Exploiting Polyphenol-Mediated Redox Reorientation in Cancer Therapy
Abstract
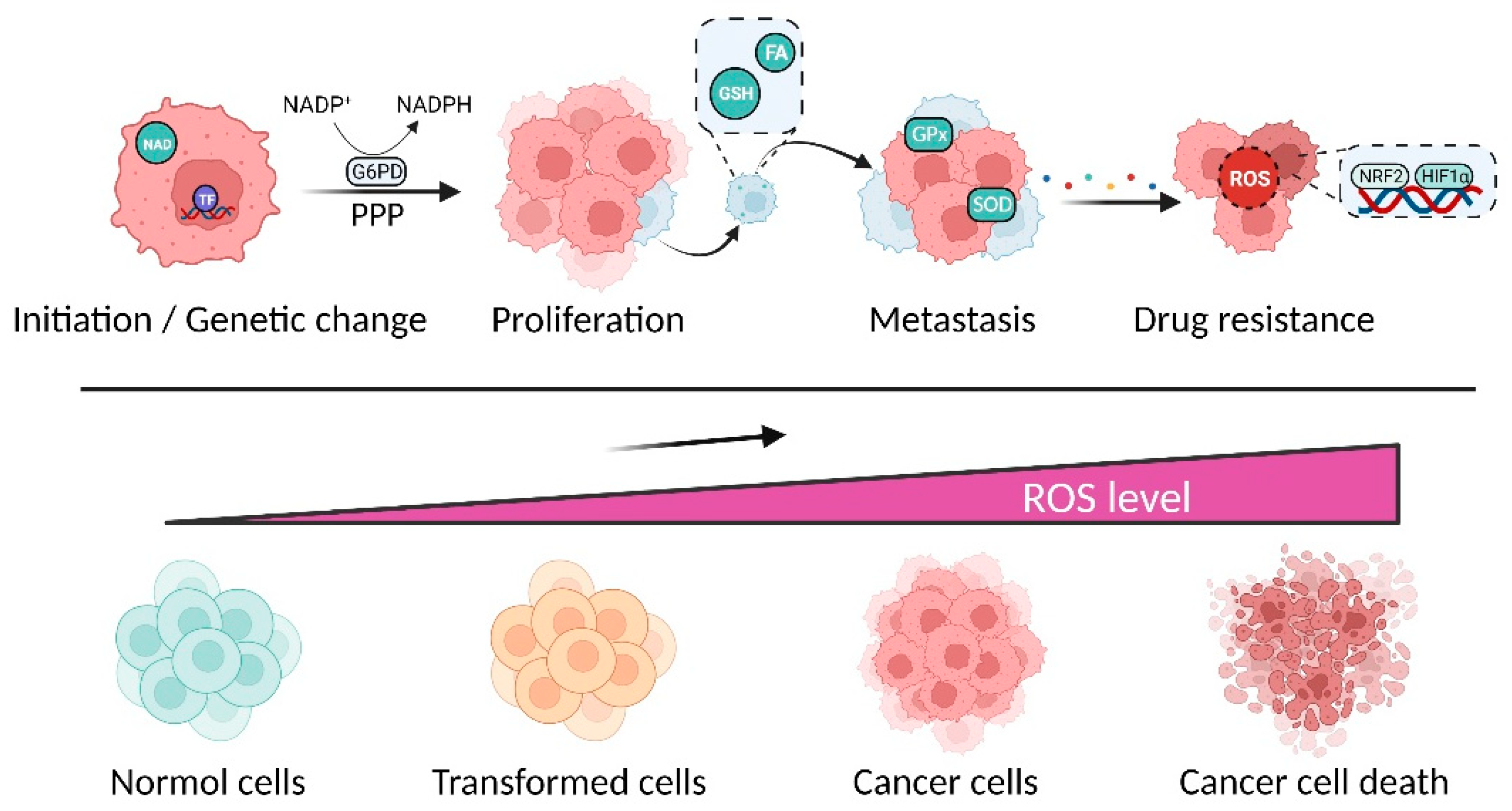
1. Introduction
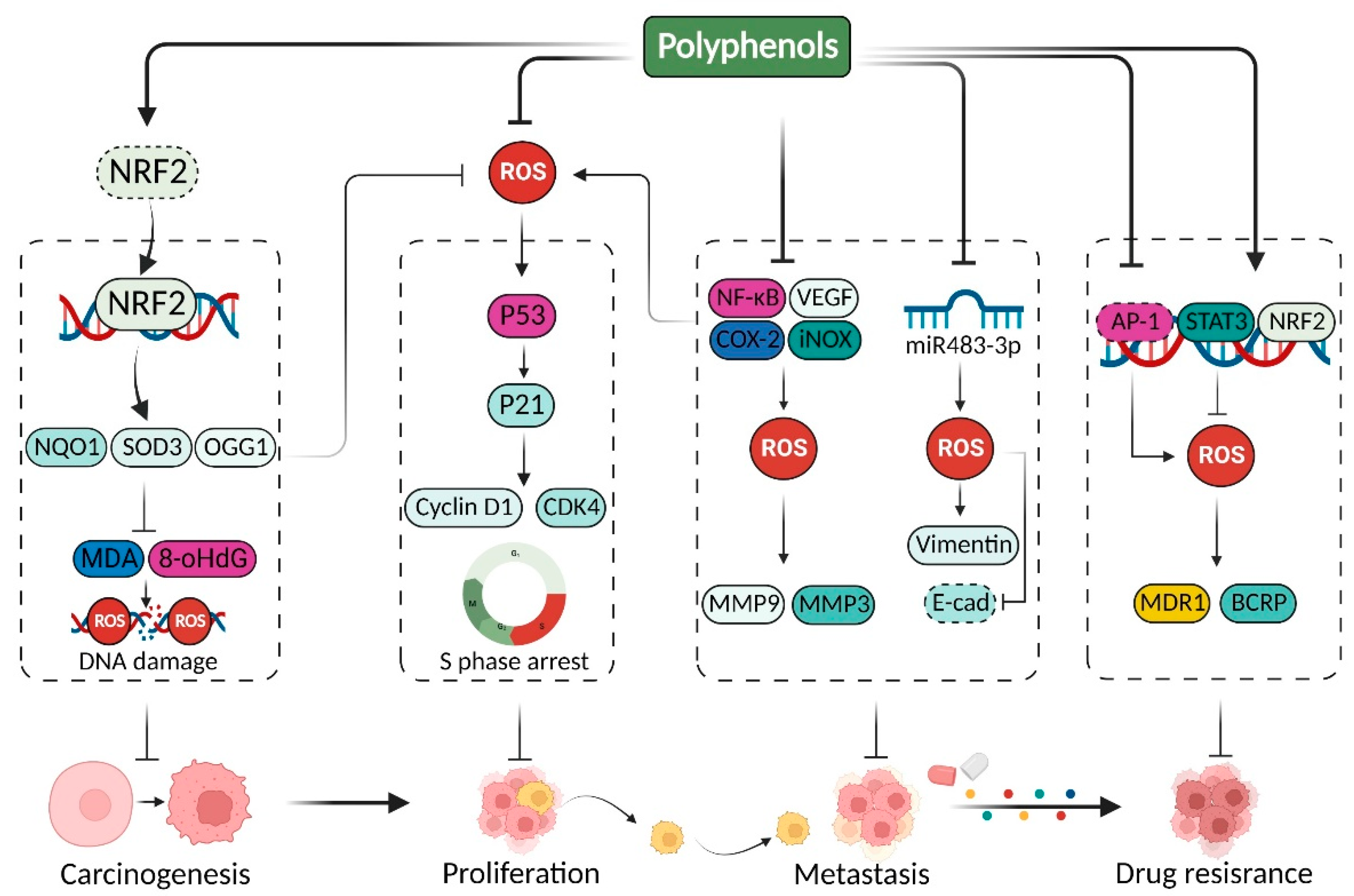
2. Polyphenols Modulate Redox Homeostasis for Cancer Therapy
2.1. Polyphenols Mediate Antioxidant Effects in Cancer Therapy
2.1.1. Kaempferol
2.1.2. Resveratrol
2.1.3. Catechins
2.1.4. Other Antioxidant Polyphenols
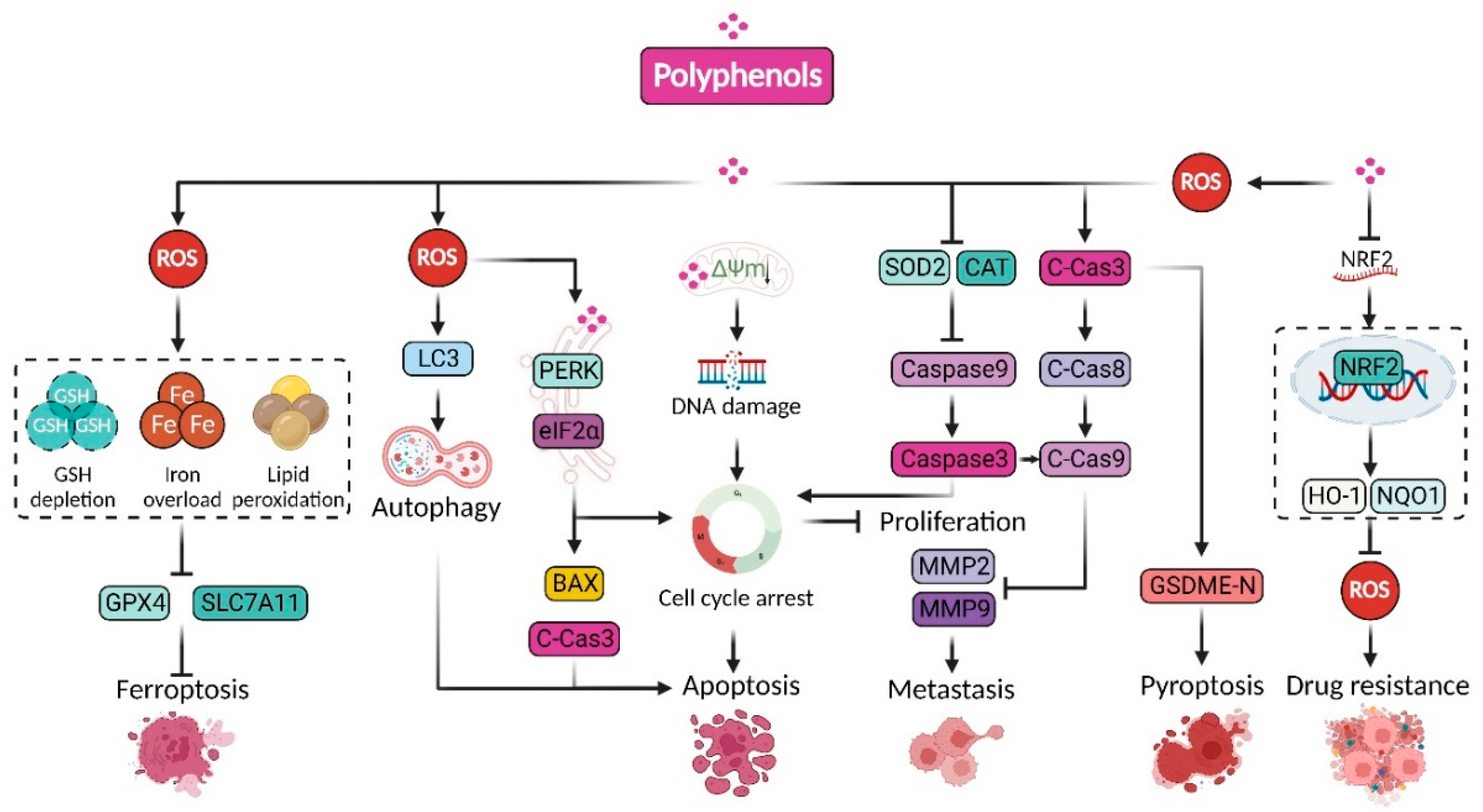
2.2. Polyphenols Suppressive Cancer by Promoting Oxidative Stress
2.2.1. Curcumin
2.2.2. Wogonin
2.2.3. Resveratrol
2.2.4. Other Prooxidant Polyphenols
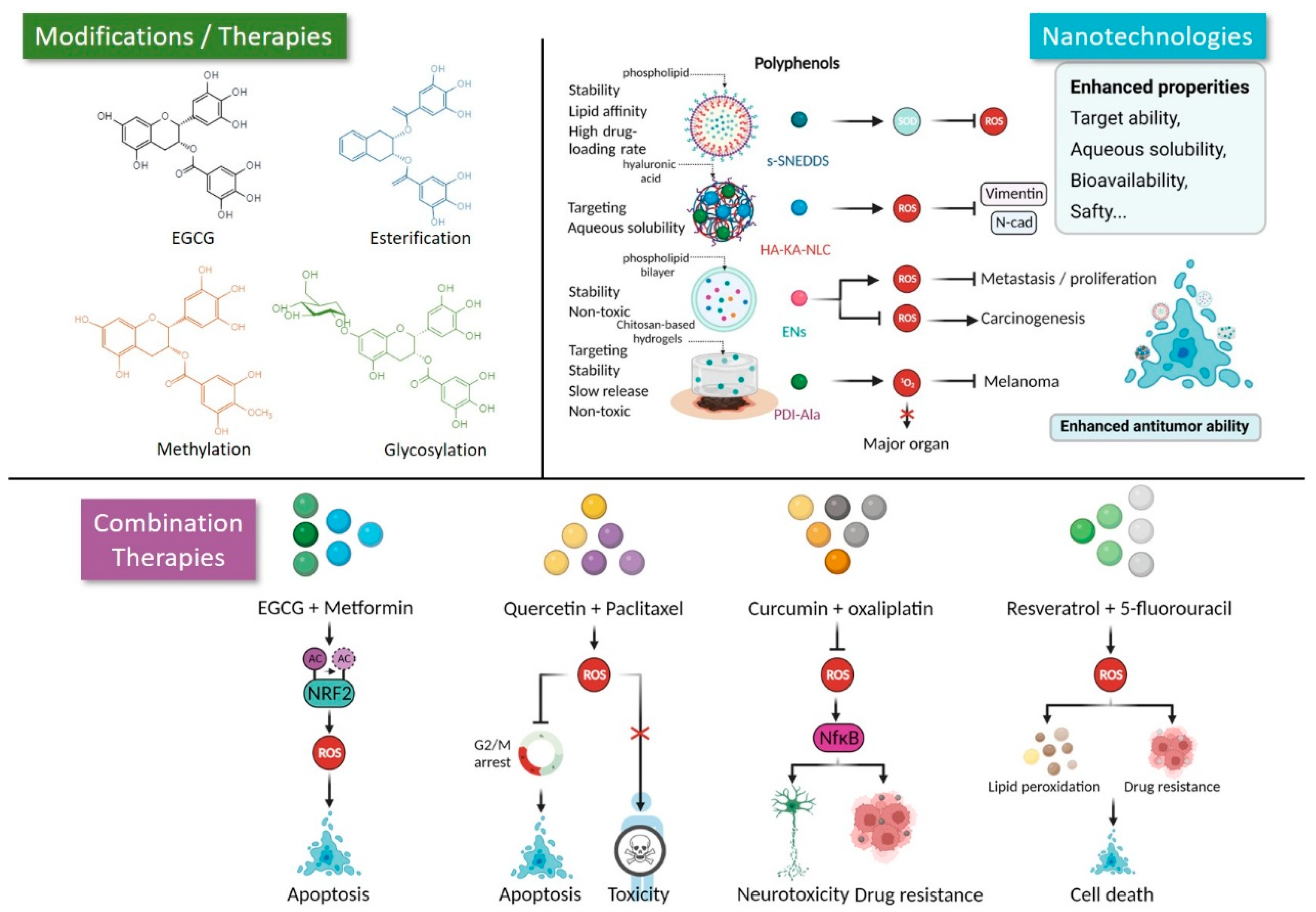
3. Novel Strategies Facilitate the Application of Polyphenols in Cancer Therapy
3.1. Modification
3.2. Nano Strategies
3.3. Combination with Other Agents
4. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Meo, F.; Valentino, A.; Petillo, O.; Peluso, G.; Filosa, S.; Crispi, S. Bioactive Polyphenols and Neuromodulation: Molecular Mechanisms in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 2564. [Google Scholar] [CrossRef] [PubMed]
- Obrador, E.; Salvador-Palmer, R.; Pellicer, B.; López-Blanch, R.; Sirerol, J.A.; Villaescusa, J.I.; Montoro, A.; Dellinger, R.W.; Estrela, J.M. Combination of natural polyphenols with a precursor of NAD(+) and a TLR2/6 ligand lipopeptide protects mice against lethal γ radiation. J. Adv. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhao, X.N.; Ma, Y.Y.; Tang, T.J.; Wang, S.S.; Wang, L.; Huang, J.L. Virtual screening analysis of natural flavonoids as trimethylamine (TMA)-lyase inhib-itors for coronary heart disease. J. Food Biochem. 2022, e14376. [Google Scholar]
- Curti, V.; Di Lorenzo, A.; Dacrema, M.; Xiao, J.; Nabavi, S.M.; Daglia, M. In Vitro polyphenol effects on apoptosis: An update of literature data. Semin. Cancer Biol. 2017, 46, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Feng, X.; Zhou, L.; He, C.; Li, H.; Xia, J.; Ge, Y.; Zhao, Y.; Song, C.; Chen, L.; et al. Heterophyllin B, a cyclopeptide from Pseudostellaria heterophylla, improves memory via immunomodulation and neurite regeneration in i.c.v.Aβ-induced mice. Food Res. Int. 2022, 158, 111576. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Duo, L.; Wang, J.; Zhula, G.; Yang, J.; Li, Z.; Tu, Y. A unique understanding of traditional medicine of pomegranate, Punica granatum L. and its current research status. J. Ethnopharmacol. 2021, 271, 113877. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Liu, X.; Zhao, C.; Yin, J.; Li, X.; Zhang, X.; Wang, J.; Wang, S. Distinctive anti-inflammatory effects of resveratrol, dihydroresveratrol, and 3-(4-hydroxyphenyl)-propionic acid on DSS-induced colitis in pseudo-germ-free mice. Food Chem. 2023, 400, 133904. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial home-ostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef]
- Hoseini, A.; Namazi, G.; Farrokhian, A.; Reiner, Ž.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. 2019, 10, 6042–6051. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Bi, K.; He, Y.; Yan, W.; Yang, C.S.; Zhang, J. Potential protective mechanisms of green tea polyphenol EGCG against COVID-19. Trends Food Sci. Technol. 2021, 114, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, Z.; Wang, J.; Weng, Q.; Chen, S.; Hu, M. Antifungal Activity of Isoliquiritin and Its Inhibitory Effect against Peronophythora litchi Chen through a Membrane Damage Mechanism. Molecules 2016, 21, 237. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R.; Turner, C.; Wong, B.; Feresin, R. Berry-Derived Polyphenols in Cardiovascular Pathologies: Mechanisms of Disease and the Role of Diet and Sex. Nutrients 2021, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shi, J.; Nice, E.C.; Huang, C.; Shi, Z. The Multifaceted Role of Flavonoids in Cancer Therapy: Leveraging Autophagy with a Double-Edged Sword. Antioxidants 2021, 10, 1138. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.S.; Ng, M.S.; Cheng, S.S.; Lo, A.W.; Xiao, Z.; Shin, T.S.; Chung, G.; Lam, H.M. Understanding the Composition, Biosynthesis, Accumulation and Transport of Fla-vonoids in Crops for the Promotion of Crops as Healthy Sources of Flavonoids for Human Consumption. Nutrients 2020, 12, 1717. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R.; Feresin, R. Protective Role of Polyphenols in Heart Failure: Molecular Targets and Cellular Mechanisms Underlying Their Therapeutic Potential. Int. J. Mol. Sci. 2021, 22, 1668. [Google Scholar] [CrossRef]
- Ngamsamer, C.; Sirivarasai, J.; Sutjarit, N. The Benefits of Anthocyanins against Obesity-Induced Inflammation. Biomolecules 2022, 12, 852. [Google Scholar] [CrossRef]
- Chialva, C.; Blein, T.; Crespi, M.; Lijavetzky, D. Insights into long non-coding RNA regulation of anthocyanin carrot root pigmentation. Sci. Rep. 2021, 11, 4093. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Lyons, J.B.; Chilaka, C.A. The Dioscorea Genus (Yam)—An Appraisal of Nutritional and Therapeutic Potentials. Foods 2020, 9, 1304. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.E.; Saleh, T.M.; Kalisch, B.E. Naturally Occurring Antioxidant Therapy in Alzheimer’s Disease. Antioxidants 2022, 11, 213. [Google Scholar] [CrossRef]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Granados, M.; Farran, A.; Cortina, J.L.; Saurina, J.; Valderrama, C. Recovery of Natural Polyphenols from Spinach and Orange By-Products by Pressure-Driven Membrane Processes. Membranes 2022, 12, 669. [Google Scholar] [CrossRef]
- Oliva, E.; Fanti, F.; Palmieri, S.; Viteritti, E.; Eugelio, F.; Pepe, A.; Compagnone, D.; Sergi, M. Predictive Multi Experiment Approach for the Determination of Conjugated Phenolic Compounds in Vegetal Matrices by Means of LC-MS/MS. Molecules 2022, 27, 3089. [Google Scholar] [CrossRef] [PubMed]
- Zeiss, D.R.; Mhlongo, M.I.; Tugizimana, F.; Steenkamp, P.A.; Dubery, I.A. Metabolomic Profiling of the Host Response of Tomato (Solanum lycopersicum) Following Infection by Ralstonia solanacearum. Int. J. Mol. Sci. 2019, 20, 3945. [Google Scholar] [CrossRef] [PubMed]
- Zálešák, F.; Bon, D.J.-Y.D.; Pospíšil, J. Lignans and Neolignans: Plant secondary metabolites as a reservoir of biologically active substances. Pharmacol. Res. 2019, 146, 104284. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Fan, S.; Hu, J.; Ma, Y.; Feng, Y.; Wang, F.; Wang, X.; Niu, L. Phytochemical Analysis Using UPLC-MS/MS Combined with Network Pharmacology Methods to Explore the Biomarkers for the Quality Control of Lingguizhugan Decoction. Evidence-Based Complement. Altern. Med. 2021, 2021, 7849032. [Google Scholar] [CrossRef]
- Wang, L.-X.; Wang, H.-L.; Huang, J.; Chu, T.-Z.; Peng, C.; Zhang, H.; Chen, H.-L.; Xiong, Y.-A.; Tan, Y.-Z. Review of lignans from 2019 to 2021: Newly reported compounds, diverse activities, structure-activity relationships and clinical applications. Phytochemistry 2022, 202, 113326. [Google Scholar] [CrossRef]
- Qayyum, Z.; Noureen, F.; Khan, M.; Khan, M.; Haider, G.; Munir, F.; Gul, A.; Amir, R. Identification and Expression Analysis of Stilbene Synthase Genes in Arachis hypogaea in Response to Methyl Jasmonate and Salicylic Acid Induction. Plants 2022, 11, 1776. [Google Scholar] [CrossRef]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Zam, W.; Kumar, M.; Cardoso, S.M.; Pereira, O.R.; Ademiluyi, A.O.; Adeleke, O.; Moreira, A.C.; Živković, J.; et al. Phenolic Bioactives as Antiplatelet Aggregation Factors: The Pivotal Ingredients in Maintaining Cardiovascular Health. Oxidative Med. Cell. Longev. 2021, 2021, 2195902. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Skinner, T.; Bridges, B.; Weber, J.T. The Pathology of Parkinson’s Disease and Potential Benefit of Dietary Polyphenols. Molecules 2020, 25, 4382. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Cheng, G.; Hardy, M.; Ouari, O.; Bennett, B.; Zielonka, J. Teaching the basics of reactive oxygen species and their relevance to cancer biology: Mitochondrial reactive oxygen species detection, redox signaling, and targeted therapies. Redox Biol. 2018, 15, 347–362. [Google Scholar] [CrossRef]
- Heurtaux, T.; Bouvier, D.S.; Benani, A.; Romero, S.H.; Frauenknecht, K.B.M.; Mittelbronn, M.; Sinkkonen, L. Normal and Pathological NRF2 Signalling in the Central Nervous System. Antioxidants 2022, 11, 1426. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Zhuang, L.; Olszewski, K.; Gan, B. NADPH debt drives redox bankruptcy: SLC7A11/xCT-mediated cystine uptake as a double-edged sword in cellular redox regulation. Genes Dis. 2021, 8, 731–745. [Google Scholar] [CrossRef]
- Qin, Z.; Xiang, C.; Zhong, F.; Liu, Y.; Dong, Q.; Li, K.; Shi, W.; Ding, C.; Qin, L.; He, F. Transketolase (TKT) activity and nuclear localization promote hepatocellular carcinoma in a metabolic and a non-metabolic manner. J. Exp. Clin. Cancer Res. 2019, 38, 1–21. [Google Scholar] [CrossRef]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef]
- Tang, Y.-A.; Chen, Y.-F.; Bao, Y.; Mahara, S.; Yatim, S.M.J.M.; Oguz, G.; Lee, P.L.; Feng, M.; Cai, Y.; Tan, E.Y.; et al. Hypoxic tumor microenvironment activates GLI2 via HIF-1α and TGF-β2 to promote chemoresistance in colorectal cancer. Proc. Natl. Acad. Sci. USA 2018, 115, E5990–E5999. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Jin, P.; Jiang, J.; Zhou, L.; Huang, Z.; Nice, E.C.; Huang, C.; Fu, L. Mitochondrial adaptation in cancer drug resistance: Prevalence, mechanisms, and management. J. Hematol. Oncol. 2022, 15, 97. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, J.; Chen, H.N.; Zhou, L.; Huang, Z.; Qin, S.; Jin, P.; Luo, M.; Li, B.; Shi, J.; et al. Redox-sensitive cyclophilin A elicits chemoresistance through realigning cellular oxi-dative status in colorectal cancer. Cell Rep. 2021, 37, 110069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qin, S.; Chen, Y.; Zhou, L.; Yang, M.; Tang, Y.; Zuo, J.; Zhang, J.; Mizokami, A.; Nice, E.C.; et al. Inhibition of NPC1L1 disrupts adaptive responses of drug-tolerant persister cells to chemotherapy. EMBO Mol. Med. 2022, 14, e14903. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Jiang, J.; Zhou, L.; Huang, Z.; Qin, S.; Chen, H.N.; Peng, L.; Zhang, Z.; Li, B.; Luo, M.; et al. Disrupting metformin adaptation of liver cancer cells by targeting the TOMM34/ATP5B axis. EMBO Mol. Med. 2022, 14, e16082. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Balasubramanian, S.; Oelschlaeger, A.T.; Grkovic, T.; Pham, N.B.; Quinn, R.J.; Hentschel, U. Potential of marine natural products against drug-resistant fungal, viral, and parasitic infections. Lancet Infect. Dis. 2017, 17, e30–e41. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jia, W.; Zhu, L.; Mao, L.; Zhang, Y. Recent advances in heterocyclic aromatic amines: An update on food safety and hazardous control from food processing to dietary intake. Compr. Rev. Food Sci. Food Saf. 2020, 19, 124–148. [Google Scholar] [CrossRef]
- Mao, X.-Y.; Jin, M.-Z.; Chen, J.-F.; Zhou, H.-H.; Jin, W.-L. Live or let die: Neuroprotective and anti-cancer effects of nutraceutical antioxidants. Pharmacol. Ther. 2018, 183, 137–151. [Google Scholar] [CrossRef]
- Olcha, P.; Winiarska-Mieczan, A.; Kwiecień, M.; Nowakowski, Ł.; Miturski, A.; Semczuk, A.; Kiczorowska, B.; Gałczyński, K. Antioxidative, Anti-Inflammatory, Anti-Obesogenic, and Antidia-betic Properties of Tea Polyphenols-The Positive Impact of Regular Tea Consumption as an Element of Prophylaxis and Pharmacotherapy Support in Endometrial Cancer. Int. J. Mol. Sci. 2022, 23, 6703. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, S.; Zhou, L.; Yu, F.; Ding, H.; Li, P.; Zhou, M.; Wang, K. Potential Mechanisms of Action of Curcumin for Cancer Prevention: Focus on Cellular Signaling Pathways and miRNAs. Int. J. Biol. Sci. 2019, 15, 1200–1214. [Google Scholar] [CrossRef] [PubMed]
- Purpura, V.; Benedetti, S.; Bondioli, E.; Scarpellini, F.; Giacometti, A.; Albertini, M.C.; Melandri, D. The Use of Quercetin to Improve the Antioxidant and Regenerative Properties of Frozen or Cryopreserved Human Amniotic Membrane. Antioxidants 2022, 11, 1250. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.R.S.; Conte-Junior, C.A. Nano-delivery systems for food bioactive compounds in cancer: Prevention, therapy, and clinical applications. Crit. Rev. Food Sci. Nutr. 2022, 1–26. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2022, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Munot, N.; Kandekar, U.; Giram, P.S.; Khot, K.; Patil, A.; Cavalu, S. A Comparative Study of Quercetin-Loaded Nanococh-leates and Liposomes: Formulation, Characterization, Assessment of Degradation and In Vitro Anticancer Potential. Pharmaceutics 2022, 14, 1601. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Rizvi, A. Understanding the Prooxidant Action of Plant Polyphenols in the Cellular Microenvironment of Malignant Cells: Role of Copper and Therapeutic Implications. Front. Pharmacol. 2022, 13, 929853. [Google Scholar] [CrossRef] [PubMed]
- Scuto, M.; Ontario, M.L.; Salinaro, A.T.; Caligiuri, I.; Rampulla, F.; Zimbone, V.; Modafferi, S.; Rizzolio, F.; Canzonieri, V.; Calabrese, E.J.; et al. Redox modulation by plant polyphenols targeting vitagenes for chemopre-vention and therapy: Relevance to novel anti-cancer interventions and mini-brain organoid technology. Free. Radic. Biol. Med. 2022, 179, 59–75. [Google Scholar] [CrossRef]
- Lendzion, K.; Gornowicz, A.; Strawa, J.W.; Bielawska, K.; Czarnomysy, R.; Popławska, B.; Bielawski, K.; Tomczyk, M.; Miltyk, W.; Bielawska, A. LC-PDA-MS and GC-MS Analysis of Scorzonera hispanica Seeds and Their Effects on Human Breast Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 11584. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, Q.; Wu, F.G.; Dai, Y.; Chen, X. Polyphenol-Containing Nanoparticles: Synthesis, Properties, and Therapeutic Delivery. Adv. Mater. 2021, 33, e2007356. [Google Scholar] [CrossRef]
- Ju, Y.; Liao, H.; Richardson, J.J.; Guo, J.; Caruso, F. Nanostructured particles assembled from natural building blocks for advanced therapies. Chem. Soc. Rev. 2022, 51, 4287–4336. [Google Scholar] [CrossRef]
- Mani, S.; Swargiary, G.; Ralph, S.J. Targeting the redox imbalance in mitochondria: A novel mode for cancer therapy. Mitochondrion 2022, 62, 50–73. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.; DeNicola, G.M.; Nixon, C.; Blyth, K.; Labuschagne, C.F.; Tuveson, D.A.; Vousden, K.H. Dynamic ROS Control by TIGAR Regulates the Initiation and Progression of Pancreatic Cancer. Cancer Cell 2020, 37, 168–182.e4. [Google Scholar] [CrossRef] [PubMed]
- Reczek, C.R.; Birsoy, K.; Kong, H.; Martínez-Reyes, I.; Wang, T.; Gao, P.; Sabatini, D.M.; Chandel, N.S. A CRISPR screen identifies a pathway required for paraquat-induced cell death. Nat. Chem. Biol. 2017, 13, 1274–1279. [Google Scholar] [CrossRef]
- Cencioni, C.; Comunanza, V.; Middonti, E.; Vallariello, E.; Bussolino, F. The role of redox system in metastasis formation. Angiogenesis 2021, 24, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Paller, C.J.; Zhou, X.C.; Heath, E.I.; Taplin, M.-E.; Mayer, T.; Stein, M.N.; Bubley, G.J.; Pili, R.; Hudson, T.; Kakarla, R.; et al. Muscadine Grape Skin Extract (MPX) in Men with Biochemically Recurrent Prostate Cancer: A Randomized, Multicenter, Placebo-Controlled Clinical Trial. Clin. Cancer Res. 2018, 24, 306–315. [Google Scholar] [CrossRef]
- vila-Gálvez, M.; González-Sarrías, A.; Martínez-Díaz, F.; Abellán, B.; Martínez-Torrano, A.J.; Fernández-López, A.J.; Giménez-Bastida, J.A.; Espín, J.C. Disposition of Dietary Polyphenols in Breast Cancer Pa-tients’ Tumors, and Their Associated Anticancer Activity: The Particular Case of Curcumin. Mol. Nutr. Food Res. 2021, 65, e2100163. [Google Scholar] [CrossRef]
- Henning, S.M.; Wang, P.; Lee, R.-P.; Trang, A.; Husari, G.; Yang, J.; Grojean, E.M.; Ly, A.; Hsu, M.; Heber, D.; et al. Prospective randomized trial evaluating blood and prostate tissue concentrations of green tea polyphenols and quercetin in men with prostate cancer. Food Funct. 2020, 11, 4114–4122. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, H.; Zhang, C.; Cheng, Y.; Hu, L.; Meng, X.; Zhao, Y. Protective effects of berberine on radiation-induced lung injury via intercellular adhesion molecular-1 and transforming growth factor-beta-1 in patients with lung cancer. Eur. J. Cancer 2008, 44, 2425–2432. [Google Scholar] [CrossRef]
- Tuli, H.S.; Mittal, S.; Aggarwal, D.; Parashar, G.; Parashar, N.C.; Upadhyay, S.K.; Barwal, T.S.; Jain, A.; Kaur, G.; Savla, R.; et al. Path of Silibinin from diet to medicine: A dietary polyphenolic flavonoid having potential anti-cancer therapeutic significance. Semin. Cancer Biol. 2021, 73, 196–218. [Google Scholar] [CrossRef]
- Rahaiee, S.; Assadpour, E.; Esfanjani, A.F.; Silva, A.S.; Jafari, S.M. Application of nano/microencapsulated phenolic compounds against cancer. Adv. Colloid Interface Sci. 2020, 279, 102153. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-F.; Huang, Y.-H.; Chiu, L.-Y.; Cherng, S.-H.; Sheu, G.-T.; Yang, T.-Y. Curcumin Induces Apoptosis of Chemoresistant Lung Cancer Cells via ROS-Regulated p38 MAPK Phosphorylation. Int. J. Mol. Sci. 2022, 23, 8248. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa, T.; Wołosewicz, K.; Jamrozik, M.; Drzeżdżon, J.; Siemińska, J.; Jacewicz, D.; Górska-Ponikowska, M.; Kołaczkowski, M.; Łaźny, R.; Kuban-Jankowska, A. Curcumin and Its New Derivatives: Correlation between Cytotoxicity against Breast Cancer Cell Lines, Degradation of PTP1B Phosphatase and ROS Generation. Int. J. Mol. Sci. 2021, 22, 10368. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fang, S.; Shao, X.; Li, Y.; Tong, Q.; Kong, B.; Chen, L.; Wang, Y.; Yang, J.; Yu, H.; et al. Curcumin Reverses NNMT-Induced 5-Fluorouracil Resistance via Increasing ROS and Cell Cycle Arrest in Colorectal Cancer Cells. Biomolecules 2021, 11, 1295. [Google Scholar] [CrossRef]
- Wang, T.; Wu, X.; Al Rudaisat, M.; Song, Y.; Cheng, H. Curcumin induces G2/M arrest and triggers autophagy, ROS gen-eration and cell senescence in cervical cancer cells. J. Cancer 2020, 11, 6704–6715. [Google Scholar] [CrossRef]
- Lin, X.; Wang, L.; Zhao, L.; Zhu, Z.; Chen, T.; Chen, S.; Tao, Y.; Zeng, T.; Zhong, Y.; Sun, H.; et al. Curcumin micelles suppress gastric tumor cell growth by upregulating ROS generation, disrupting redox equilibrium and affecting mitochondrial bioenergetics. Food Funct. 2020, 11, 4146–4159. [Google Scholar] [CrossRef]
- Mahgoub, S.; Hashad, N.; Ali, S.; Ibrahim, R.; Said, A.M.; Moharram, F.A.; Mady, M. Polyphenolic Profile of Callistemon viminalis Aerial Parts: Antioxidant, Anticancer and In Silico 5-LOX Inhibitory Evaluations. Molecules 2021, 26, 2481. [Google Scholar] [CrossRef]
- Del Mar Rivas-Chacón, L.; Yanes-Díaz, J.; de Lucas, B.; Riestra-Ayora, J.I.; Madrid-García, R.; Sanz-Fernández, R.; Sánchez-Rodríguez, C. Preventive Effect of Cocoa Flavonoids via Suppression of Oxidative Stress-Induced Apoptosis in Auditory Senescent Cells. Antioxidants 2022, 11, 1450. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Xue, J.; Mu, B.; Song, H.; Liu, Y. Exogenous Melatonin Treatment Induces Disease Resistance against Botrytis cinerea on Post-Harvest Grapes by Activating Defence Responses. Foods 2022, 11, 2231. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, F.; Mo, Y.; Liao, H.; Chen, P.; Zhang, H. Effects of Ethanol Treatment on Storage Quality and Antioxidant System of Postharvest Papaya. Front. Plant Sci. 2022, 13, 856499. [Google Scholar] [CrossRef]
- Khalil Alyahya, H.; Subash-Babu, P.; Mohammad Salamatullah, A.; Hayat, K.; Albader, N.; Alkaltham, M.S.; Ahmed, M.A.; Arzoo, S.; Bourhia, M. Quantification of Chlorogenic Acid and Vanillin from Coffee Peel Extract and its Effect on α-Amylase Activity, Immunoregulation, Mitochondrial Oxidative Stress, and Tumor Suppressor Gene Expression Levels in H(2)O(2)-Induced Human Mesenchymal Stem Cells. Front Pharmacol. 2021, 12, 760242. [Google Scholar] [CrossRef]
- Heydarzadeh, S.; Kia, S.K.; Zarkesh, M.; Pakizehkar, S.; Hosseinzadeh, S.; Hedayati, M. The Cross-Talk between Polyphenols and the Target Enzymes Related to Oxidative Stress-Induced Thyroid Cancer. Oxidative Med. Cell. Longev. 2022, 2022, 2724324. [Google Scholar] [CrossRef]
- Oláhová, M.; Veal, E.A. A peroxiredoxin, PRDX-2, is required for insulin secretion and insulin/IIS-dependent regulation of stress resistance and longevity. Aging Cell 2015, 14, 558–568. [Google Scholar] [CrossRef]
- Qi, Z.; Ji, H.; Le, M.; Li, H.; Wieland, A.; Bauer, S.; Liu, L.; Wink, M.; Herr, I. Sulforaphane promotes C. elegans longevity and healthspan via DAF-16/DAF-2 insulin/IGF-1 signaling. Aging 2021, 13, 1649–1670. [Google Scholar] [CrossRef]
- Kittimongkolsuk, P.; Roxo, M.; Li, H.; Chuchawankul, S.; Wink, M.; Tencomnao, T. Extracts of the Tiger Milk Mushroom (Lignosus rhinocerus) Enhance Stress Resistance and Extend Lifespan in Caenorhabditis elegans via the DAF-16/FoxO Sig-naling Pathway. Pharmaceuticals 2021, 14, 93. [Google Scholar] [CrossRef]
- Li, H.; Roxo, M.; Cheng, X.; Zhang, S.; Cheng, H.; Wink, M. Pro-oxidant and lifespan extension effects of caffeine and related methylxanthines in Caenorhabditis elegans. Food Chem. X 2019, 1, 100005. [Google Scholar] [CrossRef]
- Duangjan, C.; Rangsinth, P.; Gu, X.; Zhang, S.; Wink, M.; Tencomnao, T. Glochidion zeylanicum leaf extracts exhibit lifespan extending and oxidative stress resistance properties in Caenorhabditis elegans via DAF-16/FoxO and SKN-1/Nrf-2 signaling pathways. Phytomedicine 2019, 64, 153061. [Google Scholar] [CrossRef]
- Zhai, Y.; Sun, J.; Sun, C.; Zhao, H.; Li, X.; Yao, J.; Su, J.; Xu, X.; Xu, X.; Hu, J.; et al. Total flavonoids from the dried root of Tetrastigma hemsleyanum Diels et Gilg inhibit colo-rectal cancer growth through PI3K/AKT/mTOR signaling pathway. Phytother. Res. 2022, 36, 4263–4277. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, H.; Xue, J.; Yuan, J.; Cai, Z.; Wu, N.; Zou, L.; Yin, S.; Yang, W.; Liu, X.; et al. Qualitative Analysis and Componential Differences of Chemical Constituents in Lysim-achiae Herba from Different Habitats (Sichuan Basin) by UFLC-Triple TOF-MS/MS. Molecules 2022, 27, 4600. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Y.; Chen, G.; Yue, W.; Liang, Q.; Wu, Q. Optimisation of ultrasound assisted extraction of phenolic compounds from Sparganii rhizoma with response surface methodology. Ultrason. Sonochem. 2013, 20, 846–854. [Google Scholar] [CrossRef]
- Harrath, A.H.; Jalouli, M.; Oueslati, M.H.; Farah, M.A.; Feriani, A.; Aldahmash, W.; Aldawood, N.; Al-Anazi, K.; Falodah, F.; Swelum, A.; et al. The flavonoid, kaempferol-3-O-apiofuranosyl-7-O-rhamnopyranosyl, as a potential therapeutic agent for breast cancer with a promoting effect on ovarian function. Phytotherapy Res. 2021, 35, 6170–6180. [Google Scholar] [CrossRef]
- Felice, M.R.; Maugeri, A.; De Sarro, G.; Navarra, M.; Barreca, D. Molecular Pathways Involved in the Anti-Cancer Activity of Flavonols: A Focus on Myricetin and Kaempferol. Int. J. Mol. Sci. 2022, 23, 4411. [Google Scholar] [CrossRef]
- Amjad, E.; Sokouti, B.; Asnaashari, S. A systematic review of anti-cancer roles and mechanisms of kaempferol as a natural compound. Cancer Cell Int. 2022, 22, 260. [Google Scholar] [CrossRef]
- Kluska, M.; Juszczak, M.; Żuchowski, J.; Stochmal, A.; Woźniak, K. Effect of Kaempferol and Its Glycoside Derivatives on Antioxidant Status of HL-60 Cells Treated with Etoposide. Molecules 2022, 27, 333. [Google Scholar] [CrossRef]
- Wu, P.; Meng, X.; Zheng, H.; Zeng, Q.; Chen, T.; Wang, W.; Zhang, X.; Su, J. Kaempferol Attenuates ROS-Induced Hemolysis and the Molecular Mechanism of Its Induction of Apoptosis on Bladder Cancer. Molecules 2018, 23, 2592. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Wang, T.; Jiang, X.; Wang, L. Screening active components of modified Xiaoyao powder as NRF2 ago-nists. Cell Biochem. Funct. 2017, 35, 518–526. [Google Scholar] [CrossRef]
- Zeng, J.; Xu, H.; Fan, P.-Z.; Xie, J.; He, J.; Yu, J.; Gu, X.; Zhang, C.-J. Kaempferol blocks neutrophil extracellular traps formation and reduces tumour metastasis by inhibiting ROS-PAD4 pathway. J. Cell. Mol. Med. 2020, 24, 7590–7599. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, D.W.; Lim, S.R.; Sung, J.; Kim, T.H.; Min, I.S.; Choi, C.H.; Lee, S.J. Kaempferol Blocks the Skin Fibroblastic Interleukin 1β Expression and Cytotoxicity Induced by 12-O-tetradecanoylphorbol-13-acetate by Suppressing c-Jun N-terminal Kinase. Nutrients 2021, 13, 3079. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, X.; Sun, B.; Jiang, W.; Liao, C.; Dai, X.; Chen, Y.; Chen, J.; Ding, R. Pretreatment with kaempferol attenuates microglia-mediate neuroinflammation by inhibiting MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. Free. Radic. Biol. Med. 2021, 168, 142–154. [Google Scholar] [CrossRef]
- Dong, S.; Liang, S.; Cheng, Z.; Zhang, X.; Luo, L.; Li, L.; Zhang, W.; Li, S.; Xu, Q.; Zhong, M.; et al. ROS/PI3K/Akt and Wnt/β-catenin signalings activate HIF-1α-induced metabolic re-programming to impart 5-fluorouracil resistance in colorectal cancer. J. Exp. Clin. Cancer Res. 2022, 41, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhou, Q.; Huang, D.; He, L.; Zhang, H.; Hu, B.; Peng, H.; Ren, D. ROS/JNK/c-Jun axis is involved in oridonin-induced caspase-dependent apoptosis in human colorectal cancer cells. Biochem. Biophys. Res. Commun. 2019, 513, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Riahi-Chebbi, I.; Souid, S.; Othman, H.; Haoues, M.; Karoui, H.; Morel, A.; Srairi-Abid, N.; Essafi, M.; Essafi-Benkhadir, K. The Phenolic compound Kaempferol overcomes 5-fluorouracil resistance in human resistant LS174 colon cancer cells. Sci. Rep. 2019, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, G.-E.; An, H.-J.; Lee, C.-J.; Cho, E.S.; Kang, H.C.; Lee, J.Y.; Lee, H.S.; Choi, J.-S.; Kim, D.J.; et al. Kaempferol sensitizes cell proliferation inhibition in oxaliplatin-resistant colon cancer cells. Arch. Pharmacal Res. 2021, 44, 1091–1108. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, S.; Wu, X.; Zhang, J.; Chen, R.; Chen, M.; Wang, Y. Chinese herbal medicine-derived compounds for cancer therapy: A focus on hepatocellular carcinoma. J. Ethnopharmacol. 2013, 149, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.-N.; Li, X.-J. Resveratrol extracted from Chinese herbal medicines: A novel therapeutic strategy for lung diseases. Chin. Herb. Med. 2020, 12, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, A.; Hevia, D.; Mayo, J.C.; Gonzalez-Menendez, P.; Coppo, L.; Lu, J.; Holmgren, A.; Sainz, R.M. Thioredoxin 1 modulates apoptosis induced by bioactive compounds in prostate cancer cells. Redox Biol. 2017, 12, 634–647. [Google Scholar] [CrossRef]
- Dolinsky, V.W.; Chan, A.Y.; Frayne, I.R.; Light, P.E.; Rosiers, C.D.; Dyck, J.R. Resveratrol Prevents the Prohypertrophic Effects of Oxidative Stress on LKB1. Circulation 2009, 119, 1643–1652. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, W.; Wang, Z.; Wang, Z.; Jin, X.; Xu, J.; Bai, L.; Li, Y.; Cui, J.; Cai, L. Resveratrol attenuates testicular apoptosis in type 1 diabetic mice: Role of Akt-mediated Nrf2 activation and p62-dependent Keap1 degradation. Redox Biol. 2018, 14, 609–617. [Google Scholar] [CrossRef]
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An overview of its anti-cancer mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Feng, Z.; Bergan, R.; Li, B.; Qin, Y.; Zhao, L.; Zhang, Z.; Shi, M. Involvement of the PI3K/Akt/Nrf2 Signaling Pathway in Resveratrol-Mediated Reversal of Drug Resistance in HL-60/ADR Cells. Nutr. Cancer 2019, 71, 1007–1018. [Google Scholar] [CrossRef]
- Singh, B.; Shoulson, R.; Chatterjee, A.; Ronghe, A.; Bhat, N.K.; Dim, D.C.; Bhat, H.K. Resveratrol inhibits estrogen-induced breast carcinogenesis through induction of NRF2-mediated protective pathways. Carcinogenesis 2014, 35, 1872–1880. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, Y.; Huang, J.; Deng, H.; Tang, X.; Wang, X.J. Mkp-1 is required for chemopreventive activity of butylated hydroxyanisole and resveratrol against colitis-associated colon tumorigenesis. Food Chem. Toxicol. 2019, 127, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, Q.-M.; Lu, Y.-Y.; Zhang, H.; Chen, Q.-L.; Zhao, M.; Su, S.-B. Resveratrol Inhibits the Migration and Metastasis of MDA-MB-231 Human Breast Cancer by Reversing TGF-β1-Induced Epithelial-Mesenchymal Transition. Molecules 2019, 24, 1131. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yan, B.; Chen, K.; Jiang, Z.; Zhou, C.; Cao, J.; Qian, W.; Li, J.; Sun, L.; Ma, J.; et al. Resveratrol-Induced Downregulation of NAF-1 Enhances the Sensitivity of Pancreatic Cancer Cells to Gemcitabine via the ROS/Nrf2 Signaling Pathways. Oxidative Med. Cell. Longev. 2018, 2018, 9482018. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Nie, Q.; Tai, H.-C.; Song, X.-L.; Tong, Y.-F.; Zhang, L.-J.; Wu, X.-W.; Lin, Z.-H.; Zhang, Y.-Y.; Ye, D.-Y.; et al. Tea and tea drinking: China’s outstanding contributions to the mankind. Chin. Med. 2022, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.W.; Li, Y.; Chen, X.; Chu, K.O.; Zhao, Y.; Liu, Y.; Guo, X.; Man, G.C.; Wang, C.C. Green Tea Epigallocatechin-3-Gallate Regulates Autophagy in Male and Female Re-productive Cancer. Front Pharmacol. 2022, 13, 906746. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Antoniewicz, J.; Mruk, H.; Janda, K. Health Benefits and Chemical Composition of Matcha Green Tea: A Review. Molecules 2020, 26, 85. [Google Scholar] [CrossRef]
- Dai, Y.; Yang, R.; Yan, Y.; Wu, Y.; Meng, X.; Yang, A.; Wu, Z.; Shi, L.; Li, X.; Chen, H. Digestive stability and transport ability changes of β-lactoglobulin–catechin complexes by M cell model in vitro. Front. Nutr. 2022, 9, 955135. [Google Scholar] [CrossRef]
- Lou, J.; Wang, W.; Zhu, L. Occurrence, Formation, and Oxidative Stress of Emerging Disinfection Byproducts, Halobenzoquinones, in Tea. Environ. Sci. Technol. 2019, 53, 11860–11868. [Google Scholar] [CrossRef]
- Henning, S.M.; Wang, P.; Said, J.; Magyar, C.; Castor, B.; Doan, N.; Tosity, C.; Moro, A.; Gao, K.; Li, L.; et al. Polyphenols in brewed green tea inhibit prostate tumor xenograft growth by local-izing to the tumor and decreasing oxidative stress and angiogenesis. J. Nutr. Biochem. 2012, 23, 1537–1542. [Google Scholar] [CrossRef]
- Wubetu, G.Y.; Shimada, M.; Morine, Y.; Ikemoto, T.; Ishikawa, D.; Iwahashi, S.; Yamada, S.; Saito, Y.; Arakawa, Y.; Imura, S. Epigallocatechin gallate hinders human hepatoma and colon cancer sphere formation. J. Gastroenterol. Hepatol. 2016, 31, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Hayakawa, S.; Miyoshi, N. Involvement of microRNA modifications in anticancer effects of major polyphenols from green tea, coffee, wine, and curry. Crit. Rev. Food Sci. Nutr. 2022, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ali, S.; Ashraf, G.M.; Bilgrami, A.L.; Yadav, D.K.; Hassan, I. Epigallocatechin 3-gallate: From green tea to cancer therapeutics. Food Chem. 2022, 379, 132135. [Google Scholar] [CrossRef]
- Chiou, Y.S.; Sang, S.; Cheng, K.H.; Ho, C.T.; Wang, Y.J.; Pan, M.H. Peracetylated (−)-epigallocatechin-3-gallate (AcEGCG) po-tently prevents skin carcinogenesis by suppressing the PKD1-dependent signaling pathway in CD34+ skin stem cells and skin tumors. Carcinogenesis 2013, 34, 1315–1322. [Google Scholar] [CrossRef]
- Zhou, F.; Shen, T.; Duan, T.; Xu, Y.Y.; Khor, S.C.; Li, J.; Ge, J.; Zheng, Y.F.; Hsu, S.; DE Stefano, J.; et al. Antioxidant effects of lipophilic tea polyphenols on diethylnitrosa-mine/phenobarbital-induced hepatocarcinogenesis in rats. In Vivo 2014, 28, 495–503. [Google Scholar] [PubMed]
- Yuan, J.-H.; Li, Y.-Q.; Yang, X.-Y. Inhibition of Epigallocatechin Gallate on Orthotopic Colon Cancer by Upregulating the Nrf2-UGT1A Signal Pathway in Nude Mice. Pharmacology 2007, 80, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Tong, Y.; Gowd, V.; Wang, M.; Chen, F.; Cheng, K.-W. Oral administration of EGCG solution equivalent to daily achievable dosages of regular tea drinkers effectively suppresses miR483-3p induced metastasis of hepatocellular carcinoma cells in mice. Food Funct. 2021, 12, 3381–3392. [Google Scholar] [CrossRef]
- Datta, S.; Sinha, D. EGCG maintained Nrf2-mediated redox homeostasis and minimized etoposide resistance in lung cancer cells. J. Funct. Foods 2019, 62, 103553. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Y.; Wang, Y.; Rao, J.; Jiang, X.; Xu, Z. Curcumin inhibits proliferation of breast cancer cells through Nrf2-mediated down-regulation of Fen1 expression. J. Steroid Biochem. Mol. Biol. 2014, 143, 11–18. [Google Scholar] [CrossRef]
- Rajagopal, C.; Lankadasari, M.B.; Aranjani, J.M.; Harikumar, K. Targeting oncogenic transcription factors by polyphenols: A novel approach for cancer therapy. Pharmacol. Res. 2018, 130, 273–291. [Google Scholar] [CrossRef]
- Yao, J.; Zhao, L.; Zhao, Q.; Zhao, Y.; Sun, Y.; Zhang, Y.; Miao, H.; You, Q.D.; Hu, R.; Guo, Q.L. NF-κB and Nrf2 signaling pathways contribute to wogonin-mediated inhibition of in-flammation-associated colorectal carcinogenesis. Cell Death Dis. 2014, 5, e1283. [Google Scholar] [CrossRef] [PubMed]
- Sassi, N.; Mattarei, A.; Espina, V.; Liotta, L.; Zoratti, M.; Paradisi, C.; Biasutto, L. Potential anti-cancer activity of 7-O-pentyl quercetin: Efficient, membrane-targeted kinase inhibition and pro-oxidant effect. Pharmacol. Res. 2017, 124, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.W.; Hsu, S.C.; Chueh, F.S.; Chen, Y.Y.; Yang, J.S.; Lin, J.P.; Lien, J.C.; Tsai, C.H.; Chung, J.G. Quercetin inhibits migration and invasion of SAS human oral cancer cells through inhibition of NF-κB and matrix metalloproteinase-2/-9 signaling pathways. Anticancer. Res. 2013, 33, 1941–1950. [Google Scholar] [PubMed]
- Pintha, K.; Chaiwangyen, W.; Yodkeeree, S.; Suttajit, M.; Tantipaiboonwong, P. Suppressive Effects of Rosmarinic Acid Rich Fraction from Perilla on Oxidative Stress, Inflammation and Metastasis Ability in A549 Cells Exposed to PM via C-Jun, P-65-Nf-Κb and Akt Signaling Pathways. Biomolecules 2021, 11, 1090. [Google Scholar] [CrossRef]
- Sekar, V.; Anandasadagopan, S.K.; Ganapasam, S. Genistein regulates tumor microenvironment and exhibits anticancer effect in dimethyl hydrazine-induced experimental colon carcinogenesis. BioFactors 2016, 42, 623–637. [Google Scholar] [CrossRef]
- Xi, X.; Wang, J.; Qin, Y.; You, Y.; Huang, W.; Zhan, J. The Biphasic Effect of Flavonoids on Oxidative Stress and Cell Prolif-eration in Breast Cancer Cells. Antioxidants 2022, 11, 622. [Google Scholar] [CrossRef]
- Souza, K.S.; Moreira, L.S.; Silva, B.T.; Oliveira, B.P.; Carvalho, A.S.; Silva, P.S.; Verri, W.A.; Sá-Nakanishi, A.B.; Bracht, L.; Zanoni, J.N.; et al. Low dose of quercetin-loaded pectin/casein microparticles reduces the oxidative stress in arthritic rats. Life Sci. 2021, 284, 119910. [Google Scholar] [CrossRef]
- Lee, H.S.; Santana, Á.L.; Peterson, J.; Yucel, U.; Perumal, R.; De Leon, J.; Lee, S.H.; Smolensky, D. Anti-Adipogenic Activity of High-Phenolic Sorghum Brans in Pre-Adipocytes. Nutrients 2022, 14, 1493. [Google Scholar] [CrossRef]
- Han, Y.; Dong, Z.; Wang, C.; Li, Q.; Hao, Y.; Yang, Z.; Zhu, W.; Zhang, Y.; Liu, Z.; Feng, L. Ferrous ions doped calcium carbonate nanoparticles potentiate chemotherapy by in-ducing ferroptosis. J. Control. Release 2022, 348, 346–356. [Google Scholar] [CrossRef]
- Han, L.; Li, L.; Wu, G. Induction of ferroptosis by carnosic acid-mediated inactivation of Nrf2/HO-1 potentiates cisplatin responsiveness in OSCC cells. Mol. Cell. Probes 2022, 64, 101821. [Google Scholar] [CrossRef]
- Nile, A.; Nile, S.H.; Shin, J.; Park, G.; Oh, J.-W. Quercetin-3-Glucoside Extracted from Apple Pomace Induces Cell Cycle Arrest and Apoptosis by Increasing Intracellular ROS Levels. Int. J. Mol. Sci. 2021, 22, 10749. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Liu, L.; Liu, W.-W.; Li, F.; Hayashi, T.; Tashiro, S.-I.; Onodera, S.; Ikejima, T. Crosstalk of ROS/RNS and autophagy in silibinin-induced apoptosis of MCF-7 human breast cancer cells in vitro. Acta Pharmacol. Sin. 2017, 38, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.-F.; Gong, Y.-X.; Li, H.-F.; Sun, F.-L.; Li, W.-L.; Chen, D.-Q.; Xie, D.-P.; Ren, C.-X.; Guo, X.-Y.; Wang, Z.-Y.; et al. Curcumin Activates ROS Signaling to Promote Pyroptosis in Hepatocellular Carcinoma HepG2 Cells. In Vivo 2021, 35, 249–257. [Google Scholar] [CrossRef]
- Ghosh, S.; Dutta, N.; Banerjee, P.; Gajbhiye, R.L.; Sareng, H.R.; Kapse, P.; Pal, S.; Burdelya, L.; Mandal, N.C.; Ravichandiran, V.; et al. Induction of monoamine oxidase A-mediated oxidative stress and impairment of NRF2-antioxidant defence response by polyphenol-rich fraction of Bergenia ligulata sensitizes prostate cancer cells in vitro and in vivo. Free. Radic. Biol. Med. 2021, 172, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.V.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE Pathway by Dietary Flavonoids: A Friend or Foe for Cancer Management? Antioxidants 2020, 9, 973. [Google Scholar]
- Wang, C.; Song, X.; Shang, M.; Zou, W.; Zhang, M.; Wei, H.; Shao, H. Curcumin exerts cytotoxicity dependent on reactive oxygen species accumulation in non-small-cell lung cancer cells. Futur. Oncol. 2019, 15, 1243–1253. [Google Scholar] [CrossRef]
- Cao, X.; Li, Y.; Wang, Y.; Yu, T.; Zhu, C.; Zhang, X.; Guan, J. Curcumin suppresses tumorigenesis by ferroptosis in breast cancer. PLoS ONE 2022, 17, e0261370. [Google Scholar] [CrossRef]
- Tang, X.; Ding, H.; Liang, M.; Chen, X.; Yan, Y.; Wan, N.; Chen, Q.; Zhang, J.; Cao, J. Curcumin induces ferroptosis in non-small-cell lung cancer via activating autophagy. Thorac. Cancer 2021, 12, 1219–1230. [Google Scholar] [CrossRef]
- Kalinina, E.V.; Hasan, A.A.S.; Tatarskiy, V.V.; Volodina, Y.L.; Petrova, A.S.; Novichkova, M.D.; Zhdanov, D.D.; Nurmuradov, N.K.; Chernov, N.N.; Shtil, A.A. Suppression of PI3K/Akt/mTOR Signaling Pathway and Antioxidant System and Reversal of Cancer Cells Resistance to Cisplatin under the Effect of Curcumin. Bull. Exp. Biol. Med. 2022, 173, 371–375. [Google Scholar] [CrossRef]
- Obaidi, I.; Cassidy, H.; Gaspar, V.I.; McCaul, J.; Higgins, M.; Halász, M.; Reynolds, A.L.; Kennedy, B.N.; McMorrow, T. Curcumin Sensitizes Kidney Cancer Cells to TRAIL-Induced Apoptosis via ROS Mediated Activation of JNK-CHOP Pathway and Upregulation of DR4. Biology 2020, 9, 92. [Google Scholar] [CrossRef]
- Feng, C.; Xia, Y.; Zou, P.; Shen, M.; Hu, J.; Ying, S.; Pan, J.; Liu, Z.; Dai, X.; Zhuge, W.; et al. Curcumin analog L48H37 induces apoptosis through ROS-mediated endoplasmic reticulum stress and STAT3 pathways in human lung cancer cells. Mol. Carcinog. 2017, 56, 1765–1777. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cui, C. Inhibition of Lung Cancer Proliferation by Wogonin is Associated with the Activation of Apoptosis and Generation of Reactive Oxygen Species. Balk. Med. J. 2019, 37, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Azami, N.; Hamzehlou, S.; Farahani, M.V.; Hushmandi, K.; Ashrafizadeh, M.; et al. Nrf2 Signaling Pathway in Chemoprotection and Doxorubicin Resistance: Po-tential Application in Drug Discovery. Antioxidants 2021, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhang, F.; Sun, Z.; Zhou, W.; Li, Z.Y.; You, Q.D.; Guo, Q.L.; Hu, R. Drug resistance associates with activation of Nrf2 in MCF-7/DOX cells, and wogonin reverses it by down-regulating Nrf2-mediated cellular defense response. Mol. Carcinog. 2013, 52, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Wang, Y.; Zhong, Y.; Tang, J.; Zhang, J.; Li, Z.; Wang, Q.; Hu, R. Wogonin-enhanced reactive oxygen species-induced apoptosis and potentiated cyto-toxic effects of chemotherapeutic agents by suppression Nrf2-mediated signaling in HepG2 cells. Free. Radic. Res. 2014, 48, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Y.; Li, W.; Miao, H.; Zhang, H.; Zhou, Y.; Li, Z.; You, Q.; Zhao, L.; Guo, Q. Wogonin reverses multi-drug resistance of human myelogenous leukemia K562/A02 cells via downregulation of MRP1 expression by inhibiting Nrf2/ARE signaling pathway. Biochem. Pharmacol. 2014, 92, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Jang, H.; Shin, D.; Baek, S.H.; Roh, J.-L. Targeting Nrf2 with wogonin overcomes cisplatin resistance in head and neck cancer. Apoptosis 2016, 21, 1265–1278. [Google Scholar] [CrossRef]
- Chen, X.; Li, W.; Xu, C.; Wang, J.; Zhu, B.; Huang, Q.; Chen, D.; Sheng, J.; Zou, Y.; Lee, Y.M.; et al. Comparative profiling of analog targets: A case study on resveratrol for mouse melanoma metastasis suppression. Theranostics 2018, 8, 3504–3516. [Google Scholar] [CrossRef]
- Sinha, D.; Sarkar, N.; Biswas, J.; Bishayee, A. Resveratrol for breast cancer prevention and therapy: Preclinical evidence and molecular mechanisms. Semin. Cancer Biol. 2016, 40–41, 209–232. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Rout, L.; Jena, M.; Efferth, T.; Bhutia, S.K. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: Chemo-prevention, chemoprotection, drug synergism and clinical pharmacokinetics. Semin Cancer Biol. 2021, 73, 310–320. [Google Scholar] [CrossRef]
- Wang, X.X.; Li, Y.B.; Yao, H.J.; Ju, R.J.; Zhang, Y.; Li, R.J.; Yu, Y.; Zhang, L.; Lu, W.L. The use of mitochondrial targeting resveratrol liposomes modified with a dequalinium polyethylene glycol-distearoylphosphatidyl ethanolamine conjugate to induce apoptosis in resistant lung cancer cells. Biomaterials 2011, 32, 5673–5687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, F.; Li, P.; Gu, J.; Han, J.; Ni, Z.; Liu, F. Resveratrol inhibits HeLa cell proliferation by regulating mitochondrial function. Ecotoxicol. Environ. Saf. 2022, 241, 113788. [Google Scholar] [CrossRef] [PubMed]
- da Costa, P.S.; Ramos, P.S.; Ferreira, C.; Silva, J.L.; El-Bacha, T.; Fialho, E. Pro-Oxidant Effect of Resveratrol on Human Breast Cancer MCF-7 Cells is Associated with CK2 Inhibition. Nutr. Cancer 2022, 74, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Zheng, X.; Wu, M.L.; Tian, X.T.; Song, X.; Liu, Y.N.; Li, P.N.; Liu, J. Increased Reactive Oxygen Species and Distinct Oxidative Damage in Resvera-trol-suppressed Glioblastoma Cells. J. Cancer 2021, 12, 141–149. [Google Scholar] [CrossRef]
- Fouzder, C.; Mukhuty, A.; Kundu, R. Kaempferol inhibits Nrf2 signalling pathway via downregulation of Nrf2 mRNA and induces apoptosis in NSCLC cells. Arch. Biochem. Biophys. 2021, 697, 108700. [Google Scholar] [CrossRef]
- Deguchi, Y.; Ito, M. Rosmarinic acid in Perilla frutescens and perilla herb analyzed by HPLC. J. Nat. Med. 2020, 74, 341–352. [Google Scholar] [CrossRef]
- Chou, S.-T.; Ho, B.-Y.; Tai, Y.-T.; Huang, C.-J.; Chao, W.-W. Bidirect effects from cisplatin combine with rosmarinic acid (RA) or hot water extracts of Glechoma hederacea (HWG) on renal cancer cells. Chin. Med. 2020, 15, 77. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, J.; Yang, Y.; Wang, X.; Chen, G.; Shi, A.; Lu, Y.; Jia, S.; Kang, X.; Lu, L. Rosmarinic acid exerts an anticancer effect on osteosarcoma cells by inhibiting DJ-1 via regulation of the PTEN-PI3K-Akt signaling pathway. Phytomedicine 2020, 68, 153186. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, Z.; Xu, X.; Qi, H.; Cheng, Z.; Chen, L. Anticancer effects of rosmarinic acid in human oral cancer cells is mediated via endoplasmic reticulum stress, apoptosis, G2/M cell cycle arrest and inhibition of cell migration. J. Balk. Union Oncol. 2020, 25, 1245–1250. [Google Scholar]
- Majumder, D.; Das, A.; Saha, C. Catalase inhibition an anti cancer property of flavonoids: A kinetic and structural evaluation. Int. J. Biol. Macromol. 2017, 104, 929–935. [Google Scholar] [CrossRef]
- Ma, Y.; Yao, C.; Liu, H.; Yu, F.; Lin, J.; Lu, K.; Liao, C.; Chueh, F.; Chung, J. Quercetin induced apoptosis of human oral cancer SAS cells through mitochondria and endoplasmic reticulum mediated signaling pathways. Oncol. Lett. 2018, 15, 9663–9672. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.B.; Rajendiran, V.; Kasinathan, N.K.; P, A.; Venkatabalasubramanian, S.; Murali, M.R.; Devaraj, H.; Devaraj, S.N. Differential cytotoxic activity of Quercetin on colonic cancer cells depends on ROS generation through COX-2 expression. Food Chem. Toxicol. 2017, 106, 92–106. [Google Scholar] [CrossRef]
- Wu, Q.; Needs, P.W.; Lu, Y.; Kroon, P.A.; Ren, D.; Yang, X. Different antitumor effects of quercetin, quercetin-3’-sulfate and quercetin-3-glucuronide in human breast cancer MCF-7 cells. Food Funct. 2018, 9, 1736–1746. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; Li, X.; Wu, Y.; Shi, H.; Chen, Y.; Lu, G.; Shen, H.; Lu, G.; Zhou, J. Quercetin induces p53-independent cancer cell death through lysosome activation by the transcription factor EB and Reactive Oxygen Species-dependent ferroptosis. Br. J. Pharmacol. 2021, 178, 1133–1148. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.; Valente, D.; Moreira, H.R.; Pintado, M.; Costa, P. Effect of squalane-based emulsion on polyphenols skin penetration: Ex vivo skin study. Colloids Surfaces B Biointerfaces 2022, 218, 112779. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Zhu, K.; Liu, Z.; Huang, J. Prooxidant Effects of Epigallocatechin-3-Gallate in Health Benefits and Potential Adverse Effect. Oxidative Med. Cell. Longev. 2020, 2020, 9723686. [Google Scholar] [CrossRef]
- Costa, C.; Tsatsakis, A.; Mamoulakis, C.; Teodoro, M.; Briguglio, G.; Caruso, E.; Tsoukalas, D.; Margina, D.; Dardiotis, E.; Kouretas, D.; et al. Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem. Toxicol. 2017, 110, 286–299. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of poly-phenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Saunders, N.A.; Simpson, F.; Thompson, E.W.; Hill, M.M.; Endo-Munoz, L.; Leggatt, G.; Minchin, R.; Guminski, A. Role of intratumoural heterogeneity in cancer drug resistance: Molecular and clinical perspectives. EMBO Mol. Med. 2012, 4, 675–684. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Lipophilized Epigallocatechin Gallate (EGCG) Derivatives as Novel Antioxidants. J. Agric. Food Chem. 2011, 59, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, L.; Li, C.; Chen, R.; Liu, C.; Chen, M. Lipophilized Epigallocatechin Gallate Derivative Exerts Anti-Proliferation Efficacy through Induction of Cell Cycle Arrest and Apoptosis on DU145 Human Prostate Cancer Cells. Nutrients 2019, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Lambert, J.D. The catechol-O-methyltransferase inhibitor, tolcapone, increases the bioavailability of un-methylated (−)-epigallocatechin-3-gallate in mice. J. Funct Foods 2015, 17, 183–188. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Ho, C.T.; Huang, Q. Chemistry and Health Effect of Tea Polyphenol (-)-Epigallocatechin 3- O-(3- O-Methyl)gallate. J. Agric. Food Chem. 2019, 67, 5374–5378. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nguyen, T.T.H.; Kim, N.M.; Moon, Y.-H.; Ha, J.-M.; Park, N.; Lee, D.-G.; Hwang, K.-H.; Park, J.-S.; Kim, D. Functional Properties of Novel Epigallocatechin Gallate Glucosides Synthesized by Using Dextransucrase from Leuconostoc mesenteroides B-1299CB4. J. Agric. Food Chem. 2016, 64, 9203–9213. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Líter, J.A.; Pozo-Rodríguez, A.; Madruga, E.; Rubert, M.; Santana, A.G.; de Eugenio, L.I.; Sánchez, C.; Martínez, A.; Prieto, A.; Martínez, M.J. Glycosylation of Epigallocatechin Gallate by Engineered Glyco-side Hydrolases from Talaromyces amestolkiae: Potential Antiproliferative and Neuroprotective Effect of These Molecules. Antioxidants 2022, 11, 1325. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Alfonso, J.L.; Leemans, L.; Poveda, A.; Jimenez-Barbero, J.; Ballesteros, A.O.; Plou, F.J. Efficient α-Glucosylation of Epigallocatechin Gallate Catalyzed by Cyclodextrin Glucanotransferase from Thermoanaerobacter Species. J. Agric. Food Chem. 2018, 66, 7402–7408. [Google Scholar] [CrossRef]
- Boateng, I.D. Recent processing of fruits and vegetables using emerging thermal and non-thermal technologies. A critical review of their potentialities and limitations on bioactives, structure, and drying performance. Crit. Rev. 2022, 1–35. [Google Scholar] [CrossRef]
- Rambaran, T.F. A patent review of polyphenol nano-formulations and their commercialization. Trends Food Sci Tech. 2022, 120, 111–122. [Google Scholar] [CrossRef]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M.; et al. Available technologies on improving the stability of polyphenols in food processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- De Oliveira, T.V.; Stein, R.; de Andrade, D.F.; Beck, R.C.R. Preclinical studies of the antitumor effect of curcumin-loaded polymeric nanocapsules: A systematic review and meta-analysis. Phytother. Res. 2022, 36, 3202–3214. [Google Scholar] [CrossRef]
- Gugleva, V.; Ivanova, N.; Sotirova, Y.; Andonova, V. Dermal Drug Delivery of Phytochemicals with Phenolic Structure via Lipid-Based Nanotechnologies. Pharmaceuticals 2021, 14, 837. [Google Scholar] [CrossRef]
- Bohara, R.A.; Tabassum, N.; Singh, M.P.; Gigli, G.; Ragusa, A.; Leporatti, S. Recent Overview of Resveratrol’s Beneficial Effects and Its Nano-Delivery Systems. Molecules 2022, 27, 5154. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Tian, H.; Li, L.; Li, B.; Yang, M.; Zhou, L.; Jiang, H.; Li, Q.; Wang, W.; Nice, E.C.; et al. Nanoengineering a Zeolitic Imidazolate Framework-8 Capable of Manipulating Energy Me-tabolism against Cancer Chemo-Phototherapy Resistance. Small 2022, 18, e2204926. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Palazzolo, J.S.; Ju, Y.; Niego, B.; Pan, S.; Hagemeyer, C.E.; Caruso, F. Polyphenol-Functionalized Cubosomes as Thrombolytic Drug Carriers. Adv. Health Mater. 2022, 11, e2201151. [Google Scholar] [CrossRef] [PubMed]
- Hafez Ghoran, S.; Calcaterra, A.; Abbasi, M.; Taktaz, F.; Nieselt, K.; Babaei, E. Curcumin-Based Nanoformulations: A Promising Adjuvant towards Cancer Treatment. Molecules 2022, 27, 5236. [Google Scholar] [CrossRef] [PubMed]
- Soleti, R.; Andriantsitohaina, R.; Martinez, M.C. Impact of polyphenols on extracellular vesicle levels and effects and their properties as tools for drug delivery for nutrition and health. Arch. Biochem. Biophys. 2018, 644, 57–63. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, J.; Cui, X.; Hou, J.; Yu, F.; Wang, J.; Wang, X.; Chen, C.; Tong, L. Hyaluronic Acid Modified Nanostructured Lipid Carrier for Targeting Delivery of Kaempferol to NSCLC: Preparation, Optimization, Characterization, and Performance Evaluation In Vitro. Molecules 2022, 27, 4553. [Google Scholar] [CrossRef]
- Shrivastava, N.; Parikh, A.; Dewangan, R.P.; Biswas, L.; Verma, A.K.; Mittal, S.; Ali, J.; Garg, S.; Baboota, S. Solid Self-Nano Emulsifying Nanoplatform Loaded with Tamoxifen and Resveratrol for Treatment of Breast Cancer. Pharmaceutics 2022, 14, 1486. [Google Scholar] [CrossRef]
- Azadikhah, F.; Karimi, A.R.; Yousefi, G.H.; Hadizadeh, M. Dual antioxidant-photosensitizing hydrogel system: Cross-linking of chitosan with tannic acid for enhanced photodynamic efficacy. Int. J. Biol. Macromol. 2021, 188, 114–125. [Google Scholar] [CrossRef]
- Zu, M.; Xie, D.; Canup, B.S.; Chen, N.; Wang, Y.; Sun, R.; Zhang, Z.; Fu, Y.; Dai, F.; Xiao, B. ‘Green’ nanotherapeutics from tea leaves for orally targeted prevention and alleviation of colon diseases. Biomaterials 2021, 279, 121178. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, Q.; Liang, Y.; Zu, M.; Chen, N.; Canup, B.S.; Luo, L.; Wang, C.; Zeng, L.; Xiao, B. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm. Sin. B 2022, 12, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Mereles, D.; Hunstein, W. Epigallocatechin-3-gallate (EGCG) for clinical trials: More pitfalls than promises? Int. J. Mol. Sci. 2011, 12, 5592–5603. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Jiao, Y.; Xue, J.; Zhang, Q.; Yang, H.; Xing, L.; Chen, G.; Wu, J.; Zhang, S.; Zhu, W.; et al. Metformin Sensitizes Non-small Cell Lung Cancer Cells to an Epigallocatechin-3-Gallate (EGCG) Treatment by Suppressing the Nrf2/HO-1 Signaling Pathway. Int. J. Biol. Sci. 2017, 13, 1560–1569. [Google Scholar] [CrossRef]
- Du, D.; Tang, X.; Li, Y.; Gao, Y.; Chen, R.; Chen, Q.; Wen, J.; Wu, T.; Zhang, Y.; Lu, H.; et al. Senotherapy Protects against Cisplatin-Induced Ovarian Injury by Removing Senescent Cells and Alleviating DNA Damage. Oxidative Med. Cell. Longev. 2022, 2022, 9144644. [Google Scholar] [CrossRef]
- Schwingel, T.E.; Klein, C.P.; Nicoletti, N.F.; Dora, C.L.; Hädrich, G.; Bica, C.G.; Lopes, T.G.; Da Silva, V.D.; Morrone, F.B. Effects of the compounds resveratrol, rutin, quercetin, and quercetin nanoemulsion on oxaliplatin-induced hepatotoxicity and neurotoxicity in mice. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 2014, 387, 837–848. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, J.; Yu, C.; Xiang, L.; Li, L.; Shi, D.; Lin, F. Quercetin Enhanced Paclitaxel Therapeutic Effects Towards PC-3 Prostate Cancer Through ER Stress Induction and ROS Production. OncoTargets Ther. 2020, 13, 513–523. [Google Scholar] [CrossRef]
- Jalili-Nik, M.; Soltani, A.; Moussavi, S.; Ghayour-Mobarhan, M.; Ferns, G.A.; Hassanian, S.M.; Avan, A. Current status and future prospective of Curcumin as a potential therapeutic agent in the treatment of colorectal cancer. J. Cell. Physiol. 2017, 233, 6337–6345. [Google Scholar] [CrossRef]
- Zangui, M.; Atkin, S.L.; Majeed, M.; Sahebkar, A. Current evidence and future perspectives for curcumin and its analogues as promising adjuncts to oxaliplatin: State-of-the-art. Pharmacol. Res. 2019, 141, 343–356. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, Z.; Wang, X.; Sun, D.; Wang, D.; Li, Y.; Pei, B.; Ye, M.; Xu, J.; Yue, X. Curcumin Alleviates Oxaliplatin-Induced Peripheral Neuropathic Pain through In-hibiting Oxidative Stress-Mediated Activation of NF-κB and Mitigating Inflammation. Biol. Pharm. Bull. 2020, 43, 348–355. [Google Scholar] [CrossRef]
- Santandreu, F.M.; Valle, A.; Oliver, J.; Roca, P. Resveratrol potentiates the cytotoxic oxidative stress induced by chemo-therapy in human colon cancer cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2011, 28, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Le Gal, K.; Ibrahim, M.X.; Wiel, C.; Sayin, V.I.; Akula, M.K.; Karlsson, C.; Dalin, M.G.; Akyürek, L.M.; Lindahl, P.; Nilsson, J.; et al. Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med. 2015, 7, 308re308. [Google Scholar] [CrossRef] [PubMed]
- Sayin, V.I.; Ibrahim, M.X.; Larsson, E.; Nilsson, J.A.; Lindahl, P.; Bergo, M.O. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014, 6, 221ra215. [Google Scholar] [CrossRef] [PubMed]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef]
- Brunner, M.; Ziegler, S.; Di Stefano, A.F.D.; Dehghanyar, P.; Kletter, K.; Tschurlovits, M.; Villa, R.; Bozzella, R.; Celasco, G.; Moro, L.; et al. Gastrointestinal transit, release and plasma pharmacokinetics of a new oral budesonide formulation. Br. J. Clin. Pharmacol. 2006, 61, 31–38. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Jin, P.; Guan, Y.; Luo, M.; Wang, Y.; He, B.; Li, B.; He, K.; Cao, J.; Huang, C.; et al. Exploiting Polyphenol-Mediated Redox Reorientation in Cancer Therapy. Pharmaceuticals 2022, 15, 1540. https://doi.org/10.3390/ph15121540
Li L, Jin P, Guan Y, Luo M, Wang Y, He B, Li B, He K, Cao J, Huang C, et al. Exploiting Polyphenol-Mediated Redox Reorientation in Cancer Therapy. Pharmaceuticals. 2022; 15(12):1540. https://doi.org/10.3390/ph15121540
Chicago/Turabian StyleLi, Lei, Ping Jin, Yueyue Guan, Maochao Luo, Yu Wang, Bo He, Bowen Li, Kai He, Jiangjun Cao, Canhua Huang, and et al. 2022. "Exploiting Polyphenol-Mediated Redox Reorientation in Cancer Therapy" Pharmaceuticals 15, no. 12: 1540. https://doi.org/10.3390/ph15121540
APA StyleLi, L., Jin, P., Guan, Y., Luo, M., Wang, Y., He, B., Li, B., He, K., Cao, J., Huang, C., Li, J., & Shen, Z. (2022). Exploiting Polyphenol-Mediated Redox Reorientation in Cancer Therapy. Pharmaceuticals, 15(12), 1540. https://doi.org/10.3390/ph15121540







