Kaempferol Interferes with Varicella-Zoster Virus Replication in Human Foreskin Fibroblasts
Abstract
1. Introduction
2. Results
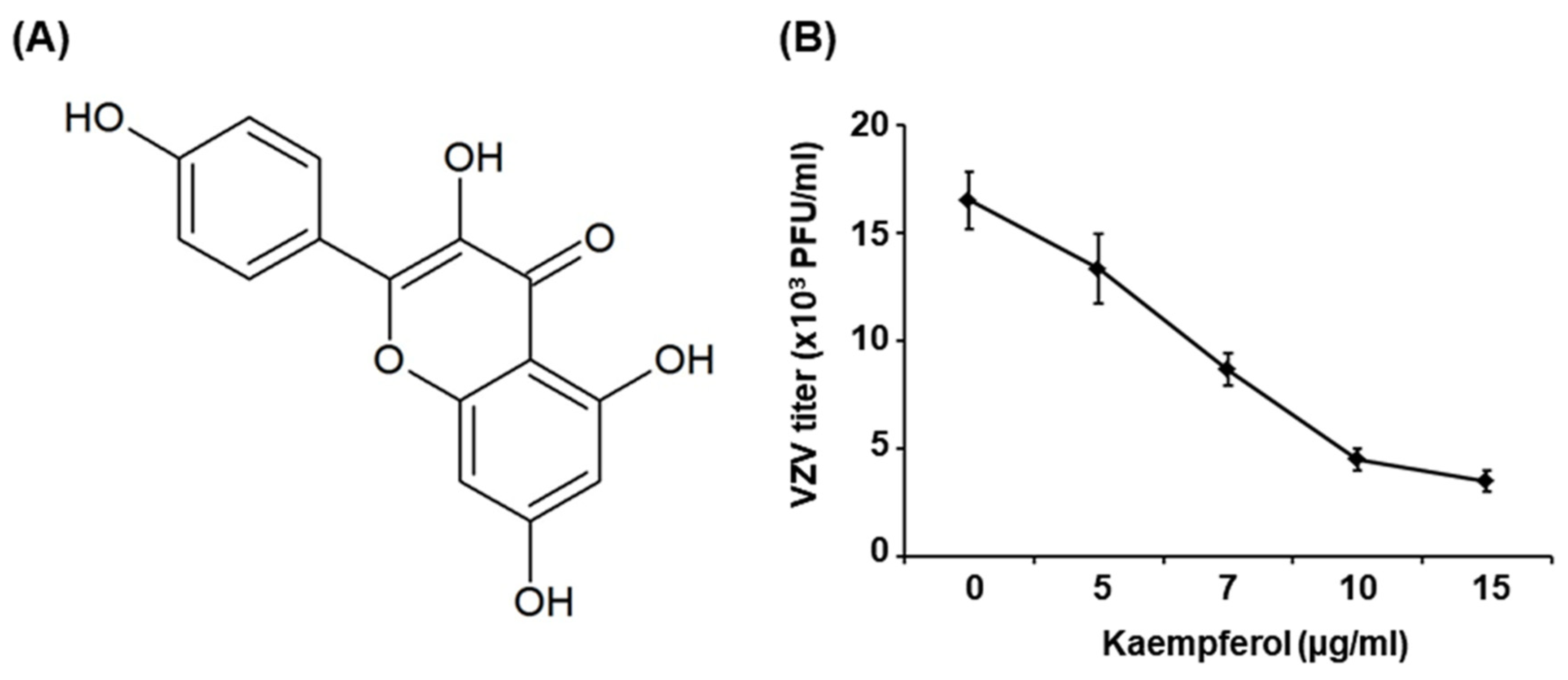
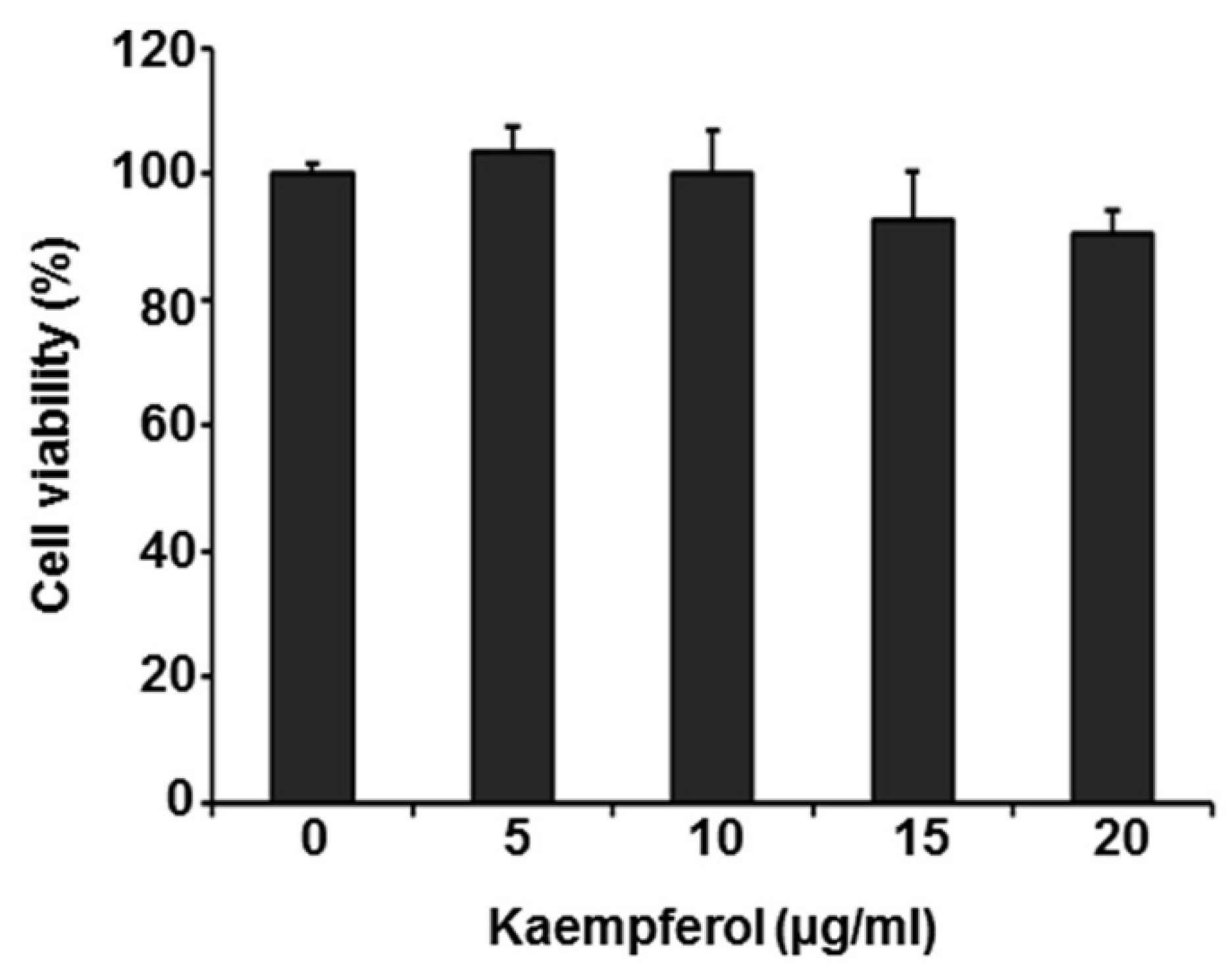
2.1. Kaempferol Inhibits VZV Replication
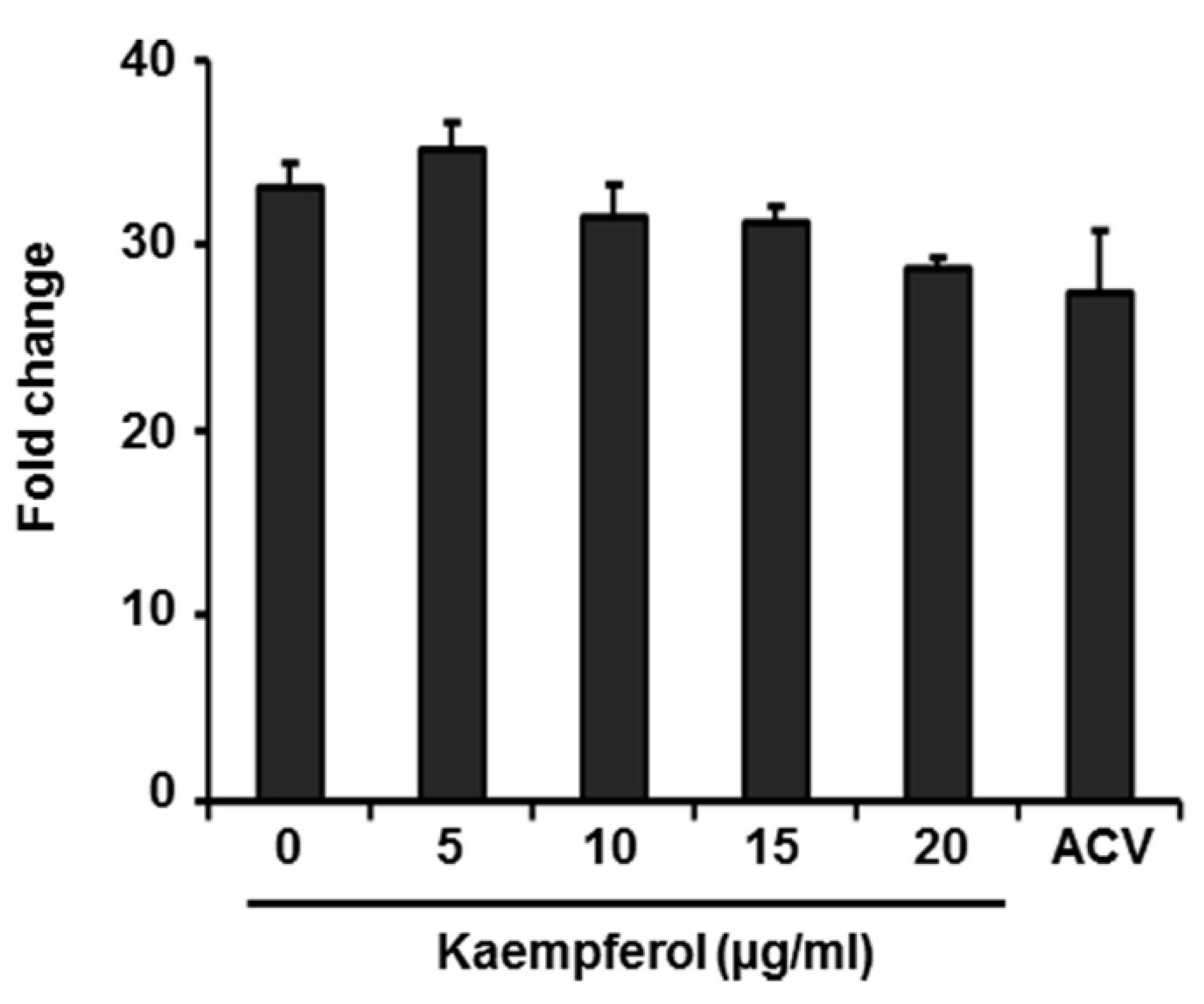
2.2. Kaempferol Has No Effect on Expression of VZV Immediate-Early Genes
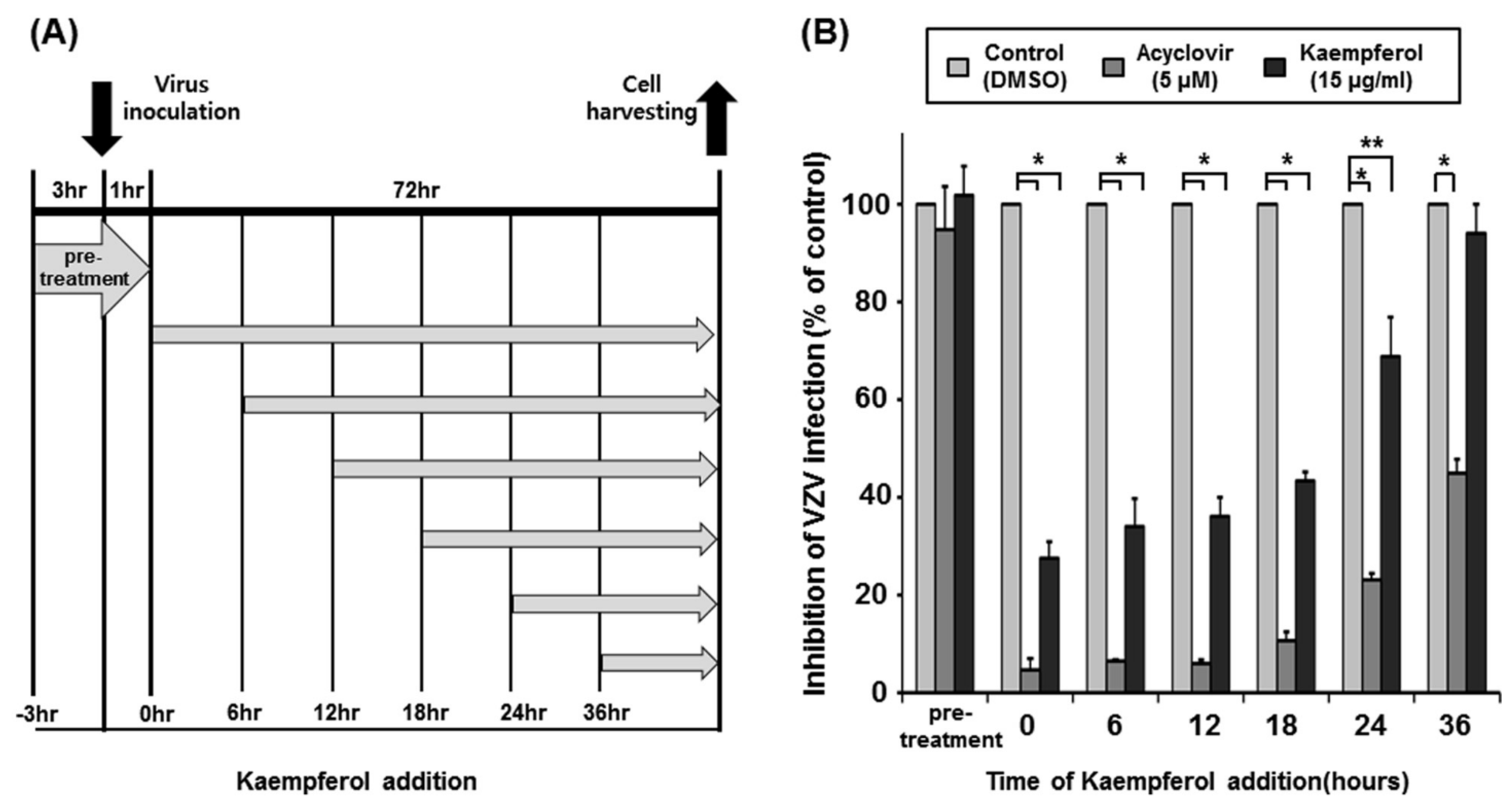
2.3. Determination of the Time Point at Which Kaempferol Suppresses VZV Infection
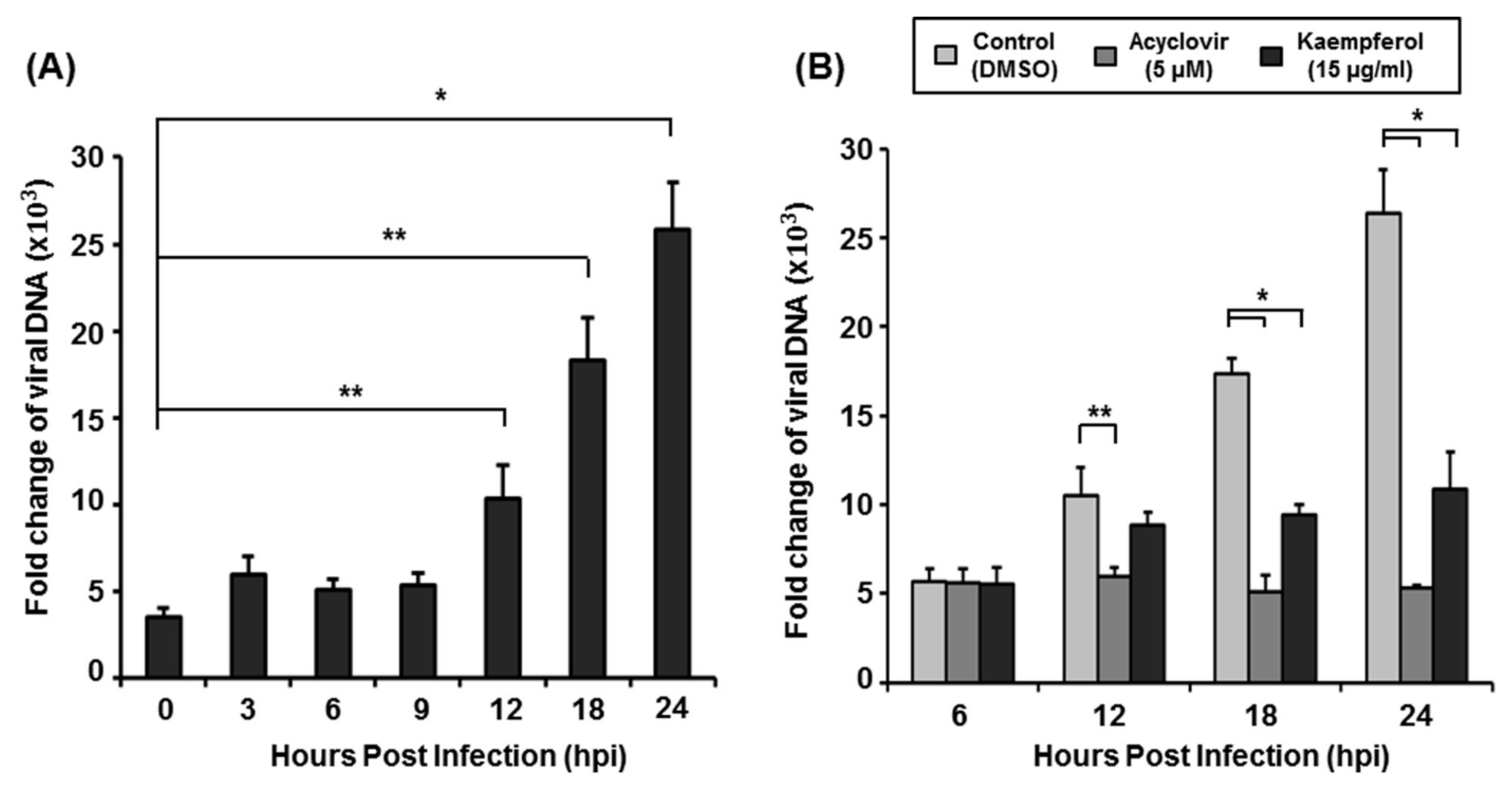
2.4. Kaempferol Inhibits VZV DNA Replication
3. Discussion
4. Materials and Methods
4.1. Cells, Viruses, and Materials
4.2. Plaque Reduction Assay
4.3. MTT Assay
4.4. Plasmids, Transfections, and Luciferase Reporter Assays
4.5. Quantitative Polymerase Chain Reaction (qPCR)
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gershon, A.A.; Breuer, J.; Cohen, J.I.; Cohrs, R.J.; Gershon, M.D.; Gilden, D.; Grose, C.; Hambleton, S.; Kennedy, P.G.; Oxman, M.N. Varicella zoster virus infection. Nat. Rev. Dis. Prim. 2015, 1, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Depledge, D.P.; Sadaoka, T.; Ouwendijk, W.J. Molecular aspects of varicella-zoster virus latency. Viruses 2018, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Gilden, M.D.; Cohrs, R.J.; Mahalingam, R.; Nagel, M.A. Neurological disease produced by varicella zoster virus reactivation without rash. Curr. Top. Microbiol. Immunol. 2010, 342, 243–253. [Google Scholar] [PubMed]
- Laing, K.J.; Ouwendijk, W.J.; Koelle, D.M.; Verjans, G.M. Immunobiology of varicella-zoster virus infection. J. Infect. Dis. 2018, 218, S68–S74. [Google Scholar] [CrossRef] [PubMed]
- Gershon, A.A.; Gershon, M.D. Pathogenesis and current approaches to control of varicella-zoster virus infections. Clin. Microbiol. Rev. 2013, 26, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.; Gershon, A.A. Clinical features of varicella-zoster virus infection. Viruses 2018, 10, 609. [Google Scholar] [CrossRef]
- Galil, K.; Brown, C.; Lin, F.; Seward, J. Hospitalizations for varicella in the United States, 1988 to 1999. Pediatr. Infect. Dis. J. 2002, 21, 931–934. [Google Scholar] [CrossRef]
- Harpaz, R.; Leung, J.W. The epidemiology of herpes zoster in the United States during the era of varicella and herpes zoster vaccines: Changing patterns among older adults. Clin. Infect. Dis. 2019, 69, 341–344. [Google Scholar] [CrossRef]
- Opstelten, W.; McElhaney, J.; Weinberger, B.; Oaklander, A.L.; Johnson, R.W. The impact of varicella zoster virus: Chronic pain. J. Clin. Virol. 2010, 48, S8–S13. [Google Scholar] [CrossRef]
- Holmes, S.J.; Reef, S.E.; Hadler, S.C.; Williams, W.W.; Wharton, M. Prevention of Varicella; Recommendations of the Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention: Atlanta, GA, USA, 1996; pp. 1–26. [Google Scholar]
- Whitley, R.J.; Middlebrooks, M.; Gnann, J.W. Acyclovir: The past ten years. Immunobiol. Prophyl. Hum. Herpesvirus Infect. 1990, 278, 243–253. [Google Scholar]
- De, S.K.; Hart, J.C.; Breuer, J. Herpes simplex virus and varicella zoster virus: Recent advances in therapy. Curr. Opin. Infect. Dis. 2015, 28, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Gunness, P.; Aleksa, K.; Bend, J.; Koren, J. Acyclovir-induced nephrotoxicity: The role of the acyclovir aldehyde metabolite. Transl. Res. 2011, 158, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Beutner, K.R. Valacyclovir: A review of its antiviral activity, pharmacokinetic properties, and clinical efficacy. Antivir. Res. 1995, 28, 281–290. [Google Scholar] [CrossRef] [PubMed]
- van der Beek, M.T.; Vermont, C.L.; Bredius, R.G.; Marijt, E.W.; van der Blij-de Brouwer, C.S.; Kroes, A.C.; Claas, E.C.; Vossen, A.C. Persistence and antiviral resistance of varicella zoster virus in hematological patients. Clin. Infect. Dis. 2013, 56, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Andrei, G.; Snoeck, R. Advances and perspectives in the management of varicella-zoster virus infections. Molecules 2011, 26, 1132. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a dietary anti-inflammatory agent: Current therapeutic standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F. Flavonols (Kaempeferol, Quercetin, Myricetin) Contents of Selected Fruits, Vegetables and Medicinal Plants. Food. Chem. 2008, 108, 879–884. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26065748 (accessed on 10 November 2022). [CrossRef]
- Arabyan, E.; Hakobyan, A.; Hakobyan, T.; Grigoryan, R.; Izmailyan, R.; Avetisyan, A.; Karalyan, Z.; Jackman, J.; Ferreira, F.C.; Elrod, C. Flavonoid library screening reveals kaempferol as a potential antiviral agent against african swine fever virus. Front. Microbiol. 2021, 12, 3165. [Google Scholar] [CrossRef]
- Liu, A.-L.; Wang, H.-D.; Lee, S.M.; Wang, Y.-T.; Du, G.-H. Structure–activity relationship of flavonoids as influenza virus neuraminidase inhibitors and their in vitro anti-viral activities. Bioorganic Med. Chem. 2008, 16, 7141–7147. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, Z.; Du, J.; Hu, Y.; Liu, L.; Yang, F.; Jin, Q. Anti-japanese-encephalitis-viral effects of kaempferol and daidzin and their rna-binding characteristics. PLoS ONE 2012, 7, e30259. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.Y.; Rhim, J.Y.; Park, W.B. Antiherpetic activities of flavonoids against herpes simplex virus type 1 (hsv-1) and type 2 (hsv-2) in vitro. Arch. Pharmacal Res. 2005, 28, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.C.; Chang, Y.H.; Chien, Y.; Lee, T.L. Type i interferon inhibits varicella-zoster virus replication by interfering with the dynamic interaction between mediator and ie62 within replication compartments. Cell Biosci. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Matsushita, M.; Fukui, Y.; Yamada, S.; Tsuda, M.; Higashi, C.; Kaneko, K.; Hasegawa, H.; Yamaguchi, T. Identification of a varicella-zoster virus replication inhibitor that blocks capsid assembly by interacting with the floor domain of the major capsid protein. J. Virol. 2012, 86, 12198–12207. [Google Scholar]
- Bai, N.; He, K.; Roller, M.; Lai, C.S.; Shao, X.; Pan, M.H.; Ho, C.T. Flavonoids and Phenolic Compounds from Rosmarinus Officinalis. J. Agric. Food. Chem. 2010, 58, 5363–5367. Available online: https://www.ncbi.nlm.nih.gov/pubmed/20397728 (accessed on 10 November 2022). [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. Available online: https://www.ncbi.nlm.nih.gov/pubmed/31557798 (accessed on 10 November 2022). [CrossRef]
- Behbahani, M.; Sayedipour, S.; Pourazar, A.; Shanehsazzadeh, M. In vitro anti-hiv-1 activities of kaempferol and kaempferol-7-o-glucoside isolated from securigera securidaca. Res. Pharm. Sci. 2014, 9, 463. [Google Scholar]
- Huang, W.W.; Tsai, S.C.; Peng, S.F.; Lin, M.W.; Chiang, J.H.; Chiu, Y.J.; Fushiya, S.; Tseng, M.T.; Yang, J.S. Kaempferol induces autophagy through ampk and akt signaling molecules and causes g2/m arrest via downregulation of cdk1/cyclin b in sk-hep-1 human hepatic cancer cells. Int. J. Oncol. 2013, 42, 2069–2077. [Google Scholar] [CrossRef]
- Girsch, J.H.; Walters, K.; Jackson, W.; Grose, C. Progeny varicella-zoster virus capsids exit the nucleus but never undergo secondary envelopment during autophagic flux inhibition by bafilomycin a1. J. Virol. 2019, 93, e00505–e00519. [Google Scholar] [CrossRef]
- Jeon, J.S.; Won, Y.H.; Kim, I.K.; Ahn, J.H.; Shin, O.S.; Kim, J.H.; Lee, C.H. Analysis of single nucleotide polymorphism among varicella-zoster virus and identification of vaccine-specific sites. Virology 2016, 496, 277–286. [Google Scholar] [CrossRef]
- Dong, W.; Wei, X.; Zhang, F.; Hao, J.; Huang, F.; Zhang, C.; Liang, W. A dual character of flavonoids in influenza a virus replication and spread through modulating cell-autonomous immunity by mapk signaling pathways. Sci. Rep. 2014, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kang, S.C.; Song, Y.J. Inhibition of human cytomegalovirus immediate-early gene expression and replication by the ethyl acetate (etoac) fraction of elaeocarpus sylvestris in vitro. BMC Complement. Altern. Med. 2017, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bari, W.; Song, Y.J.; Yoon, S.S. Suppressed Induction of Proinflammatory Cytokines by a Unique Metabolite Produced by Vibrio Cholerae o1 el tor Biotype in Cultured Host Cells. Infect. Immun. 2011, 79, 3149–3158. Available online: https://www.ncbi.nlm.nih.gov/pubmed/21576340 (accessed on 10 November 2022). [CrossRef] [PubMed]
- Bae, S.; Kim, S.Y.; Do, M.H.; Lee, C.H.; Song, Y.J. 1, 2, 3, 4, 6-penta-o-galloyl-ss-d-glucose, a bioactive compound in elaeocarpus sylvestris extract, inhibits varicella-zoster virus replication. Antivir. Res. 2017, 144, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.E.; Kim, D.K.; Song, Y.J. SARS-CoV-2 Nonstructural Proteins 1 and 13 Suppress Caspase-1 and the nlrp3 Inflammasome Activation. Microorganisms 2021, 9, 494. Available online: https://www.ncbi.nlm.nih.gov/pubmed/33652815 (accessed on 10 November 2022). [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Kim, N.-E.; Park, B.J.; Kwon, H.C.; Song, Y.-J. Kaempferol Interferes with Varicella-Zoster Virus Replication in Human Foreskin Fibroblasts. Pharmaceuticals 2022, 15, 1582. https://doi.org/10.3390/ph15121582
Park S, Kim N-E, Park BJ, Kwon HC, Song Y-J. Kaempferol Interferes with Varicella-Zoster Virus Replication in Human Foreskin Fibroblasts. Pharmaceuticals. 2022; 15(12):1582. https://doi.org/10.3390/ph15121582
Chicago/Turabian StylePark, Subin, Na-Eun Kim, Bang Ju Park, Hak Cheol Kwon, and Yoon-Jae Song. 2022. "Kaempferol Interferes with Varicella-Zoster Virus Replication in Human Foreskin Fibroblasts" Pharmaceuticals 15, no. 12: 1582. https://doi.org/10.3390/ph15121582
APA StylePark, S., Kim, N.-E., Park, B. J., Kwon, H. C., & Song, Y.-J. (2022). Kaempferol Interferes with Varicella-Zoster Virus Replication in Human Foreskin Fibroblasts. Pharmaceuticals, 15(12), 1582. https://doi.org/10.3390/ph15121582





