Insight into the Prospects for Tumor Therapy Based on Photodynamic Immunotherapy
Abstract
1. Introduction
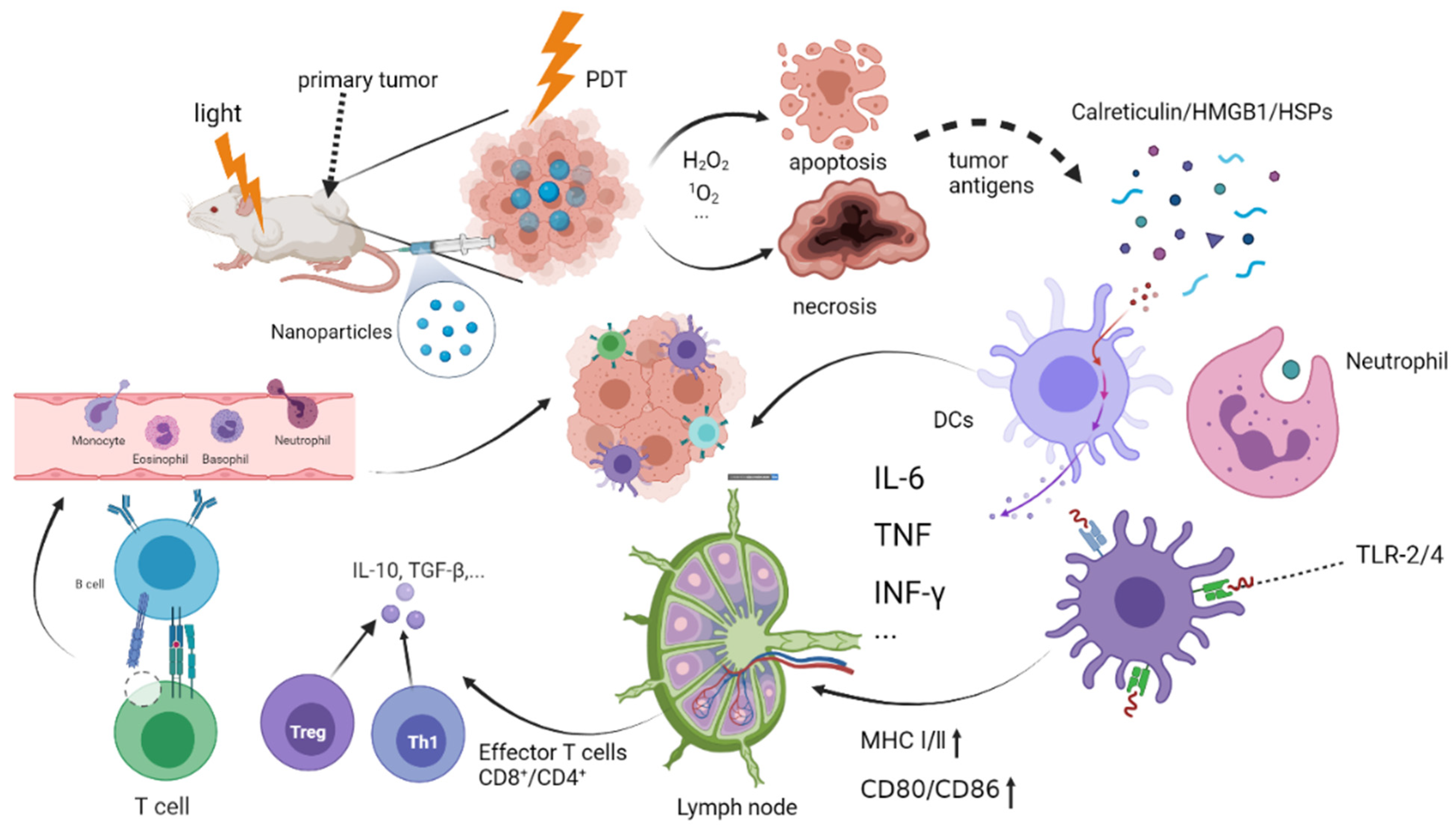
2. PDT Triggers Host Immunity
2.1. The Mechanism of Photodynamic Triggering Immunotherapy
2.2. The Basic Process of Photodynamic Immune Response
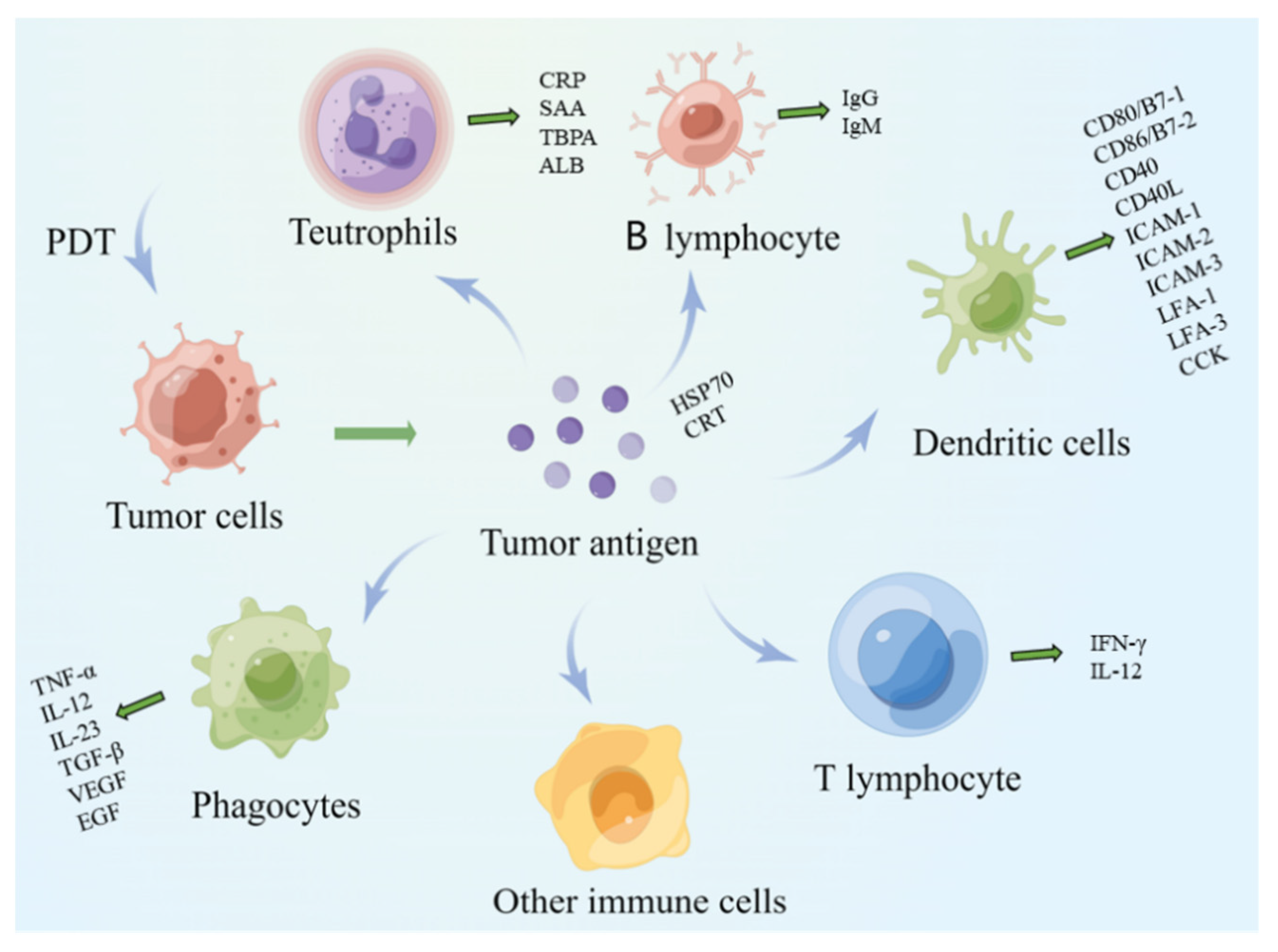
2.2.1. Antigen Recognition Stage
2.2.2. Lymphocyte Activation Stage
- Macrophages
- B lymphocytes
- Dendritic cells
- Natural killer cells
- Neutrophils
2.2.3. Antigen Clearance Phase
2.3. Innate and Adaptive Immune Responses in Anticancer PDT
2.3.1. Innate Response of Innate Immune Cells
2.3.2. Adaptive Immune Response
3. The Methods to Improve PIT
3.1. Immune Stimulators
3.1.1. Exogenous Immune Stimulators
- Chitosan
- CpG oligodeoxynucleotide
- Nanoparticles
- Immune checkpoint inhibitors
3.1.2. Endogenous Immune Stimulators
- Low-dose cyclophosphamide
- Enzyme Stimuli
- Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factors
3.2. TAAs
3.2.1. HSPs
3.2.2. Damage-Associated Molecular Patterns
3.2.3. Cluster of Differentiation
3.3. Immune Cells
3.3.1. Tumor Associated Macrophage
3.3.2. Neutrophils
3.3.3. T Lymphocytes
4. Application of Photodynamic Immune Response
4.1. Photoimmunotherapy
4.2. PDT Combined with Adaptive Immunotherapy
4.3. Cancer Vaccines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | The full name |
| PS | PS |
| PDT | Photodynamic Therapy |
| PIT | Photoimmunotherapy |
| ROS | Reactive Oxygen Species |
| mAb | Monoclonal Antibody |
| DC | Dendritic Cell |
| CRT | Calreticulin |
| HSP | Heat Shock Proteins |
| HSP70 | Heat Shock Proteins 70 |
| HSP90 | Heat Shock Proteins 90 |
| HMGB1 | High Mobility Group Protein 1 |
| ER | Endoplasmic Reticulum |
| MHC | Major Histocompatibility Complex |
| MHCⅠ/Ⅱ | Major Histocompatibility Class Ⅰ/Ⅱ |
| TNF | Tumor Necrosis Factor |
| TNF-α | Tumor Necrosis Factor-A |
| TA-As | Tumor-Associated Antigens |
| DCs-α | Dendritic Cells A |
| IL | Interleukin |
| IL-6 | Interleukin- 6 |
| IL-12 | Interleukin-12 |
| IL-23 | Interleukin-23 |
| TLR4 | Toll-Like Receptors-4 |
| Treg | Regulatory T |
| TGF-β | Transforming Growth Factor Beta |
| VEGF | Vascular Endothelial Growth Factor |
| EGF | Epidermal Growth Factor |
| ICAM-1 | Intercellular Cell Adhesion Molecule-1 |
| ICAM-2 | Intercellular Cell Adhesion Molecule-2 |
| ICAM-3, | Intercellular Cell Adhesion Molecule-3 |
| LFA-1 | Lymphocyte Function-Associated Antigen-1 |
| LFA-3 | Lymphocyte Function-Associated Antigen-3 |
| APCs | Antigen-Presenting Cells |
| CTLIFN-γ | Cytotoxic T LymphocytesInterferon-Γ |
| DAMPs | Damage-Associated Molecular Patterns |
| cDAMPs | Cell-Death-Associated Molecular Patterns |
| TRL2/4 | Toll-Like Receptors 2 And 4 |
| CRP | C Reactive Protein |
| SAA | Serum Amyloid A |
| TBPA | Transferrin-Binding Protein A |
| ALB | Albumin |
| CCK | Chemotactic Cytokines |
| CD80/B7-1 | Recombinant Human Activation B7-1 Antigen |
| CD86/B7-2 | B-Lymphocyte Antigen B7-2 |
| CD40 | Clusters Of Differentiation 40 |
| CD40L | Clusters Of Differentiation 40 Ligand |
| CD91 | Low-Density Lipoprotein Receptor-Related Protein 1 |
| ODN | Oligodeoxynucleotide |
| DNA | Deoxyribonucleic Acid |
| TLR9 | Toll-Like Receptor 9 |
| NPs | Nanoparticles |
| GO | Functionalized Graphene Oxide |
| HK | His-Lys |
| HPPH | Photochlor |
| SPECT | Single Photon Emission Computed Tomography |
| CT | Computed Tomography |
| CD | Cluster Of Differentiation |
| PD-1 | Programmed Cell Death Protein 1 |
| CTLA-4 | Cytotoxic T-Lymphocyte-Associated Protein 4 |
| G-CSF | Granulocyte-Colony Stimulating Factors |
| GM-CSF | Granulocyte Macrophage-Colony Stimulating Factors |
| FDA | The Us Food and Drug Administration |
| HSP | Heat Shock Protein |
| HMGB-1 | High-Mobility Group Protein 1 |
| ATP | Adenosine Triphosphate |
| HER2 | Human Epidermal Growth Factor Receptor-2 |
| TAMs | Tumor-Associated Macrophages |
| ADCC | Antibody-Dependent Cell-Mediated Cytotoxicity |
| HA | Hyaluronic Acid |
| BP | Black Phosphorus |
| MPO | Myeloperoxidase |
| IBR | Ibrutinib |
| DiR | Dialkylcarbocyanine 1,1’-Dioctadecyl-3,3,3’,3’-Tetramethylindotricarbocyanine Iodide |
| CAFs | Cancer-Associated Fibroblasts |
| CXCL | Chemokine (C-X-C Motif) Ligand |
| MMP | Matrix Metalloproteinases |
| PPa | Pyropheophorbide-a |
| scFvs | Single-Chain Fv Fragments |
| ADC | Antibody Conjured Drug |
| VEGF | Vascular Endothelial Growth Factor |
| DAF | Decay-Accelerating Factor |
| HMME-PDT | Hematoporphyrin-PDT |
| ICD | Immunogenic Cell Death |
References
- Zhang, Y.; Wang, B.; Zhao, R.; Zhang, Q.; Kong, X. Multifunctional nanoparticles as photosensitizer delivery carriers for enhanced photodynamic cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 111099. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic Therapy and Anti-Tumour Immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef]
- Kaleta-Richter, M.; Kawczyk-Krupka, A.; Aebisher, D.; Bartusik-Aebisher, D.; Czuba, Z.; Cieślar, G. The capability and potential of new forms of personalized colon cancer treatment: Immunotherapy and Photodynamic Therapy. Photodiagn. Photodyn. Ther. 2019, 25, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xie, Z.; Li, W.; Wu, X.; Jiang, X.; Li, G.; Cao, L.; Zhang, D.; Wang, Q.; Xue, P. Photodynamic immunotherapy of cancers based on nanotechnology: Recent advances and future challenges. J. Nanobiotechnol. 2021, 19, 160. [Google Scholar] [CrossRef]
- Demaria, O.; Cornen, S.; Daëron, M.; Morel, Y.; Medzhitov, R.; Vivier, E. Harnessing innate immunity in cancer therapy. Nature 2019, 574, 45–56. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, L. Photodynamic combinational therapy in cancer treatment. J. BUON 2018, 23, 561–567. [Google Scholar]
- Rao, E.; Hou, Y.; Huang, X.; Wang, L.; Wang, J.; Zheng, W.; Yang, H.; Yu, X.; Yang, K.; Bugno, J.; et al. All-trans retinoic acid overcomes solid tumor radioresistance by inducing inflammatory macrophages. Sci. Immunol. 2021, 6, aba8426. [Google Scholar] [CrossRef]
- Li, F.; Mao, C.; Xin, J.; Shi, Q.; Wu, X. Advances of using photoimmunotherapy for anticancer treatment. Sheng Wu Gong Cheng Xue Bao 2021, 37, 3088–3100. [Google Scholar]
- Slovak, R.; Ludwig, J.M.; Gettinger, S.N.; Herbst, R.S.; Kim, H.S. Immuno-thermal ablations—Boosting the anticancer immune response. J. Immunother. Cancer 2017, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- den Brok, M.H.; Sutmuller, R.P.; van der Voort, R.; Bennink, E.J.; Figdor, C.G.; Ruers, T.J.; Adema, G.J. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004, 64, 4024–4029. [Google Scholar] [CrossRef]
- Yan, J.; Wang, C.; Jiang, X.; Wei, Y.; Wang, Q.; Cui, K.; Xu, X.; Wang, F.; Zhang, L. Application of phototherapeutic-based nanoparticles in colorectal cancer. Int. J. Biol. Sci. 2021, 17, 1361–1381. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, Y.; Cao, W.; Xia, F.; Liu, B.; Niu, J.; Alfranca, G.; Sun, X.; Ma, L.; de la Fuente, J.M.; et al. A tumor microenvironment responsive biodegradable CaCO(3)/MnO(2)- based nanoplatform for the enhanced photodynamic therapy and improved PD-L1 immunotherapy. Theranostics 2019, 9, 6867–6884. [Google Scholar] [CrossRef] [PubMed]
- Takaki, H.; Cornelis, F.; Kako, Y.; Kobayashi, K.; Kamikonya, N.; Yamakado, K. Thermal ablation and immunomodulation: From preclinical experiments to clinical trials. Diagn. Interv. Imaging 2017, 98, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Buytaert, E.; Breyssens, H.; Hendrickx, N. Regulatory pathways in photodynamic therapy induced apoptosis. Photochem. Photobiol. Sci. 2004, 3, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Moor, A.C. Signaling pathways in cell death and survival after photodynamic therapy. J. Photochem. Photobiol. B 2000, 57, 1–13. [Google Scholar] [CrossRef]
- Hou, X.; Tao, Y.; Pang, Y.; Li, X.; Jiang, G.; Liu, Y. Nanoparticle-based photothermal and photodynamic immunotherapy for tumor treatment. Int. J. Cancer 2018, 143, 3050–3060. [Google Scholar] [CrossRef]
- Reginato, E.; Wolf, P.; Hamblin, M.R. Immune response after photodynamic therapy increases anti-cancer and anti-bacterial effects. World J. Immunol. 2014, 4, 1–11. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.; Luo, L.; Jiang, M.; Qin, B.; Yin, H.; Zhu, C.; Yuan, X.; Zhang, J.; Luo, Z.; et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat. Commun. 2019, 10, 3349. [Google Scholar] [CrossRef]
- Bravis, V.; Kaur, A.; Walkey, H.C.; Godsland, I.F.; Misra, S.; Bingley, P.J.; Williams, A.J.K.; Dunger, D.B.; Dayan, C.M.; Peakman, M.; et al. Relationship between islet autoantibody status and the clinical characteristics of children and adults with incident type 1 diabetes in a UK cohort. BMJ Open 2018, 8, e020904. [Google Scholar] [CrossRef]
- Adkins, I.; Fucikova, J.; Garg, A.D.; Agostinis, P.; Špíšek, R. Physical modalities inducing immunogenic tumor cell death for cancer immunotherapy. Oncoimmunology 2014, 3, e968434. [Google Scholar] [CrossRef]
- Showalter, A.; Limaye, A.; Oyer, J.L.; Igarashi, R.; Kittipatarin, C.; Copik, A.J.; Khaled, A.R. Cytokines in immunogenic cell death: Applications for cancer immunotherapy. Cytokine 2017, 97, 123–132. [Google Scholar] [CrossRef]
- Yenari, M.A.; Liu, J.; Zheng, Z.; Vexler, Z.S.; Lee, J.E.; Giffard, R.G. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann. N. Y. Acad. Sci. 2005, 1053, 74–83. [Google Scholar] [CrossRef]
- Nowis, D.; Makowski, M.; Stokłosa, T.; Legat, M.; Issat, T.; Gołab, J. Direct tumor damage mechanisms of photodynamic therapy. Acta Biochim. Pol. 2005, 52, 339–352. [Google Scholar] [CrossRef]
- Radogna, F.; Diederich, M. Stress-induced cellular responses in immunogenic cell death: Implications for cancer immunotherapy. Biochem. Pharmacol. 2018, 153, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Patente, T.A.; Pinho, M.P.; Oliveira, A.A.; Evangelista, G.C.M.; Bergami-Santos, P.C.; Barbuto, J.A.M. Human Dendritic Cells: Their Heterogeneity and Clinical Application Potential in Cancer Immunotherapy. Front. Immunol. 2018, 9, 3176. [Google Scholar] [CrossRef]
- Shevtsov, M.A.; Kim, A.V.; Samochernych, K.A.; Romanova, I.V.; Margulis, B.A.; Guzhova, I.V.; Yakovenko, I.V.; Ischenko, A.M.; Khachatryan, W.A. Pilot study of intratumoral injection of recombinant heat shock protein 70 in the treatment of malignant brain tumors in children. Onco Targets Ther. 2014, 7, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Krysko, D.V.; Vandenabeele, P.; Agostinis, P. DAMPs and PDT-mediated photo-oxidative stress: Exploring the unknown. Photochem. Photobiol. Sci. 2011, 10, 670–680. [Google Scholar] [CrossRef]
- Korbelik, M. Induction of tumor immunity by photodynamic therapy. J. Clin. Laser. Med. Surg. 1996, 14, 329–334. [Google Scholar] [CrossRef]
- Ai, X.; Hu, M.; Wang, Z.; Lyu, L.; Zhang, W.; Li, J.; Yang, H.; Lin, J.; Xing, B. Enhanced Cellular Ablation by Attenuating Hypoxia Status and Reprogramming Tumor-Associated Macrophages via NIR Light-Responsive Upconversion Nanocrystals. Bioconjug. Chem. 2018, 29, 928–938. [Google Scholar] [CrossRef]
- Fujiu, K.; Manabe, I.; Nagai, R. Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J. Clin. Investig. 2011, 121, 3425–3441. [Google Scholar] [CrossRef]
- Wynn, T.A.; Barron, L.; Thompson, R.W.; Madala, S.K.; Wilson, M.S.; Cheever, A.W.; Ramalingam, T. Quantitative assessment of macrophage functions in repair and fibrosis. Curr. Protoc. Immunol. 2011, 93, 14–22. [Google Scholar]
- de Vree, W.J.; Fontijne-Dorsman, A.N.; Koster, J.F.; Sluiter, W. Photodynamic treatment of human endothelial cells promotes the adherence of neutrophils in vitro. Br. J. Cancer 1996, 73, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Fan, Z.; Zhou, F.; Wang, X.; Shi, L.; Zhang, H.; Wang, P.; Yang, D.; Zhang, L.; Chen, W.R.; et al. Improvement of DC vaccine with ALA-PDT induced immunogenic apoptotic cells for skin squamous cell carcinoma. Oncotarget 2015, 6, 17135–17146. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef] [PubMed]
- Jalili, A.; Makowski, M.; Switaj, T.; Nowis, D.; Wilczynski, G.M.; Wilczek, E.; Chorazy-Massalska, M.; Radzikowska, A.; Maslinski, W.; Biały, L.; et al. Effective photoimmunotherapy of murine colon carcinoma induced by the combination of photodynamic therapy and dendritic cells. Clin. Cancer Res. 2004, 10, 4498–4508. [Google Scholar] [CrossRef]
- Pletinckx, K.; Vaeth, M.; Schneider, T.; Beyersdorf, N.; Hünig, T.; Berberich-Siebelt, F.; Lutz, M.B. Immature dendritic cells convert anergic nonregulatory T cells into Foxp3- IL-10+ regulatory T cells by engaging CD28 and CTLA-4. Eur. J. Immunol. 2015, 45, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.N.; Lorenz, K.; Lotfi, R.; Fürst, D.; Tsamadou, C.; Jaekle, S.; Mytilineos, J.; Brunner, C.; Theodorakis, J.; Hoffmann, T.K.; et al. Influence of photodynamic therapy on peripheral immune cell populations and cytokine concentrations in head and neck cancer. Photodiagn. Photodyn. Ther. 2017, 19, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Kabingu, E.; Vaughan, L.; Owczarczak, B.; Ramsey, K.D.; Gollnick, S.O. CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells. Br. J. Cancer 2007, 96, 1839–1848. [Google Scholar] [CrossRef]
- Shaul, M.E.; Fridlender, Z.G. Neutrophils as active regulators of the immune system in the tumor microenvironment. J. Leukoc. Biol. 2017, 102, 343–349. [Google Scholar] [CrossRef]
- Morgan, M.C.; Rashid, R.M. The effect of phototherapy on neutrophils. Int. Immunopharmacol. 2009, 9, 383–388. [Google Scholar] [CrossRef]
- Cecic, I.; Stott, B.; Korbelik, M. Acute phase response-associated systemic neutrophil mobilization in mice bearing tumors treated by photodynamic therapy. Int. Immunopharmacol. 2006, 6, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Fanger, N.A.; Liu, C.; Guyre, P.M.; Wardwell, K.; O’Neil, J.; Guo, T.L.; Christian, T.P.; Mudzinski, S.P.; Gosselin, E.J. Activation of human T cells by major histocompatability complex class II expressing neutrophils: Proliferation in the presence of superantigen, but not tetanus toxoid. Blood 1997, 89, 4128–4135. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, L.; Wang, C.; Yang, R.; Zhuang, Q.; Han, X.; Dong, Z.; Zhu, W.; Peng, R.; Liu, Z. Near-Infrared-Triggered Photodynamic Therapy with Multitasking Upconversion Nanoparticles in Combination with Checkpoint Blockade for Immunotherapy of Colorectal Cancer. ACS Nano 2017, 11, 4463–4474. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-Czop, K.; Kotlinska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy - mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, X.; Liu, X.; Yu, J.; Bai, X.; Wu, X.; Guo, X.; Liu, Z.; Liu, X. Combination of phototherapy with immune checkpoint blockade: Theory and practice in cancer. Front. Immunol. 2022, 13, 955920. [Google Scholar] [CrossRef]
- Garg, A.D.; Nowis, D.; Golab, J.; Agostinis, P. Photodynamic therapy: Illuminating the road from cell death towards anti-tumour immunity. Apoptosis 2010, 15, 1050–1071. [Google Scholar] [CrossRef]
- Korbelik, M. PDT-associated host response and its role in the therapy outcome. Lasers Surg. Med. 2006, 38, 500–508. [Google Scholar] [CrossRef]
- Gollnick, S.O.; Evans, S.S.; Baumann, H.; Owczarczak, B.; Maier, P.; Vaughan, L.; Wang, W.C.; Unger, E.; Henderson, B.W. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br. J. Cancer 2003, 88, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Dejima, H.; Hu, X.; Chen, R.; Zhang, J.; Fujimoto, J.; Parra, E.R.; Haymaker, C.; Hubert, S.M.; Duose, D.; Solis, L.M.; et al. Immune evolution from preneoplasia to invasive lung adenocarcinomas and underlying molecular features. Nat. Commun. 2021, 12, 2722. [Google Scholar] [CrossRef]
- Kousis, P.C.; Henderson, B.W.; Maier, P.G.; Gollnick, S.O. Photodynamic therapy enhancement of antitumor immunity is regulated by neutrophils. Cancer Res. 2007, 67, 10501–10510. [Google Scholar] [CrossRef] [PubMed]
- Vabulas, R.M.; Wagner, H.; Schild, H. Heat shock proteins as ligands of toll-like receptors. Curr. Top. Microbiol. Immunol. 2002, 270, 169–184. [Google Scholar] [PubMed]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar]
- Thimme, R.; Binder, M.; Bartenschlager, R. Failure of innate and adaptive immune responses in controlling hepatitis C virus infection. FEMS Microbiol. Rev. 2012, 36, 663–683. [Google Scholar] [CrossRef]
- Thompson, E.A.; Loré, K. Non-human primates as a model for understanding the mechanism of action of toll-like receptor-based vaccine adjuvants. Curr. Opin. Immunol. 2017, 47, 1–7. [Google Scholar] [CrossRef] [PubMed]
- St Denis, T.G.; Aziz, K.; Waheed, A.A.; Huang, Y.Y.; Sharma, S.K.; Mroz, P.; Hamblin, M.R. Combination approaches to potentiate immune response after photodynamic therapy for cancer. Photochem. Photobiol. Sci. 2011, 10, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Feng, J.; Chen, F.; Guo, C.; Sun, L.; Li, L. Synergistic effect of glycated chitosan and photofrin photodynamic therapy on different breast tumor model. Photodiagn. Photodyn. Ther. 2020, 31, 101842. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, B.; Zhou, W.; Liu, Y. High-Efficiency Synergistic Effect of Supramolecular Nanoparticles Based on Cyclodextrin Prodrug on Cancer Therapy. Biomacromolecules 2020, 21, 4998–5007. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Dong, X.; Liu, Z.; Liu, G.; Liu, Y. Controllable Singlet Oxygen Generation in Water Based on Cyclodextrin Secondary Assembly for Targeted Photodynamic Therapy. Biomacromolecules 2020, 21, 5369–5379. [Google Scholar] [CrossRef] [PubMed]
- Phua, S.Z.F.; Yang, G.; Lim, W.Q.; Verma, A.; Chen, H.; Thanabalu, T.; Zhao, Y. Catalase-Integrated Hyaluronic Acid as Nanocarriers for Enhanced Photodynamic Therapy in Solid Tumor. ACS Nano 2019, 13, 4742–4751. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Guo, J.; Wang, P.; Sun, K.; Chen, J.; Ren, W.; Wei, T.; Yang, Y.; Li, J.; Liu, X.; et al. A self-designed CpG ODN enhanced the anti-melanoma effect of pimozide. Int. Immunopharmacol. 2020, 83, 106397. [Google Scholar] [CrossRef]
- Xia, Y.; Gupta, G.K.; Castano, A.P.; Mroz, P.; Avci, P.; Hamblin, M.R. CpG oligodeoxynucleotide as immune adjuvant enhances photodynamic therapy response in murine metastatic breast cancer. J. Biophoton. 2014, 7, 897–905. [Google Scholar] [CrossRef]
- Kajiwara, A.; Doi, H.; Eguchi, J.; Ishii, S.; Hiraide-Sasagawa, A.; Sakaki, M.; Omori, R.; Hiroishi, K.; Imawari, M. Interleukin-4 and CpG oligonucleotide therapy suppresses the outgrowth of tumors by activating tumor-specific Th1-type immune responses. Oncol. Rep. 2012, 27, 1765–1771. [Google Scholar] [PubMed]
- Ishii, K.J.; Kawakami, K.; Gursel, I.; Conover, J.; Joshi, B.H.; Klinman, D.M.; Puri, R.K. Antitumor therapy with bacterial DNA and toxin: Complete regression of established tumor induced by liposomal CpG oligodeoxynucleotides plus interleukin-13 cytotoxin. Clin. Cancer Res. 2003, 9, 6516–6522. [Google Scholar] [PubMed]
- Poinard, B.; Neo, S.Z.Y.; Yeo, E.L.L.; Heng, H.P.S.; Neoh, K.G.; Kah, J.C.Y. Polydopamine Nanoparticles Enhance Drug Release for Combined Photodynamic and Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 21125–21136. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Gao, D.; Gao, L.; Lai, J.; Zhang, C.; Zhao, Y.; Zhong, L.; Jia, B.; Wang, F.; Chen, X.; et al. Inhibiting Metastasis and Preventing Tumor Relapse by Triggering Host Immunity with Tumor-Targeted Photodynamic Therapy Using Photosensitizer-Loaded Functional Nanographenes. ACS Nano 2017, 11, 10147–10158. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C. The adoptive transfer of cultured T cells for patients with metastatic melanoma. Clin. Dermatol. 2013, 31, 209–219. [Google Scholar] [CrossRef]
- Haugh, A.M.; Probasco, J.C.; Johnson, D.B. Neurologic complications of immune checkpoint inhibitors. Expert Opin. Drug. Saf. 2020, 19, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Khan, F.; Qari, H.A.; Upadhyay, T.K.; Alkhateeb, A.F.; Oves, M. Revolutionization in Cancer Therapeutics via Targeting Major Immune Checkpoints PD-1, PD-L1 and CTLA-4. Pharmaceuticals 2022, 15, 335. [Google Scholar] [CrossRef] [PubMed]
- Kiss, B.; van den Berg, N.S.; Ertsey, R.; McKenna, K.; Mach, K.E.; Zhang, C.A.; Volkmer, J.P.; Weissman, I.L.; Rosenthal, E.L.; Liao, J.C. CD47-Targeted Near-Infrared Photoimmunotherapy for Human Bladder Cancer. Clin. Cancer Res. 2019, 25, 3561–3571. [Google Scholar] [CrossRef]
- Zhong, H.; Lai, Y.; Zhang, R.; Daoud, A.; Feng, Q.; Zhou, J.; Shang, J. Low Dose Cyclophosphamide Modulates Tumor Microenvironment by TGF-β Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 957. [Google Scholar] [CrossRef]
- Elmslie, R.E.; Glawe, P.; Dow, S.W. Metronomic therapy with cyclophosphamide and piroxicam effectively delays tumor recurrence in dogs with incompletely resected soft tissue sarcomas. J. Vet. Intern. Med. 2008, 22, 1373–1379. [Google Scholar] [CrossRef]
- Keklik, M.; Karakus, E.; Kaynar, L.; Akyol, G.; Guven, Z.T.; Celik, S.; Baydar, M.; Sanlı, N.; Unal, A.; Cetin, M. Low-dose cyclophosphamide and granulocyte colony-stimulating factor are sufficient for peripheral blood stem cell mobilization in patients with multiple myeloma. Transfus. Apher. Sci. 2020, 59, 102844. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Lin, J.; Huang, P.; Chen, X. Development of endogenous enzyme-responsive nanomaterials for theranostics. Chem. Soc. Rev. 2018, 47, 5554–5573. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Adhikari, N.; Banerjee, S.; Amin, S.A.; Jha, T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur. J. Med. Chem. 2020, 194, 112260. [Google Scholar] [CrossRef]
- MacPherson, D.S.; McPhee, S.A.; Zeglis, B.M.; Ulijn, R.V. The Impact of Tyrosine Iodination on the Aggregation and Cleavage Kinetics of MMP-9-Responsive Peptide Sequences. ACS Biomater. Sci. Eng. 2022, 8, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Price, T.H.; Chatta, G.S.; Dale, D.C. Effect of recombinant granulocyte colony-stimulating factor on neutrophil kinetics in normal young and elderly humans. Blood 1996, 88, 335–340. [Google Scholar] [CrossRef] [PubMed]
- de Vree, W.J.; Essers, M.C.; Koster, J.F.; Sluiter, W. Role of interleukin 1 and granulocyte colony-stimulating factor in photofrin-based photodynamic therapy of rat rhabdomyosarcoma tumors. Cancer Res. 1997, 57, 2555–2558. [Google Scholar] [PubMed]
- Barreda, D.R.; Hanington, P.C.; Belosevic, M. Regulation of myeloid development and function by colony stimulating factors. Dev. Comp. Immunol. 2004, 28, 509–554. [Google Scholar] [CrossRef]
- Metcalf, D. The colony-stimulating factors and cancer. Nat. Rev. Cancer 2010, 10, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Gutschalk, C.M.; Yanamandra, A.K.; Linde, N.; Meides, A.; Depner, S.; Mueller, M.M. GM-CSF enhances tumor invasion by elevated MMP-2, -9, and -26 expression. Cancer Med. 2013, 2, 117–129. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Xue, T.; Cheng, Q.; Ye, X.; Wang, C.; Yu, Y.; Ji, X.; Wu, M.; Zhang, X.; et al. Liposomes Encapsulating Neoantigens and Black Phosphorus Quantum Dots for Enhancing Photothermal Immunotherapy. J. Biomed. Nanotechnol. 2020, 16, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Abreu, T.R.; Fonseca, N.A.; Gonçalves, N.; Moreira, J.N. Current challenges and emerging opportunities of CAR-T cell therapies. J. Control. Release 2020, 319, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Cocco, C.; Pistoia, V.; Airoldi, I. New perspectives for melanoma immunotherapy: Role of IL-12. Curr. Mol. Med. 2009, 9, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Yang, H.; Zhang, R.; Zhao, J.J.; Hao, D.J. Tumour-associated antigens and their anti-cancer applications. Eur. J. Cancer Care 2017, 26, e12446. [Google Scholar] [CrossRef] [PubMed]
- Harao, M.; Mittendorf, E.A.; Radvanyi, R.G. Peptide-based vaccination and induction of CD8+ T-cell responses against tumor antigens in breast cancer. BioDrugs 2015, 29, 15–30. [Google Scholar] [CrossRef]
- Yun, C.W.; Kim, H.J.; Lim, J.H.; Lee, S.H. Heat Shock Proteins: Agents of Cancer Development and Therapeutic Targets in Anti-Cancer Therapy. Cells 2019, 9, 60. [Google Scholar] [CrossRef]
- Ogawa, M.; Tomita, Y.; Nakamura, Y.; Lee, M.J.; Lee, S.; Tomita, S.; Nagaya, T.; Sato, K.; Yamauchi, T.; Iwai, H.; et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget 2017, 8, 10425–10436. [Google Scholar] [CrossRef]
- Turubanova, V.D.; Mishchenko, T.A.; Balalaeva, I.V.; Efimova, I.; Peskova, N.N.; Klapshina, L.G.; Lermontova, S.A.; Bachert, C.; Krysko, O.; Vedunova, M.V.; et al. Novel porphyrazine-based photodynamic anti-cancer therapy induces immunogenic cell death. Sci. Rep. 2021, 11, 7205. [Google Scholar] [CrossRef]
- Ahmed, A.; Tait, S.W.G. Targeting immunogenic cell death in cancer. Mol. Oncol. 2020, 14, 2994–3006. [Google Scholar] [CrossRef]
- Yatim, N.; Cullen, S.; Albert, M.L. Dying cells actively regulate adaptive immune responses. Nat. Rev. Immunol. 2017, 17, 262–275. [Google Scholar] [CrossRef]
- Land, W.G.; Agostinis, P.; Gasser, S.; Garg, A.D.; Linkermann, A. DAMP-Induced Allograft and Tumor Rejection: The Circle Is Closing. Am. J. Transpl. 2016, 16, 3322–3337. [Google Scholar] [CrossRef] [PubMed]
- Madheswaran, S.; Mungra, N.; Biteghe, F.A.N.; De la Croix Ndong, J.; Arowolo, A.T.; Adeola, H.A.; Ramamurthy, D.; Naran, K.; Khumalo, N.P.; Barth, S. Antibody-Based Targeted Interventions for the Diagnosis and Treatment of Skin Cancers. Anticancer Agents Med. Chem. 2021, 21, 162–186. [Google Scholar] [CrossRef] [PubMed]
- Mączyńska, J.; Da Pieve, C.; Burley, T.A.; Raes, F.; Shah, A.; Saczko, J.; Harrington, K.J.; Kramer-Marek, G. Immunomodulatory activity of IR700-labelled affibody targeting HER2. Cell Death Dis. 2020, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Muntimadugu, E.; Kumar, R.; Saladi, S.; Rafeeqi, T.A.; Khan, W. CD44 targeted chemotherapy for co-eradication of breast cancer stem cells and cancer cells using polymeric nanoparticles of salinomycin and paclitaxel. Colloids Surf. B Biointerfaces 2016, 143, 532–546. [Google Scholar] [CrossRef]
- Kretschmer, K.; Apostolou, I.; Jaeckel, E.; Khazaie, K.; von Boehmer, H. Making regulatory T cells with defined antigen specificity: Role in autoimmunity and cancer. Immunol. Rev. 2006, 212, 163–169. [Google Scholar] [CrossRef]
- Maruoka, Y.; Furusawa, A.; Okada, R.; Inagaki, F.; Fujimura, D.; Wakiyama, H.; Kato, T.; Nagaya, T.; Choyke, P.L.; Kobayashi, H. Combined CD44- and CD25-Targeted Near-Infrared Photoimmunotherapy Selectively Kills Cancer and Regulatory T Cells in Syngeneic Mouse Cancer Models. Cancer Immunol. Res. 2020, 8, 345–355. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, K.; Li, J.; Tang, X.; Lin, J.; Lu, X.; Huang, R.; Yang, B.; Shi, Y.; Ye, D.; et al. Melatonin attenuates choroidal neovascularization by regulating macrophage/microglia polarization via inhibition of RhoA/ROCK signaling pathway. J. Pineal. Res. 2020, 69, e12660. [Google Scholar] [CrossRef]
- Hao, S.; Meng, J.; Zhang, Y.; Liu, J.; Nie, X.; Wu, F.; Yang, Y.; Wang, C.; Gu, N.; Xu, H. Macrophage phenotypic mechanomodulation of enhancing bone regeneration by superparamagnetic scaffold upon magnetization. Biomaterials 2017, 140, 16–25. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, J.; Li, C.; Lu, Y.; Cheng, L.; Liu, J. A targeting black phosphorus nanoparticle based immune cells nano-regulator for photodynamic/photothermal and photo-immunotherapy. Bioact. Mater. 2021, 6, 472–489. [Google Scholar] [CrossRef]
- Fingar, V.H.; Wieman, T.J.; Wiehle, S.A.; Cerrito, P.B. The role of microvascular damage in photodynamic therapy: The effect of treatment on vessel constriction, permeability, and leukocyte adhesion. Cancer Res. 1992, 52, 4914–4921. [Google Scholar] [PubMed]
- Sun, J.; Cecic, I.; Parkins, C.S.; Korbelik, M. Neutrophils as inflammatory and immune effectors in photodynamic therapy-treated mouse SCCVII tumours. Photochem. Photobiol. Sci. 2002, 1, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Li, C.; Yan, X.; Zhang, H.; Luo, X.; Gao, X.; Liu, X.; Song, Y.; Deng, Y. Photodynamic/photothermal therapy enhances neutrophil-mediated ibrutinib tumor delivery for potent tumor immunotherapy: More than one plus one? Biomaterials 2021, 269, 120652. [Google Scholar] [CrossRef]
- Kishton, R.J.; Sukumar, M.; Restifo, N.P. Metabolic Regulation of T Cell Longevity and Function in Tumor Immunotherapy. Cell Metab. 2017, 26, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Tang, W.; Wang, M.; Zhou, S.; Wang, H.; Wu, Z.; Hao, Z.; Li, Z.; Liu, L.; Xie, J. Protein Nanocage Mediated Fibroblast-Activation Protein Targeted Photoimmunotherapy to Enhance Cytotoxic T Cell Infiltration and Tumor Control. Nano Lett. 2017, 17, 862–869. [Google Scholar] [CrossRef]
- Oh, D.S.; Kim, H.; Oh, J.E.; Jung, H.E.; Lee, Y.S.; Park, J.H.; Lee, H.K. Intratumoral depletion of regulatory T cells using CD25-targeted photodynamic therapy in a mouse melanoma model induces antitumoral immune responses. Oncotarget 2017, 8, 47440–47453. [Google Scholar] [CrossRef]
- Reginato, E.; Lindenmann, J.; Langner, C.; Schweintzger, N.; Bambach, I.; Smolle-Jüttner, F.; Wolf, P. Photodynamic therapy downregulates the function of regulatory T cells in patients with esophageal squamous cell carcinoma. Photochem. Photobiol. Sci. 2014, 13, 1281–1289. [Google Scholar] [CrossRef]
- Pizova, K.; Tomankova, K.; Daskova, A.; Binder, S.; Bajgar, R.; Kolarova, H. Photodynamic therapy for enhancing antitumour immunity. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2012, 156, 93–102. [Google Scholar] [CrossRef]
- Kwitniewski, M.; Juzeniene, A.; Glosnicka, R.; Moan, J. Immunotherapy: A way to improve the therapeutic outcome of photodynamic therapy? Photochem. Photobiol. Sci. 2008, 7, 1011–1017. [Google Scholar] [CrossRef]
- Yarmush, M.L.; Thorpe, W.P.; Strong, L.; Rakestraw, S.L.; Toner, M.; Tompkins, R.G. Antibody Targeted Photolysis. Crit. Rev. Ther. Drug Carr. Syst. 1993, 10, 197–252. [Google Scholar]
- Savellano, M.D.; Hasan, T. Targeting cells that overexpress the epidermal growth factor receptor with polyethylene glycolated BPD verteporfin photosensitizer immunoconjugates. Photochem. Photobiol. 2003, 77, 431–439. [Google Scholar] [CrossRef]
- Bhatti, M.; Yahioglu, G.; Milgrom, L.R.; Garcia-Maya, M.; Chester, K.A.; Deonarain, M.P. Targeted photodynamic therapy with multiply-loaded recombinant antibody fragments. Int. J. Cancer 2008, 122, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, S.O.; Vaughan, L.; Henderson, B.W. Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res. 2002, 62, 1604–1608. [Google Scholar] [PubMed]
- Korbelik, M.; Sun, J. Cancer treatment by photodynamic therapy combined with adoptive immunotherapy using genetically altered natural killer cell line. Int. J. Cancer 2001, 93, 269–274. [Google Scholar] [CrossRef]
- Korbelik, M.; Cecic, I. Enhancement of tumour response to photodynamic therapy by adjuvant mycobacterium cell-wall treatment. J. Photochem. Photobiol. B 1998, 44, 151–158. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, X.; Kalkanis, S.N.; Zhang, Z.; Yang, H.; Katakowski, M.; Hong, X.; Zheng, X.; Zhu, Z.; Chopp, M. Combination therapy with antiangiogenic treatment and photodynamic therapy for the nude mouse bearing U87 glioblastoma. Photochem. Photobiol. 2008, 84, 128–137. [Google Scholar] [CrossRef]
- Bhuvaneswari, R.; Thong, P.S.; Gan, Y.Y.; Soo, K.C.; Olivo, M. Evaluation of hypericin-mediated photodynamic therapy in combination with angiogenesis inhibitor bevacizumab using in vivo fluorescence confocal endomicroscopy. J. Biomed. Opt. 2010, 15, 11114. [Google Scholar] [CrossRef]
- Korbelik, M.; Cecic, I. Complement activation cascade and its regulation: Relevance for the response of solid tumors to photodynamic therapy. J. Photochem. Photobiol. B 2008, 93, 53–59. [Google Scholar] [CrossRef]
- Hwang, H.S.; Shin, H.; Han, J.; Na, K. Combination of photodynamic therapy (PDT) and anti-tumor immunity in cancer therapy. J. Pharm. Investig. 2018, 48, 143–151. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef]
- Korbelik, M.; Sun, J. Photodynamic therapy-generated vaccine for cancer therapy. Cancer Immunol. Immunother. 2006, 55, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Hashmi, J.T.; Huang, Y.Y.; Lange, N.; Hamblin, M.R. Stimulation of anti-tumor immunity by photodynamic therapy. Expert Rev. Clin. Immunol. 2011, 7, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M. Photodynamic therapy-generated cancer vaccines. Methods Mol. Biol. 2010, 635, 147–153. [Google Scholar] [PubMed]
- Gollnick, S.O.; Owczarczak, B.; Maier, P. Photodynamic therapy and anti-tumor immunity. Lasers Surg. Med. 2006, 38, 509–515. [Google Scholar] [CrossRef]
- Korbelik, M.; Stott, B.; Sun, J. Photodynamic therapy-generated vaccines: Relevance of tumour cell death expression. Br. J. Cancer 2007, 97, 1381–1387. [Google Scholar] [CrossRef]
- Shixiang, Y.; Xi, S.; Junliang, L.; Shanyi, Z.; Xingke, X.; Meiguang, Z.; Kai, W.; Fangcheng, L. Antitumor efficacy of a photodynamic therapy-generated dendritic cell glioma vaccine. Med. Oncol. 2011, 28 (Suppl. 1), S453–S461. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Wei, Y.; Jiang, X.; Wang, C.; Liu, M.; Yan, J.; Zhang, L.; Zhou, Y. Insight into the Prospects for Tumor Therapy Based on Photodynamic Immunotherapy. Pharmaceuticals 2022, 15, 1359. https://doi.org/10.3390/ph15111359
Cheng X, Wei Y, Jiang X, Wang C, Liu M, Yan J, Zhang L, Zhou Y. Insight into the Prospects for Tumor Therapy Based on Photodynamic Immunotherapy. Pharmaceuticals. 2022; 15(11):1359. https://doi.org/10.3390/ph15111359
Chicago/Turabian StyleCheng, Xiaoxia, Yiqu Wei, Xiaomei Jiang, Chunli Wang, Mengyu Liu, Jiaxin Yan, Lei Zhang, and Yaqi Zhou. 2022. "Insight into the Prospects for Tumor Therapy Based on Photodynamic Immunotherapy" Pharmaceuticals 15, no. 11: 1359. https://doi.org/10.3390/ph15111359
APA StyleCheng, X., Wei, Y., Jiang, X., Wang, C., Liu, M., Yan, J., Zhang, L., & Zhou, Y. (2022). Insight into the Prospects for Tumor Therapy Based on Photodynamic Immunotherapy. Pharmaceuticals, 15(11), 1359. https://doi.org/10.3390/ph15111359





