Oleanolic Acid Alleviates Cerebral Ischemia/Reperfusion Injury via Regulation of the GSK-3β/HO-1 Signaling Pathway
Abstract
:1. Introduction
2. Results
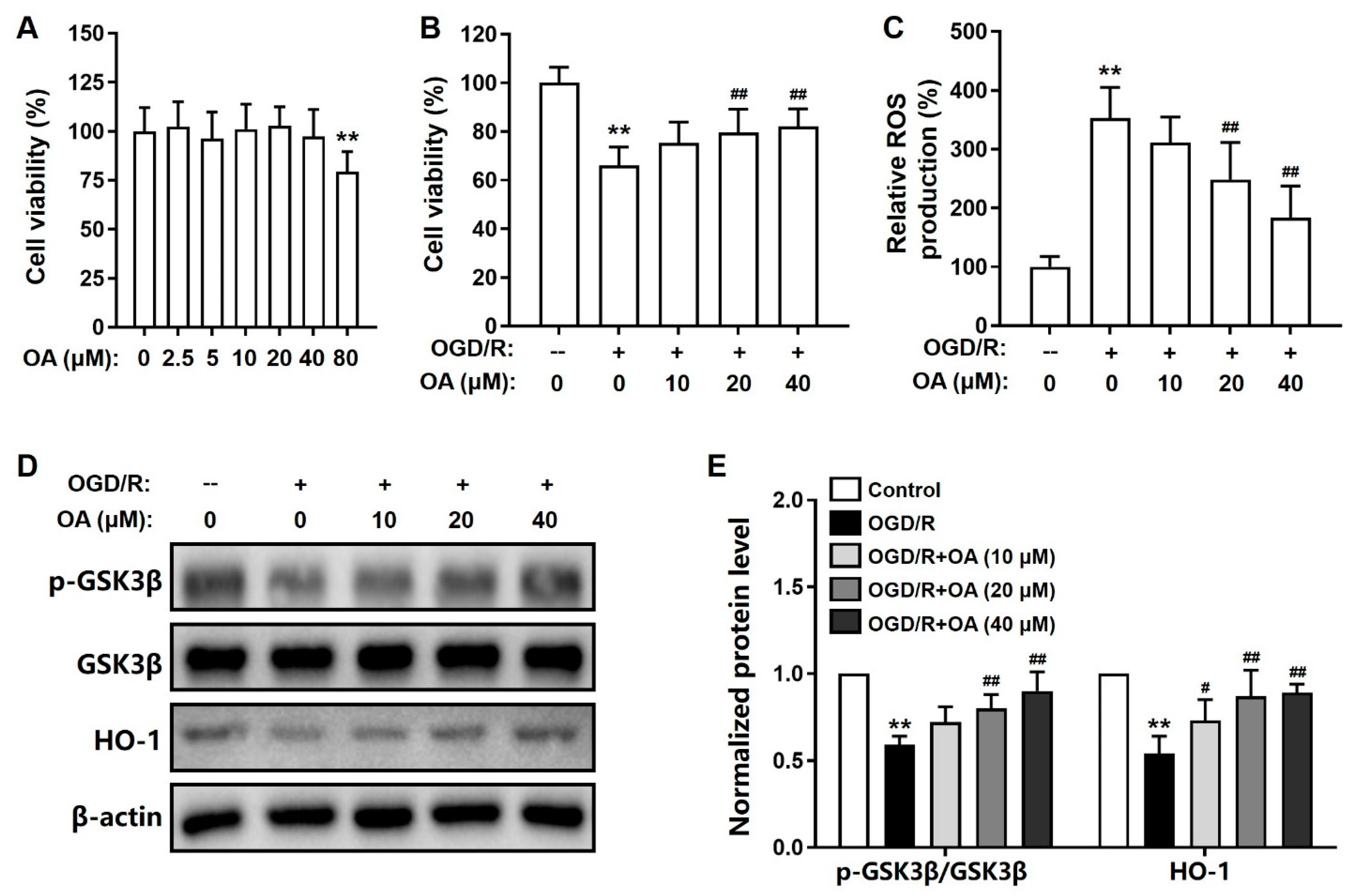
2.1. OA-Mediated Suppression of OGD/R-Induced Toxicity in SH-SY5Y Cells
2.2. OA Regulates GSK-3β/HO-1 Pathway in OGD/R-Induced SH-SY5Y Cells
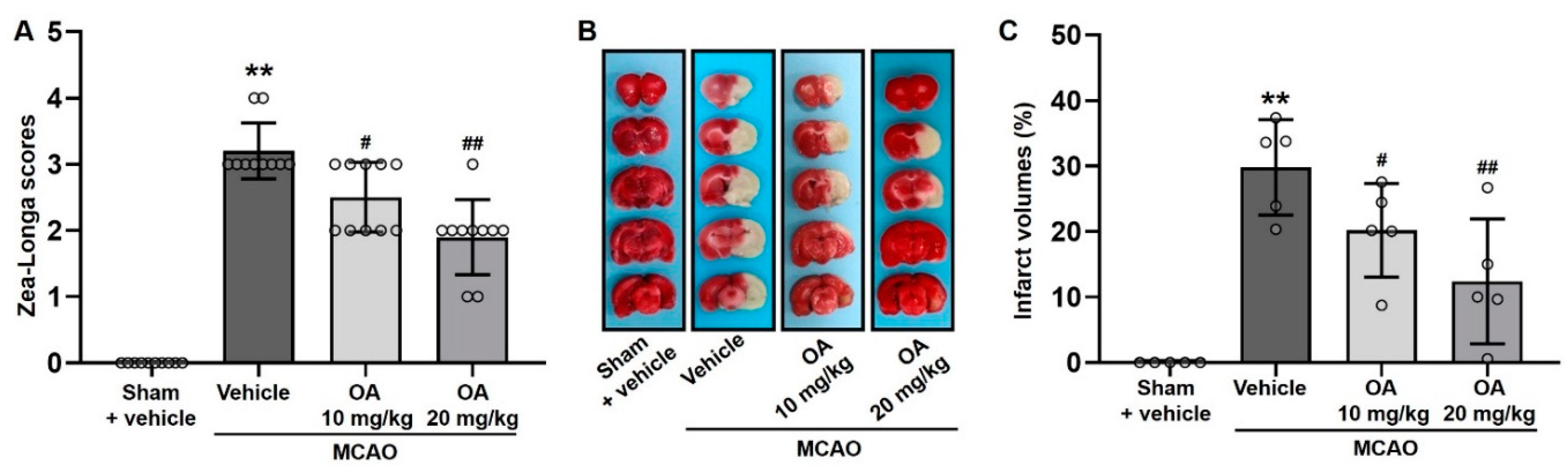
2.3. OA Administration Attenuated Neurological Deficits and Cerebral Infarction in MCAO Rats
2.4. OA Administration Reduced Neuronal Damage in MCAO Rats
2.5. OA Administration Reduced Cellular Apoptosis in MCAO Rats
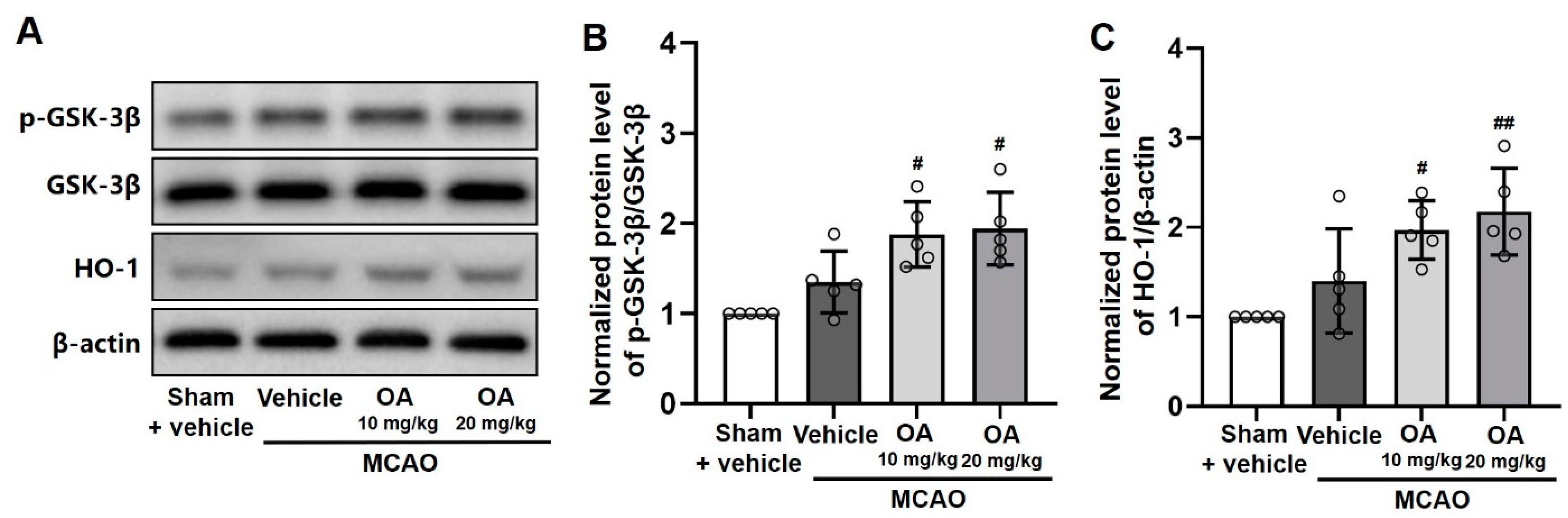
2.6. OA Administration Regulated GSK-3β/HO-1 Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Culture and the OGD/R Model
4.2. MTT Assay
4.3. Measurement of ROS in Cells
4.4. Western Blot Assay
4.5. Animals and OA Administration
4.6. MCAO Procedure
4.7. Neurological Deficit Assessment and Brain Tissue Collection
4.8. TTC Staining
4.9. Nissl and Immunofluorescent Staining
4.10. TUNEL Staining
4.11. ROS Quantification in Tissue
4.12. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, P.; Wu, S.P.; Wang, N.; Seto, S.; Chang, D. Hydroxysafflor yellow A alleviates cerebral ischemia reperfusion injury by suppressing apoptosis via mitochondrial permeability transition pore. Phytomedicine 2021, 85, 153532. [Google Scholar]
- Abe, H.; Jitsuki, S.; Takahashi, T. Pharmacological enhancement of stroke rehabilitation. Stroke 2019, 50, 3323–3329. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.Q.; Lui, C.N.P.; Zhu, P.L.; Zhang, Z.; Lin, K.L.; Dai, Y.W.; Yung, K.K.L. Traditional Chinese medicine-based neurorestorative therapy for Alzheimer’s and Parkinson’s disease. J. Neurorestoratol. 2019, 7, 207–222. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Anderson, G.A.; Fernandez, T.G.; Doré, S. Efficacy and mechanism of Panax Ginseng in experimental stroke. Front. Neurosci. 2019, 13, 294. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Lee, M.; An, H.J. Oleanolic acid protects against mast cell-mediated allergic responses by suppressing Akt/NF-κB and STAT1 activation. Phytomedicine 2021, 80, 153340. [Google Scholar] [CrossRef] [PubMed]
- Menon, B.; Ramalingam, K.; Kumar, R. Evaluating the role of oxidative stress in acute ischemic stroke. J. Neurosci. Rural Pract. 2020, 11, 156–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, Z.T.; Gong, X.J.; Sun, H.B.; Li, Y.M.; Ji, H. Protective effects of oleanolic acid on cerebral ischemic damage in vivo and H2O2-induced injury in vitro. Pharm. Biol. 2011, 49, 78–85. [Google Scholar] [CrossRef]
- Gu, S. Oleanolic acid improved inflammatory response and apoptosis of PC12 cells induced by OGD/R through downregulating miR-142-5p. Nat. Prod. Commun. 2021, 16, 1–7. [Google Scholar] [CrossRef]
- Caltana, L.; Nieto, M.L.; Brusco, A. Oleanolic acid: A promising neuroprotective agent for cerebral ischemia. Neural Regen. Res. 2015, 10, 540–541. [Google Scholar]
- Bereczki, D., Jr.; Balla, J.; Bereczki, D. Heme oxygenase-1: Clinical relevance in ischemic stroke. Curr. Pharm. Des. 2018, 24, 2229–2235. [Google Scholar] [CrossRef]
- Qin, Z.; Kong, B.; Zheng, J.; Wang, X.; Li, L. Alprostadil injection attenuates coronary microembolization-induced myocardial injury through GSK-3β/HO-1 signaling-mediated apoptosis inhibition. Drug Des. Devel. Ther. 2020, 14, 4407–4422. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, X.; Miao, J.; Yuan, H.; Liu, E.; Liang, T.; Li, Q. Farrerol directly targets GSK-3β to activate Nrf2-ARE pathway and protect EA.hy926 cells against oxidative stress-induced injuries. Oxid. Med. Cell Longev. 2020, 2020, 5967434. [Google Scholar] [CrossRef] [Green Version]
- Pang, T.; Wang, Y.J.; Gao, Y.X.; Xu, Y.; Li, Q.; Zhou, Y.B.; Xu, L.; Huang, Z.J.; Liao, H.; Zhang, L.Y.; et al. A novel GSK-3β inhibitor YQ138 prevents neuronal injury induced by glutamate and brain ischemia through activation of the Nrf2 signaling pathway. Acta Pharmacol. Sin. 2016, 37, 741–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.J.; Sun, L.L.; Ji, X.; Shi, R.; Xu, F.; Gu, J.H. Neuroprotective effects of oleanolic acid against cerebral ischemia-reperfusion injury in mice. Exp. Neurol. 2021, 343, 113785. [Google Scholar] [CrossRef] [PubMed]
- Gui, B.; Hua, F.; Chen, J.; Xu, Z.; Sun, H.; Qian, Y. Protective effects of pretreatment with oleanolic acid in rats in the acute phase of hepatic ischemia-reperfusion injury: Role of the PI3K/Akt pathway. Mediators Inflamm. 2014, 2014, 451826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Wu, B.; Liu, M.; Chen, Z.; Wang, W.; Anderson, C.S.; Sandercock, P.; Wang, Y.; Huang, Y.; Cui, L.; et al. Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019, 18, 394–405. [Google Scholar] [CrossRef]
- Dhir, N.; Medhi, B.; Prakash, A.; Goyal, M.K.; Modi, M.; Mohindra, S. Pre-clinical to clinical translational failures and current status of clinical trials in stroke therapy: A brief review. Curr. Neuropharmacol. 2020, 18, 596–612. [Google Scholar] [CrossRef]
- Modrego, P.J. The risk of symptomatic intracranial hemorrhage after thrombolysis for acute stroke: Current concepts and perspectives. Ann. Indian Acad. Neurol. 2019, 22, 336–340. [Google Scholar] [CrossRef]
- Yamashita, T.; Abe, K. Recent progress in therapeutic strategies for ischemic stroke. Cell Transpl. 2016, 25, 893–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; He, Y.; Chen, S.; Qi, S.; Shen, J. Therapeutic targets of oxidative/nitrosative stress and neuroinflammation in ischemic stroke: Applications for natural product efficacy with omics and systemic biology. Pharmacol. Res. 2020, 158, 104877. [Google Scholar] [CrossRef]
- Park, S.K.; Hyun, S.H.; In, G.; Park, C.K.; Kwak, Y.S.; Jang, Y.J.; Kim, B.; Kim, J.H.; Han, C.K. The antioxidant activities of Korean Red Ginseng (Panax ginseng) and ginsenosides: A systemic review through in vivo and clinical trials. J. Ginseng Res. 2021, 45, 41–47. [Google Scholar] [CrossRef]
- Han, Y.W.; Liu, X.J.; Zhao, Y.; Li, X.M. Role of Oleanolic acid in maintaining BBB integrity by targeting p38MAPK/VEGF/Src signaling pathway in rat model of subarachnoid hemorrhage. Eur. J. Pharmacol. 2018, 839, 12–20. [Google Scholar] [CrossRef]
- Lin, K.L.; Sze, S.C.W.; Liu, B.; Zhang, Z.; Zhang, Z.; Zhu, P.L.; Wong, Y.; Deng, Q.D.; Yung, K.K.L.; Zhang, S.Q. 20(S)-protopanaxadiol and oleanolic acid ameliorate cognitive deficits in APP/PS1 transgenic mice by enhancing hippocampal neurogenesis. J. Ginseng Res. 2021, 45, 325–333. [Google Scholar] [CrossRef]
- Wang, K.; Sun, W.; Zhang, L.; Guo, W.; Xu, J.; Liu, S.; Zhou, Z.; Zhang, Y. Oleanolic acid ameliorates Aβ25-35 injection-induced memory deficit in Alzheimer’s disease model rats by maintaining synaptic plasticity. CNS Neurol. Disord. Drug Targets 2018, 17, 389–399. [Google Scholar] [CrossRef]
- Dong, S.Q.; Wang, S.S.; Zhu, J.X.; Mu, R.H.; Li, C.F.; Geng, D.; Liu, Q.; Yi, L.T. Oleanolic acid decreases SGK1 in the hippocampus in corticosterone-induced mice. Steroids 2019, 149, 108419. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y.F.; Zhang, Y.; Wu, K.C.; Fan, F.; Klaassen, C.D. Oleanolic acid alters bile acid metabolism and produces cholestatic liver injury in mice. Toxicol. Appl. Pharmacol. 2013, 272, 816–824. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.J.; Zhao, X.S.; Fan, T.P.; Qi, H.X.; Li, D. Glycine improves ischemic stroke through miR-19a-3p/AMPK/GSK-3β/HO-1 pathway. Drug Des. Devel. Ther. 2020, 14, 2021–2031. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Zhu, J.; Lei, S.; Dong, Y.; Li, L.; Jiang, B.; Tan, L.; Wu, J.; Yu, S.; et al. GSK-3β downregulates Nrf2 in cultured cortical neurons and in a rat model of cerebral ischemia-reperfusion. Sci. Rep. 2016, 6, 20196. [Google Scholar] [CrossRef] [Green Version]
- To, C.; Roy, A.; Chan, E.; Prado, M.A.M.; Di Guglielmo, G.M. Synthetic triterpenoids inhibit GSK3β activity and localization and affect focal adhesions and cell migration. Biochim. Biophys. Acta. Mol. Cell Res. 2017, 1864, 1274–1284. [Google Scholar] [CrossRef]
- Ahamed, K.B.; Gowdru, H.B.; Rajashekarappa, S.; Malleshappa, K.S.; Krishna, V. Molecular docking of glycogen synthase kinase3-β inhibitor oleanolic acid and its wound-healing activity in rats. Med. Chem. Res. 2013, 22, 156–164. [Google Scholar] [CrossRef]
- Salcedo-Tello, P.; Ortiz-Matamoros, A.; Arias, C. GSK3 function in the brain during development, neuronal plasticity, and neurodegeneration. Int. J. Alzheimers Dis. 2011, 2011, 189728. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.B.; Wang, R.X.; Deng, H.J.; Wang, Y.H.; Tang, J.D.; Cao, F.Y.; Wang, J.H. Protective effects of oleanolic acid on oxidative stress and the expression of cytokines and collagen by the AKT/NF-κB pathway in silicotic rats. Mol. Med. Rep. 2017, 15, 3121–3128. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; He, J.; Zhang, H.; Yao, L.; Li, H. Oleanolic acid alleviates oxidative stress in Alzheimer’s disease by regulating stanniocalcin-1 and uncoupling protein-2 signalling. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1263–1271. [Google Scholar] [CrossRef]
- Bao, J.; Yan, W.; Xu, K.; Chen, M.; Chen, Z.; Ran, J.; Xiong, Y.; Wu, L. Oleanolic acid decreases IL-1β-induced activation of fibroblast-like synoviocytes via the SIRT3-NF-κB axis in osteoarthritis. Oxid. Med. Cell. Longev. 2020, 2020, 7517219. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell. Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Jiang, X.L.; Wang, Y.; Lin, K.L.; Zhang, Z.; Zhang, Z.; Ng, M.L.; Qu, S.G.; Sze, S.C.W.; Yung, K.K.L. Protective effect of An-Gong-Niu-Huang wan pre-treatment against experimental cerebral ischemia injury via regulating GSK-3β/HO-1 pathway. Front. Pharmacol. 2021, 16, 640297. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.; Zhang, Z.; Zhang, Z.; Zhu, P.; Jiang, X.; Wang, Y.; Deng, Q.; Lam Yung, K.K.; Zhang, S. Oleanolic Acid Alleviates Cerebral Ischemia/Reperfusion Injury via Regulation of the GSK-3β/HO-1 Signaling Pathway. Pharmaceuticals 2022, 15, 1. https://doi.org/10.3390/ph15010001
Lin K, Zhang Z, Zhang Z, Zhu P, Jiang X, Wang Y, Deng Q, Lam Yung KK, Zhang S. Oleanolic Acid Alleviates Cerebral Ischemia/Reperfusion Injury via Regulation of the GSK-3β/HO-1 Signaling Pathway. Pharmaceuticals. 2022; 15(1):1. https://doi.org/10.3390/ph15010001
Chicago/Turabian StyleLin, Kaili, Zhang Zhang, Zhu Zhang, Peili Zhu, Xiaoli Jiang, Ying Wang, Qiudi Deng, Ken Kin Lam Yung, and Shiqing Zhang. 2022. "Oleanolic Acid Alleviates Cerebral Ischemia/Reperfusion Injury via Regulation of the GSK-3β/HO-1 Signaling Pathway" Pharmaceuticals 15, no. 1: 1. https://doi.org/10.3390/ph15010001
APA StyleLin, K., Zhang, Z., Zhang, Z., Zhu, P., Jiang, X., Wang, Y., Deng, Q., Lam Yung, K. K., & Zhang, S. (2022). Oleanolic Acid Alleviates Cerebral Ischemia/Reperfusion Injury via Regulation of the GSK-3β/HO-1 Signaling Pathway. Pharmaceuticals, 15(1), 1. https://doi.org/10.3390/ph15010001




