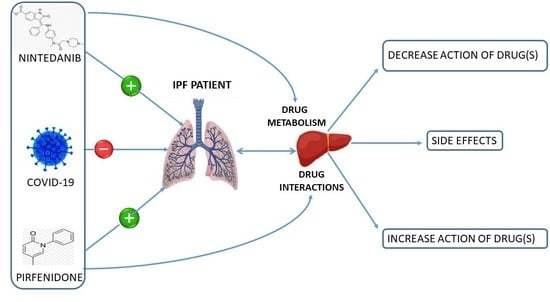
Pharmacological Interactions of Nintedanib and Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis in Times of COVID-19 Pandemic
Abstract
:1. Background
2. COVID-19 and ILD
3. Pharmacovigilance
Drug Interactions
- 1.
- Pharmacodynamic: They take place at biologically active sites, such as receptors, and produce changes in pharmacological activity. They do not usually affect pharmacokinetic parameters, but they alter the patient’s response to the drug. These interactions are as clinically important as pharmacokinetic interactions but much more difficult to study systematically since they usually take place affecting pairs of medications, which makes it difficult to establish common mechanisms explaining the effects on both drugs. Two types of pharmacodynamic interactions can be defined [26]:
- -
- Synergistic: Two drugs with the same pharmacological effect are administered together;
- -
- Antagonistic: Two drugs that are administered together have opposite actions.
- 2.
4. Pirfenidone
5. Nintedanib
6. Managing the Adverse Effects of Antifibrotic Therapy
7. Concomitant Administration of Nintedanib and Pirfenidone
8. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, K.K.; Martinez, F.J.; Walsh, S.L.; Thannickal, V.J.; Prasse, A.; Schlenker-Herceg, R.; Goeldner, R.-G.; Clerisme-Beaty, E.; Tetzlaff, K.; Cottin, V.; et al. The natural history of progressive fibrosing interstitial lung diseases. Eur. Respir. J. 2020, 55, 2000085. [Google Scholar] [CrossRef] [PubMed]
- Wijsenbeek, M.; Cottin, V. Spectrum of Fibrotic Lung Diseases. N. Engl. J. Med. 2020, 383, 958–968. [Google Scholar] [CrossRef]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.-F.; Flaherty, K.R.; Lasky, J.A.; et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef]
- Hutchinson, J.P.; Fogarty, A.; Hubbard, R.B.; McKeever, T. Global incidence and mortality of idiopathic pulmonary fibrosis: A systematic review. Eur. Respir. J. 2015, 46, 795–806. [Google Scholar] [CrossRef] [Green Version]
- Vasarmidi, E.; Tsitoura, E.; Spandidos, D.A.; Tzanakis, N.; Antoniou, K.M. Pulmonary fibrosis in the aftermath of the Covid-19 era (Review). Exp. Ther. Med. 2020, 20, 2557–2560. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, P.; Balestro, E.; Aliberti, S.; Cocconcelli, E.; Biondini, D.; Della Casa, G.; Sverzellati, N.; Maher, T. Pulmonary fibrosis secondary to COVID-19: A call to arms? Lancet Respir. Med. 2020, 8, 750–752. [Google Scholar] [CrossRef]
- Ojo, A.S.; Balogun, S.A.; Williams, O.T.; Ojo, O.S. Pulmonary Fibrosis in COVID-19 Survivors: Predictive Factors and Risk Reduction Strategies. Pulm. Med. 2020, 2020, 6175964. [Google Scholar] [CrossRef] [PubMed]
- Nasser, M.; Larrieu, S.; Si-Mohamed, S.; Ahmad, K.; Boussel, L.; Brevet, M.; Chalabreysse, L.; Fabre, C.; Marque, S.; Revel, D.; et al. Progressive fibrosing interstitial lung disease: A clinical cohort (the PROGRESS study). Eur. Respir. J. 2021, 57, 2002718. [Google Scholar] [CrossRef] [PubMed]
- Caminati, A.; Madotto, F.; Conti, S.; Cesana, G.; Mantovani, L.; Harari, S. The natural history of idiopathic pulmonary fibrosis in a large European population: The role of age, sex and comorbidities. Intern. Emerg. Med. 2021, 1–10. [Google Scholar] [CrossRef]
- Snyder, L.D.; Mosher, C.; Holtze, C.H.; Lancaster, L.H.; Flaherty, K.R.; Noth, I.; Neely, M.L.; Hellkamp, A.S.; Bender, S.; Conoscenti, C.S.; et al. Time to diagnosis of idiopathic pulmonary fibrosis in the IPF-PRO Registry. BMJ Open Respir. Res. 2020, 7, e000567. [Google Scholar] [CrossRef]
- Kaunisto, J.; Salomaa, E.-R.; Hodgson, U.; Kaarteenaho, R.; Kankaanranta, H.; Koli, K.; Vahlberg, T.; Myllärniemi, M. Demographics and survival of patients with idiopathic pulmonary fibrosis in the FinnishIPF registry. ERJ Open Res. 2019, 5, 00170–02018. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- The Idiopathic Pulmonary Fibrosis Clinical Research Network Prednisone, Azathioprine, and N-Acetylcysteine for Pulmonary Fibrosis. N. Engl. J. Med. 2012, 366, 1968–1977. [CrossRef] [PubMed]
- OFEV (Nintedanib) Summary of Product Characteristics. Available online: https://ec.europa.eu/health/documents/communityregister/2015/20150115130436/anx_130436_en.pdf (accessed on 16 April 2021).
- ESBRIET (Pirfenidone) Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/esbriet-epar-product-information_en.pdf (accessed on 16 April 2021).
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.; Lau, E.H.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- John Hopkins University of Medicine Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/ (accessed on 25 February 2021).
- Eapen, M.S.; Lu, W.; Gaikwad, A.V.; Bhattarai, P.; Chia, C.; Hardikar, A.; Haug, G.; Sohal, S.S. Endothelial to mesenchymal transition: A precursor to post-COVID-19 interstitial pulmonary fibrosis and vascular obliteration? Eur. Respir. J. 2020, 56, 2003167. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wu, Q.; Chen, Z.; Xiong, Z.; Wang, K.; Tian, J.; Zhang, S. The potential indicators for pulmonary fibrosis in survivors of severe COVID-19. J. Infect. 2021, 82, e5–e7. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Huang, M.-Y.; Xiao, Z.-W.; Yang, S.; Chen, X.-Q. Lactate dehydrogenase elevations is associated with severity of COVID-19: A meta-analysis. Crit. Care 2020, 24, 1–3. [Google Scholar] [CrossRef] [PubMed]
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Alunno, A.; Najm, A.; Machado, P.M.; Bertheussen, H.; Burmester, G.R.; Carubbi, F.; De Marco, G.; Giacomelli, R.; Hermine, O.; Isaacs, J.D.; et al. EULAR points to consider on pathophysiology and use of immunomodulatory therapies in COVID-19. Ann. Rheum. Dis. 2021, 80, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Khouri, C.; Petit, C.; Tod, M.; Lepelley, M.; Revol, B.; Roustit, M.; Cracowski, J.-L. Adverse drug reaction risks obtained from meta-analyses and pharmacovigilance disproportionality analyses are correlated in most cases. J. Clin. Epidemiol. 2021, 134, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Huang, C. Adverse drug reactions in primary care: A scoping review. BMC Health Serv. Res. 2020, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Insani, W.N.; Whittlesea, C.; Alwafi, H.; Man, K.K.C.; Chapman, S.; Wei, L. Prevalence of adverse drug reactions in the primary care setting: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0252161. [Google Scholar] [CrossRef]
- Cascorbi, I. Drug Interactions. Dtsch. Aerzteblatt Online 2012, 109, 546–556. [Google Scholar] [CrossRef]
- Zheng, W.Y.; Richardson, L.C.; Li, L.; Day, R.; Westbrook, J.; Baysari, M. Drug-drug interactions and their harmful effects in hospitalised patients: A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2018, 74, 15–27. [Google Scholar] [CrossRef]
- Klomp, F.; Wenzel, C.; Drozdzik, M.; Oswald, S. Drug–Drug Interactions Involving Intestinal and Hepatic CYP1A Enzymes. Pharmaceutics 2020, 12, 1201. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.E.; Spiers, G.; Kingston, A.; Todd, A.; Adamson, J.; Hanratty, B. Adverse Outcomes of Polypharmacy in Older People: Systematic Review of Reviews. J. Am. Med. Dir. Assoc. 2020, 21, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Khezrian, M.; McNeil, C.J.; Murray, A.; Myint, P.K. An overview of prevalence, determinants and health outcomes of polypharmacy. Ther. Adv. Drug Saf. 2020, 11, 2042098620933741. [Google Scholar] [CrossRef]
- Fried, T.R.; Street, R.L.; Cohen, A.B. Chronic Disease Decision Making and “What Matters Most". J. Am. Geriatr. Soc. 2020, 68, 474–477. [Google Scholar] [CrossRef]
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glassberg, M.K.; Kardatzke, D.; King, T.E.; Lancaster, L.; Sahn, S.A.; Szwarcberg, J.; et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet 2011, 377, 1760–1769. [Google Scholar] [CrossRef]
- King, T.E.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A Phase 3 Trial of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behr, J.; Prasse, A.; Kreuter, M.; Johow, J.; Rabe, K.F.; Bonella, F.; Bonnet, R.; Grohe, C.; Held, M.; Wilkens, H.; et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2021, 9, 476–486. [Google Scholar] [CrossRef]
- Richeldi, L.; Costabel, U.; Selman, M.; Kim, D.S.; Hansell, D.M.; Nicholson, A.G.; Brown, K.K.; Flaherty, K.R.; Noble, P.W.; Raghu, G.; et al. Efficacy of a Tyrosine Kinase Inhibitor in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2011, 365, 1079–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richeldi, L.; Du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Distler, O.; Highland, K.B.; Gahlemann, M.; Azuma, A.; Fischer, A.; Mayes, M.D.; Raghu, G.; Sauter, W.; Girard, M.; Alves, M.; et al. Nintedanib for Systemic Sclerosis–Associated Interstitial Lung Disease. N. Engl. J. Med. 2019, 380, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials. Efficacy and Safety of Nintedanib in Patients with Progressive Fibrosing Interstitial Lung Disease (PF-ILD) (INBUILD®). Available online: https://clinicaltrials.gov/ct2/show/record/NCT02999178 (accessed on 20 July 2021).
- Vancheri, C.; Kreuter, M.; Richeldi, L.; Ryerson, C.J.; Valeyre, D.; Grutters, J.C.; Wiebe, S.; Stansen, W.; Quaresma, M.; Stowasser, S.; et al. Nintedanib with Add-on Pirfenidone in Idiopathic Pulmonary Fibrosis. Results of the INJOURNEY Trial. Am. J. Respir. Crit. Care Med. 2018, 197, 356–363. [Google Scholar] [CrossRef]
- Albera, C.; Costabel, U.; Fagan, E.A.; Glassberg, M.K.; Gorina, E.; Lancaster, L.; Lederer, D.; Nathan, S.D.; Spirig, D.; Swigris, J.J. Efficacy of pirfenidone in patients with idiopathic pulmonary fibrosis with more preserved lung function. Eur. Respir. J. 2016, 48, 843–851. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Wu, J.; Chen, H.; Chen, H.; Wu, J.; Zeng, F. Single- and Multiple-Dose Pharmacokinetics of Pirfenidone, an Antifibrotic Agent, in Healthy Chinese Volunteers. J. Clin. Pharmacol. 2007, 47, 1268–1276. [Google Scholar] [CrossRef]
- Richeldi, L.; Cottin, V.; du Bois, R.M.; Selman, M.; Kimura, T.; Bailes, Z.; Schlenker-Herceg, R.; Stowasser, S.; Brown, K.K. Nintedanib in patients with idiopathic pulmonary fibrosis: Combined evidence from the TOMORROW and INPULSIS® trials. Respir. Med. 2016, 113, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; Du Bois, R.M.; Fagan, E.A.; Fishman, R.S.; Glaspole, I.; Glassberg, M.K.; Lancaster, L.; et al. Pirfenidone for idiopathic pulmonary fibrosis: Analysis of pooled data from three multinational phase 3 trials. Eur. Respir. J. 2015, 47, 243–253. [Google Scholar] [CrossRef]
- Verma, N.; Kumar, P.; Mitra, S.; Taneja, S.; Dhooria, S.; Das, A.; Duseja, A.; Dhiman, R.K.; Chawla, Y. Drug idiosyncrasy due to pirfenidone presenting as acute liver failure: Case report and mini-review of the literature. Hepatol. Commun. 2018, 2, 142–147. [Google Scholar] [CrossRef]
- Corte, T.J.; Bonella, F.; Crestani, B.; Demedts, M.G.; Richeldi, L.; Coeck, C.; Pelling, K.; Quaresma, M.; Lasky, J.A. Safety, tolerability and appropriate use of nintedanib in idiopathic pulmonary fibrosis. Respir. Res. 2015, 16, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathan, S.D.; Lancaster, L.H.; Albera, C.; Glassberg, M.K.; Swigris, J.J.; Gilberg, F.; Kirchgaessler, K.-U.; Limb, S.L.; Petzinger, U.; Noble, P.W. Dose modification and dose intensity during treatment with pirfenidone: Analysis of pooled data from three multinational phase III trials. BMJ Open Respir. Res. 2018, 5, e000323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toellner, H.; Hughes, G.; Beswick, W.; Crooks, M.G.; Donaldson, C.; Forrest, I.; Hart, S.P.; Leonard, C.; Major, M.; Simpson, A.J.; et al. Early clinical experiences with nintedanib in three UK tertiary interstitial lung disease centres. Clin. Transl. Med. 2017, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Noth, I.; Oelberg, D.; Kaul, M.; Conoscenti, C.S.; Raghu, G. Safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis in the USA. Eur. Respir. J. 2018, 52, 1702106. [Google Scholar] [CrossRef] [Green Version]
- Cottin, V.; Koschel, D.; Günther, A.; Albera, C.; Azuma, A.; Sköld, C.M.; Tomassetti, S.; Hormel, P.; Stauffer, J.L.; Strombom, I.; et al. Long-term safety of pirfenidone: Results of the prospective, observational PASSPORT study. ERJ Open Res. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Galli, J.A.; Pandya, A.; Vega-Olivo, M.; Dass, C.; Zhao, H.; Criner, G.J. Pirfenidone and nintedanib for pulmonary fibrosis in clinical practice: Tolerability and adverse drug reactions. Respirology 2017, 22, 1171–1178. [Google Scholar] [CrossRef] [Green Version]
- Hughes, G.; Toellner, H.; Morris, H.; Leonard, C.; Chaudhuri, N. Real World Experiences: Pirfenidone and Nintedanib are Effective and Well Tolerated Treatments for Idiopathic Pulmonary Fibrosis. J. Clin. Med. 2016, 5, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzouvelekis, A.; Karampitsakos, T.; Ntolios, P.; Tzilas, V.; Bouros, E.; Markozannes, E.; Malliou, I.; Anagnostopoulos, A.; Granitsas, A.; Steiropoulos, P.; et al. Longitudinal “Real-World” Outcomes of Pirfenidone in Idiopathic Pulmonary Fibrosis in Greece. Front. Med. 2017, 4, 213. [Google Scholar] [CrossRef] [Green Version]
- Tzouvelekis, A.; Karampitsakos, T.; Kontou, M.; Granitsas, A.; Malliou, I.; Anagnostopoulos, A.; Ntolios, P.; Tzilas, V.; Bouros, E.; Steiropoulos, P.; et al. Safety and efficacy of nintedanib in idiopathic pulmonary fibrosis: A real-life observational study in Greece. Pulm. Pharmacol. Ther. 2018, 49, 61–66. [Google Scholar] [CrossRef]
- Ogura, T.; Taniguchi, H.; Azuma, A.; Inoue, Y.; Kondoh, Y.; Hasegawa, Y.; Bando, M.; Abe, S.; Mochizuki, Y.; Chida, K.; et al. Safety and pharmacokinetics of nintedanib and pirfenidone in idiopathic pulmonary fibrosis. Eur. Respir. J. 2015, 45, 1382–1392. [Google Scholar] [CrossRef] [Green Version]
- Richeldi, L.; Fletcher, S.; Adamali, H.; Chaudhuri, N.; Wiebe, S.; Wind, S.; Hohl, K.; Baker, A.; Schlenker-Herceg, R.; Stowasser, S.; et al. No relevant pharmacokinetic drug–drug interaction between nintedanib and pirfenidone. Eur. Respir. J. 2019, 53, 1801060. [Google Scholar] [CrossRef] [PubMed]
- Dallinger, C.; Trommeshauser, D.; Marzin, K.; Liesener, A.; Kaiser, R.; Stopfer, P. Pharmacokinetic Properties of Nintedanib in Healthy Volunteers and Patients with Advanced Cancer. J. Clin. Pharmacol. 2016, 56, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Maher, T. Long-term clinical and real-world experience with pirfenidone in the treatment of idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2015, 24, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Brunnemer, E.; Wälscher, J.; Tenenbaum, S.; Hausmanns, J.; Schulze, K.; Seiter, M.; Heussel, C.P.; Warth, A.; Herth, F.J.; Kreuter, M. Real-World Experience with Nintedanib in Patients with Idiopathic Pulmonary Fibrosis. Respiration 2018, 95, 301–309. [Google Scholar] [CrossRef]
- Sgalla, G.; Lerede, M.; Richeldi, L. Emerging drugs for the treatment of idiopathic pulmonary fibrosis: 2020 phase II clinical trials. Expert Opin. Emerg. Drugs 2021, 26, 93–101. [Google Scholar] [CrossRef]
- Ali, I.; Alharbi, O.M. COVID-19: Disease, management, treatment, and social impact. Sci. Total Environ. 2020, 728, 138861. [Google Scholar] [CrossRef]
- Van der Sar-van der Brugge, S.; Talman, S.; Winter, L.B.-D.; de Mol, M.; Hoefman, E.; van Etten, R.W.; De Backer, I.C. Pulmonary function and health-related quality of life after COVID-19 pneumonia. Respir. Med. 2020, 176, 106272. [Google Scholar] [CrossRef] [PubMed]
- Powell, P.; Saggu, R.; Jones, S.; Clari, M.; Saraiva, I.; Hardavella, G.; Hansen, K.; Pinnock, H. Discussing treatment burden. Breathe 2021, 17, 200284. [Google Scholar] [CrossRef]
- García-Caballero, T.M.; Lojo, J.; Menéndez, C.; Fernández-Álvarez, R.; Mateos, R.; Garcia-Caballero, A. Polimedication: Applicability of a computer tool to reduce polypharmacy in nursing homes. Int. Psychogeriatr. 2018, 30, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Gentizon, J.; Büla, C.; Mabire, C. Medication literacy in older patients: Skills needed for self-management of medications. Rev. Med. Suisse 2020, 16, 2165–2168. [Google Scholar] [PubMed]
- McFarland, M.S.; Buck, M.L.; Crannage, E.; Armistead, L.T.; Ourth, H.; Finks, S.W.; McClurg, M.R. Assessing the Impact of Comprehensive Medication Management on Achievement of the Quadruple Aim. Am. J. Med. 2021, 134, 456–461. [Google Scholar] [CrossRef]
| Study (References) | Design | Treatment | Main Endpoints | Patients |
|---|---|---|---|---|
| CAPACITY 004 [32] | Phase 3 Randomized Parallel Assignment Double-Blind | Pirfenidone (2403 mg or 1197 mg) versus Placebo | Absolute Change in Percentage of predicted FVC Mean Change in Percent Predicted FVC as measured from baseline to week 72 | 435 |
| CAPACITY 006 [32] | Phase 3 Randomized Parallel Assigment Double-Blind | Pirfenidone (2403 mg) versus Placebo | Change in percentage of predicted FVC at week 72 | 344 |
| ASCEND [33] | Phase 3 Randomized Parallel Assigment Double-Blind | Pirfenidone (2403 mg) versus Placebo | Change in FVC or death at week 52 | 555 |
| RELIEF [34] | Phase 2 Randomized Parallel assignment Double blinded | Pirfenidone (267 mg or 534 mg or 801 mg) versus placebo | Absolute change in percentage of predicted FVC at week 48 | 127 |
| TOMORROW [35] | Phase 2 Randomized Parallel assignment Double blinded | Nintedanib (50 mg,100 mg, 200 mg or 300 mg) versus Placebo | Annual rate of decline in FVC over 52 weeks | 432 |
| INPULSIS 1- INPULSIS 2 [36] | Phase 3 Randomized Parallel assignment Double blinded | Nintedanib (200 mg or 300 mg) versus Placebo | Annual rate of decline in FVC over 52 weeks | 1066 |
| SENSCIS [37] | Phase 3 Randomized Parallel assigment Double blinded | Nintedanib (150 mg) versus placebo | Annual rate of decline in FVC over 52 weeks | 576 |
| INBUILD [38] | Phase 3 Randomized Parallel assigment Double blinded | Nintedanib (150 mg) versus placebo | Annual rate of decline in FVC over 52 weeks | 663 |
| INJOURNEY [39] | Phase 4 Randomized Parallel assignment Open-label | Nintedanib (150 mg) versus Pirfenidone (2403 mg) | Percentage of patients with on-treatment gastrointestinal AEs from baseline to week 12 | 105 |
| Pirfenidone | Nintedanib | |
|---|---|---|
| Pharmaceutical form (orally) | Capsules Tablets | Capsules |
| Half-life (hours) | 3 | 9.5 |
| Side effects | Bloating, dizziness, diarrhoea, dyspepsia, gastroesophageal reflux, nausea, vomiting, fatigue, weight loss, photosensitivity reactions and rash | Increased liver enzymes, abdominal pain, diarrhoea, nausea, vomiting weight loss |
| Major pharmacological Interactions * | Aminolevulinic acid, amiodarone, enoxacin, fluvoxamine, leflunomide, mibefradil, mipomersen, rucaparib, teriflunomide, vemurafenib | Carbamazepine, dexamethasone, drotrecogin alfa, phenytoin, leflunomide, lomitapide, mipomersen, mitotane, phenobarbital, primidone, rifampicin, St. John’s wort, tripanavir, teriflunomide |
| Contraindications | Smoking Kidney failure Liver failure | Thromboembolic disease Lung toxicity Gastric perforation Smoking Kidney failure Liver failure |
| Pregnancy Category (FDA) | C | D |
| Pirfenidone | Nintedanib | ||||
|---|---|---|---|---|---|
| Type of AE | Gastrointestinal | Cutaneous | Hepatic | Gastrointestinal | Hepatic |
| AE prevention | Take pirfenidone with plenty of food. Titration for 4 weeks instead of 2. | Avoid exposure to sunlight or intense artificial light. Applications of complete protection cream every 2 h. Use of sunglasses and protective clothing. Avoid use of phototoxic drugs. | Monitor liver bio-chemistry (ALT, AST and bilirubin) at baseline, monthly for 6 months and then every 3 months. | Take nintedanib with food. | Monitor liver biochemistry (ALT, AST, bilirubin) at baseline, monthly for the first 3 months and then periodically. |
| AE treatment | Prokinetics and proton pump inhibitors. | Steroids or sulphadiazine if severe phototoxicity. | Antidiarrheal (loperamide). Antiemetics. Proper hydration. | ||
| Dose reduction | Reduce doses to 1–2 capsules 2–3 times daily. Make the reduction at the time point in which the AE is most pronounced. | Reduce dose to 1 capsule every 8 h for one week. | If AST and ALT are increased (>3 to 5× ULN) or there are symptoms or hyperbilirubinemia, reduce doses until values recovery. | Reduce to 100 mg/12 h if persistent diarrhoea. | If AST/ALT are increased (>3 to 5× ULN) reduce dosage until values recovery. Then, re-scale doses up to max tolerated. |
| Dose interruption | If AE persists, temporarily discontinue therapy until symptom resolution. | Discontinue doses for 14 days if rash persists and subsequently re-escalate. Do not re-escalate if the rash does not subside. | Permanently discontinue if the elevations of AST and ALT are accompanied by symptoms of hyperbilirubinemia or if the elevations are >5× ULN. | Stop doses if severe diarrhoea for one week. Discontinue permanently if there is no improvement. | Permanently stop doses if elevations are accompanied by severe symptoms of liver damage. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra López-Matencio, J.M.; Gómez, M.; Vicente-Rabaneda, E.F.; González-Gay, M.A.; Ancochea, J.; Castañeda, S. Pharmacological Interactions of Nintedanib and Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis in Times of COVID-19 Pandemic. Pharmaceuticals 2021, 14, 819. https://doi.org/10.3390/ph14080819
Serra López-Matencio JM, Gómez M, Vicente-Rabaneda EF, González-Gay MA, Ancochea J, Castañeda S. Pharmacological Interactions of Nintedanib and Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis in Times of COVID-19 Pandemic. Pharmaceuticals. 2021; 14(8):819. https://doi.org/10.3390/ph14080819
Chicago/Turabian StyleSerra López-Matencio, José M., Manuel Gómez, Esther F. Vicente-Rabaneda, Miguel A. González-Gay, Julio Ancochea, and Santos Castañeda. 2021. "Pharmacological Interactions of Nintedanib and Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis in Times of COVID-19 Pandemic" Pharmaceuticals 14, no. 8: 819. https://doi.org/10.3390/ph14080819
APA StyleSerra López-Matencio, J. M., Gómez, M., Vicente-Rabaneda, E. F., González-Gay, M. A., Ancochea, J., & Castañeda, S. (2021). Pharmacological Interactions of Nintedanib and Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis in Times of COVID-19 Pandemic. Pharmaceuticals, 14(8), 819. https://doi.org/10.3390/ph14080819