Bcl-XL but Not Bcl-2 Is a Potential Target in Medulloblastoma Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Growth Conditions
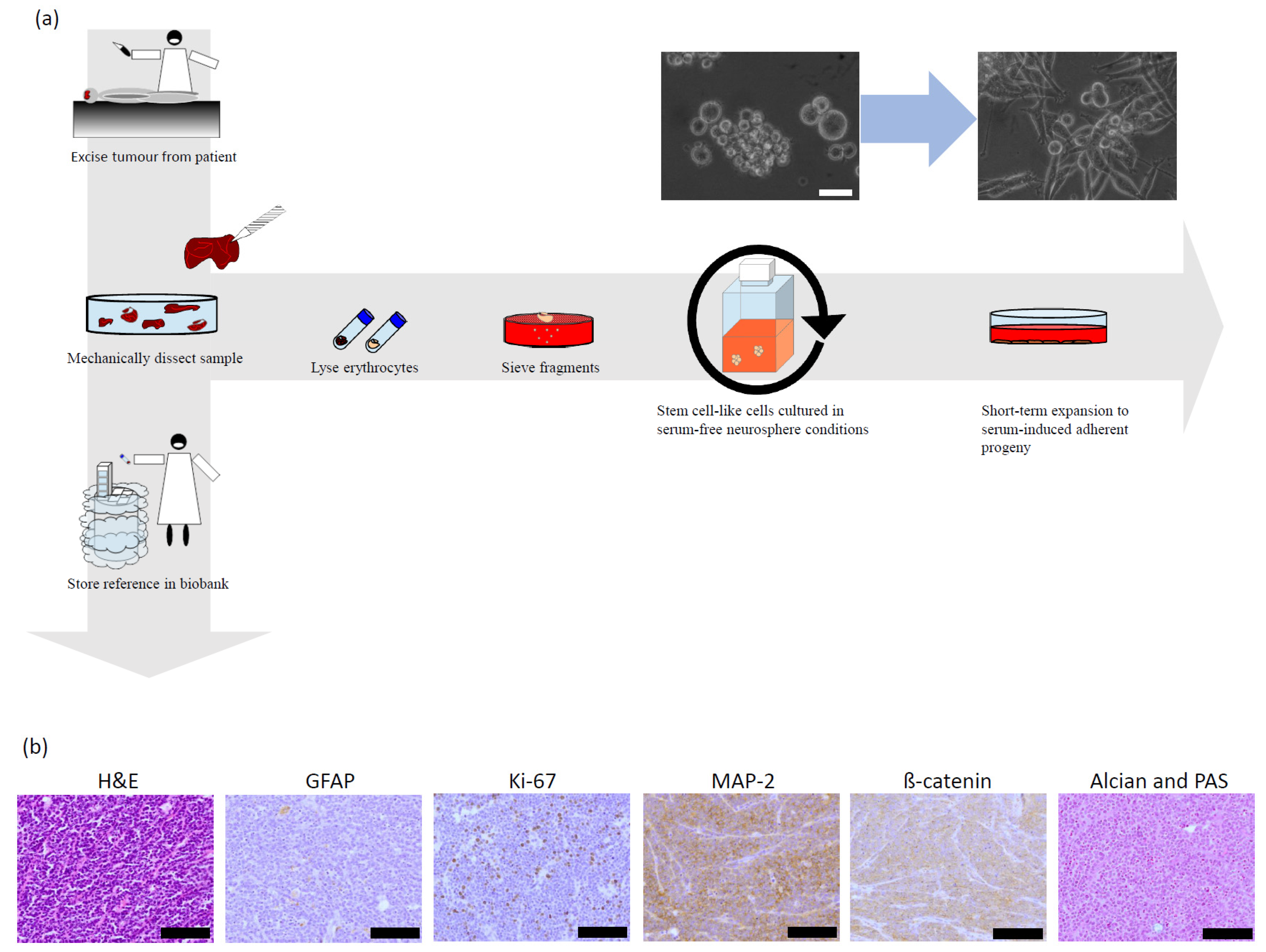
2.2. Primary Patient Material
2.3. Reagents
- Concentrations used for established cell lines:
- ABT-199 10 nM
- ABT-263 1 µM
- Doxorubicin 5 nM (Daoy) or 10 nM (all others)
- Vincristine 1 nM (Daoy) or 0.5 nM (all others)
- Growth factor reduction: 1.5% FCS (Daoy) or 5% FCS (the others)
- Concentrations used for primary patient-derived cells:
- ABT-199 10 nM
- ABT-263 1 µM
- Doxorubicin 5 nM and 10 nM (lower concentration for subsequent experiments)
- Vincristine 0.5 nM and 1 nM (lower concentration for subsequent experiments)
- Growth factor reduction: 50% of original concentration for primary cells,
- 25% of original concentration for their differentiated progeny.
2.4. Cell Viability Assay
2.5. Western Blot Analysis
2.6. Changes in Cell Number (‘CASY’)
2.7. Determination of Apoptosis
2.8. Pathology of the Original Tumour
2.9. Characterisation of the Stem Cell-like Cells and Their Differentiated Progenies
2.10. Chorioallantoic Membrane (CAM) Assay
2.11. Statistical Analysis
3. Results
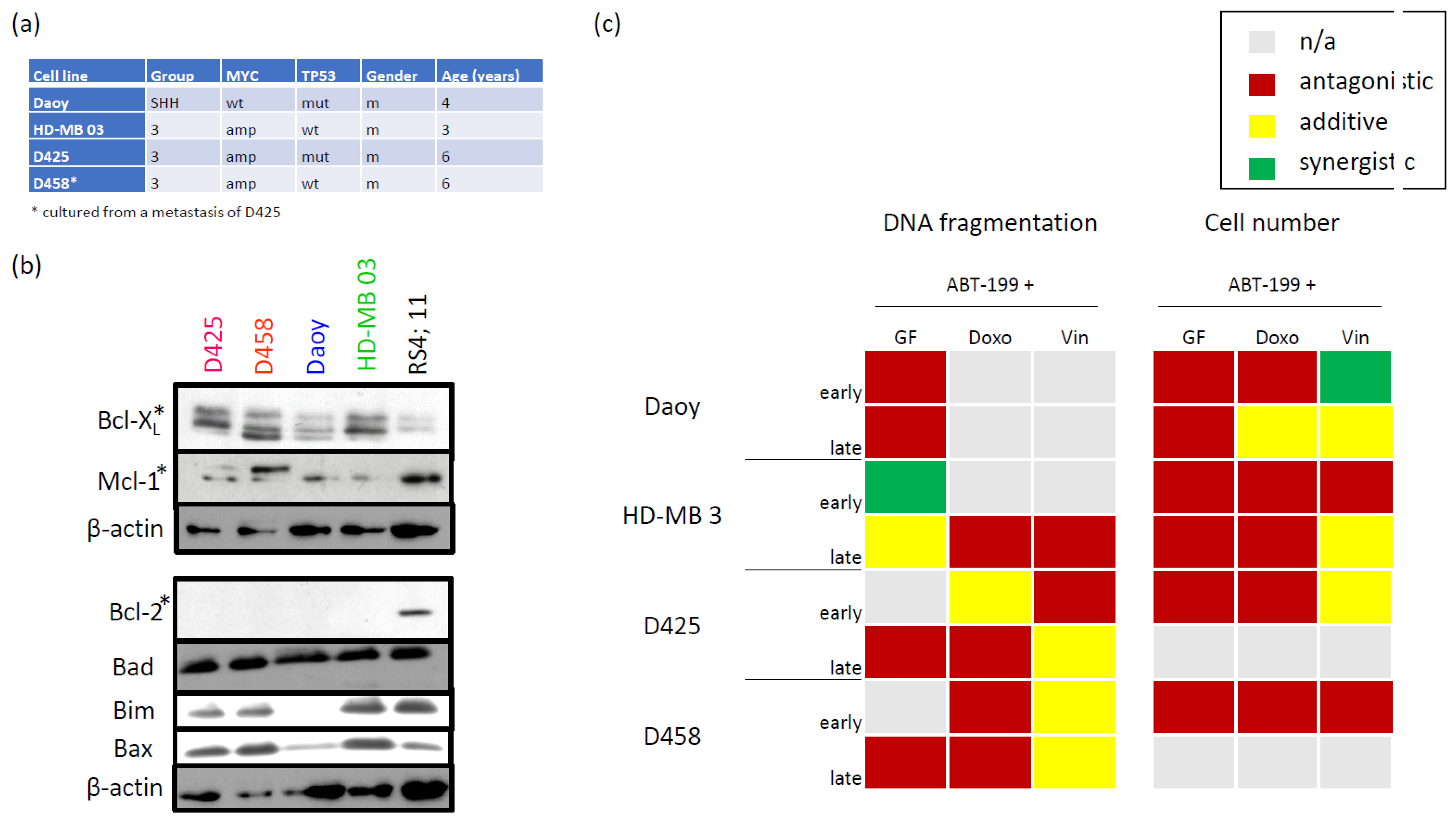
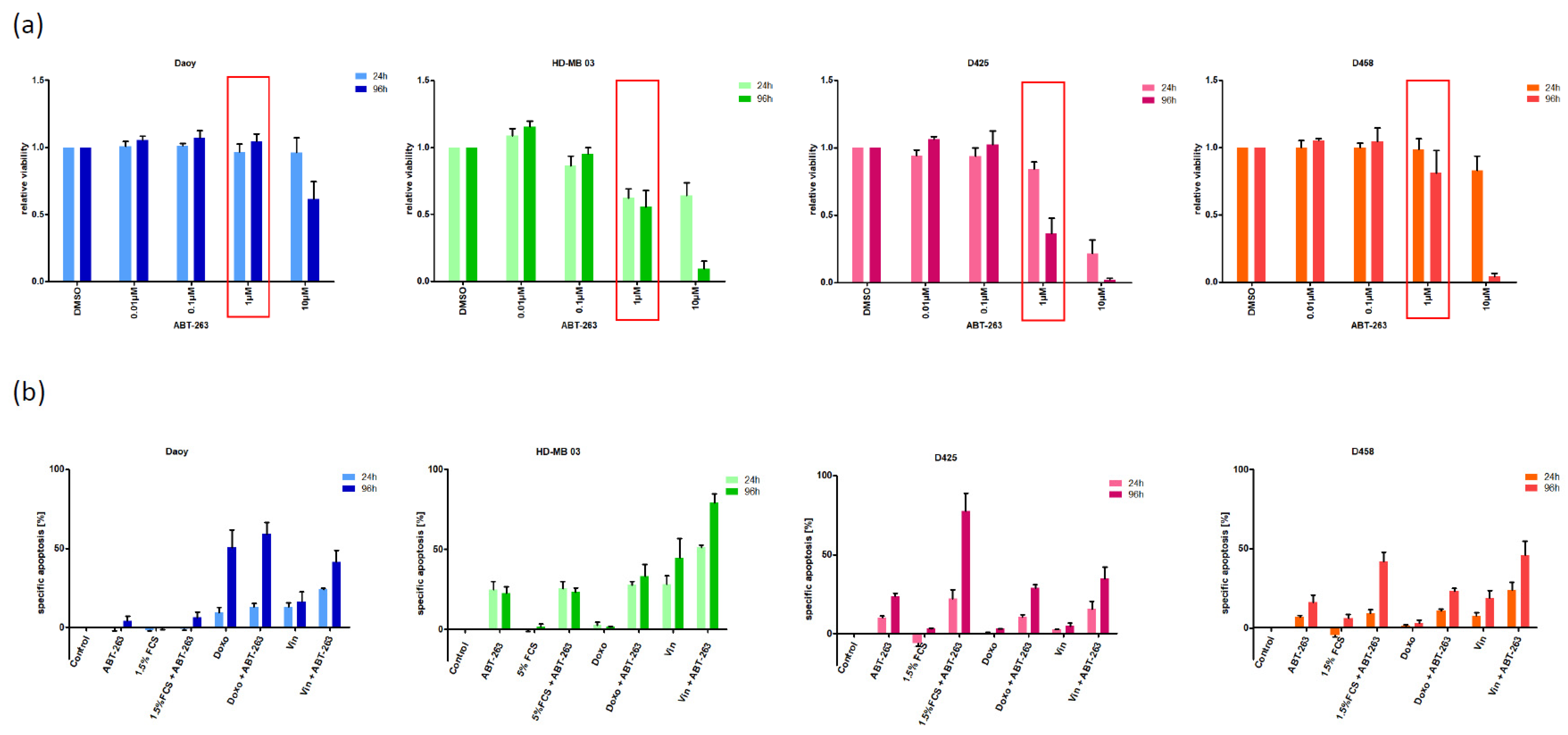
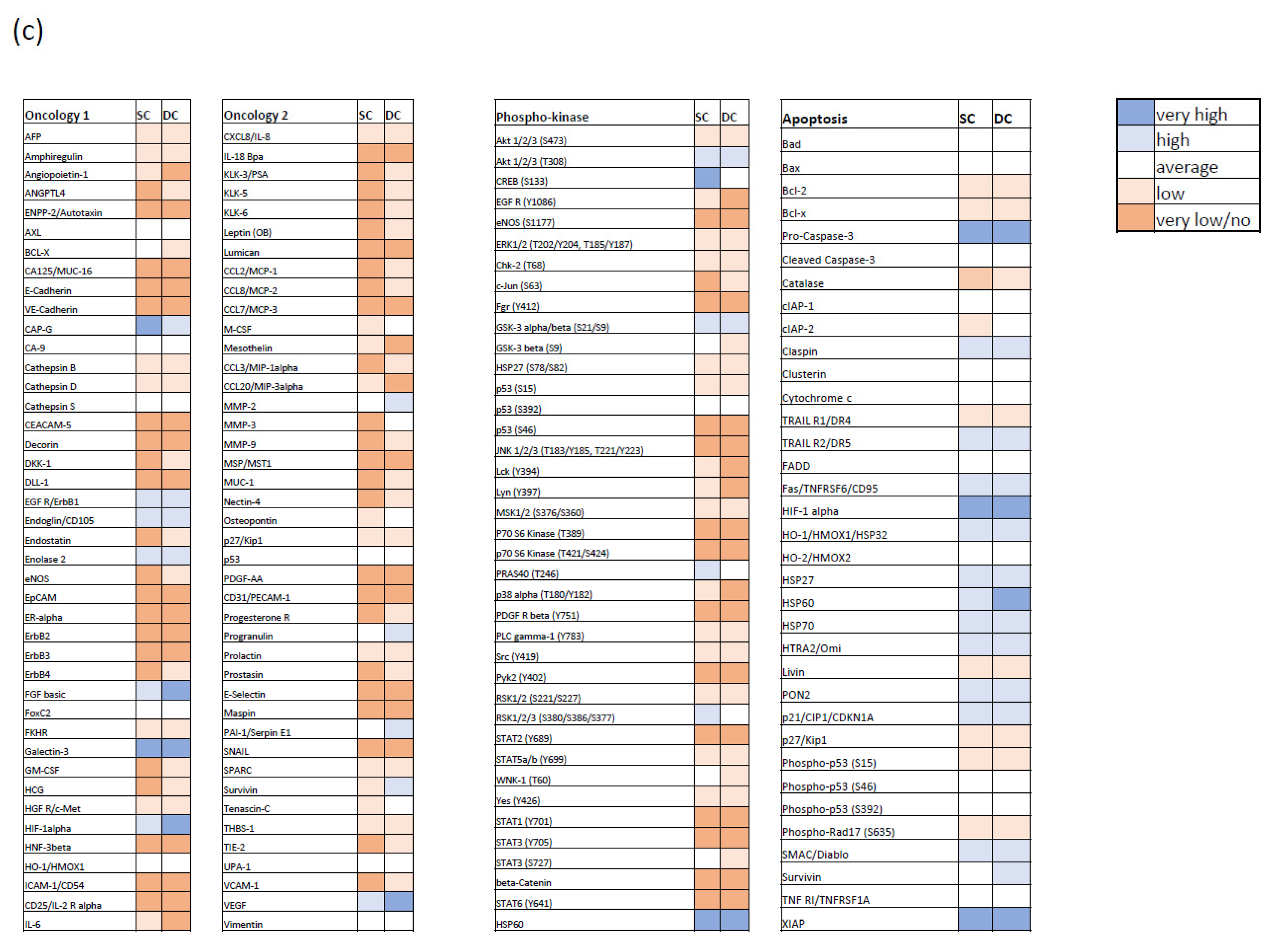
3.1. Characterizing the Expression of the Bcl-2 Family in Medulloblastoma Cell Lines and Identifying Bcl-XL as Potential Therapeutic Target
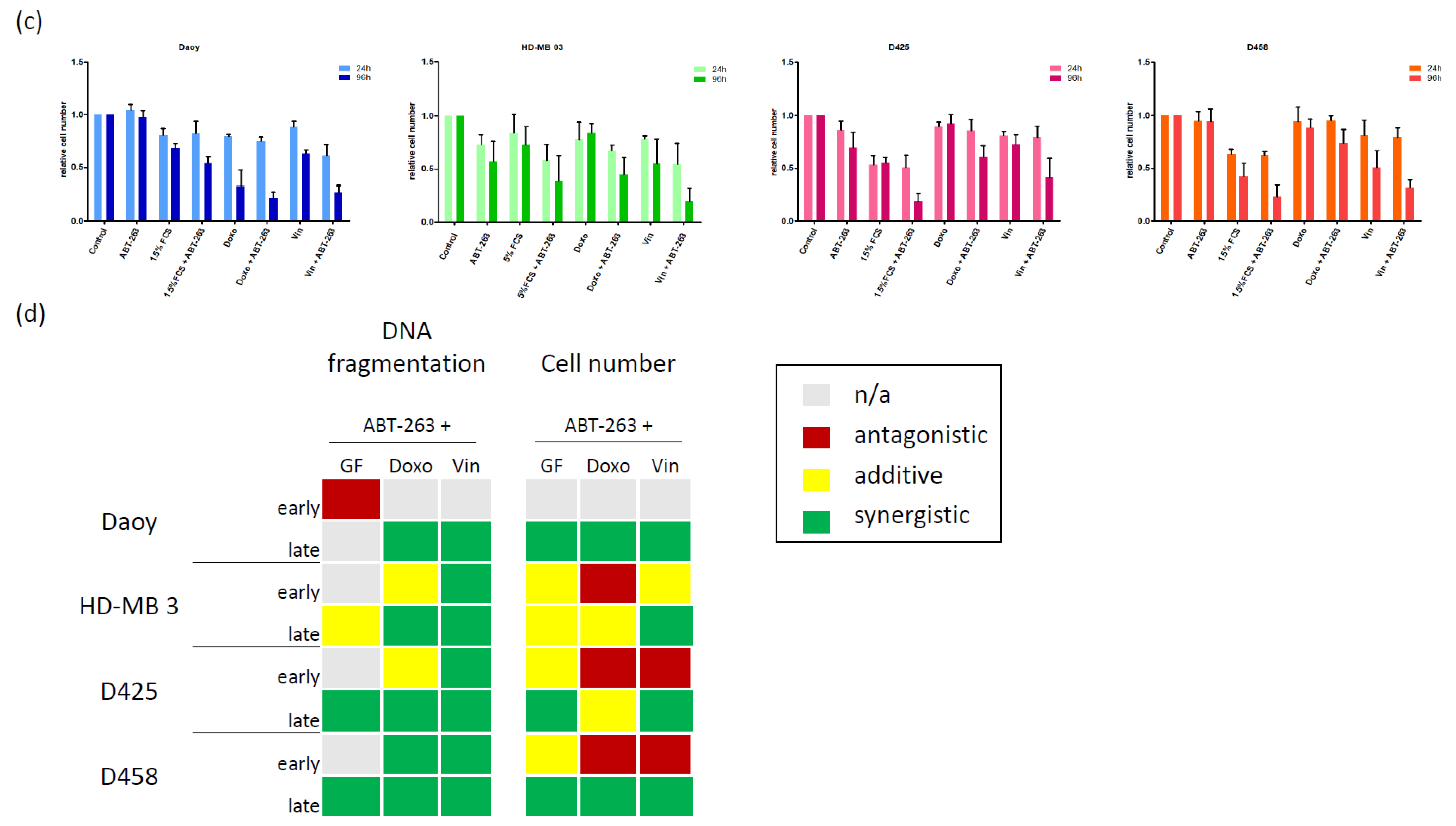
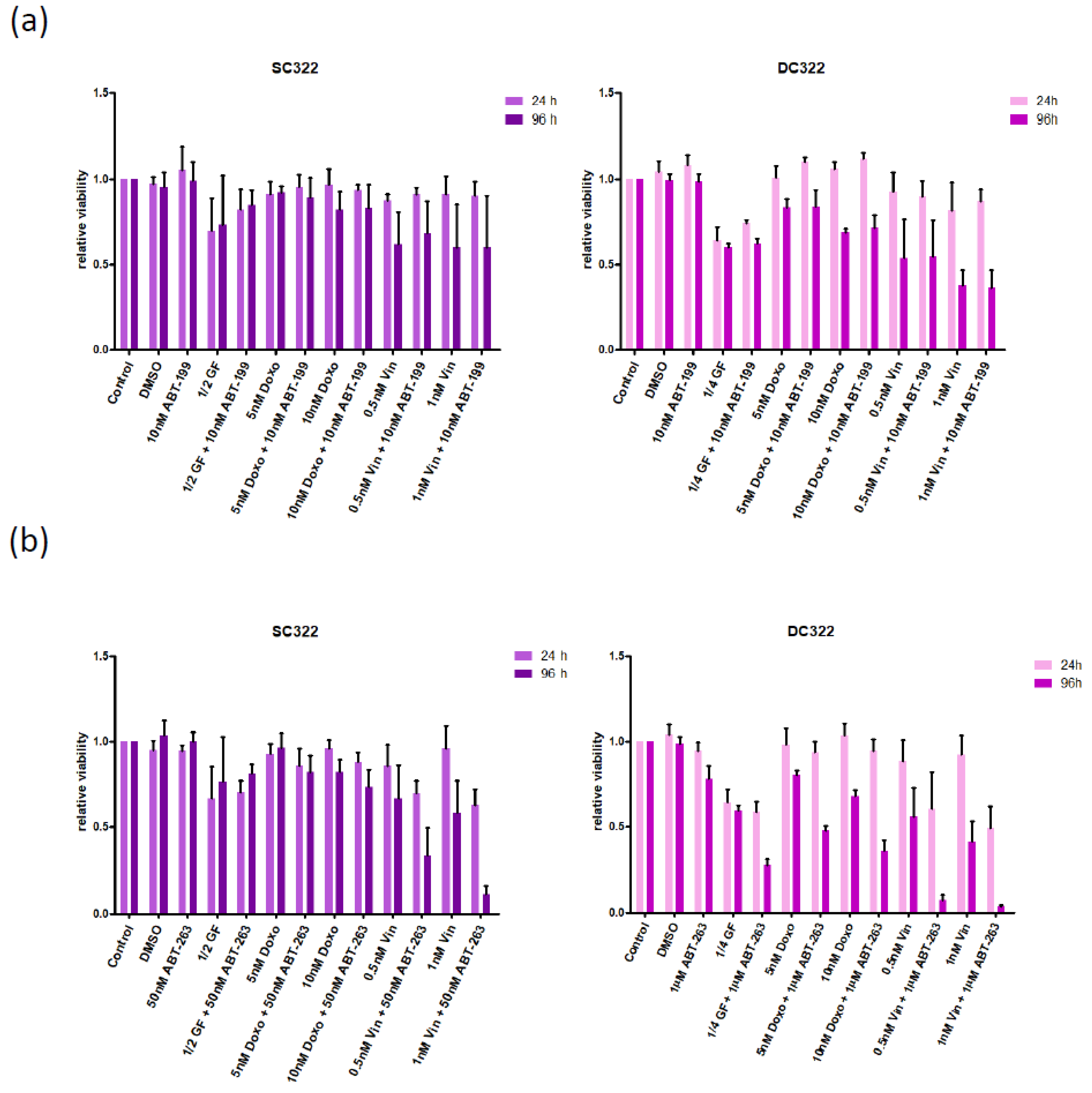
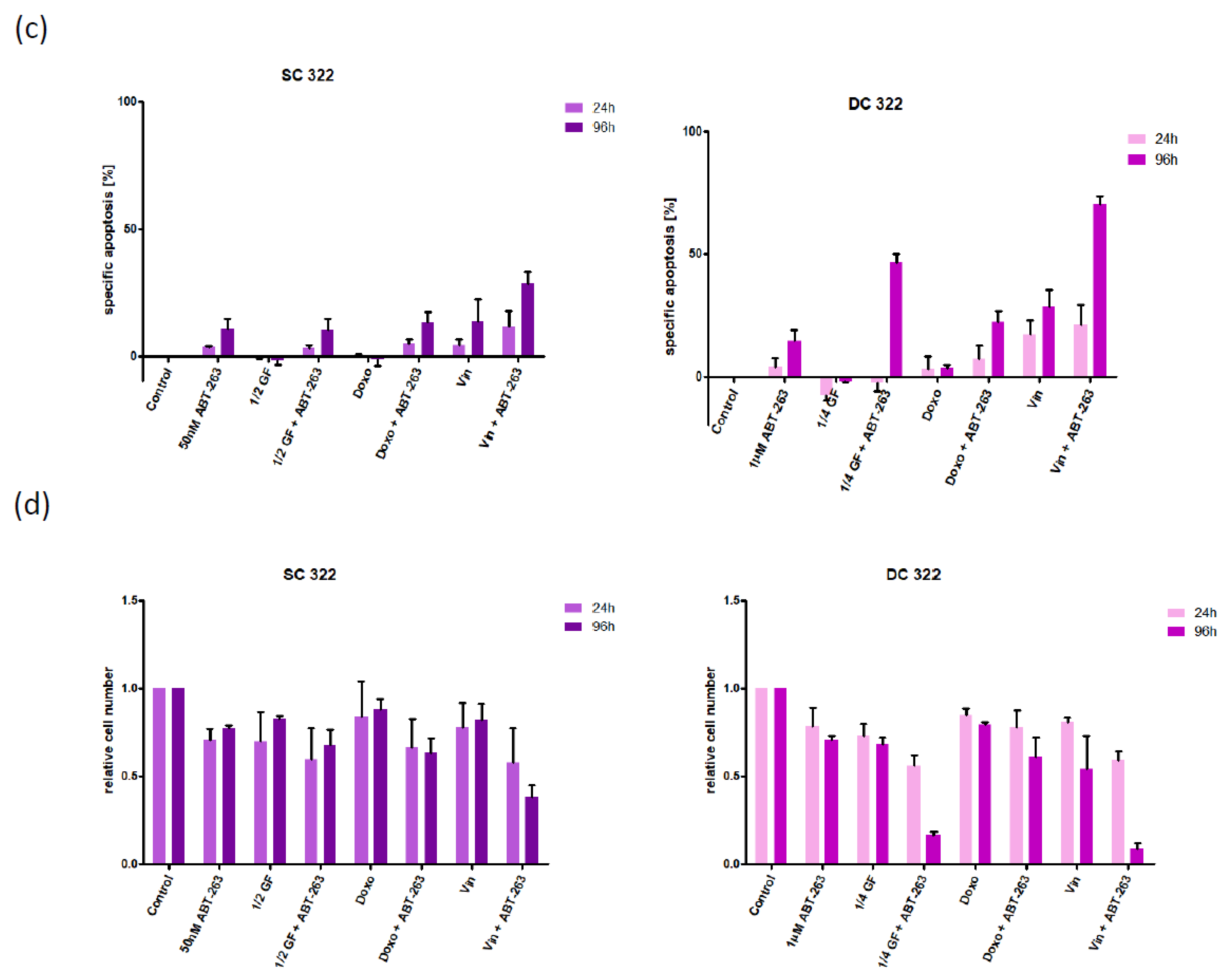
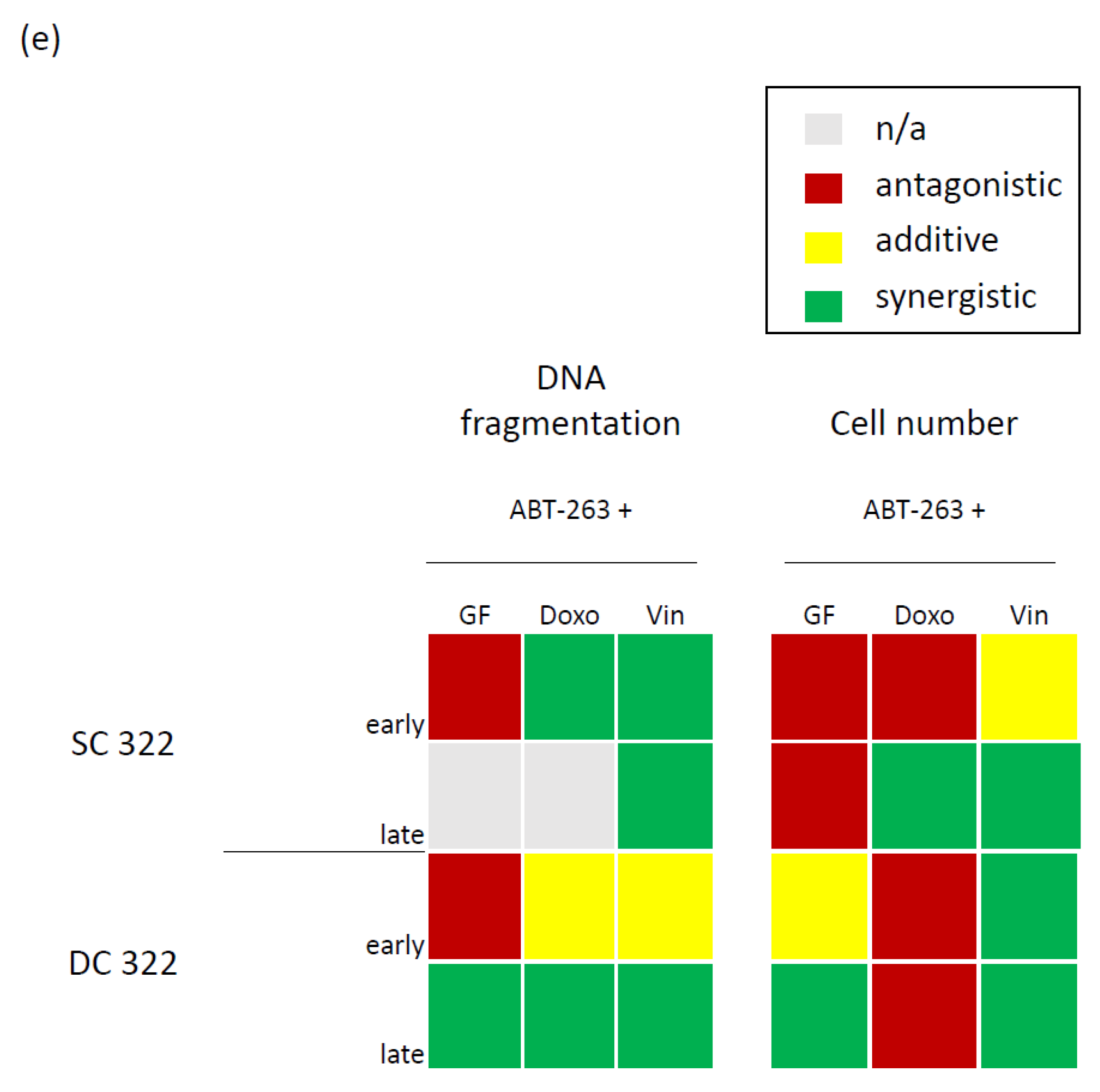
3.2. Bcl-XL Modulation Is Also a Promising Therapeutic Approach in Primary Material
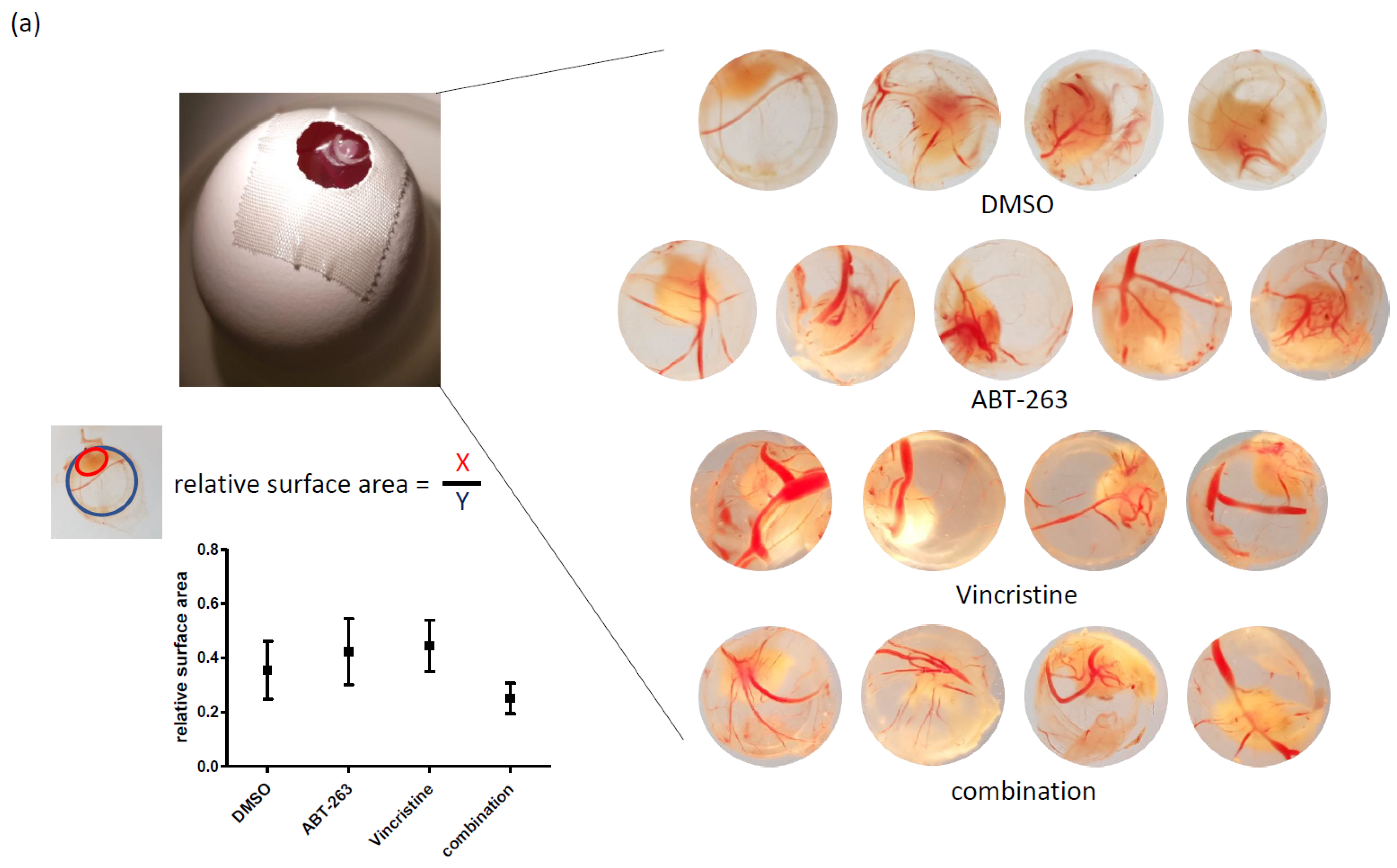
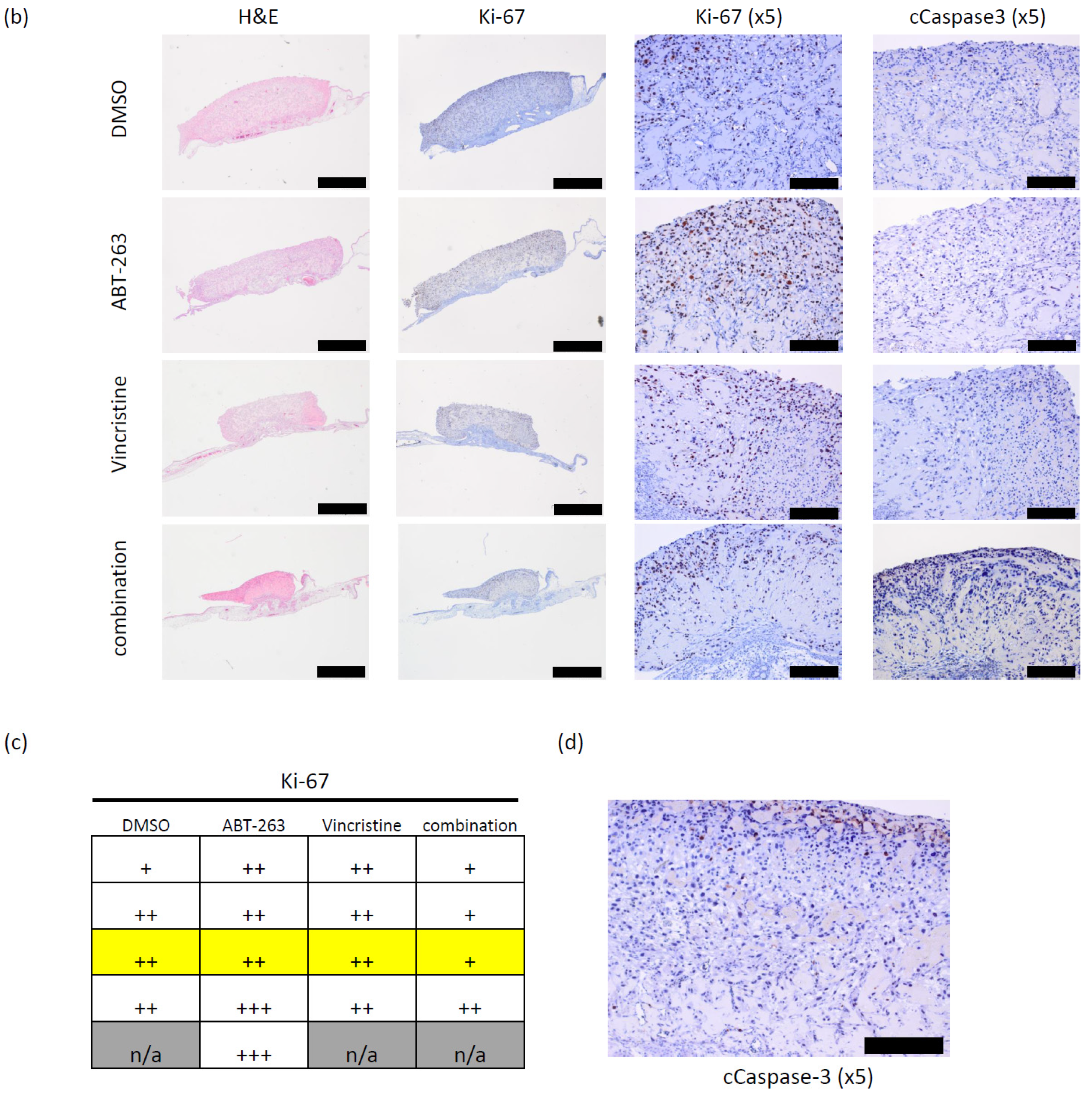
3.3. Bcl-XL Modulation Also Show Effects in an Artificial Tumour Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pizer, B.L.; Clifford, S.C. The potential impact of tumour biology on improved clinical practice for medulloblastoma: Progress towards biologically driven clinical trials. Br. J. Neurosurg. 2009, 23, 364–375. [Google Scholar] [CrossRef]
- Khanna, V.; Achey, R.L.; Ostrom, Q.; Block-Beach, H.; Kruchko, C.; Barnholtz-Sloan, J.S.; de Blank, P. Incidence and survival trends for medulloblastomas in the United States from 2001 to 2013. J. Neuro-Oncol. 2017, 135, 433–441. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, T.; Haberler, C. Update on the integrated histopathological and genetic classification of medulloblastoma—A practical diagnostic guideline. Clin. Neuropathol. 2016, 35, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.W.; Dalton, J.; Kocak, M.; Nicholson, S.L.; Fraga, C.; Neale, G.; Kenney, A.M.; Brat, D.J.; Perry, A.; Yong, W.H.; et al. Medulloblastoma: Clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011, 121, 381–396. [Google Scholar] [CrossRef]
- Tabori, U.; Baskin, B.; Shago, M.; Alon, N.; Taylor, M.D.; Ray, P.N.; Bouffet, E.; Malkin, D.; Hawkins, C. Universal Poor Survival in Children With Medulloblastoma Harboring Somatic TP53 Mutations. J. Clin. Oncol. 2010, 28, 1345–1350. [Google Scholar] [CrossRef]
- Westhoff, M.-A.; Marschall, N.; Grunert, M.; Karpel-Massler, G.; Burdach, S.; Debatin, K.-M. Cell death-based treatment of childhood cancer. Cell Death Dis. 2018, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Kattner, P.; Strobel, H.; Khoshnevis, N.; Grunert, M.; Bartholomae, S.; Pruss, M.; Fitzel, R.; Halatsch, M.-E.; Schilberg, K.; Siegelin, M.D.; et al. Compare and contrast: Pediatric cancer versus adult malignancies. Cancer Metastasis Rev. 2019, 38, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Cory, S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007, 26, 1324–1337. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Ströbele, S.; Nonnenmacher, L.; Siegelin, M.D.; Tepper, M.; Stroh, S.; Hasslacher, S.; Enzenmüller, S.; Strauss, G.; Baumann, B.; et al. A paired comparison between glioblastoma “stem cells” and differentiated cells. Int. J. Cancer 2015, 138, 1709–1718. [Google Scholar] [CrossRef]
- Oltersdorf, T.; Elmore, S.W.; Shoemaker, A.R.; Armstrong, R.C.; Augeri, D.J.; Belli, B.A.; Bruncko, M.; Deckwerth, T.L.; Dinges, J.; Hajduk, P.J.; et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005, 435, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.; Shoemaker, A.R.; Adickes, J.; Anderson, M.G.; Chen, J.; Jin, S.; Johnson, E.F.; Marsh, K.C.; Mitten, M.J.; Nimmer, P.; et al. ABT-263: A Potent and Orally Bioavailable Bcl-2 Family Inhibitor. Cancer Res. 2008, 68, 3421–3428. [Google Scholar] [CrossRef] [PubMed]
- Lessene, G.; Czabotar, P.E.; Colman, P.M. BCL-2 family antagonists for cancer therapy. Nat. Rev. Drug Discov. 2008, 7, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Karpel-Massler, G.; Shu, C.; Chau, L.; Banu, M.; Halatsch, M.-E.; Westhoff, M.-A.; Ramirez, Y.; Ross, A.H.; Bruce, J.N.; Canoll, P.; et al. Combined inhibition of Bcl-2/Bcl-xL and Usp9X/Bag3 overcomes apoptotic resistance in glioblastoma in vitro and in vivo. Oncotarget 2015, 6, 14507–14521. [Google Scholar] [CrossRef][Green Version]
- Karpel-Massler, G.; Bâ, M.; Shu, C.; Halatsch, M.-E.; Westhoff, M.-A.; Bruce, J.N.; Canoll, P.; Siegelin, M.D. TIC10/ONC201 synergizes with Bcl-2/Bcl-xL inhibition in glioblastoma by suppression of Mcl-1 and its binding partners in vitro and in vivo. Oncotarget 2015, 6, 36456–36471. [Google Scholar] [CrossRef]
- Ishida, C.T.; Bianchetti, E.; Shu, C.; Halatsch, M.-E.; Westhoff, M.A.; Karpel-Massler, G.; Siegelin, M.D. BH3-mimetics and BET-inhibitors elicit enhanced lethality in malignant glioma. Oncotarget 2017, 8, 29558–29573. [Google Scholar] [CrossRef]
- Ströbele, S.; Schneider, M.; Schneele, L.; Siegelin, M.D.; Nonnenmacher, L.; Zhou, S.; Karpel-Massle, G.; Westhoff, M.-A.; Halatsch, M.-E.; Debatin, K.-M. A Potential Role for the Inhibition of PI3K Signaling in Glioblastoma Therapy. PLoS ONE 2015, 10, e0131670. [Google Scholar] [CrossRef] [PubMed]
- Hasslacher, S.; Schneele, L.; Stroh, S.; Langhans, J.; Zeiler, K.; Kattner, P.; Karpel-Massler, G.; Siegelin, M.D.; Schneider, M.; Zhou, S.; et al. Inhibition of PI3K signalling increases the efficiency of radiotherapy in glioblastoma cells. Int. J. Oncol. 2018, 53, 1881–1896. [Google Scholar] [CrossRef]
- Westhoff, M.-A.; Zhou, S.; Nonnenmacher, L.; Karpel-Massler, G.; Jennewein, C.; Schneider, M.; Halatsch, M.-E.; Carragher, N.; Baumann, B.; Krause, A.; et al. Inhibition of NF-κB Signaling Ablates the Invasive Phenotype of Glioblastoma. Mol. Cancer Res. 2013, 11, 1611–1623. [Google Scholar] [CrossRef] [PubMed]
- Faber, A.C.; Farago, A.F.; Costa, C.; Dastur, A.; Gomez-Caraballo, M.; Robbins, R.; Wagner, B.L.; Rideout, W.M.; Jakubik, C.T.; Ham, J.; et al. Assessment of ABT-263 activity across a cancer cell line collection leads to a potent combination therapy for small-cell lung cancer. Proc. Natl. Acad. Sci. USA 2015, 112, E1288–E1296. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, D.P.; Coyle, B.; Walker, D.A.; Grabowska, A.M. In vitro models of medulloblastoma: Choosing the right tool for the job. J. Biotechnol. 2016, 236, 10–25. [Google Scholar] [CrossRef]
- Johnsen, J.I.; Kogner, P.; Albihn, A.; Henriksson, M.A. Embryonal neural tumours and cell death. Apoptosis 2009, 14, 424–438. [Google Scholar] [CrossRef]
- Kang, M.H.; Reynolds, C.P. Bcl-2 Inhibitors: Targeting Mitochondrial Apoptotic Pathways in Cancer Therapy. Clin. Cancer Res. 2009, 15, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Luca, C.G.; Ziazadeh, D.; McEachern, N.; Zhao, Y.; Sun, W.; Debussche, L.; Wang, S. Elucidation of Acquired Resistance to Bcl-2 and MDM2 Inhibitors in Acute Leukemia In Vitro and In Vivo. Clin. Cancer Res. 2015, 21, 2558–2568. [Google Scholar] [CrossRef] [PubMed]
- Halatsch, M.-E.; Kast, R.E.; Dwucet, A.; Hlavac, M.; Heiland, T.; Westhoff, M.-A.; Debatin, K.-M.; Wirtz, C.R.; Siegelin, M.D.; Karpel-Massler, G. Bcl-2/Bcl-xL inhibition predominantly synergistically enhances the anti-neoplastic activity of a low-dose CUSP9 repurposed drug regime against glioblastoma. Br. J. Pharmacol. 2019, 176, 3681–3694. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; Huang, D.; Roberts, A. Targeting BCL2 for the Treatment of Lymphoid Malignancies. Semin. Hematol. 2014, 51, 219–227. [Google Scholar] [CrossRef]
- Alabaster, O.; Cassidy, M. Flow Microfluorometric Analysis of P3SS Murine Leukemia after Administration of Vincristine and Maytansine In Vivo. J. Natl. Cancer Inst. 1978, 60, 649–652. [Google Scholar] [CrossRef]
- Chang, D.; Chen, F.; Zhang, F.; McKay, B.C.; Ljungman, M. Dose-dependent effects of DNA-damaging agents on p53-mediated cell cycle arrest. Cell Growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res. 1999, 10, 155–162. [Google Scholar]
- Coppe, A.; Ferrari, F.; Bisognin, A.; Danieli, G.A.; Ferrari, S.; Bicciato, S.; Bortoluzzi, S. Motif discovery in promoters of genes co-localized and co-expressed during myeloid cells differentiation. Nucleic Acids Res. 2008, 37, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Ewald, J.A.; Desotelle, J.A.; Wilding, G.; Jarrard, D.F. Therapy-Induced Senescence in Cancer. JNCI J. Natl. Cancer Inst. 2010, 102, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Sikora, E.; Mosieniak, G.; Śliwińska, M. Morphological and Functional Characteristic of Senescent Cancer Cells. Curr. Drug Targets 2016, 17, 377–387. [Google Scholar] [CrossRef]
- Zeng, J.; Wu, Y.; Zhuang, S.; Qin, L.; Hua, S.; Mungur, R.; Pan, J.; Zhu, Y.; Zhan, R. Identification of the role of TRPM8 in glioblastoma and its effect on proliferation, apoptosis and invasion of the U251 human glioblastoma cell line. Oncol. Rep. 2019, 42, 1517–1526. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2017, 217, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kohli, J.; Demaria, M. Senescent Cells in Cancer Therapy: Friends or Foes? Trends Cancer 2020, 6, 838–857. [Google Scholar] [CrossRef] [PubMed]
- Salama, R.; Sadaie, M.; Hoare, M.; Narita, M. Cellular senescence and its effector programs. Genes Dev. 2014, 28, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tao, Q.; Pan, Y.; Wanyan, Z.; Zhu, F.; Xu, X.; Wang, H.; Yi, L.; Zhou, M.; Zhai, Z. Stress-induced premature senescence activated by the SENEX gene mediates apoptosis resistance of diffuse large B-cell lymphoma via promoting immunosuppressive cells and cytokines. Immun. Inflamm. Dis. 2020, 8, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Liu, L.; Zeng, S. Senescence and Cancer. Cancer Transl. Med. 2018, 4, 70–74. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; de Jong, T.V.; Melov, S.; Guryev, V.; Campisi, J.; DeMaria, M. Unmasking Transcriptional Heterogeneity in Senescent Cells. Curr. Biol. 2017, 27, 2652–2660.e4. [Google Scholar] [CrossRef]
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
- Kipps, T.J.; Eradat, H.; Grosicki, S.; Catalano, J.; Cosolo, W.; Dyagil, I.S.; Yalamanchili, S.; Chai, A.; Sahasranaman, S.; Punnoose, E.; et al. A phase 2 study of the BH3 mimetic BCL2 inhibitor navitoclax (ABT-263) with or without rituximab, in previously untreated B-cell chronic lymphocytic leukemia. Leuk. Lymphoma 2015, 56, 2826–2833. [Google Scholar] [CrossRef]
- Roberts, A.W.; Advani, R.H.; Kahl, B.S.; Persky, D.; Sweetenham, J.W.; Carney, D.A.; Yang, J.; Busman, T.B.; Enschede, S.H.; Humerickhouse, R.A.; et al. Phase 1 study of the safety, pharmacokinetics, and antitumour activity of the BCL2 inhibitor navitoclax in combination with rituximab in patients with relapsed or refractory CD20+lymphoid malignancies. Br. J. Haematol. 2015, 170, 669–678. [Google Scholar] [CrossRef]
- Rudin, C.M.; Hann, C.L.; Garon, E.B.; De Oliveira, M.R.; Bonomi, P.D.; Camidge, D.R.; Chu, Q.; Giaccone, G.; Khaira, D.; Ramalingam, S.S.; et al. Phase II Study of Single-Agent Navitoclax (ABT-263) and Biomarker Correlates in Patients with Relapsed Small Cell Lung Cancer. Clin. Cancer Res. 2012, 18, 3163–3169. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Lorusso, P.; Arzt, J.; Busman, T.A.; Lian, G.; Rudersdorf, N.S.; Vanderwal, C.A.; Waring, J.F.; Yang, J.; Holen, K.D.; et al. Safety, efficacy, and pharmacokinetics of navitoclax (ABT-263) in combination with irinotecan: Results of an open-label, phase 1 study. Cancer Chemother. Pharmacol. 2015, 76, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.H.; O’Connor, O.A.; Czuczman, M.S.; LaCasce, A.S.; Gerecitano, J.F.; Leonard, J.P.; Tulpule, A.; Dunleavy, K.; Xiong, H.; Chiu, Y.-L.; et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: A phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010, 11, 1149–1159. [Google Scholar] [CrossRef]
- Mason, K.D.; Carpinelli, M.R.; Fletcher, J.I.; Collinge, J.E.; Hilton, A.A.; Ellis, S.; Kelly, P.N.; Ekert, P.G.; Metcalf, D.; Roberts, A.W.; et al. Programmed Anuclear Cell Death Delimits Platelet Life Span. Cell 2007, 128, 1173–1186. [Google Scholar] [CrossRef] [PubMed]
- Balachander, S.B.; Criscione, S.W.; Byth, K.F.; Cidado, J.; Adam, A.; Lewis, P.; MacIntyre, T.; Wen, S.; Lawson, D.; Burke, K.; et al. AZD4320, A Dual Inhibitor of Bcl-2 and Bcl-xL, Induces Tumor Regression in Hematologic Cancer Models without Dose-limiting Thrombocytopenia. Clin. Cancer Res. 2020, 26, 6535–6549. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westhoff, M.-A.; Schuler-Ortoli, M.; Zerrinius, D.; Hadzalic, A.; Schuster, A.; Strobel, H.; Scheuerle, A.; Wong, T.; Wirtz, C.R.; Debatin, K.-M.; et al. Bcl-XL but Not Bcl-2 Is a Potential Target in Medulloblastoma Therapy. Pharmaceuticals 2022, 15, 91. https://doi.org/10.3390/ph15010091
Westhoff M-A, Schuler-Ortoli M, Zerrinius D, Hadzalic A, Schuster A, Strobel H, Scheuerle A, Wong T, Wirtz CR, Debatin K-M, et al. Bcl-XL but Not Bcl-2 Is a Potential Target in Medulloblastoma Therapy. Pharmaceuticals. 2022; 15(1):91. https://doi.org/10.3390/ph15010091
Chicago/Turabian StyleWesthoff, Mike-Andrew, Marie Schuler-Ortoli, Daniela Zerrinius, Amina Hadzalic, Andrea Schuster, Hannah Strobel, Angelika Scheuerle, Tiana Wong, Christian Rainer Wirtz, Klaus-Michael Debatin, and et al. 2022. "Bcl-XL but Not Bcl-2 Is a Potential Target in Medulloblastoma Therapy" Pharmaceuticals 15, no. 1: 91. https://doi.org/10.3390/ph15010091
APA StyleWesthoff, M.-A., Schuler-Ortoli, M., Zerrinius, D., Hadzalic, A., Schuster, A., Strobel, H., Scheuerle, A., Wong, T., Wirtz, C. R., Debatin, K.-M., & Peraud, A. (2022). Bcl-XL but Not Bcl-2 Is a Potential Target in Medulloblastoma Therapy. Pharmaceuticals, 15(1), 91. https://doi.org/10.3390/ph15010091







