Danazol in Refractory Autoimmune Hemolytic Anemia or Immune Thrombocytopenia: A Case Series Report and Literature Review
Abstract
1. Introduction
2. Results
2.1. Case Series
2.2. Literature Review
3. Discussion
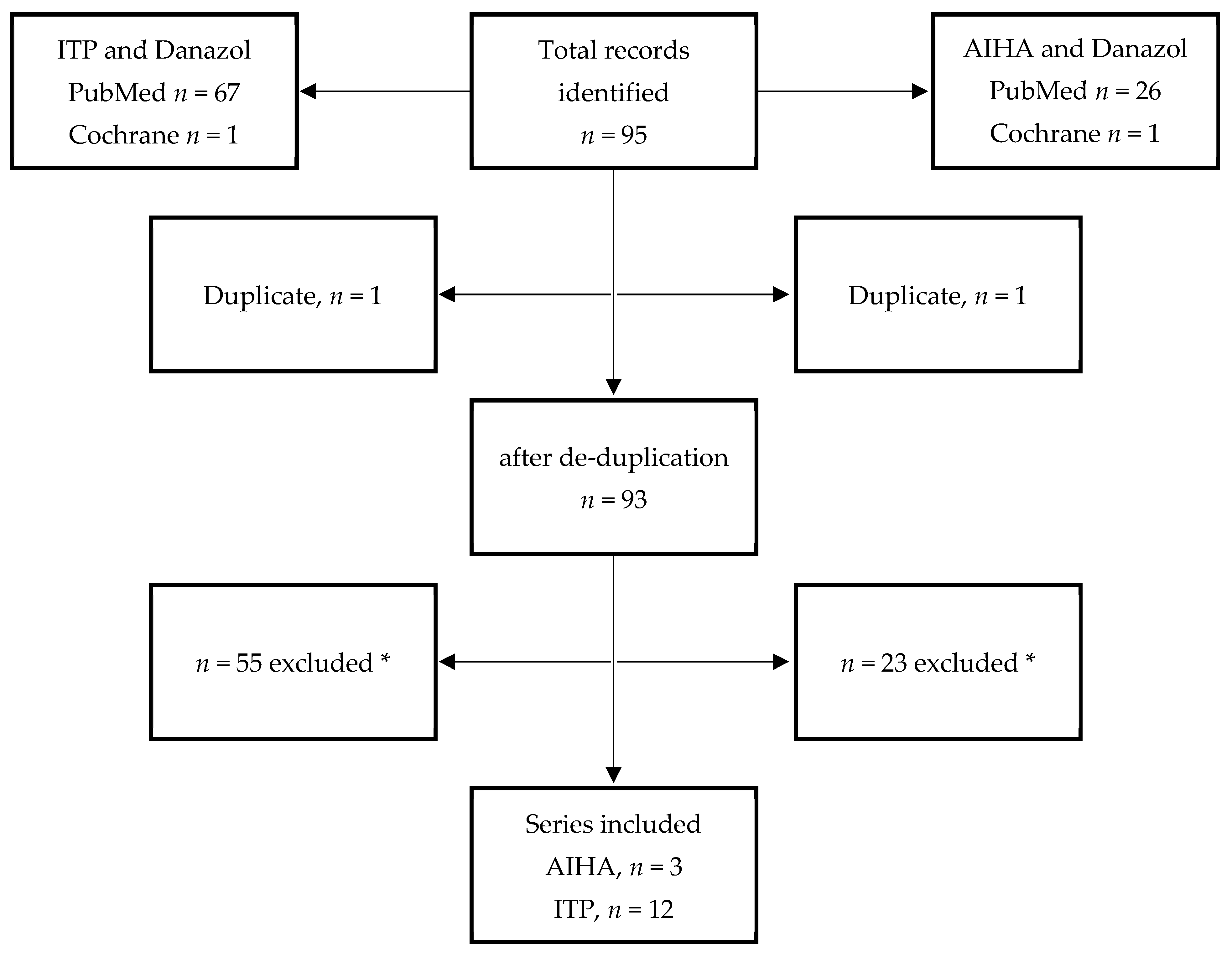
4. Materials and Methods
Study Design and Ethical Approval
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lechner, K.; Jäger, U. How I treat autoimmune hemolytic anemias in adults. Blood 2010, 116, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, C.A.; Pell, J.; Hill, Q.; Bagot, C.; Cooper, N.; Ingram, J.; Breheny, K.; Kandiyali, R.; Rayment, R.; Evans, G.; et al. Mycophenolate Mofetil for First-Line Treatment of Immune Thrombocytopenia. N. Engl. J. Med. 2021, 385, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Fattizzo, B.; Cantoni, S.; Giannotta, J.A.; Bandiera, L.; Zavaglia, R.; Bortolotti, M.; Barcellini, W. Efficacy and safety of cyclosporine A treatment in autoimmune cytopenias: The experience of two Italian reference centers. Ther. Adv. Hematol. 2022, 13, 20406207221097780. [Google Scholar] [CrossRef] [PubMed]
- Roumier, M.; Loustau, V.; Guillaud, C.; Languille, L.; Mahevas, M.; Khellaf, M.; Limal, N.; Noizat-Pirenne, F.; Godeau, B.; Michel, M. Characteristics and outcome of warm autoimmune hemolytic anemia in adults: New insights based on a single-center experience with 60 patients. Am. J. Hematol. 2014, 89, E150–E155. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, S.; Charpak, Y.; Chastang, C.; Tobelem, G. Low doses v conventional doses of corticoids in immune thrombocytopenic purpura (ITP): Results of a randomized clinical trial in 160 children, 223 adults. Blood 1988, 71, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Terriou, L.; Roudot-Thoraval, F.; Hamidou, M.; Ebbo, M.; Le Guenno, G.; Galicier, L.; Audia, S.; Royer, B.; Morin, A.-S.; et al. A randomized and double-blind controlled trial evaluating the safety and efficacy of rituximab for warm auto-immune hemolytic anemia in adults (the RAIHA study). Am. J. Hematol. 2016, 92, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Birgens, H.; Frederiksen, H.; Hasselbalch, H.C.; Rasmussen, I.H.; Nielsen, O.J.; Kjeldsen, L.; Larsen, H.; Mourits-Andersen, T.; Plesner, T.; Rønnov-Jessen, D.; et al. A phase III randomized trial comparing glucocorticoid monotherapy versus glucocorticoid and rituximab in patients with autoimmune haemolytic anaemia. Br. J. Haematol. 2013, 163, 393–399. [Google Scholar] [CrossRef]
- Ghanima, W.; Khelif, A.; Waage, A.; Michel, M.; E Tjønnfjord, G.; Ben Romdhan, N.; Kahrs, J.; Darne, B.; Holme, P.A. Rituximab as second-line treatment for adult immune thrombocytopenia (the RITP trial): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015, 385, 1653–1661. [Google Scholar] [CrossRef]
- Gudbrandsdottir, S.; Birgens, H.S.; Frederiksen, H.; Jensen, B.A.; Jensen, M.K.; Kjeldsen, L.; Klausen, T.W.; Larsen, H.; Mourits-Andersen, H.T.; Nielsen, C.H.; et al. Rituximab and dexamethasone vs dexamethasone monotherapy in newly diagnosed patients with primary immune thrombocytopenia. Blood 2013, 121, 1976–1981. [Google Scholar] [CrossRef]
- Bonelli, M.M.; Mrak, D.; Perkmann, T.; Haslacher, H.; Aletaha, D. SARS-CoV-2 vaccination in rituximab-treated patients: Evidence for impaired humoral but inducible cellular immune response. Ann. Rheum. Dis. 2021, 80, 1355–1356. [Google Scholar] [CrossRef]
- Pasquale, R.; Giannotta, J.A.; Barcellini, W.; Fattizzo, B. Bortezomib in autoimmune hemolytic anemia and beyond. Ther. Adv. Hematol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Kuter, D.J.; Bussel, J.B.; Lyons, R.M.; Pullarkat, V.; Gernsheimer, T.B.; Senecal, F.M.; Aledort, L.M.; George, J.N.; Kessler, C.M.; Sanz, M.A.; et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: A double-blind randomised controlled trial. Lancet 2008, 371, 395–403. [Google Scholar] [CrossRef]
- Cheng, G.; Saleh, M.N.; Marcher, C.; Vasey, S.; Mayer, B.; Aivado, M.; Arning, M.; Stone, N.L.; Bussel, J.B. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): A 6-month, randomised, phase 3 study. Lancet 2011, 377, 393–402. [Google Scholar] [CrossRef]
- Jurczak, W.; Chojnowski, K.; Mayer, J.; Krawczyk, K.; Jamieson, B.D.; Tian, W.; Allen, L.F. Phase 3 randomised study of avatrombopag, a novel thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia. Br. J. Haematol. 2018, 183, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Saleh, M.N.; Khelif, A.; Salama, A.; Portella, M.S.O.; Burgess, P.; Bussel, J.B. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: Final results of the EXTEND study. Blood 2017, 130, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Kuter, D.J.; Bussel, J.B.; Newland, A.; Baker, R.; Lyons, R.M.; Wasser, J.; Viallard, J.-F.; Macik, G.; Rummel, M.; Nie, K.; et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: Safety and efficacy. Br. J. Haematol. 2013, 161, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.S.; Harrington, W.J.; Mylvaganam, R.; Ayub, J.; Pall, L.M. Danazol Therapy for Autoimmune Hemolytic Anemia. Ann. Intern. Med. 1985, 102, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, A. Danazol Therapy in Patients with Immune Cytopenias. Aust. N. Z. J. Med. 1987, 17, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.-M.; Poirson, E.; Rochant, H. Danazol in autoimmune haemolytic anaemia. Br. J. Haematol. 1993, 83, 343–345. [Google Scholar] [CrossRef]
- Ahn, Y.S.; Rocha, R.; Mylvaganam, R.; Garcia, R.; Duncan, R.; Harrington, W.J. Long-Term Danazol Therapy in Autoimmune Thrombocytopenia: Unmaintained Remission and Age-Dependent Response in Women. Ann. Intern. Med. 1989, 111, 723–729. [Google Scholar] [CrossRef]
- Edelmann, D.Z.; Knobel, B.; Virag, I.; Meytes, D. Danazol in non-splenectomized patients with refractory idiopathic thrombocytopenic purpura. Postgrad. Med. J. 1990, 66, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Iseki, T.; Goto, S.; Takaso, T.; Ohto, M.; Okuda, K. Danazol therapy in idiopathic thrombocytopenic purpura: The efficacy of low-medium dose therapy. Int. J. Hematol. 1992, 55, 293–300. [Google Scholar] [PubMed]
- Schiavotto, C.; Castaman, G.; Rodeghiero, F. Treatment of idiopathic thrombocytopenic purpura (ITP) in patients with refractoriness to or with contraindication for corticosteroids and/or splenectomy with immunosuppressive therapy and danazol. Haematologica 1993, 78, 29–34. [Google Scholar] [PubMed]
- Arnal, C.; Piette, J.-C.; Léone, J.; Taillan, B.; Hachulla, E.; Roudot-Thoraval, F.; Papo, T.; Schaeffer, A.; Bierling, P.; Godeau, B. Treatment of severe immune thrombocytopenia associated with systemic lupus erythematosus: 59 cases. J. Rheumatol. 2002, 29, 75–83. [Google Scholar] [PubMed]
- Andrès, E.; Zimmer, J.; Noel, E.; Kaltenbach, G.; Koumarianou, A.; Maloisel, F. Idiopathic Thrombocytopenic Purpura: A retrospective analysis in 139 patients of the influence of age on the response to corticosteroids, splenectomy and danazol. Drugs Aging 2003, 20, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, J.; Andres, E.; Noel, E.; Koumarianou, A.; Blickle, J.-F.; Maloisel, F. Current management of adult idiopathic thrombocytopenic purpura in practice: A cohort study of 201 patients from a single center1. Int. J. Lab. Hematol. 2004, 26, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Maloisel, F.; Andrès, E.; Zimmer, J.; Noel, E.; Zamfir, A.; Koumarianou, A.; Dufour, P. Danazol therapy in patients with chronic idiopathic thrombocytopenic purpura: Long-term results. Am. J. Med. 2004, 116, 590–594. [Google Scholar] [CrossRef]
- Daou, S.; Federici, L.; Zimmer, J.; Maloisel, F.; Serraj, K.; Andrès, E. Idiopathic thrombocytopenic purpura in elderly patients: A study of 47 cases from a single reference center. Eur. J. Intern. Med. 2008, 19, 447–451. [Google Scholar] [CrossRef]
- Liu, W.; Gu, X.; Fu, R.; Li, Y.; Lv, M.; Sun, T.; Lv, C.; Liu, X.; Xue, F.; Zhang, L.; et al. The Effect of Danazol in Primary Immune Thrombocytopenia: An Analysis of a Large Cohort from a Single Center in China. Clin. Appl. Thromb. 2015, 22, 727–733. [Google Scholar] [CrossRef]
- Feng, F.-E.; Feng, R.; Wang, M.; Zhang, J.-M.; Jiang, H.; Jiang, Q.; Lu, J.; Liu, H.; Peng, J.; Hou, M.; et al. Oral all-trans retinoic acid plus danazol versus danazol as second-line treatment in adults with primary immune thrombocytopenia: A multicentre, randomised, open-label, phase 2 trial. Lancet Haematol. 2017, 4, e487–e496. [Google Scholar] [CrossRef]
- Mylvaganam, R.; Ahn, Y.S.; Harrington, W.J.; Kim, C.I. Immune modulation by danazol in autoimmune thrombocytopenia. Clin. Immunol. Immunopathol. 1987, 42, 281–287. [Google Scholar] [CrossRef]
- Schreiber, A.D.; Chien, P.; Tomaski, A.; Cines, D.B. Effect of Danazol in Immune Thrombocytopenic Purpura. N. Engl. J. Med. 1987, 316, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.S.; Fernandez, L.F.; Kim, C.I.; Mylvaganam, R.; Temple, J.D., Jr.; Cayer, M.L.; Harrington, W.J. Danazol therapy renders red cells resistant to osmotic lysis. FASEB J. 1989, 3, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Horstman, L.L.; Jy, W.; Schultz, D.R.; Mao, W.W.; Ahn, Y.S. Complement-mediated fragmentation and lysis of opsonized platelets: Ender differences in sensitivity. J. Lab. Clin. Med. 1994, 123, 515–525. [Google Scholar] [PubMed]
- López-Vidal, H.; Peña, C.; Gajardo, C.; Valladares, X.; Cabrera, C.M. Autoimmune hemolytic anemia. Review of 43 cases. Rev. Med. Chil. 2019, 147, 836–841. [Google Scholar] [CrossRef]
- Segal, J.B.; Powe, N.R. Prevalence of immune thrombocytopenia: Analyses of administrative data. J. Thromb. Haemost. 2006, 4, 2377–2383. [Google Scholar] [CrossRef]
- Letchumanan, P.; Thumboo, J. Danazol in the Treatment of Systemic Lupus Erythematosus: A Qualitative Systematic Review. Semin. Arthritis Rheum. 2011, 40, 298–306. [Google Scholar] [CrossRef]
- Bork, K.; Bygum, A.; Hardt, J. Benefits and risks of danazol in hereditary angioedema: A long-term survey of 118 patients. Ann. Allergy Asthma Immunol. 2008, 100, 153–161. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Kuter, D.J. Optimal use of thrombopoietin receptor agonists in immune thrombocytopenia. Ther. Adv. Hematol. 2019, 10, 2040620719841735. [Google Scholar] [CrossRef]
- Neunert, C.; Lim, W.; Crowther, M.A.; Cohen, A.; Solberg, L., Jr.; Crowther, M.A. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011, 117, 4190–4207. [Google Scholar] [CrossRef]
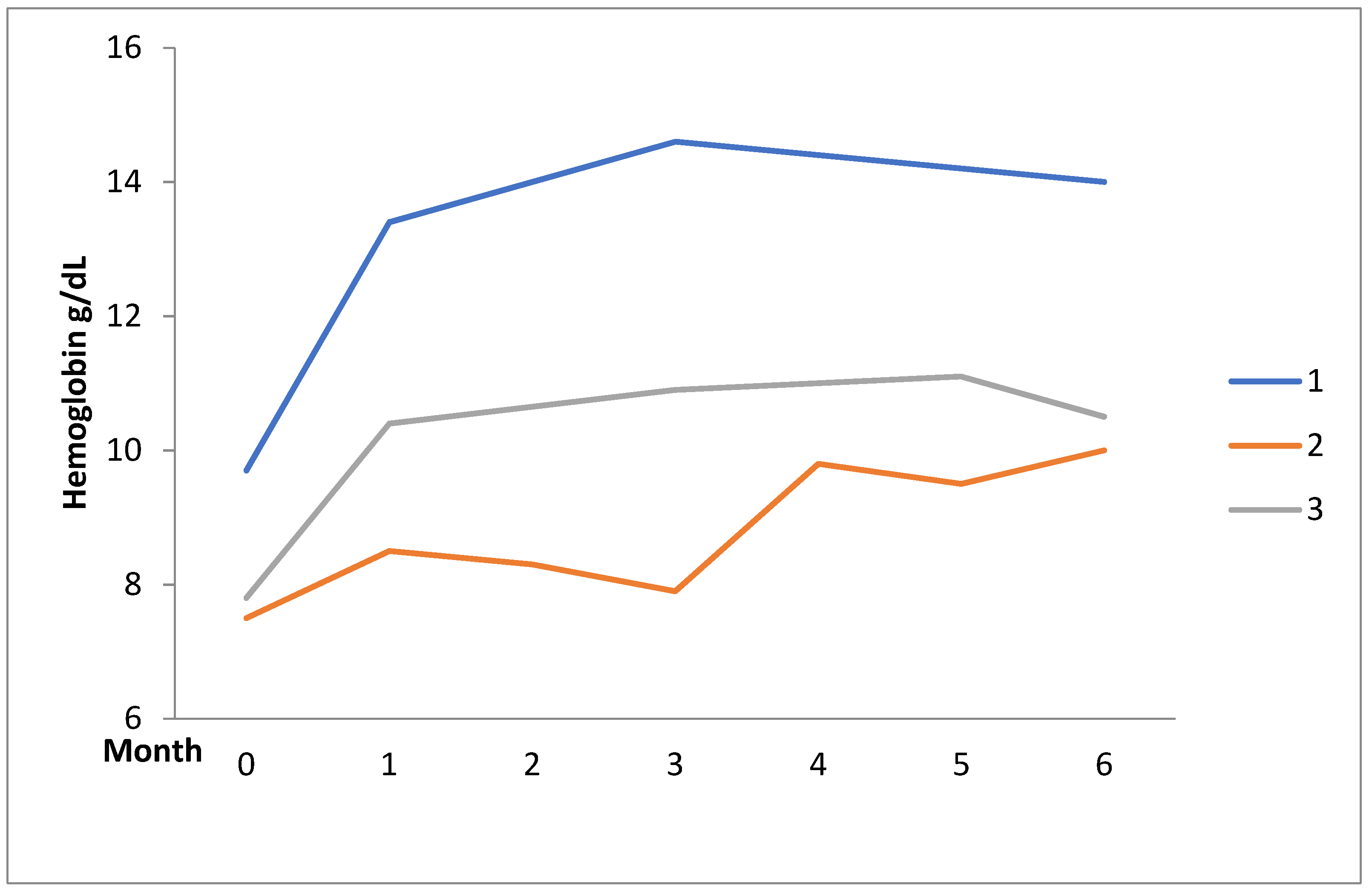
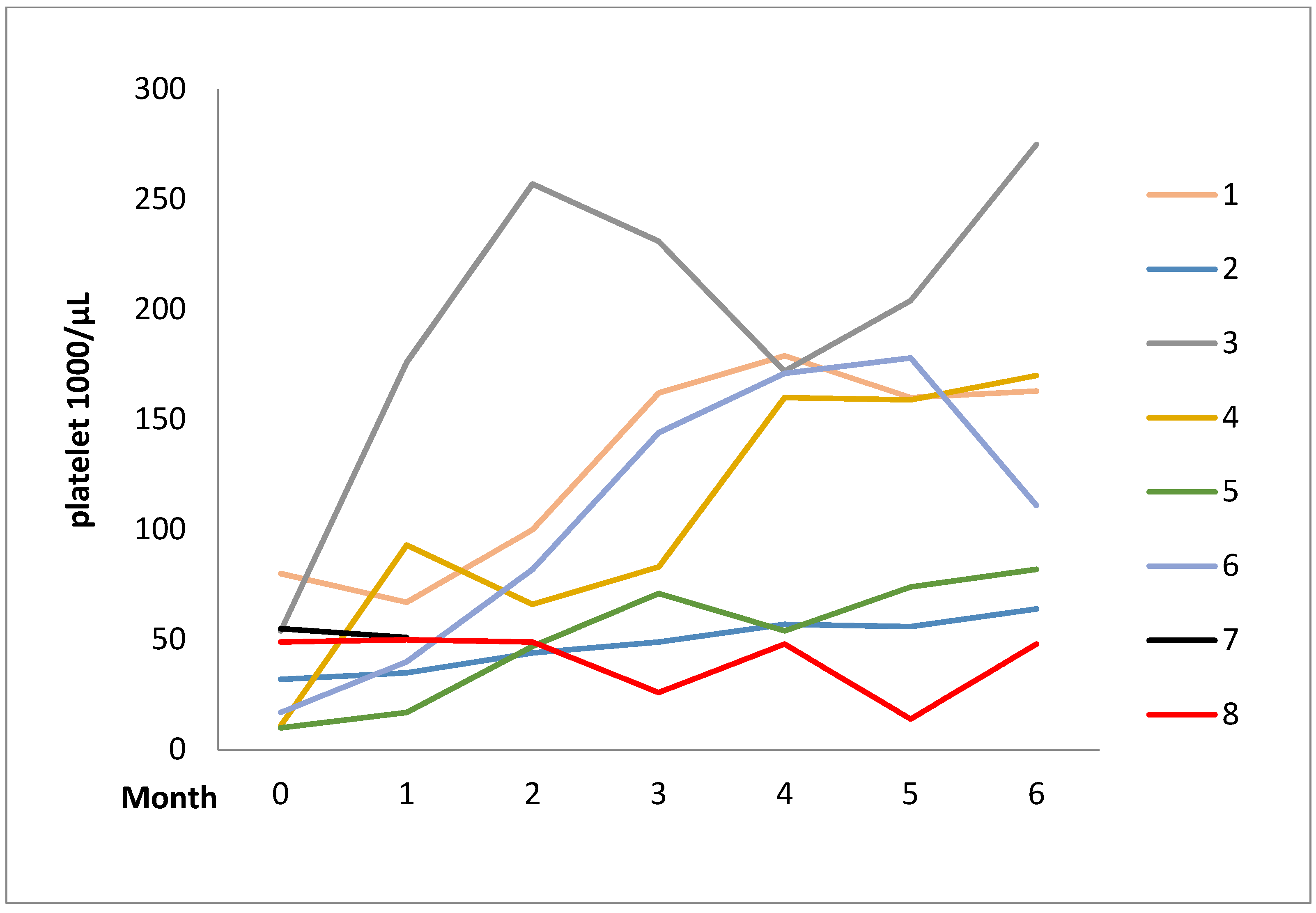
| Patients | Disease | Sex | Age | Concomitant Diseases | Disease Duration (Year) | Previous Therapy | Danazol Daily Dose | Response | Adverse Events | |
|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Mean (6 mo) | |||||||||
| 1 | AIHA | F | 41 | SLE | 4 | GC, AZA | 200 | 167 | Complete | Acne |
| 2 | AIHA | F | 60 | Chronic ischemic heart disease, SLE | 20 | GC, AZA, MMF | 400 | 200 | Partial | - |
| 3 | AIHA | F | 48 | SLE | 6 | GC, AZA, CsA, MMF, CYC (IV/Oral), | 200 | 200 | Partial | - |
| 4 | ITP | F | 43 | Hepatitis b, liver cirrhosis, SLE | 14 | GC, AZA, CYC (oral), | 200 | 200 | Complete | - |
| 5 | ITP | F | 49 | SLE, aplastic anemia | 11 | GC, AZA, CsA, | 400 | 233.3 | Partial | - |
| 6 | ITP | F | 63 | DM, CKD, SLE | 17 | GC, AZA | 200 | 200 | Complete | - |
| 7 | ITP | F | 43 | SLE | 5 | GC, CsA CYC (IV/Oral) | 400 | 233.3 | Complete | Liver Function impairment |
| 8 | ITP | F | 74 | MGUS, Hypothyroidism, DM | 21 | GC, RTX, CYC (Oral) | 400 | 400 | Partial | - |
| 9 | ITP | F | 50 | - | 4 | GC, AZA, MMF, CYC (IV), RTX, splenectomy | 400 | 333.3 | Complete | - |
| 10 | ITP | F | 59 | Hyperthyroidism, SLE | 31 | GC, AZA, CsA | 400 | - * | No | Acne |
| 11 | ITP | F | 49 | SLE | 15 | GC, CsA | 400 | 366.7 | No | - |
| Author | Diseases | Cases | Female (%) | Age | Disease Duration (Year) | Previous Therapies | Initial Danazol Dose/Day | Response, % |
|---|---|---|---|---|---|---|---|---|
| Ahn 1985 [17] | AIHA | 12 | 91.7 | 52.2 | 4.1 | GC, CYC, AZA, splenectomy | 600–800 | 75.0 |
| Manoharan 1987 [18] | AIHA | 3 | 66.7 | 63.3 | 0.4 | GC, splenectomy | 600 | 100 |
| Pignon 1993 [19] | AIHA | 17 | 58.8 | 53.1 | - | GC, splenectomy, immunosuppressants | 400–600 | 70.6 |
| Current series | AIHA | 3 | 100 | 49.7 | 10 | GC, AZA, CsA, MMF, CYC | 200–400 | 100 |
| Manoharan 1987 [18] | ITP | 5 | 80 | 55.8 | 2.7 | GC, splenectomy, CYC, VCR, AZA | 600 | 80.0 |
| Ahn 1989 [20] | ITP | 96 | 62.5 | 52 | - | GC, splenectomy | 400–800 | 61.4 |
| Edelmann 1990 [21] | ITP | 7 | 57.1 | 64 | 6.3 | GC, AZA VCR, colchicine | 800 | 57.1 |
| Kondo 1992 [22] | ITP | 14 | 78.6 | 54 | - | GC, AZA, IVIG, splenectomy | 100–400 | 78.6 |
| Schiavotto 1993 [23] | ITP | 17 | - | - | - | - | 400–800 | 56.0 |
| Arnal 2002 [24] | SLE + ITP | 18 | - | - | 2.2 | GC, AZA, CYC, HCQ, IVIG, | 50–600 | 50.0 |
| Andrès E 2003 [25] | ITP | 33 | 60 | - | - | GC, splenectomy | 600 | 72.0 |
| Zimmer 2004 [26] | ITP | 37 | - | - | - | 600 | 73.0 | |
| Maloisel 2004 [27] | ITP | 57 | 63 | 54 | 2 | GC, IVIG, splenectomy | 600 | 67.0 |
| Daou 2008 [28] | ITP | 15 | - | - | - | GC, splenectomy | 400 | 60.0 |
| Liu W 2016 [29] | ITP | 319 | 69.9 | 51 | - | GC | 100–300 | 65.0 |
| Feng 2017 [30] | ITP | 48 | 60.4 | 32 | - | GC, AZA, CsA, MMF, IVIG, RTX, VCR, rHuTPO | 400 | 43.8 |
| Current series | ITP | 8 | 100 | 53.8 | 14.8 | GC, AZA, CsA, MMF, CYC, RTX, splenectomy | 200–400 | 75.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-E.; Lin, K.-M.; Lin, J.-C.; Lin, Y.-T.; He, H.-R.; Wang, Y.-W.; Yu, S.-F.; Chen, J.-F.; Cheng, T.-T. Danazol in Refractory Autoimmune Hemolytic Anemia or Immune Thrombocytopenia: A Case Series Report and Literature Review. Pharmaceuticals 2022, 15, 1377. https://doi.org/10.3390/ph15111377
Huang H-E, Lin K-M, Lin J-C, Lin Y-T, He H-R, Wang Y-W, Yu S-F, Chen J-F, Cheng T-T. Danazol in Refractory Autoimmune Hemolytic Anemia or Immune Thrombocytopenia: A Case Series Report and Literature Review. Pharmaceuticals. 2022; 15(11):1377. https://doi.org/10.3390/ph15111377
Chicago/Turabian StyleHuang, Hsu-En, Ko-Ming Lin, Jing-Chi Lin, Yu-Ting Lin, Hsiao-Ru He, Yu-Wei Wang, Shan-Fu Yu, Jia-Feng Chen, and Tien-Tsai Cheng. 2022. "Danazol in Refractory Autoimmune Hemolytic Anemia or Immune Thrombocytopenia: A Case Series Report and Literature Review" Pharmaceuticals 15, no. 11: 1377. https://doi.org/10.3390/ph15111377
APA StyleHuang, H.-E., Lin, K.-M., Lin, J.-C., Lin, Y.-T., He, H.-R., Wang, Y.-W., Yu, S.-F., Chen, J.-F., & Cheng, T.-T. (2022). Danazol in Refractory Autoimmune Hemolytic Anemia or Immune Thrombocytopenia: A Case Series Report and Literature Review. Pharmaceuticals, 15(11), 1377. https://doi.org/10.3390/ph15111377






