Copaiba Oil Resin Exerts an Additive Effect to Babassu Oil on Behavioral Changes in Human Endometriotic Cell Cultures
Abstract
1. Introduction
2. Results
2.1. SNEDDS-18 and SNEDDS-18/COPA Nanoemulsions Preparation
2.2. SNEDDS-18 and SNEDDS-18/COPA Does Not Show Toxicity In Vivo
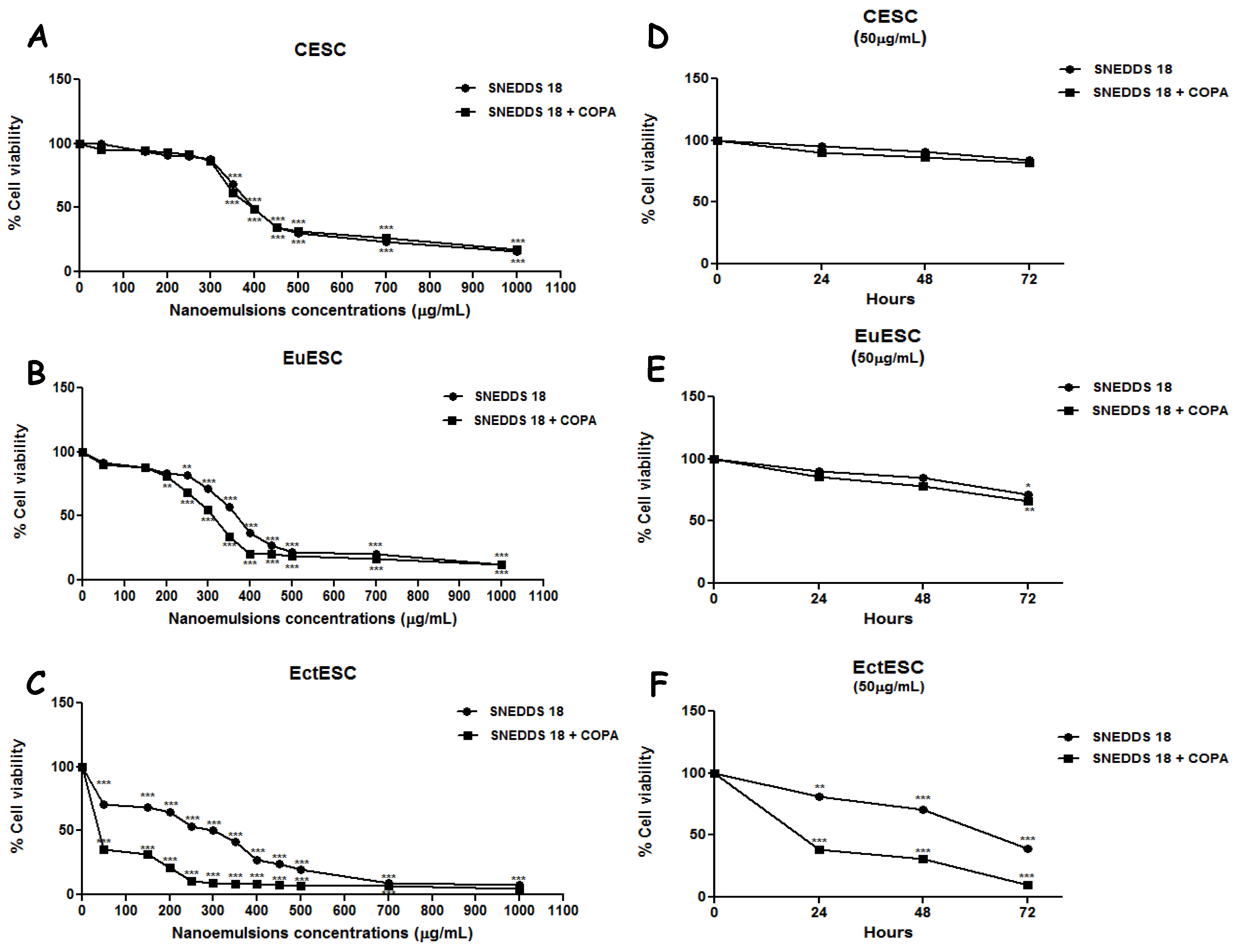
2.3. SNEDDS-18 and SNEDDS-18/COPA Reduce Cell Viability in EctESC Cultures
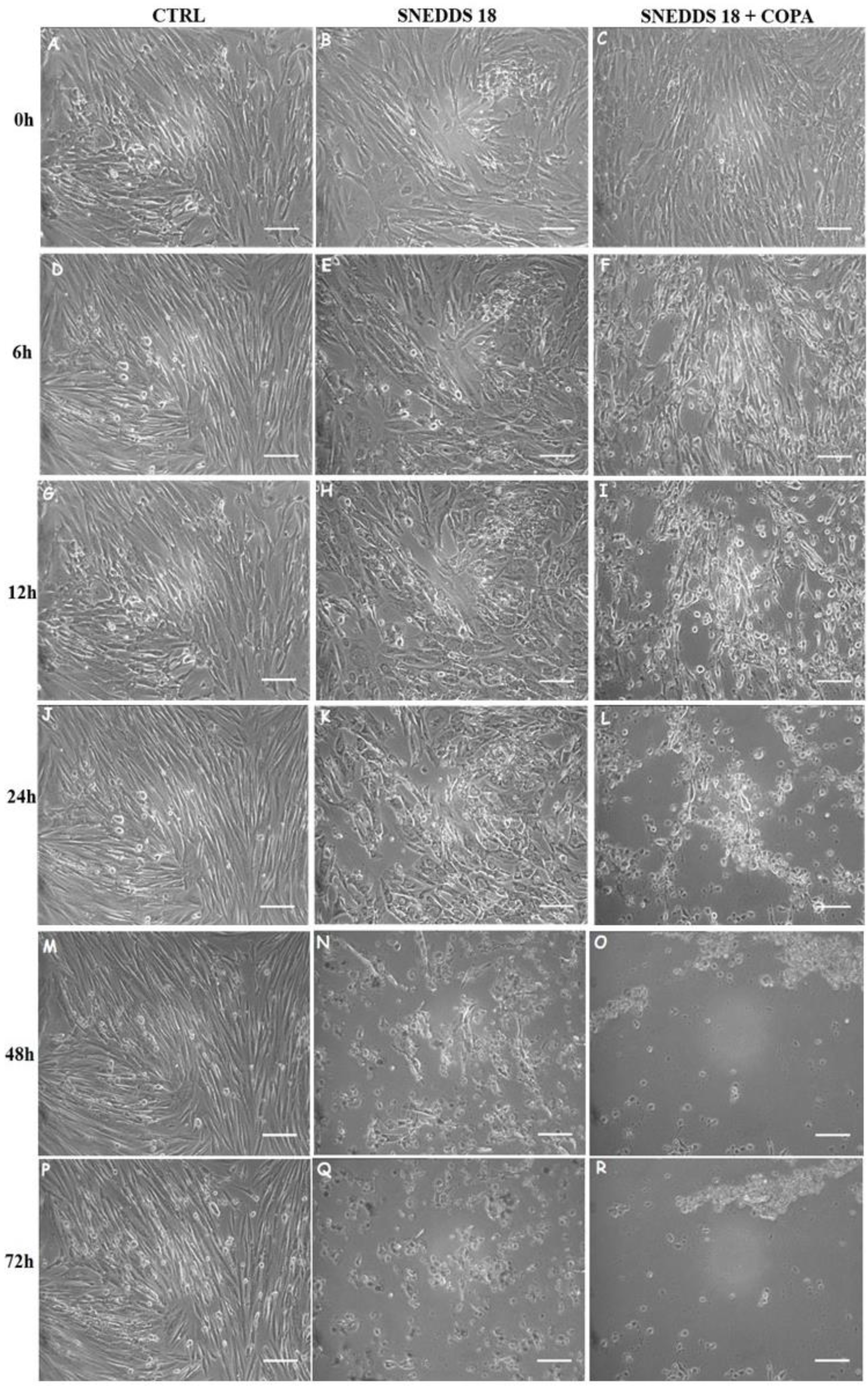
2.4. SNEDDS-18 and SNEDDS-18/COPA Reduce Cell Proliferation in EctESC Cultures
2.5. SNEDDS-18 and SNEDDS-18/COPA Alters EctESC Motility
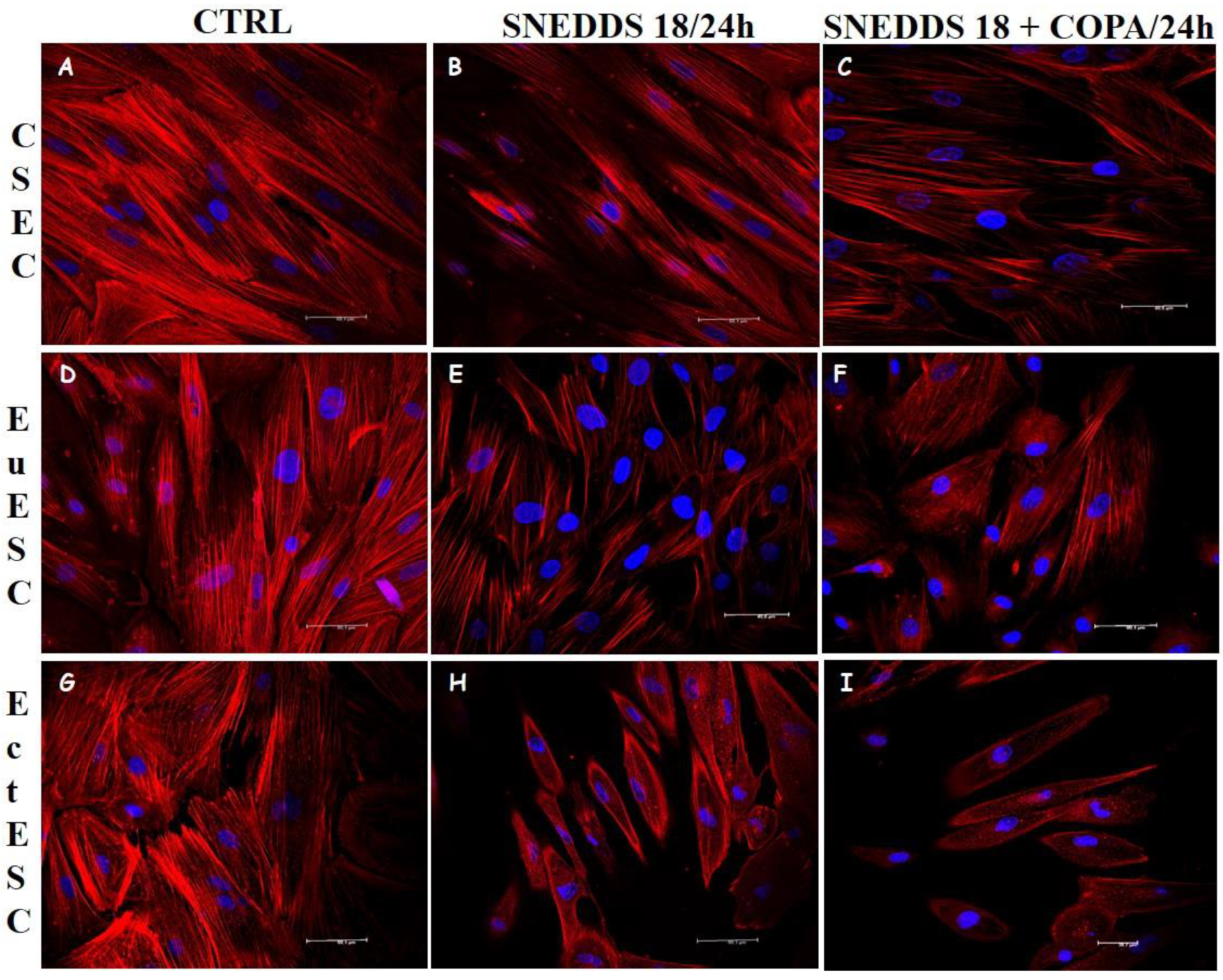
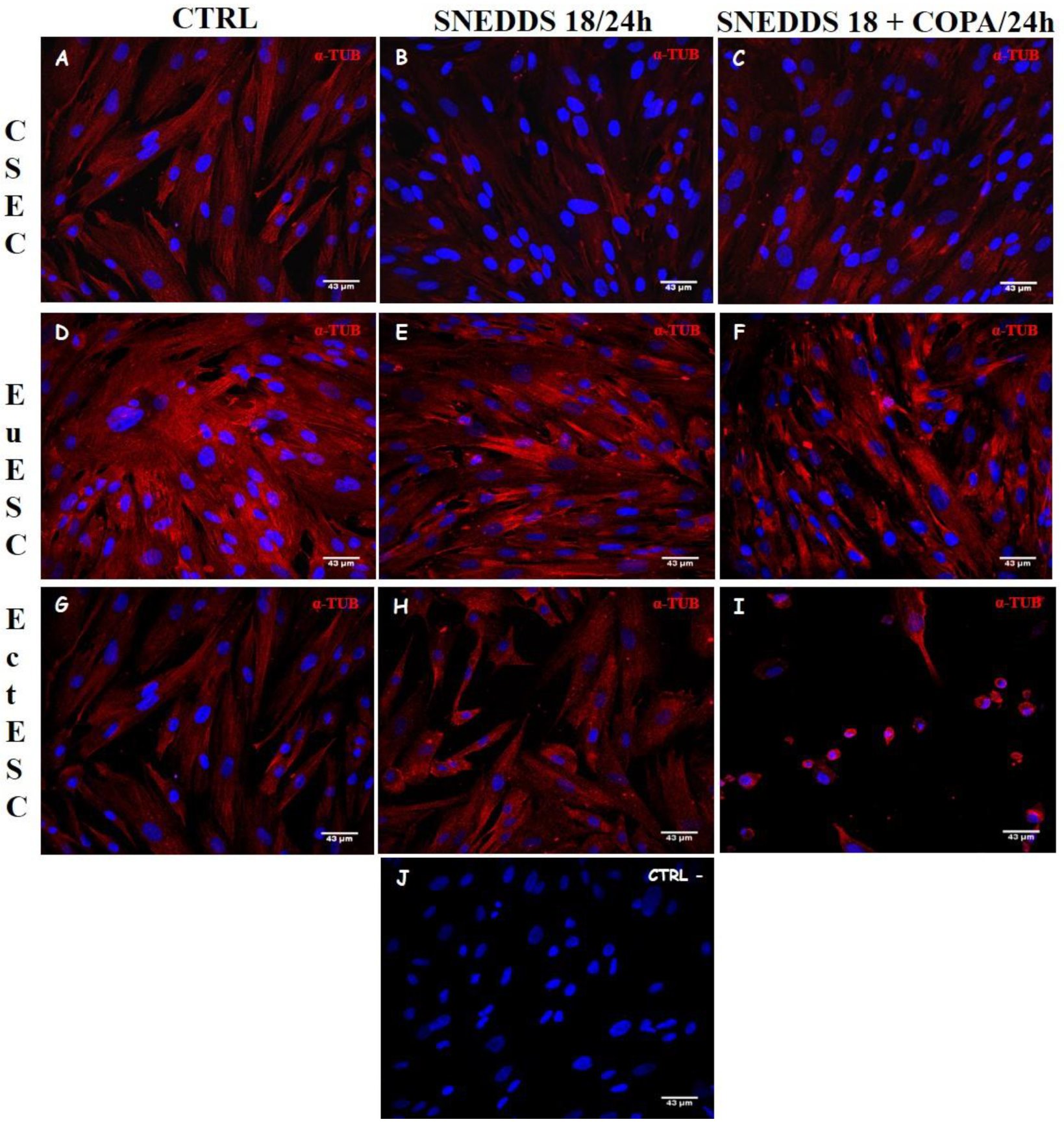
2.6. Nanoemulsions Disturbs the Cytoskeleton of the EctESCs Cultures
2.7. Nanoemulsions Induce Changes in Cell Adhesion Process in EctESC Cultures
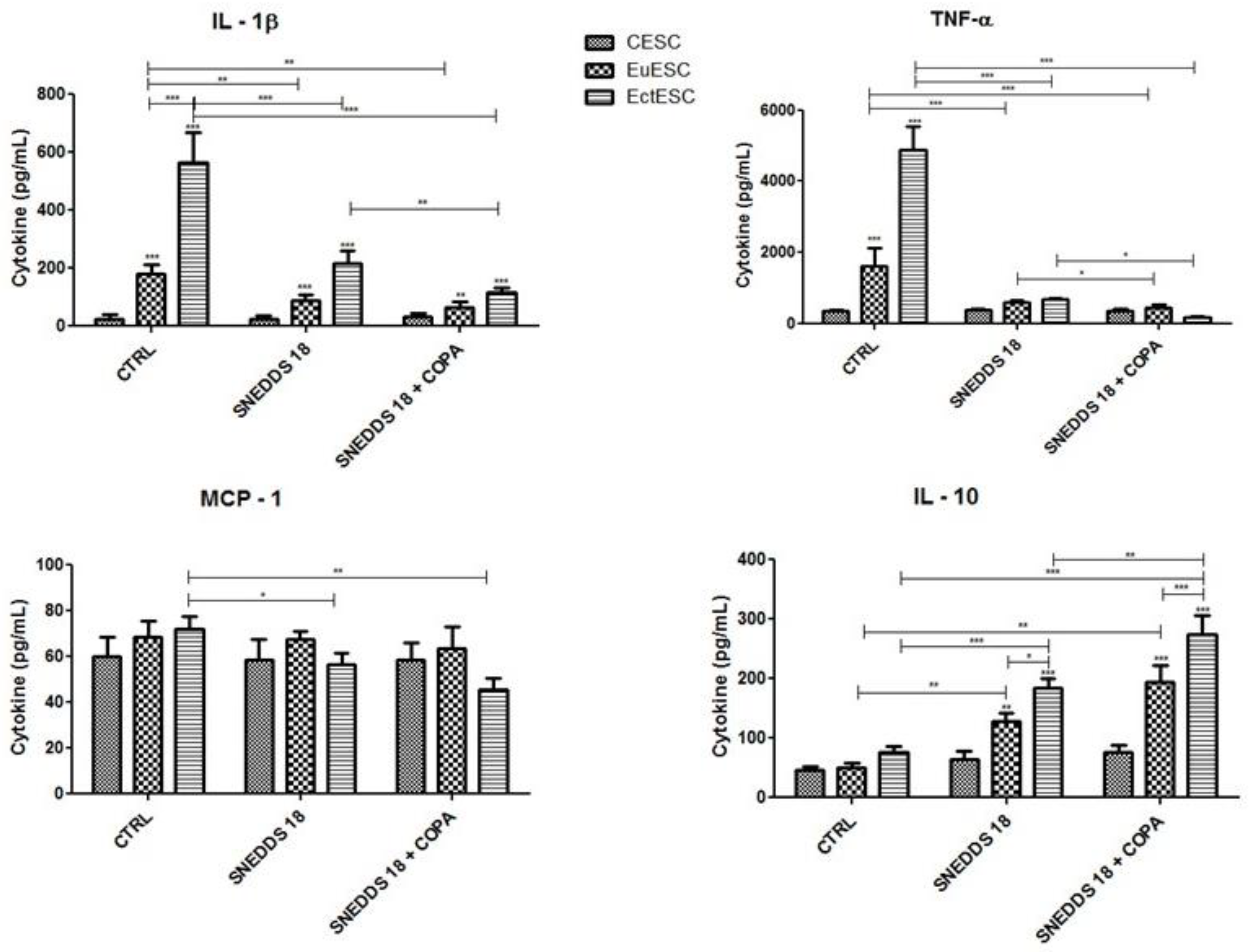
2.8. SNEDDS-18 and SNEDDS-18/COPA Affect the Secretion of Cytokines by EctESC Cultures
3. Discussion
4. Materials and Methods
4.1. Preparation of SNEDDS-18 and SNEDDS-18/COPA
4.2. Endometrial Tissue and Endometriotic Lesion Samples
4.3. Acute Toxicity Test
4.4. Cell Viability Assay
4.5. Evaluation of Cell Morphology and Proliferation
4.6. Analysis of the Cellular Motility
4.7. Analysis of Cellular Adhesion
4.8. Cytokine Measurement
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peres, L.C.; Risch, H.; Terry, K.L.M.; Webb, P.; Goodman, M.T.; Wu, A.H.; Alberg, A.J.; Bandera, E.V.; Barnholtz-Sloan, J.; Bondy, M.L.; et al. Racial/ethnic differences in the epidemiology of ovarian cancer: A pooled analysis of 12 case-control studies. Int. J. Epidemiol. 2018, 47, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.K.; Chapron, C.; Giudice, L.C.; Laufer, M.R.; Levland, N.; Missmer, S.A.; Singh, S.S.; Taylor, H.S. Clinical diagnosis of endometriosis: A call to action. Am. J. Obst. Gyn. 2019, 220, e1–e354. [Google Scholar] [CrossRef] [PubMed]
- Comptour, A.; Chauvet, P.; Canis, M.; Grémeau, A.S.; Pouly, J.L.; Rabischong, B.; Pereira, B.; Bourdel, N. Patient quality of life and symptoms after surgical treatment for endometriosis. J. Min. Inv. Gynecol. 2019, 26, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.S.; Han, S.J. Endometriosis-associated angiogenesis and anti-angiogenic therapy for endometriosis. Front. Glob. Womens Health 2022, 3, 856316. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, S.; Vijayakumar, S.; Prabhu, S.; Morvin Yabesh, J.E. Traditional plants used for the treatment of gynaecological disorders in Vedaranyam taluk, South India—An ethnomedicinal survey. J. Tradit. Complement. Med. 2018, 8, 308–323. [Google Scholar] [CrossRef]
- Edi, R.; Cheng, T. Endometriosis: Evaluation and treatment. Am. Fam. Physician 2022, 106, 397–404. [Google Scholar]
- Johnson, P.J.; Kozhimannil, K.B.; Jou, J.; Ghildayal, N.; Rockwood, T.H. Complementary and alternative medicine use among women of reproductive age in the United States. Women’s Health Issues 2016, 26, 40–47. [Google Scholar] [CrossRef]
- Trindade, R.; Silva, J.K.; Setzer, W.N. Copaifera of the neotropics: A review of the phytochemistry and pharmacology. Int. J. Mol. Sci. 2018, 19, 1511. [Google Scholar] [CrossRef]
- Sousa, V.P.; Crean, J.; De Almeida Borges, V.R.; Rodrigues, C.R.; Tajber, L.; Boylan, F.; Cabral, L.M. Nanostructured systems containing babassu (Orbignya speciosa) oil as a potential alternative therapy for benign prostatic hyperplasia. Int. J. Nanomed. 2013, 8, 3129–3139. [Google Scholar] [CrossRef][Green Version]
- Souza, P.A.; Palumbo, A.; Alves, L.M.; De Souza, V.P.; Cabral, L.M.; Fernandes, P.D.; Takiya, C.M.; Menezes, F.S.; Nasciutti, L.E. Effects of a nanocomposite containing Orbignya speciosa lipophilic extract on Benign Prostatic Hyperplasia. J. Ethnopharmacol. 2011, 135, 135–146. [Google Scholar] [CrossRef]
- Guimarães-Santos, A.; Santos, D.S.; Santos, I.R.; Lima, R.R.; Pereira, A.; De Moura, L.S.; Carvalho, R.N.; Lameira, O.; Gomes-Leal, W. Copaiba oil-resin treatment is neuroprotective and reduces neutrophil recruitment and microglia activation after motor cortex excitotoxic injury. Evid. Based Comp. Altern. Med. 2012, 9, 2012. [Google Scholar] [CrossRef]
- Gomes, N.M.; Rezende, C.M.; Fontes, S.P.; Matheus, M.E.; Fernandes, P.D. Antinociceptive activity of Amazonian Copaiba oils. J. Ethnopharmacol. 2007, 12, 486–492. [Google Scholar] [CrossRef]
- Pessoa, R.S.; França, E.L.; Ribeiro, E.B.; Lanes, P.K.; Chaud, N.G.; Moraes, L.C.; Honorio-França, A.C. Microemulsion of babassu oil as a natural product to improve human immune system function. Drug Design Develop. Ther. 2015, 9, 21–31. [Google Scholar]
- Nogueira-Neto, J.; Lindoso, M.J.S.; Coelho, L.F.; Carvalho, R.A.F.; Rodrigues, T.G.P.M.; Araújo, A.G.P.; Girão, M.J.B.C.; Schor, E. Changes in the volume and histology of endometriosis foci in rats treated with copaiba oil (Copaifera langsdorffii). Acta Cirurgica Bras. 2011, 26, 20–24. [Google Scholar] [CrossRef]
- Silva, B.P.; Parente, J.P. An anti-inflammatory and immunomodulatory polysaccharide from Orbignya phalerata. Fitoterapia 2001, 72, 887–893. [Google Scholar] [CrossRef]
- Renno, M.N.; Barbosa, G.N.; Zancan, P.; Veiga, V.F.; Alviano, C.S.; Sola-Penna, M. Crude ethanol extract from babassu (Orbignya speciosa): Cytotoxicity on tumoral and non-tumoral cell lines. An. Ac. Bras. Cien. 2008, 80, 467–476. [Google Scholar] [CrossRef]
- Souza, M.H.; Monteiro, C.A.; Figueredo, P.M.; Nascimento, F.R.; Guerra, R.N. Ethnopharmacological use of babassu (Orbignya phalerata Mart) in communities of babassu nut breakers in Maranhão, Brazil. J. Ethnopharmacol. 2011, 133, 1–5. [Google Scholar] [CrossRef]
- Amaral, L.H.; Barros, R.; Barbosa, S.; Abreu, L.; Carmo, F.; Castro, H.; Nasciutti, L.E.; Rodrigues, C.; Sousa, V.; Cabral, L.M. Development of new babassu oil lipidic nanostructured systems; potential alternative for benign prostatic hyperplasia therapy. Curr. Nanosci. 2014, 10, 786–795. [Google Scholar] [CrossRef]
- Henriques-Da-Silva, J.; Borges, V.R.; Pereira, L.D.A.C.; Ferrari, R.; De Mattos, R.M.; Barros, E.G.; Palmero, C.Y.; Fernandes, P.D.; De Carvalho, P.R.; Pereira De Sousa, V.; et al. The oil-resin of the tropical rainforest tree Copaifera langsdorffii reduces cell viability, changes cell morphology and induces cell death in human endometriotic stromal cultures. J. Pharm. Pharmacol. 2015, 67, 1744–1755. [Google Scholar] [CrossRef]
- Ghosh, V.; Saranya, S.; Mukherjee, A.; Chandrasekaran, N. Antibacterial microemulsion prevents sepsis and triggers healing of wound in wistar rats. Colloids Surf. B Biointerfaces 2013, 105, 152–157. [Google Scholar] [CrossRef]
- Alexander, A.; Patel, R.J.; Saraf, S.; Saraf, S. Recent expansion of pharmaceutical nanotechnologies and targeting strategies in the field of phytopharmaceuticals for the delivery of herbal extracts and bioactives. J. Cont. Release 2016, 241, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Singh, D. Self-nanoemulsifying drug delivery system: A versatile carrier for lipophilic drugs. Pharm. Nanotechnol. 2021, 9, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Abreu, L.C.L.; Furtado, P.S.; Honorio, T.S.; Pádula, M.; Carmo, F.A.; Rodrigues, C.R.; Cabral, L.M. A synergistic nanoformulation of babassu and copaiba oils as natural alternative for prevention of benign prostatic hyperplasia. J. Drug Del. Sci. Technol. 2018, 47, 167–175. [Google Scholar] [CrossRef]
- Krstić, M.; Medarević, Đ.; Đuriš, J.; Ibrić, S. Self-nanoemulsifying drug delivery systems (SNEDDS) and self-microemulsifying drug delivery systems (SMEDDS) as lipid nanocarriers for improving dissolution rate and bioavailability of poorly soluble drugs. In Lipid Nanocarriers for Drug Targeting; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 473–508. [Google Scholar]
- Dai, Y.; Li, X.; Shi, J.; Leng, J. A review of the risk factors, genetics and treatment of endometriosis in Chinese women: A comparative update. Reprod. Health 2018, 15, 82. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; Da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (-)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef]
- Senedese, J.M.; Rinaldi-Neto, F.; Furtado, R.A.; Nicollela, H.D.; de Souza, L.D.R.; Ribeiro, A.B.; Ferreira, L.S.; Magalhães, G.M.; Carlos, I.Z.; da Silva, J.J.M.; et al. Chemopreventive role of Copaifera reticulata Ducke oleoresin in colon carcinogenesis. Biomed. Pharmacother. 2019, 111, 331–337. [Google Scholar] [CrossRef]
- Cardoso, P.C.S.; Rocha, C.A.M.; Leal, M.F.; Bahia, M.O.; Alcântara, D.D.F.A.; Santos, R.A.; Gonçalves, N.S.; Ambrósio, R.S.; Cavalcanti, B.C.; Moreira-Nunes, C.A.; et al. Effect of diterpenoid kaurenoic acid on genotoxicity and cell cycle progression in gastric cancer cell lines. Biomed. Pharmacother. 2017, 89, 772–780. [Google Scholar] [CrossRef]
- Yuan, M.; Ding, S.; Meng, T.; Lu, B.; Shao, S.; Zhang, X.; Yuan, H.; Hu, F. Effect of A-317491 delivered by glycolipid-like polymer micelles on endometriosis pain. Int. J. Nanomed. 2017, 12, 8171–8183. [Google Scholar] [CrossRef]
- Antsiferova, Y.; Sotnikova, N.; Parfenyuk, E. Different effects of the immunomodulatory drug GMDP immobilized onto aminopropyl modified and unmodified mesoporous silica nanoparticles upon peritoneal macrophages of women with endometriosis. Biomed Res. Int. 2013, 2013, 924362. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, P.; Liu, L.; Ma, G.; Ma, J.; Liu, X.; Liu, Y.; Lin, W.; Zhu, Y. Evaluation of PLGA containing anti-CTLA4 inhibited endometriosis progression by regulating CD4+CD25+Treg cells in peritoneal fluid of mouse endometriosis model. Eur. J. Pharmac. Sci. 2017, 96, 542–550. [Google Scholar] [CrossRef]
- Mohammadi, R.K.; Arablou, T. Resveratrol and endometriosis: In vitro and animal studies and underlying mechanisms (Review). Biomed. Pharmacother. 2017, 91, 220–228. [Google Scholar] [CrossRef]
- Khazaei, M.R.; Rashidi, Z.; Chobsaz, F.; Niromand, E.; Khazaei, M. Inhibitory effect of resveratrol on the growth and angiogenesis of human endometrial tissue in an in vitro three-dimensional model of endometriosis. Reprod. Biol. 2020, 20, 484–490. [Google Scholar] [CrossRef]
- Demirel, M.A.; Suntar, I.; Ilhan, M.; Keles, H.; Kupeli Akkol, E. Experimental endometriosis remission in rats treated with Achillea biebersteinii Afan.: Histopathological evaluation and determination of cytokine levels. Eur. J. Obst. Gynecol. Rep. Biol. 2014, 175, 172–177. [Google Scholar] [CrossRef]
- Hyung-Kim, K.; Park, J.K.; Choi, Y.W.; Kim, Y.H.; Lee, E.N.; Lee, J.R.; Kim, H.S.; Baek, S.Y.; Kim, B.S.; Lee, K.S.; et al. Hexane extract of aged black garlic reduces cell proliferation and attenuates the expression of ICAM-1 and VCAM 1 in TNF-α-activated human endometrial stromal cells. Int. J. Mol. Med. 2013, 32, 67–78. [Google Scholar]
- Kim, J.H.; Yang, Y.I.; Ahn, J.H.; Lee, J.G.; Lee, K.T.; Choi, J.H. Deer (Cervus elaphus) antler extract suppresses adhesion and migration of endometriotic cells and regulates MMP-2 and MMP-9 expression. J. Ethnopharmacol. 2012, 140, 391–397. [Google Scholar] [CrossRef]
- Kim, J.H.; Jung, S.H.; Yang, Y.I.; Ahn, J.H.; Cho, J.G.; Lee, K.T.; Baek, N.I.; Choi, J.H. Artemisia leaf extract induces apoptosis in human endometriotic cells through regulation of the p38 and NFκB pathways. J. Ethnopharmacol. 2013, 145, 767–775. [Google Scholar] [CrossRef]
- Kim, J.H.; Woo, J.H.; Kim, H.M.; Oh, M.S.; Jang, D.S.; Choi, J.H. Anti-endometriotic effects of Pueraria flower extract in human endometriotic cells and mice. Nutrients 2017, 9, 212. [Google Scholar] [CrossRef]
- Kolahdouz-Mohammadi, R.; Delbandi, A.A.; Khodaverdi, S.; Arefi, S.; Arablou, T.; Shidfar, F. The effects of resveratrol treatment on Bcl-2 and Bax gene expression in endometriotic compared with non-endometriotic stromal cells. Iran J. Public Health 2020, 49, 1546–1554. [Google Scholar] [CrossRef]
- Cao, H.; Wei, Y.X.; Zhou, Q.; Zhang, Y.; Guo, X.P.; Zhang, J. Inhibitory effect of curcumin in human endometriosis endometrial cells via downregulation of vascular endothelial growth factor. Mol. Med. Rep. 2017, 16, 5611–5617. [Google Scholar] [CrossRef]
- Matasariu, D.R.; Lozneanu, L.; Dumitraşcu, I.; Grigore, M.; Cristofor, A.E.; Mandici, C.E.; Bujor, I.E.; Ursache, A.; Brăila, A.D.; Bauşic, A.; et al. Hormonal, apoptotic, proliferative and inflammatory markers’ expression in Desogestrel-treated women with ovarian endometriosis. Rom. J. Morphol. Embryol. 2022, 63, 137–144. [Google Scholar] [CrossRef]
- Sun, X.H. Protective effects of marrubiin improve endometriosis through suppression of the expression of RANTES. Mol. Med. Rep. 2017, 16, 3339–3344. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, Y.; Xu, L.; Wang, Z.; Wang, Y.; Shi, W.; Ma, K. Traditional Chinese medicine prescription Guizhi Fuling Pills in the treatment of endometriosis. Int. J. Med. Sci. 2021, 18, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.; Das Mahapatra, P.; Saha, P.; Srivastava, A.K.; Swarnakar, S. Interleukin-1β activated c-FOS transcription factor binds preferentially to a specific allele of the matrix metalloproteinase-13 promoter and increases susceptibility to endometriosis. J. Cell Physiol. 2022, 237, 3095–3108. [Google Scholar] [CrossRef] [PubMed]
- Nagayasu, M.; Imanaka, S.; Kimura, M.; Maruyama, S.; Kobayashi, H. Nonhormonal treatment for endometriosis focusing on redox imbalance. Gynecol. Obstet. Investig. 2021, 86, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Delbandi, A.A.; Mahmoudi, M.; Shervin, A.; Zarnani, A.H. 1,25-Dihydroxy vitamin D3 modulates endometriosis-related features of human endometriotic stromal cells. Am. J. Rep. Immunol. 2016, 75, 461–473. [Google Scholar] [CrossRef]
- Chen, F.Y.; Wang, X.; Tang, R.Y.; Guo, Z.X.; Deng, Y.Z.; Yu, Q. New therapeutic approaches for endometriosis besides hormonal therapy. New therapeutic approaches for endometriosis besides hormonal therapy. Chin. Med. J. 2019, 132, 2984–2993. [Google Scholar] [CrossRef]
- Khazaei, M.R.; Rashidi, Z.; Chobsaz, F.; Khazaei, M. Apoptosis induction of human endometriotic epithelial and stromal cells by noscapine. Iran. J. Basic Med. Sci. 2016, 19, 940–945. [Google Scholar]
- Ricci, A.G.; Olivares, C.N.; Bilotas, M.A.; Bastón, J.I.; Singla, J.J.; Meresman, G.F.; Barañao, R.I. Natural therapies assessment for the treatment of endometriosis. Human Reprod. 2013, 28, 178–188. [Google Scholar] [CrossRef]
- Taguchi, A.; Koga, K.; Kawana, K.; Makabe, T.; Sue, F.; Miyashita, M.; Yoshida, M.; Urata, Y.; Izumi, G.; Tkamura, M.; et al. Resveratrol enhances apoptosis in endometriotic stromal cells. Am. J. Reprod. Immunol. 2016, 75, 486–492. [Google Scholar] [CrossRef]
- Amro, B.; Aristondo, M.E.R.; Alsuwaidi, S.; Almaamari, B.; Hakim, Z.; Tahlak, M.; Wattiez, A.; Koninckx, P.R. New understanding of diagnosis, treatment and prevention of endometriosis. Int. J. Environ. Res. Public Health 2022, 19, 6725. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y. Curcumin and endometriosis. Int. J. Mol. Sci. 2020, 21, 2440. [Google Scholar] [CrossRef]
- Yun, B.H.; Kim, S.; Chon, S.J.; Kim, G.H.; Choi, Y.S.; Cho, S.; Lee, B.S.; Seo, S.K. High mobility group box-1 promotes inflammation in endometriotic stromal cells through Toll-like receptor 4/nuclear factor-kappa B. Am. J. Transl. Res. 2021, 13, 1400–1410. [Google Scholar]
- Silveira, C.G.; Finas, D.; Hunold, P.; Köster, F.; Stroschein, K.; Canny, G.O.; Moldenhauer, G.; Altevogt, P.; Rody, A.; Hornung, D. L1 cell adhesion molecule as a potential therapeutic target in murine models of endometriosis using a monoclonal antibody approach. PLoS ONE 2013, 8, e82512. [Google Scholar] [CrossRef]
- Zhang, J.J.; Xu, Z.M.; Zhang, C.M.; Dai, H.Y.; Ji, X.Q.; Wang, X.F.; Li, C. Pyrrolidine dithiocarbamate inhibits nuclear factor-κB pathway activation, and regulates adhesion, migration, invasion and apoptosis of endometriotic stromal cells. Mol. Hum. Reprod. 2011, 17, 175–181. [Google Scholar] [CrossRef]
- Jørgensen, H.; Hill, A.S.; Beste, M.T.; Kumar, M.P.; Chiswick, E.; Fedorcsak, P.; Isaacson, K.B.; Lauffenburger, D.A.; Griffith, L.G.; Qvigstad, E. Peritoneal fluid cytokines related to endometriosis in patients evaluated for infertility. Fertil. Steril. 2017, 107, 1191–1199. [Google Scholar] [CrossRef]
- Králíčková, M.; Vetvicka, V. Immunological aspects of endometriosis: A review. Ann. Transl. Med. 2015, 3, 153–158. [Google Scholar]
- Chopyak, V.V.; Koval, H.D.; Havrylyuk, A.M.; Lishchuk-Yakymovych, K.A.; Potomkina, H.A.; Kurpisz, M.K. Immunopathogenesis of endometriosis-a novel look at an old problem. Cent. Eur. J. Immunol. 2022, 47, 109–116. [Google Scholar] [CrossRef]
- Birt, J.A.; Nabli, H.; Stilley, J.A.; Windham, E.A.; Frazier, S.R.; Sharpe-Timms, K.L. Elevated peritoneal fluid TNF-α incites ovarian early growth response factor 1 expression and downstream protease mediators: A correlation with ovulatory dysfunction in endometriosis. Reprod. Sci. 2013, 20, 514–523. [Google Scholar] [CrossRef]
- Lee, M.Y.; Kim, S.H.; Oh, Y.S.; Heo, S.H.; Kim, K.H.; Chae, H.D.; Kim, C.H.; Kang, B.M. Role of interleukin-32 in the pathogenesis of endometriosis: In vitro, human and transgenic mouse data. Hum. Reprod. 2018, 33, 807–816. [Google Scholar] [CrossRef]
- Miller, J.E.; Monsanto, S.P.; Ahn, S.H.; Khalaj, K.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Koti, M.; Tayade, C. Interleukin-33 modulates inflammation in endometriosis. Sci. Rep. 2017, 7, 17903. [Google Scholar] [CrossRef]
- Ouyang, Z.; Sun, J.P.; Tian, X.L.; Chen, M.X.; Zhai, J.J. The expressions and the roles of SDF-1/CXCR-4 and SDF-1/CXCR-4 in human endometriosis. Zhonghua Yi Xue Za Zhi 2018, 98, 1854–1858. [Google Scholar] [PubMed]
- Wang, X.M.; Ma, Z.Y.; Song, N. Inflammatory cytokines IL-6, IL-10, IL-13, TNF-α and peritoneal fluid flora were associated with infertility in patients with endometriosis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2513–2518. [Google Scholar] [PubMed]
- Cakmak, H.; Seval-Celik, Y.; Arlier, S.; Guzeloglu-Kayisli, O.; Schatz, F.; Arici, A.; Kayisli, U.A. p38 Mitogen-activated protein kinase is involved in the pathogenesis of endometriosis by modulating inflammation, but not cell survival. Reprod. Sci. 2018, 25, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Bedaiwy, M.A. Endometrial cytokines, endometriosis and infertility: A deeper dive into the endometrial immune microenvironment. Fertil. Steril. 2022, 117, 641–642. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.; Fedorcsak, P.; Isaacson, K.; Tevonian, E.; Xiao, A.; Beste, M.; Qvigstad, E.; Lauffenburger, D.; Griffith, L. Endometrial cytokines in patients with and without endometriosis evaluated for infertility. Fertil. Steril. 2022, 117, 629–640. [Google Scholar] [CrossRef]
- Kolanska, K.; Alijotas-Reig, J.; Cohen, J.; Cheloufi, M.; Selleret, L.; d’Argent, E.; Kayem, G.; Valverde, E.E.; Fain, O.; Bornes, M.; et al. Endometriosis with infertility: A comprehensive review on the role of immune deregulation and immunomodulation therapy. Am. J. Reprod. Immunol. 2021, 85, e13384. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Chen, H.Y.; Chen, W.; Liu, Y.N.; Fu, Y.; Wang, L.N. Expression of inflammatory cytokines in serum and peritoneal fluid from patients with different stages of endometriosis. Gynecol. Endocrinol. 2018, 34, 507–512. [Google Scholar] [CrossRef]
- Sipak-Szmigiel, O.; Włodarski, P.; Ronin-Walknowska, E.; Niedzielski, A.; Karakiewicz, B.; Słuczanowska-Głąbowska, S.; Laszczyńska, M.; Malinowski, W. Serum and peritoneal fluid concentrations of soluble human leukocyte antigen, tumor necrosis factor alpha and interleukin 10 in patients with selected ovarian pathologies. J. Ovarian Res. 2017, 10, 25. [Google Scholar] [CrossRef]
- Suen, J.L.; Chang, Y.; Chiu, P.R.; Hsieh, T.H.; Hsi, E.; Chen, Y.C.; Chen, Y.F.; Tsai, E.M. Serum level of IL-10 is increased in patients with endometriosis, and IL-10 promotes the growth of lesions in a murine model. Am. J. Pathol. 2014, 184, 464–471. [Google Scholar] [CrossRef]
- Olivares, C.; Bilotas, M.; Buquet, R.; Borghi, M.; Sueldo, C.; Tesone, M.; Meresman, G. Effects of a selective cyclooxygenase-2 inhibitor on endometrial epithelial cells from patients with endometriosis. Hum. Reprod. 2008, 23, 2701–2708. [Google Scholar] [CrossRef]
- Lorke, D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.H.d.; Abreu, L.C.L.d.; Ferrari, R.; Quintana, C.Y.P.; Barros, E.G.d.O.; Cordeiro, N.d.M.; Pontes, B.; Sousa, V.P.d.; Cabral, L.M.; Fernandes, P.D.; et al. Copaiba Oil Resin Exerts an Additive Effect to Babassu Oil on Behavioral Changes in Human Endometriotic Cell Cultures. Pharmaceuticals 2022, 15, 1414. https://doi.org/10.3390/ph15111414
Silva JHd, Abreu LCLd, Ferrari R, Quintana CYP, Barros EGdO, Cordeiro NdM, Pontes B, Sousa VPd, Cabral LM, Fernandes PD, et al. Copaiba Oil Resin Exerts an Additive Effect to Babassu Oil on Behavioral Changes in Human Endometriotic Cell Cultures. Pharmaceuticals. 2022; 15(11):1414. https://doi.org/10.3390/ph15111414
Chicago/Turabian StyleSilva, Julianna Henriques da, Leticia Coli Louvisse de Abreu, Renato Ferrari, Celia Yelimar Palmero Quintana, Eliane Gouvêa de Oliveira Barros, Natália de Moraes Cordeiro, Bruno Pontes, Valeria Pereira de Sousa, Lucio Mendes Cabral, Patricia Dias Fernandes, and et al. 2022. "Copaiba Oil Resin Exerts an Additive Effect to Babassu Oil on Behavioral Changes in Human Endometriotic Cell Cultures" Pharmaceuticals 15, no. 11: 1414. https://doi.org/10.3390/ph15111414
APA StyleSilva, J. H. d., Abreu, L. C. L. d., Ferrari, R., Quintana, C. Y. P., Barros, E. G. d. O., Cordeiro, N. d. M., Pontes, B., Sousa, V. P. d., Cabral, L. M., Fernandes, P. D., & Nasciutti, L. E. (2022). Copaiba Oil Resin Exerts an Additive Effect to Babassu Oil on Behavioral Changes in Human Endometriotic Cell Cultures. Pharmaceuticals, 15(11), 1414. https://doi.org/10.3390/ph15111414






