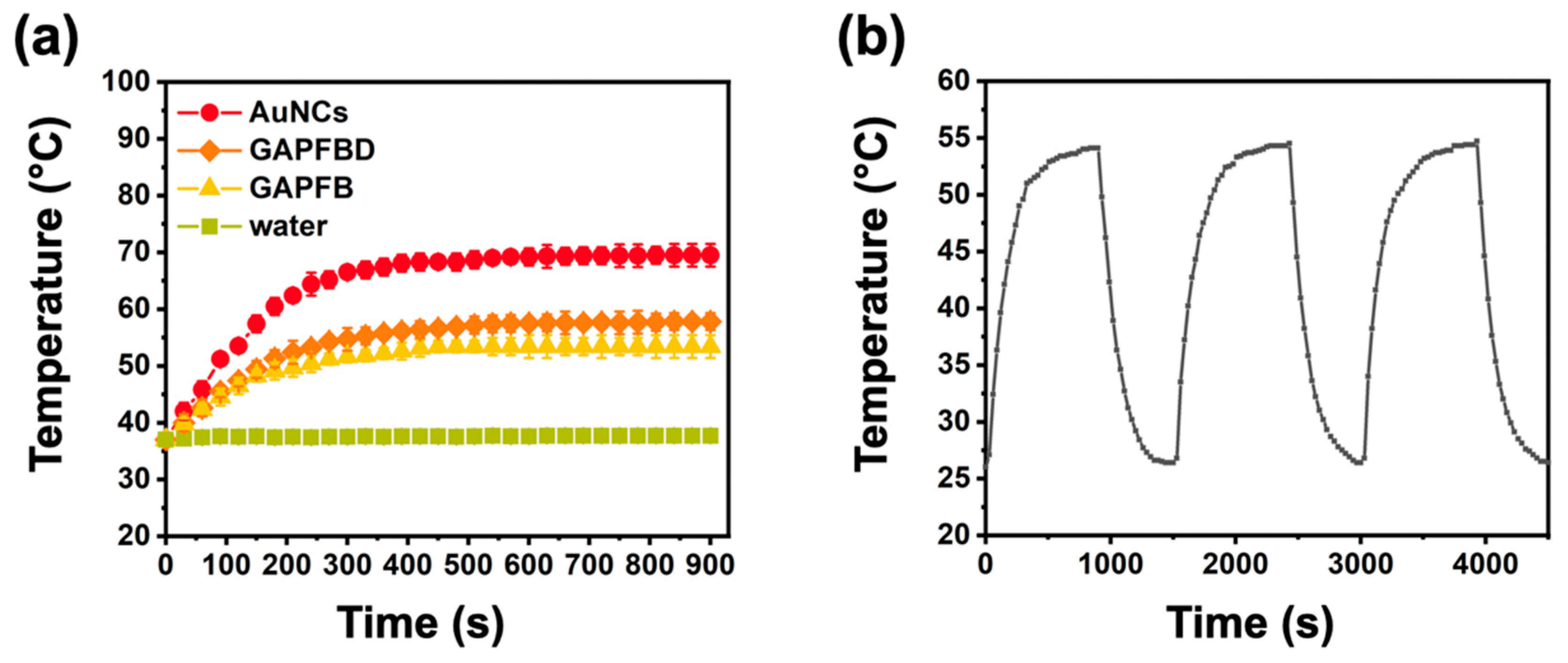
2.2. Thermally Dependent NO Production and pH-Responsive Drug Release
2.3. Cellular Uptake
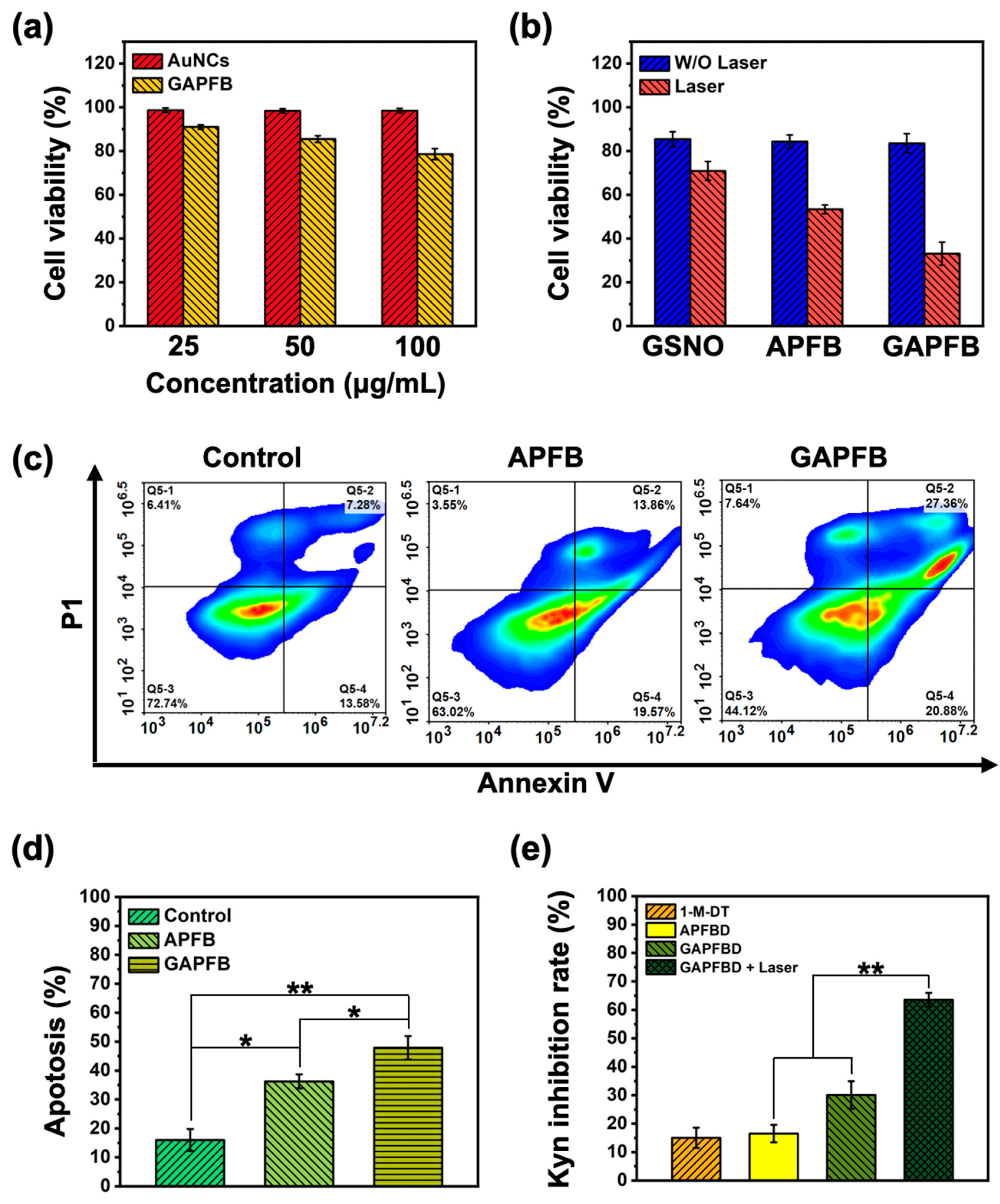
2.4. In Vitro NO/Photothermal Treatment and Apoptosis Analysis
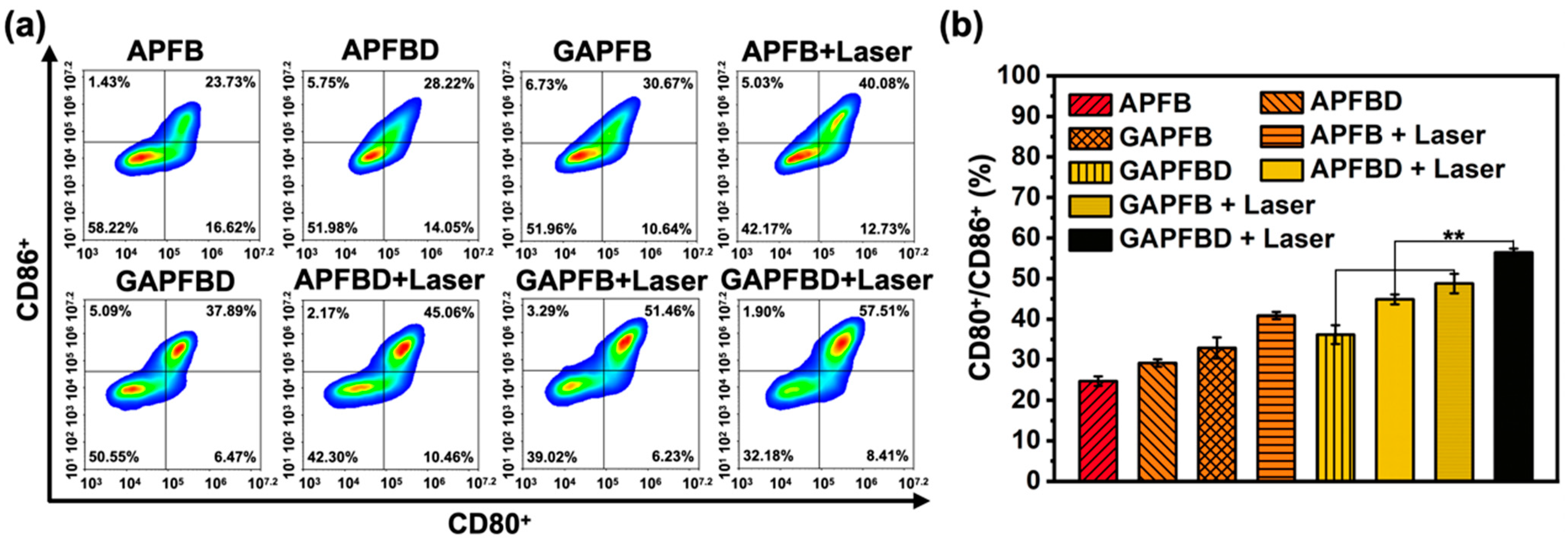
2.5. In Vitro DCs Maturation Efficiency of PTT/NO/IDO Synergy
3. Materials and Methods
3.1. Materials
3.2. Preparation of Gold Nanocages (AuNCs)
3.3. Synthesis of GSNO-AuNCs-PAA (GAP)
3.4. Synthesis of GSNO-AuNCs-PAA-Fe3O4-Betanin-1-M-DT (GAPFBD)
3.5. Characterization of GAPFBD
3.6. Photothermic Effect and Stability of GAPFBD
3.7. Drug Loading Ability
3.8. NO Production of GAPFB
3.9. GSNO Release of GAPFB
3.10. 1-M-DT Release of GAPFBD
3.11. Cell Culture
3.12. Cytotoxicity Assay of Hep55.1c Treated with Nanoparticle
3.13. Cell Uptake
3.14. NO/Photothermal Treatment of Hep55.1c Cell
3.15. Apoptosis Analysis of GAPFB
3.16. Kyn Inhibition
3.17. Analysis of Mature DCs Surface-Marker Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qian, S.; Zhang, M.; Chen, Q.; He, Y.; Wang, W.; Wang, Z. IDO as a drug target for cancer immunotherapy: Recent developments in IDO inhibitors discovery. RSC Adv. 2016, 6, 7575–7581. [Google Scholar] [CrossRef]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Fallarino, F.; Grohmann, U.; Hwang, K.W.; Orabona, C.; Vacca, C.; Bianchi, R.; Belladonna, M.L.; Fioretti, M.C.; Alegre, M.-L.; Puccetti, P. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003, 4, 1206–1212. [Google Scholar] [CrossRef]
- Jans, H.; Jans, K.; Lagae, L.; Borghs, G.; Maes, G.; Huo, Q. Poly(acrylic acid)-stabilized colloidal gold nanoparticles: Synthesis and properties. Nanotechnology 2010, 21, 455702. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, R.; Huang, Y.; Zhao, W.; Li, J.; Zhang, X.; Wang, P.; Venkataramanan, R.; Fan, J.; Xie, W.; et al. An immunostimulatory dual-functional nanocarrier that improves cancer immunochemotherapy. Nat. Commun. 2016, 7, 13443. [Google Scholar] [CrossRef]
- Xing, L.; Gong, J.-H.; Wang, Y.; Zhu, Y.; Huang, Z.-J.; Zhao, J.; Li, F.; Wang, J.-H.; Wen, H.; Jiang, H.-L. Hypoxia alleviation-triggered enhanced photodynamic therapy in combination with IDO inhibitor for preferable cancer therapy. Biomaterials 2019, 206, 170–182. [Google Scholar] [CrossRef]
- Vong, L.; Nagasaki, Y. Nitric Oxide Nano-Delivery Systems for Cancer Therapeutics: Advances and Challenges. Antioxidants 2020, 9, 791. [Google Scholar] [CrossRef]
- Choudhari, S.K.; Chaudhary, M.; Bagde, S.; Gadbail, A.R.; Joshi, V. Nitric oxide and cancer: A review. World J. Surg. Oncol. 2013, 11, 118. [Google Scholar] [CrossRef]
- Rapozzi, V.; Della Pietra, E.; Bonavida, B. Dual roles of nitric oxide in the regulation of tumor cell response and resistance to photodynamic therapy. Redox Biol. 2015, 6, 311–317. [Google Scholar] [CrossRef]
- Goedegebuure, R.S.A.; De Klerk, L.K.; Bass, A.J.; Derks, S.; Thijssen, V.L.J.L. Combining Radiotherapy With Anti-angiogenic Therapy and Immunotherapy; A Therapeutic Triad for Cancer? Front. Immunol. 2019, 9, 3107. [Google Scholar] [CrossRef]
- Thomas, S.; Mohr, D.; Stocker, R. Nitric oxide inhibits indoleamine 2,3-dioxygenase activity in interferon-gamma primed mononuclear phagocytes. J. Biol. Chem. 1994, 269, 14457–14464. [Google Scholar] [CrossRef]
- Alimoradi, H.; Greish, K.; Gamble, A.B.; Giles, G.I. Controlled Delivery of Nitric Oxide for Cancer Therapy. Pharm. Nanotechnol. 2019, 7, 279–303. [Google Scholar] [CrossRef]
- Xu, W.; Liu, L.Z.; Loizidou, M.; Ahmed, M.; Charles, I.G. The role of nitric oxide in cancer. Cell Res. 2002, 12, 311–320. [Google Scholar] [CrossRef]
- Broniowska, K.A.; Diers, A.R.; Hogg, N. S-Nitrosoglutathione. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2013, 1830, 3173–3181. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Z.; Li, L.-L. Advanced nitric oxide donors: Chemical structure of NO drugs, NO nanomedicines and biomedical applications. Nanoscale 2020, 13, 444–459. [Google Scholar] [CrossRef]
- Rapozzi, V.; Della Pietra, E.; Zorzet, S.; Zacchigna, M.; Bonavida, B.; Xodo, L. Nitric oxide-mediated activity in anti-cancer photodynamic therapy. Nitric Oxide 2013, 30, 26–35. [Google Scholar] [CrossRef]
- Wu, W.; Chen, M.; Luo, T.; Fan, Y.; Zhang, J.; Zhang, Y.; Zhang, Q.; Sapin-Minet, A.; Gaucher, C.; Xia, X. ROS and GSH-responsive S-nitrosoglutathione functionalized polymeric nanoparticles to overcome multidrug resistance in cancer. Acta Biomater. 2020, 103, 259–271. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, G.; Yin, W.; Wang, L.; Zheng, X.; Yan, L.; Li, J.; Su, H.; Chen, C.; Gu, Z.; et al. Controllable Generation of Nitric Oxide by Near-Infrared-Sensitized Upconversion Nanoparticles for Tumor Therapy. Adv. Funct. Mater. 2015, 25, 3049–3056. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Chen, J.; Au, L.; Lu, X.; Li, X.; Xia, Y. Gold Nanocages for Biomedical Applications. Adv. Mater. 2007, 19, 3177–3184. [Google Scholar] [CrossRef]
- Nejati, K.; Dadashpour, M.; Gharibi, T.; Mellatyar, H.; Akbarzadeh, A. Biomedical Applications of Functionalized Gold Nanoparticles: A Review. J. Clust. Sci. 2021, 33, 1–16. [Google Scholar] [CrossRef]
- Huang, X.; Xu, F.; Hou, H.; Hou, J.; Wang, Y.; Zhou, S. Stimuli-responsive nitric oxide generator for light-triggered synergistic cancer photothermal/gas therapy. Nano Res. 2019, 12, 1361–1370. [Google Scholar] [CrossRef]
- Nurkeeva, Z.S.; Khutoryanskiy, V.V.; Mun, G.A.; Sherbakova, M.V.; Ivaschenko, A.T.; Aitkhozhina, N.A. Polycomplexes of poly(acrylic acid) with streptomycin sulfate and their antibacterial activity. Eur. J. Pharm. Biopharm. 2004, 57, 245–249. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Wagner, A.E.; Schiniatbeyo, V.B.; Rimbach, G. Betanin-A food colorant with biological activity. Mol. Nutr. Food Res. 2014, 59, 36–47. [Google Scholar] [CrossRef]
- Chen, J.; Glaus, C.; Laforest, R.; Zhang, Q.; Yang, M.; Gidding, M.; Welch, M.J.; Xia, Y. Gold Nanocages as Photothermal Transducers for Cancer Treatment. Small 2010, 6, 811–817. [Google Scholar] [CrossRef]
- Lin, J.-T.; Chiang, Y.-S.; Lin, G.-H.; Lee, H.; Liu, H.-W. In Vitro Photothermal Destruction of Cancer Cells Using Gold Nanorods and Pulsed-Train Near-Infrared Laser. J. Nanomater. 2012, 2012, 861385. [Google Scholar] [CrossRef]
- Yuan, A.; Wu, J.; Tang, X.; Zhao, L.; Xu, F.; Hu, Y. Application of Near-Infrared Dyes for Tumor Imaging, Photothermal, and Photodynamic Therapies. J. Pharm. Sci. 2013, 102, 6–28. [Google Scholar] [CrossRef]
- Fan, J.; He, N.; He, Q.; Liu, Y.; Ma, Y.; Fu, X.; Liu, Y.; Huang, P.; Chen, X. A novel self-assembled sandwich nanomedicine for NIR-responsive release of NO. Nanoscale 2015, 7, 20055–20062. [Google Scholar] [CrossRef]
- Hou, D.-Y.; Muller, A.; Sharma, M.D.; DuHadaway, J.; Banerjee, T.; Johnson, M.; Mellor, A.L.; Prendergast, G.C.; Munn, D. Inhibition of Indoleamine 2,3-Dioxygenase in Dendritic Cells by Stereoisomers of 1-Methyl-Tryptophan Correlates with Antitumor Responses. Cancer Res. 2007, 67, 792–801. [Google Scholar] [CrossRef]
- Liu, X.; Shin, N.; Koblish, H.K.; Yang, G.; Wang, Q.; Wang, K.; Leffet, L.; Hansbury, M.J.; Thomas, B.; Rupar, M.; et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood 2010, 115, 3520–3530. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Dilioglou, S.; Cruse, J.M.; Lewis, R.E. Function of CD80 and CD86 on monocyte- and stem cell-derived dendritic cells. Exp. Mol. Pathol. 2003, 75, 217–227. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Au, L.; Li, X.; Xia, Y. Facile synthesis of Ag nanocubes and Au nanocages. Nat. Protoc. 2007, 2, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Cheng, Y.; Chang, Y.; Jian, H.; Zheng, R.; Wu, X.; Xu, K.; Wang, L.; Ma, X.; Li, X.; et al. Time-staggered delivery of erlotinib and doxorubicin by gold nanocages with two smart polymers for reprogrammable release and synergistic with photothermal therapy. Biomaterials 2019, 217, 119327. [Google Scholar] [CrossRef]
- Chen, L.-F.; Chen, L.-C.; Chen, C. The Manufacture and Effectiveness Evaluation of Liposome Incorporated with Silk Protein. J. Agric. Forestry. 2015, 64, 1–10. [Google Scholar]
- Zhu, Y.; Yang, Z.; Dong, Z.; Gong, Y.; Hao, Y.; Tian, L.; Yang, X.; Liu, Z.; Feng, L. CaCO3-Assisted Preparation of pH-Responsive Immune-Modulating Nanoparticles for Augmented Chemo-Immunotherapy. Nano-Micro Lett. 2020, 13, 29. [Google Scholar] [CrossRef]
- Madaan, A.; Verma, R.; Singh, A.T.; Jain, S.K.; Jaggi, M. A stepwise procedure for isolation of murine bone marrow and generation of dendritic cells. J. Biol. Methods 2014, 1, e1. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsao, H.-Y.; Cheng, H.-W.; Kuo, C.-C.; Chen, S.-Y. Dual-Sensitive Gold-Nanocubes Platform with Synergistic Immunotherapy for Inducing Immune Cycle Using NIR-Mediated PTT/NO/IDO. Pharmaceuticals 2022, 15, 138. https://doi.org/10.3390/ph15020138
Tsao H-Y, Cheng H-W, Kuo C-C, Chen S-Y. Dual-Sensitive Gold-Nanocubes Platform with Synergistic Immunotherapy for Inducing Immune Cycle Using NIR-Mediated PTT/NO/IDO. Pharmaceuticals. 2022; 15(2):138. https://doi.org/10.3390/ph15020138
Chicago/Turabian StyleTsao, Hsin-Yi, Hung-Wei Cheng, Chia-Chi Kuo, and San-Yuan Chen. 2022. "Dual-Sensitive Gold-Nanocubes Platform with Synergistic Immunotherapy for Inducing Immune Cycle Using NIR-Mediated PTT/NO/IDO" Pharmaceuticals 15, no. 2: 138. https://doi.org/10.3390/ph15020138
APA StyleTsao, H.-Y., Cheng, H.-W., Kuo, C.-C., & Chen, S.-Y. (2022). Dual-Sensitive Gold-Nanocubes Platform with Synergistic Immunotherapy for Inducing Immune Cycle Using NIR-Mediated PTT/NO/IDO. Pharmaceuticals, 15(2), 138. https://doi.org/10.3390/ph15020138