In Vitro Investigation of the Anticancer Properties of Ammodaucus Leucotrichus Coss. & Dur.
Abstract
1. Introduction
2. Results and Discussion
2.1. Qualitative and Quantitative Characterization of Ammolactone-A and R-Perillaldehyde in GC-MS and HPLC-DAD
2.2. A. Leucotrichus Extract and Perillaldehyde Exhibit Cytotoxicity on Different Cancer Cell Lines
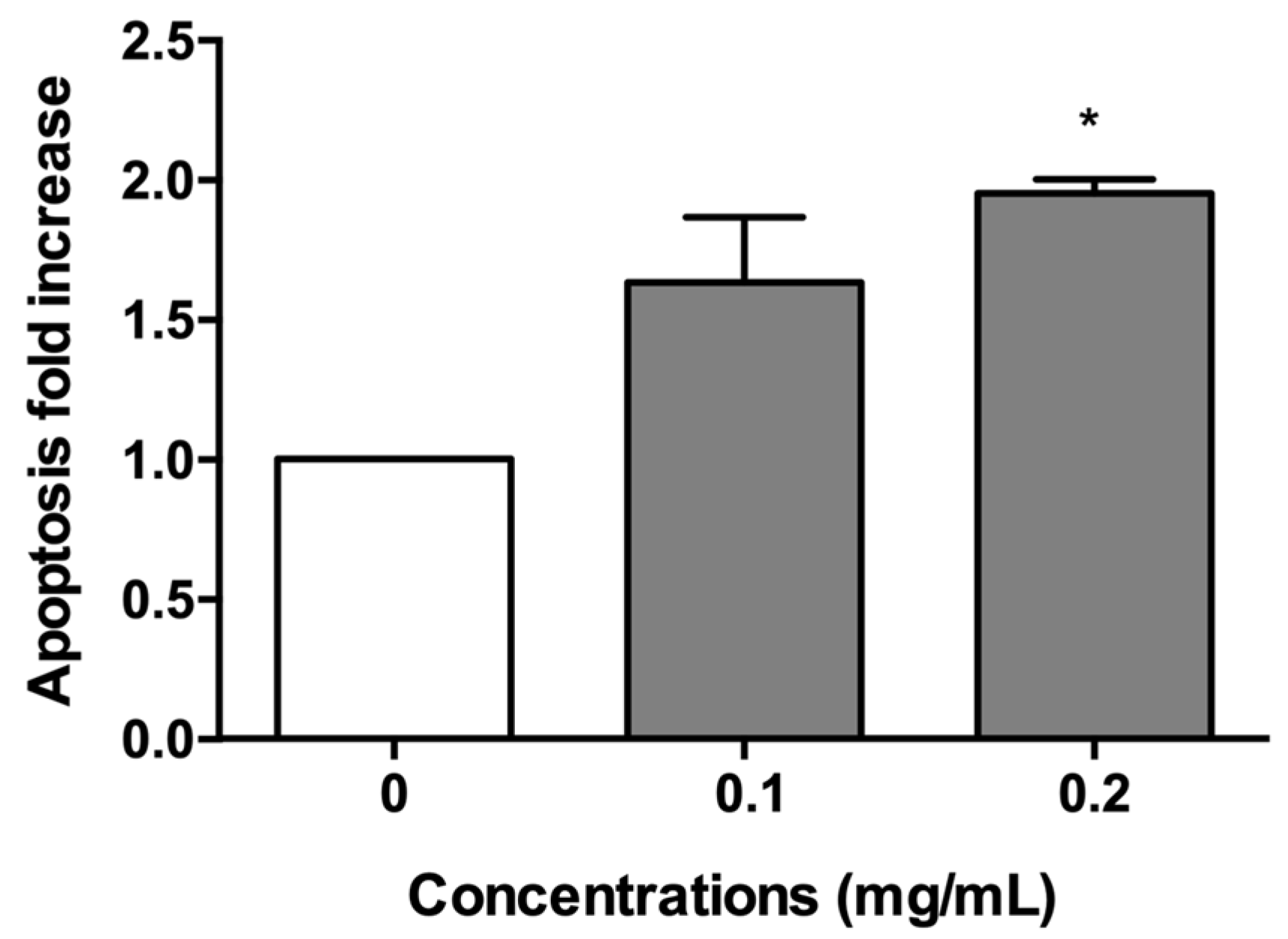
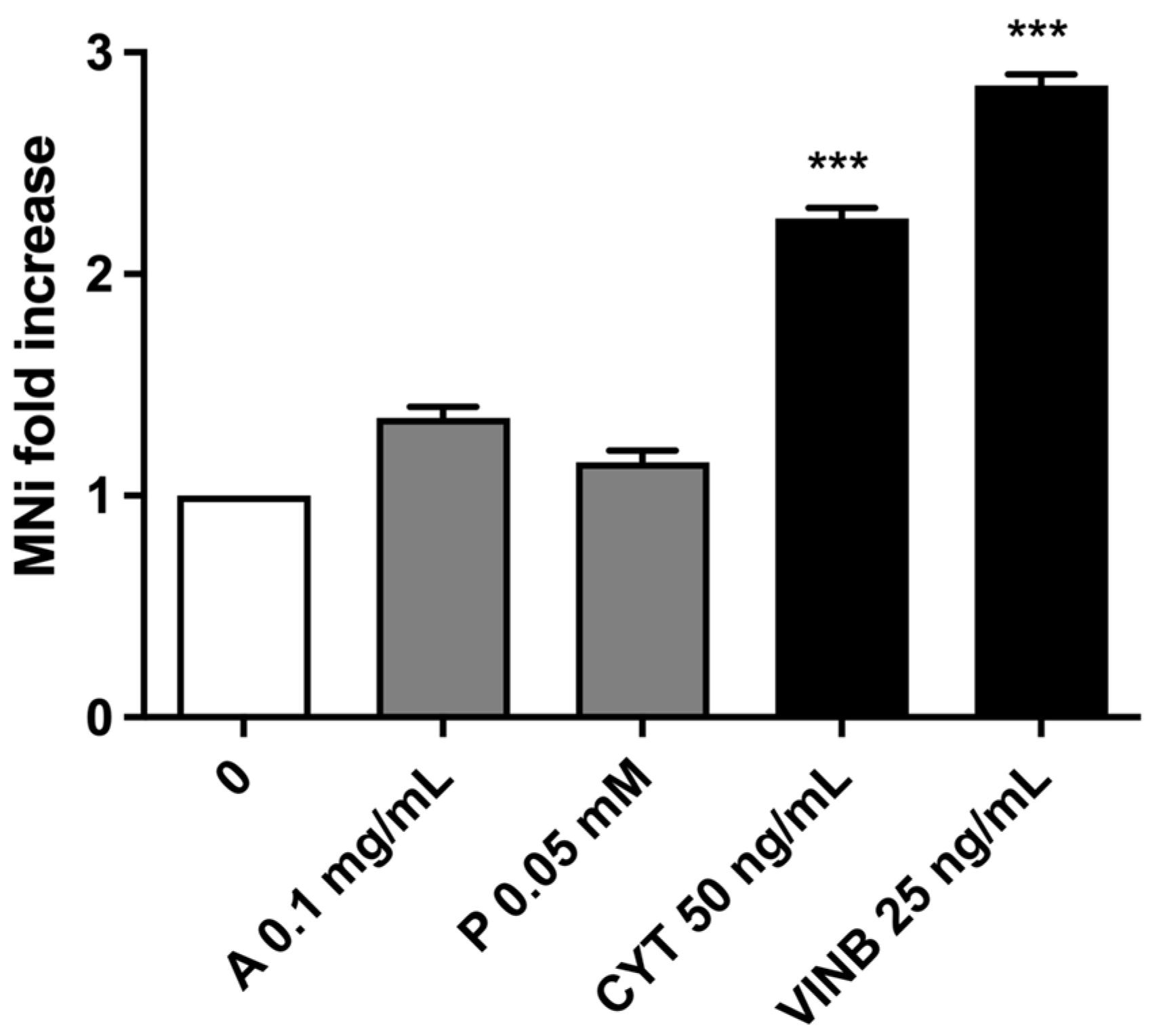
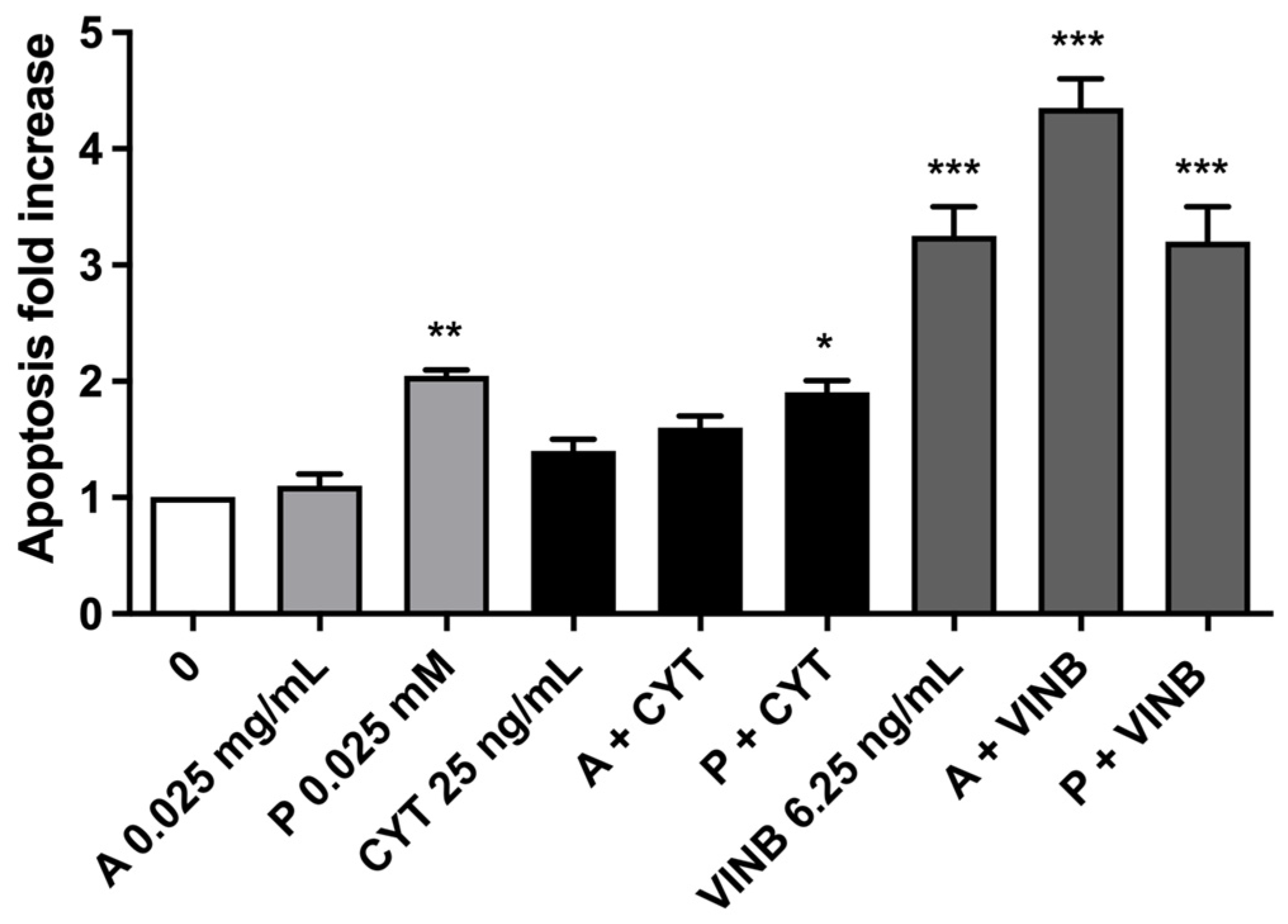
2.3. A. Leucotrichus Extract and Perillaldehyde Do Not Increase the Frequency of MNi and Induces Apoptosis on TK6 Cells
2.3.1. Short-Term Treatment
2.3.2. Long-Term Treatment
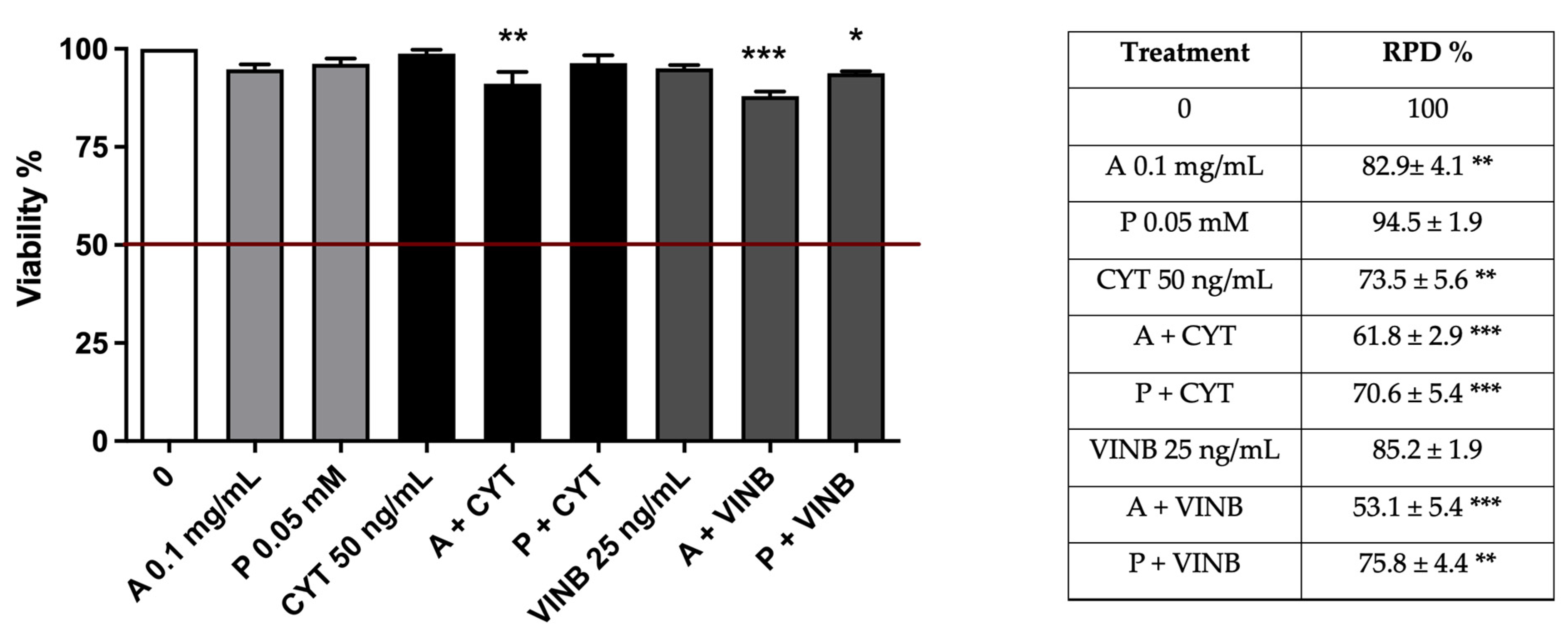
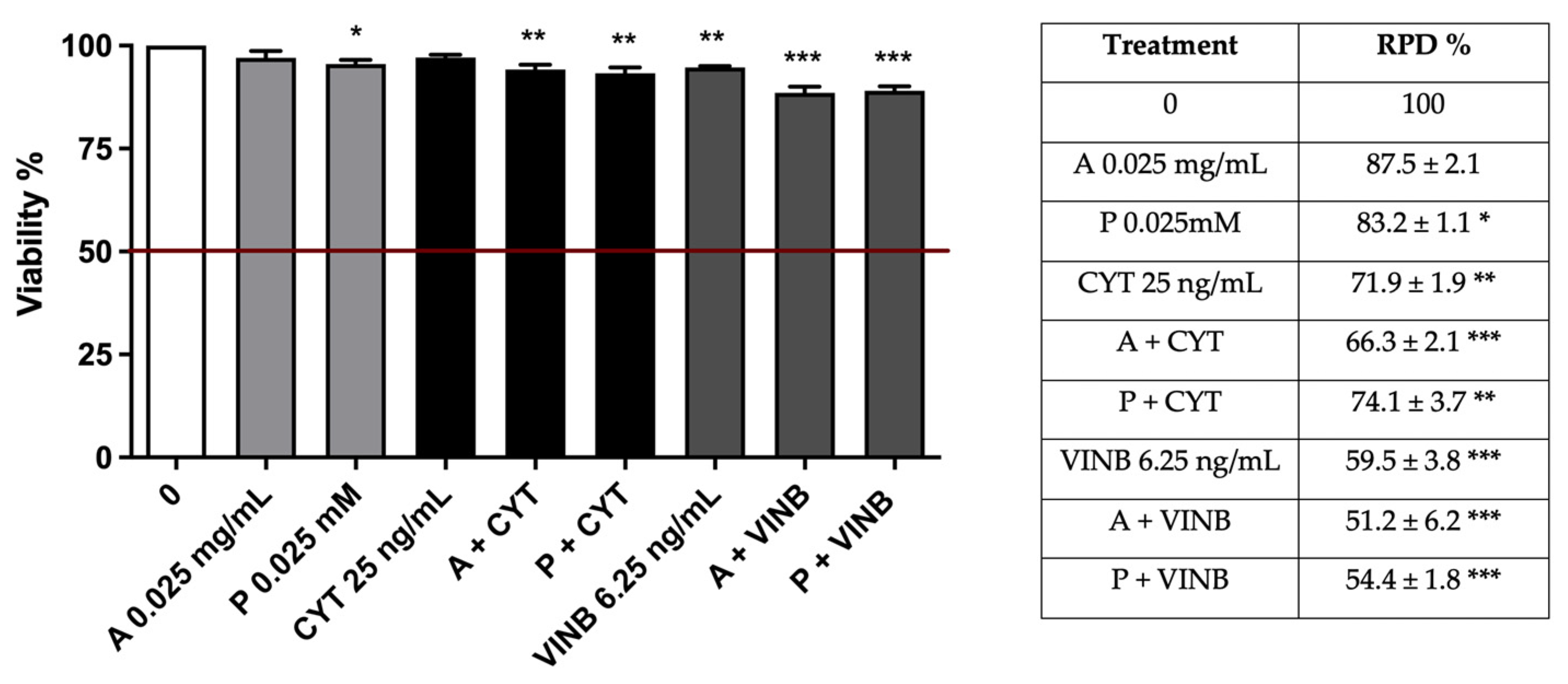
2.4. A. Leucotrichus Extract and Perillaldehyde Modulate the Mutagenicity of Different Compounds
2.4.1. Short-Term Treatment
2.4.2. Long-Term Treatment
2.5. A. Leucotrichus Extract and Perillaldehyde Do Not Affect the Differentiation of HL60 Cells
3. Materials and Methods
3.1. Reagents
3.2. Plant Material
3.3. Preparation of Ethanolic Extract and Chemical Characterization
3.4. Cell Cultures and Treatments
3.5. Flow Cytometry
3.6. Cytotoxicity Analysis
3.7. Cytostasis Analysis
3.8. Apoptosis Analysis
3.9. MNi Frequency Analysis
3.10. Analysis of Cell Differentiation
3.10.1. α-Naphthyl Acetate Esterase Activity
3.10.2. NBT Reduction Assay
3.10.3. Adherence to the Plastic Substrate
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greco, G.; Catanzaro, E.; Fimognari, C. Natural Products as Inducers of Non-Canonical Cell Death: A Weapon against Cancer. Cancers 2021, 13, 304. [Google Scholar] [CrossRef]
- Turrini, E.; Catanzaro, E.; Muraro, M.G.; Governa, V.; Trella, E.; Mele, V.; Calcabrini, C.; Morroni, F.; Sita, G.; Hrelia, P.; et al. Hemidesmus Indicus Induces Immunogenic Death in Human Colorectal Cancer Cells. Oncotarget 2018, 9, 24443–24456. [Google Scholar] [CrossRef]
- Turrini, E.; Calcabrini, C.; Tacchini, M.; Efferth, T.; Sacchetti, G.; Guerrini, A.; Paganetto, G.; Catanzaro, E.; Greco, G.; Fimognari, C. In Vitro Study of the Cytotoxic, Cytostatic, and Antigenotoxic Profile of Hemidesmus Indicus (L.) R.Br. (Apocynaceae) Crude Drug Extract on T Lymphoblastic Cells. Toxins 2018, 10, 70. [Google Scholar] [CrossRef]
- Fimognari, C.; Lenzi, M.; Ferruzzi, L.; Turrini, E.; Scartezzini, P.; Poli, F.; Gotti, R.; Guerrini, A.; Carulli, G.; Ottaviano, V.; et al. Mitochondrial Pathway Mediates the Antileukemic Effects of Hemidesmus Indicus, a Promising Botanical Drug. PLoS ONE 2011, 6, e21544. [Google Scholar] [CrossRef]
- Lenzi, M.; Malaguti, M.; Cocchi, V.; Hrelia, S.; Hrelia, P. Castanea Sativa Mill. Bark Extract Exhibits Chemopreventive Properties Triggering Extrinsic Apoptotic Pathway in Jurkat Cells. BMC Complement. Altern Med. 2017, 17, 251. [Google Scholar] [CrossRef]
- Lenzi, M.; Cocchi, V.; Malaguti, M.; Barbalace, M.C.; Marchionni, S.; Hrelia, S.; Hrelia, P. 6-(Methylsulfonyl) Hexyl Isothiocyanate as Potential Chemopreventive Agent: Molecular and Cellular Profile in Leukaemia Cell Lines. Oncotarget 2017, 8, 111697–111714. [Google Scholar] [CrossRef]
- Catanzaro, E.; Greco, G.; Potenza, L.; Calcabrini, C.; Fimognari, C. Natural Products to Fight Cancer: A Focus on Juglans Regia. Toxins 2018, 10, 469. [Google Scholar] [CrossRef]
- Fimognari, C.; Berti, F.; Cantelli-Forti, G.; Hrelia, P. Effect of Sulforaphane on Micronucleus Induction in Cultured Human Lymphocytes by Four Different Mutagens. Environ. Mol. Mutagen. 2005, 46, 260–267. [Google Scholar] [CrossRef]
- Turrini, E.; Maffei, F.; Milelli, A.; Calcabrini, C.; Fimognari, C. Overview of the Anticancer Profile of Avenanthramides from Oat. Int. J. Mol. Sci. 2019, 20, 4536. [Google Scholar] [CrossRef]
- Velasco-Negueruela, A.; Pérez-Alonso, M.J.; Pérez de Paz, P.L.; Palá-Paúl, J.; Sanz, J. Analysis by Gas Chromatography–Mass Spectrometry of the Volatiles from the Fruits of Ammodaucus Leucotrichus Subsp. Leucotrichus and Subsp. Nanocarpus Grown in North Africa and the Canary Islands, Respectively. J. Chromatogr. A 2006, 1108, 273–275. [Google Scholar] [CrossRef]
- Benhouhou, S. Ammodaucus Leucotrichus Coss. &Dur. In Guide to Medicinal Plants in North Africa; International Union for Conservation of Nature and Centre for Mediterranean Cooperation: Malaga, Spain, 2005; pp. 31–32. [Google Scholar]
- Sebaa, A.; Marouf, A.; Kambouche, N.; Derdour, A. Phytochemical Composition, Antioxidant and Antimicrobial Activities of Ammodaucus Leucotrichus Fruit from Algerian Sahara. Orient. J. Chem. 2018, 34, 519–525. [Google Scholar] [CrossRef]
- Merzouki, A.; Ed-derfoufi, F.; Molero Mesa, J. Contribution to the Knowledge of Rifian Traditional Medicine. II: Folk Medicine in Ksar Lakbir District (NW Morocco). Fitoterapia 2000, 71, 278–307. [Google Scholar] [CrossRef]
- Hammiche, V.; Maiza, K. Traditional Medicine in Central Sahara: Pharmacopoeia of Tassili N’ajjer. J. Ethnopharmacol. 2006, 105, 358–367. [Google Scholar] [CrossRef]
- El-Mansouri, L.; Ennabili, A.; Bousta, D. Socioeconomic Interest and Valorization of Medicinal Plants from the Rissani Oasis (SE of Morocco). Bol. Latinoamer Caribe Plantas Med. Arom 2011, 10, 30–45. [Google Scholar]
- Jouad, H.; Haloui, M.; Rhiouani, H.; El Hilaly, J.; Eddouks, M. Ethnobotanical Survey of Medicinal Plants Used for the Treatment of Diabetes, Cardiac and Renal Diseases in the North Centre Region of Morocco (Fez–Boulemane). J. Ethnopharmacol. 2001, 77, 175–182. [Google Scholar] [CrossRef]
- Hmamouchi, M. Plantes Alimentaires, Aromatiques, Condimentaires, Médicinales et Toxiques Au Maroc. In Identification of Wild Food and Non-Food Plants of the Mediterranean Region; Heywood, V.H., Skoula, M., Eds.; Cahiers Options Méditerranéennes; CIHEAM: Chania, Greece, 1997; Volume 23, pp. 89–108. [Google Scholar]
- Nassif, F.; Tanji, A. Gathered Food Plants in Morocco: The Long-Forgotten Species in Ethnobotanical Research. Life Sci. 2013, 3, 17–54. [Google Scholar]
- Beghalia, M.; Ghalem, S.; Allali, H.; Belouatek, A.; Marouf, A. Inhibition of Calcium Oxalate Monohydrate Crystal Growth Using Algerian Medicinal Plants. J. Med. Plant Res. 2008, 2, 066–070. [Google Scholar]
- El-Ouady, F.; Eddouks, M. Glucose Lowering Activity of Aqueous Ammodaucus Leucotrichus Extract in Diabetic Rats. Cardiovasc Hematol. Disord. Drug Targets 2020, 20, 152–159. [Google Scholar] [CrossRef]
- Bouknana, S.; Daoudi, N.E.; Bouhrim, M.; Ziyyat, A.; Legssyer, A.; Mekhfi, H.; Bnouham, M. Ammodaucus Leucotrichus Coss. & Durieu: Antihyperglycemic Activity via the Inhibition of α-Amylase, α-Glucosidase, and Intestinal Glucose Absorption Activities and Its Chemical Composition. J. Pharm. Pharmacogn. Res. 2022, 10, 94–103. [Google Scholar] [CrossRef]
- Gherraf, N.; Zellagui, A.; Kabouche, A.; Lahouel, M.; Salhi, R.; Rhouati, S. Chemical Constituents and Antimicrobial Activity of Essential Oils of Ammodaucus Leucotricus. Arab. J. Chem. 2017, 10, S2476–S2478. [Google Scholar] [CrossRef]
- Louail, Z.; Kameli, A.; Benabdelkader, T.; Bouti, K.; Hamza, K.; Krimat, S. Antimicrobial and Antioxidant Activity of Essential Oil of Ammodaucus Leucotrichus Coss. & Dur. Seeds. J. Mater. Environ. Sci. 2016, 7, 2328–2334. [Google Scholar]
- Selama, G.; Cadi, H.E.; Ramdan, B.; Majdoub, Y.O.E.; Dugo, P.; Mondello, L.; Cacciola, F.; Nhiri, M. Determination of the Polyphenolic Content of Ammodaucus Leucotrichus Cosson and Durieu by Liquid Chromatography Coupled with Mass Spectrometry and Evaluation of the Antioxidant and Antiglycation Properties. J. Sep. Sci. 2022, 45, 3301–3309. [Google Scholar] [CrossRef]
- Btissam, R.; Hafssa, E.C.; Mohamed, N. Increased Non-Enzymatic Glycation Reported in Apiaceae Family Extracts. Pak. J. Bot. 2022, 54, 1549–1556. [Google Scholar] [CrossRef]
- Dahmane, D.; Dob, T.; Krimat, S.; Nouasri, A.; Metidji, H.; Ksouri, A. Chemical Composition, Antioxidant and Antibacterial Activities of the Essential Oils of Medicinal Plant Ammodaucus Leucotrichus from Algeria. J. Essent Oil Res. 2017, 29, 48–55. [Google Scholar] [CrossRef]
- Khaldi, A.; Meddah, B.; Moussaoui, A.; Sonnet, P. Anti-Mycotoxin Effect and Antifungal Properties of Essential Oil from Ammodaucus Leucotrichus Coss. & Dur. on Aspergillus Flavus and Aspergillus Ochraceus. J. Essent Oil-Bear Plants 2017, 20, 36–44. [Google Scholar] [CrossRef]
- Abu Zarga, M.H.; Al-Jaber, H.I.; Baba Amer, Z.Y.; Sakhrib, L.; Al-Qudah, M.A.; Al-humaidi, J.Y.G.; Abaza, I.F.; Afifi, F.U. Chemical Composition, Antimicrobial and Antitumor Activities of Essential Oil of Ammodaucus Leucotrichus Growing in Algeria. J. Biol. Act Prod. Nat. 2013, 3, 224–231. [Google Scholar] [CrossRef]
- Abdi Bellau, M.L.; Chiurato, M.A.; Maietti, A.; Fantin, G.; Tedeschi, P.; Marchetti, N.; Tacchini, M.; Sacchetti, G.; Guerrini, A. Nutrients and Main Secondary Metabolites Characterizing Extracts and Essential Oil from Fruits of Ammodaucus Leucotrichus Coss. & Dur. (Western Sahara). Molecules 2022, 27, 5013. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, S.Y.; Chen, C. Increasing Antioxidant Activity and Reducing Decay of Blueberries by Essential Oils. J. Agric. Food Chem. 2008, 56, 3587–3592. [Google Scholar] [CrossRef]
- Tian, J.; Zeng, X.; Lü, A.; Zhu, A.; Peng, X.; Wang, Y. Perillaldehyde, a Potential Preservative Agent in Foods: Assessment of Antifungal Activity against Microbial Spoilage of Cherry Tomatoes. Food Sci. Technol. 2015, 60, 63–70. [Google Scholar] [CrossRef]
- Ito, N.; Nagai, T.; Oikawa, T.; Yamada, H.; Hanawa, T. Antidepressant-like Effect of l-Perillaldehyde in Stress-Induced Depression-like Model Mice through Regulation of the Olfactory Nervous System. Evid. Based Complement. Alternat. Med. 2011, 2011, 512697. [Google Scholar] [CrossRef]
- Catanzaro, E.; Turrini, E.; Kerre, T.; Sioen, S.; Baeyens, A.; Guerrini, A.; Bellau, M.L.A.; Sacchetti, G.; Paganetto, G.; Krysko, D.V.; et al. Perillaldehyde Is a New Ferroptosis Inducer with a Relevant Clinical Potential for Acute Myeloid Leukemia Therapy. Biomed. Pharmacother. 2022, 154, 113662. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, S.; LingHu, X.; Wang, Y.; Wang, B.; Zhong, S.; Xie, S.; Xu, X.; Yu, A.; Nagai, A.; et al. Perillaldehyde Inhibits Bone Metastasis and Receptor Activator of Nuclear Factor-ΚB Ligand (RANKL) Signaling-Induced Osteoclastogenesis in Prostate Cancer Cell Lines. Bioengineered 2022, 13, 2710–2719. [Google Scholar] [CrossRef]
- Karlson, J.; Borg-Karlson, A.-K.; Unelius, R.; Shoshan, M.C.; Wilking, N.; Ringborg, U.; Linder, S. Inhibition of Tumor Cell Growth by Monoterpenes in Vitro: Evidence of a Ras-Independent Mechanism of Action. Anticancer Drugs 1996, 7, 422–429. [Google Scholar] [CrossRef]
- Test No. 487: In Vitro Mammalian Cell Micronucleus Test. Available online: https://read.oecd-ilibrary.org/environment/test-no-487-in-vitro-mammalian-cell-micronucleus-test_9789264264861-en (accessed on 19 April 2021).
- Ahmed, N.; Williams, J.F.; Weidemann, M.J. The Human Promyelocytic HL60 Cell Line: A Model of Myeloid Cell Differentiation Using Dimethylsulphoxide, Phorbol Ester and Butyrate. Biochem. Int. 1991, 23, 591–602. [Google Scholar]
- Kajstura, M.; Halicka, H.D.; Pryjma, J.; Darzynkiewicz, Z. Discontinuous Fragmentation of Nuclear DNA during Apoptosis Revealed by Discrete “Sub-G1” Peaks on DNA Content Histograms. Cytometry 2007, 71A, 125–131. [Google Scholar] [CrossRef]
- Honma, M. An Assessment of Mutagenicity of Chemical Substances by (Quantitative) Structure–Activity Relationship. Genes Environ. 2020, 42, 23. [Google Scholar] [CrossRef]
- Honma, M.; Kitazawa, A.; Kasamatsu, T.; Sugiyama, K. Screening for Ames Mutagenicity of Food Flavor Chemicals by (Quantitative) Structure-Activity Relationship. Genes Environ. 2020, 42, 32. [Google Scholar] [CrossRef]
- Witz, G. Biological Interactions of α,β-Unsaturated Aldehydes. Free Radic. Biol. Med. 1989, 7, 333–349. [Google Scholar] [CrossRef]
- Honma, M.; Yamada, M.; Yasui, M.; Horibata, K.; Sugiyama, K.; Masumura, K. In Vivo and in Vitro Mutagenicity of Perillaldehyde and Cinnamaldehyde. Genes Environ. 2021, 43, 30. [Google Scholar] [CrossRef]
- Elhajouji, A.; Van Hummelen, P.; Kirsch-Volders, M. Indications for a Threshold of Chemically-Induced Aneuploidy in Vitro in Human Lymphocytes. Environ. Mol. Mutagen. 1995, 26, 292–304. [Google Scholar] [CrossRef]
- Hernández, L.G.; van Benthem, J.; Johnson, G.E. A Mode-of-Action Spproach for the Identification of Genotoxic Carcinogens. PLoS ONE 2013, 8, e64532. [Google Scholar] [CrossRef]
- Enane, F.O.; Saunthararajah, Y.; Korc, M. Differentiation Therapy and the Mechanisms That Terminate Cancer Cell Proliferation without Harming Normal Cells. Cell Death Dis. 2018, 9, 912. [Google Scholar] [CrossRef]
- Kong, G.; Jeen Lee, S.; Jun Kim, H.; Surh, Y.-J.; Doo Kim, N. Induction of Granulocytic Differentiation in Acute Promyelocytic Leukemia Cells (HL-60) by 2-(Allylthio) Pyrazine. Cancer Lett. 1999, 144, 1–8. [Google Scholar] [CrossRef]
- Hou, W.; Wang, Z.-Y.; Lin, J.; Chen, W.-M. Induction of Differentiation of the Acute Myeloid Leukemia Cell Line (HL-60) by a Securinine Dimer. Cell Death Discov. 2020, 6, 123. [Google Scholar] [CrossRef]
- Martin, S.J.; Bradley, J.G.; Cotter, T.G. HL-60 Cells Induced to Differentiate towards Neutrophils Subsequently Die via Apoptosis. Clin. Exp. Pharmacol. Immunol. 1990, 79, 448–453. [Google Scholar] [CrossRef]
- Attaallah, A.; Lenzi, M.; Marchionni, S.; Bincoletto, G.; Cocchi, V.; Croco, E.; Hrelia, P.; Hrelia, S.; Sell, C.; Lorenzini, A. A pro Longevity Role for Cellular Senescence. GeroScience 2020, 42, 867–879. [Google Scholar] [CrossRef]
- Lenzi, M.; Cocchi, V.; Hrelia, P. Flow Cytometry vs Optical Microscopy in the Evaluation of the Genotoxic Potential of Xenobiotic Compounds. Cytom. Part B Clin. Cytom. 2018, 94, 852–862. [Google Scholar] [CrossRef]
- Cocchi, V.; Hrelia, P.; Lenzi, M. Antimutagenic and Chemopreventive Properties of 6-(Methylsulfinyl) Hexyl Isothiocyanate on TK6 Human Cells by Flow Cytometry. Front. Pharmacol. 2020, 11, 1242. [Google Scholar] [CrossRef]
- Lenzi, M.; Cocchi, V.; Cavazza, L.; Bilel, S.; Hrelia, P.; Marti, M. Genotoxic Properties of Synthetic Cannabinoids on TK6 Human Cells by Flow Cytometry. Int. J. Mol. Sci. 2020, 21, 1150. [Google Scholar] [CrossRef]
- Cocchi, V.; Gasperini, S.; Hrelia, P.; Tirri, M.; Marti, M.; Lenzi, M. Novel Psychoactive Phenethylamines: Impact on Genetic Material. Int. J. Mol. Sci. 2020, 21, 9616. [Google Scholar] [CrossRef]
- Lenzi, M.; Cocchi, V.; Gasperini, S.; Arfè, R.; Marti, M.; Hrelia, P. Evaluation of Cytotoxic and Mutagenic Effects of the Synthetic Cathinones Mexedrone, α-PVP and α-PHP. Int. J. Mol. Sci. 2021, 22, 6320. [Google Scholar] [CrossRef]
- Ferruzzi, L.; Turrini, E.; Burattini, S.; Falcieri, E.; Poli, F.; Mandrone, M.; Sacchetti, G.; Tacchini, M.; Guerrini, A.; Gotti, R.; et al. Hemidesmus Indicus Induces Apoptosis as Well as Differentiation in a Human Promyelocytic Leukemic Cell Line. J. Ethnopharmacol. 2013, 147, 84–91. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Norman, A.W.; Lübbert, M.; Collins, E.D.; Uskokovic, M.R.; Koeffler, H.P. Novel Vitamin D Analogs That Modulate Leukemic Cell Growth and Differentiation with Little Effect on Either Intestinal Calcium Absorption or Bone Mobilization. Blood 1989, 74, 82–93. [Google Scholar]
- Catino, J.J.; Miceli, L.A. Microtiter Assay Useful for Screening of Cell-Differentiation Agents. J. Natl. Cancer Inst. 1988, 80, 962–966. [Google Scholar] [CrossRef]

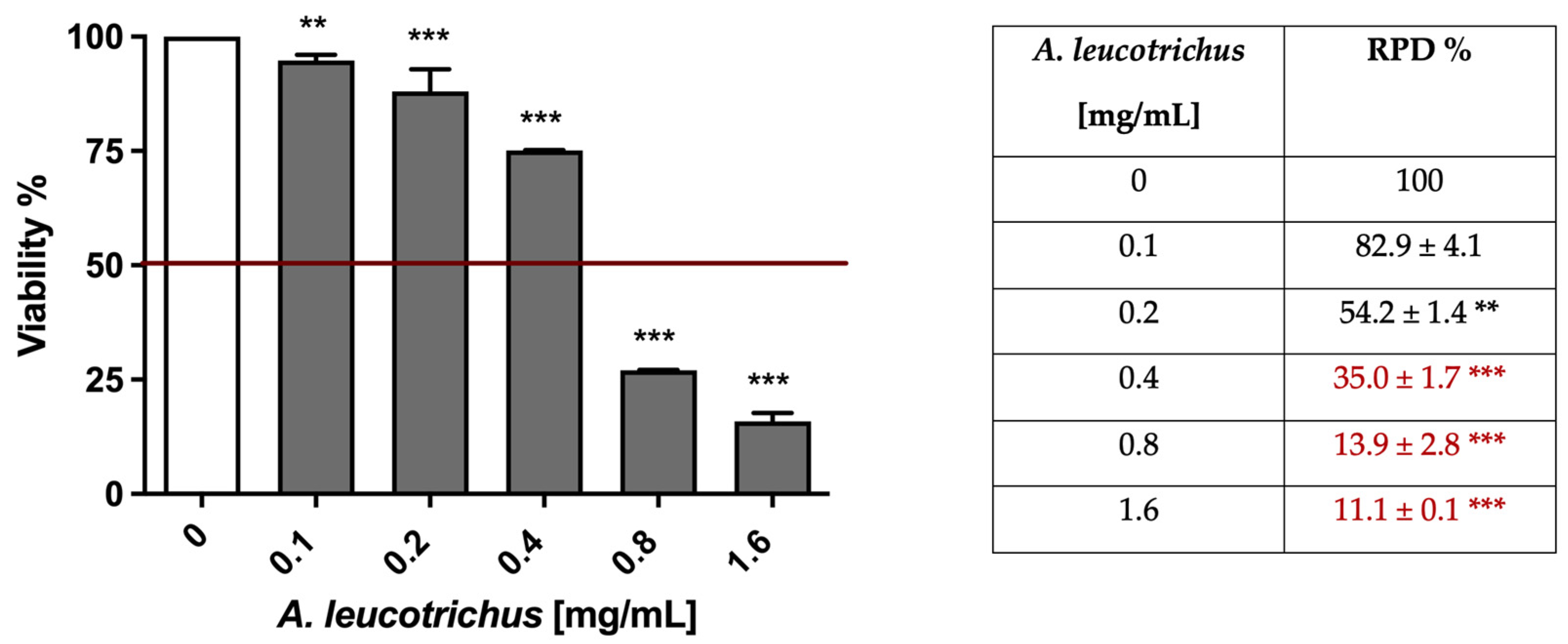
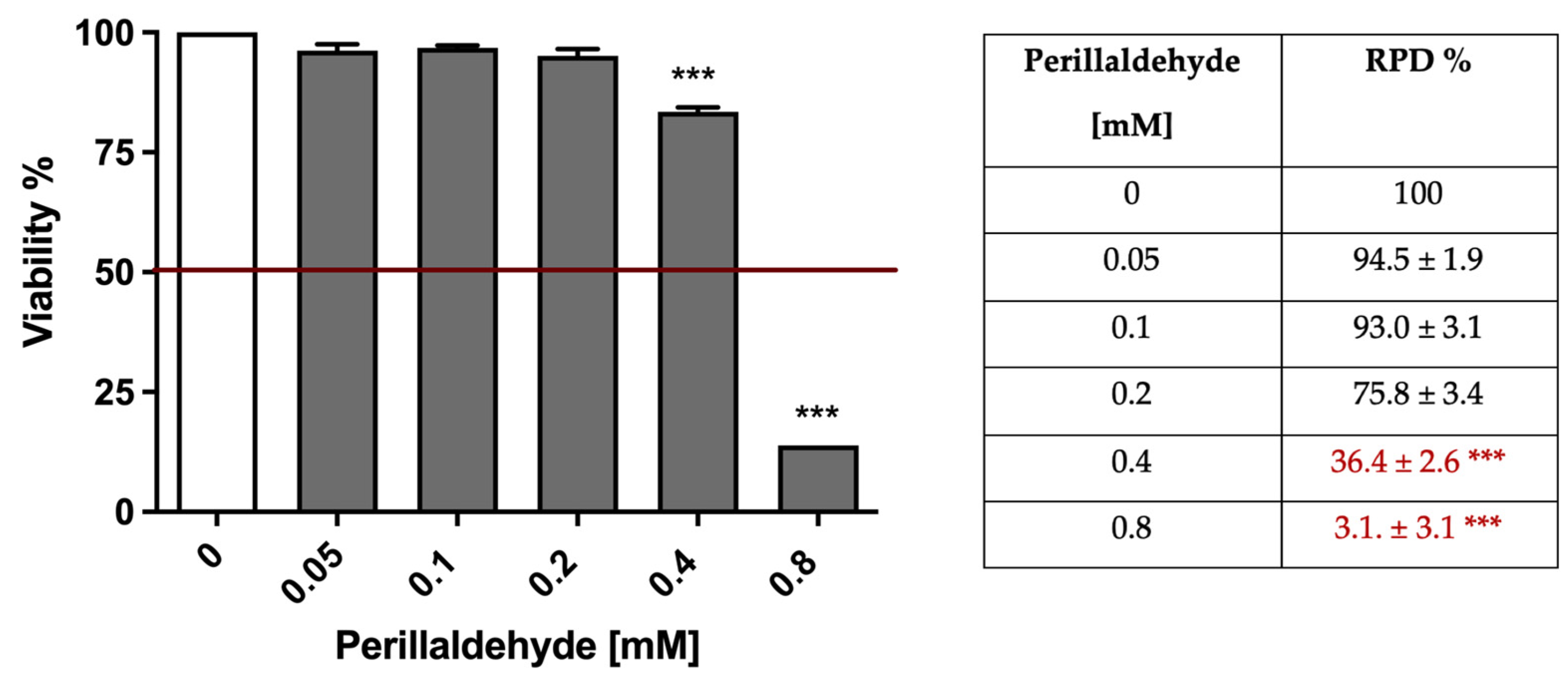




| Cell Line | IC50 (mg/mL) |
|---|---|
| HL60 | 0.354 |
| TK6 | 0.223 |
| DLD1 | 0.389 |
| SHSY5Y | 0.340 |
| Viability % | RPD % | Apoptosis Fold Increase | MNi Fold Increase | |
|---|---|---|---|---|
| Untreated cultures | 100 | 100 | 1 | 1 |
| A. leucotrichus 0.025 mg/mL | 97.0 ± 1.7 | 87.5 ± 2.1 | 1.1 ± 0.1 | 1.1 ± 0.1 |
| Perillaldehyde 0.025 mM | 95.5 ± 1.0 | 83.2 ± 1.1 * | 2.0 ± 0.1 | 0.9 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenzi, M.; Turrini, E.; Catanzaro, E.; Cocchi, V.; Guerrini, A.; Hrelia, P.; Gasperini, S.; Stefanelli, C.; Abdi Bellau, M.L.; Pellicioni, V.; et al. In Vitro Investigation of the Anticancer Properties of Ammodaucus Leucotrichus Coss. & Dur. Pharmaceuticals 2022, 15, 1491. https://doi.org/10.3390/ph15121491
Lenzi M, Turrini E, Catanzaro E, Cocchi V, Guerrini A, Hrelia P, Gasperini S, Stefanelli C, Abdi Bellau ML, Pellicioni V, et al. In Vitro Investigation of the Anticancer Properties of Ammodaucus Leucotrichus Coss. & Dur. Pharmaceuticals. 2022; 15(12):1491. https://doi.org/10.3390/ph15121491
Chicago/Turabian StyleLenzi, Monia, Eleonora Turrini, Elena Catanzaro, Veronica Cocchi, Alessandra Guerrini, Patrizia Hrelia, Sofia Gasperini, Claudio Stefanelli, Mohamed Lamin Abdi Bellau, Valentina Pellicioni, and et al. 2022. "In Vitro Investigation of the Anticancer Properties of Ammodaucus Leucotrichus Coss. & Dur." Pharmaceuticals 15, no. 12: 1491. https://doi.org/10.3390/ph15121491
APA StyleLenzi, M., Turrini, E., Catanzaro, E., Cocchi, V., Guerrini, A., Hrelia, P., Gasperini, S., Stefanelli, C., Abdi Bellau, M. L., Pellicioni, V., Tacchini, M., Greco, G., & Fimognari, C. (2022). In Vitro Investigation of the Anticancer Properties of Ammodaucus Leucotrichus Coss. & Dur. Pharmaceuticals, 15(12), 1491. https://doi.org/10.3390/ph15121491











