Evaluation of the Organotellurium Compound AS101 for Treating Colistin- and Carbapenem-Resistant Klebsiella pneumoniae
Abstract
1. Introduction
2. Results
2.1. In Vitro AS101 Antibacterial Activity
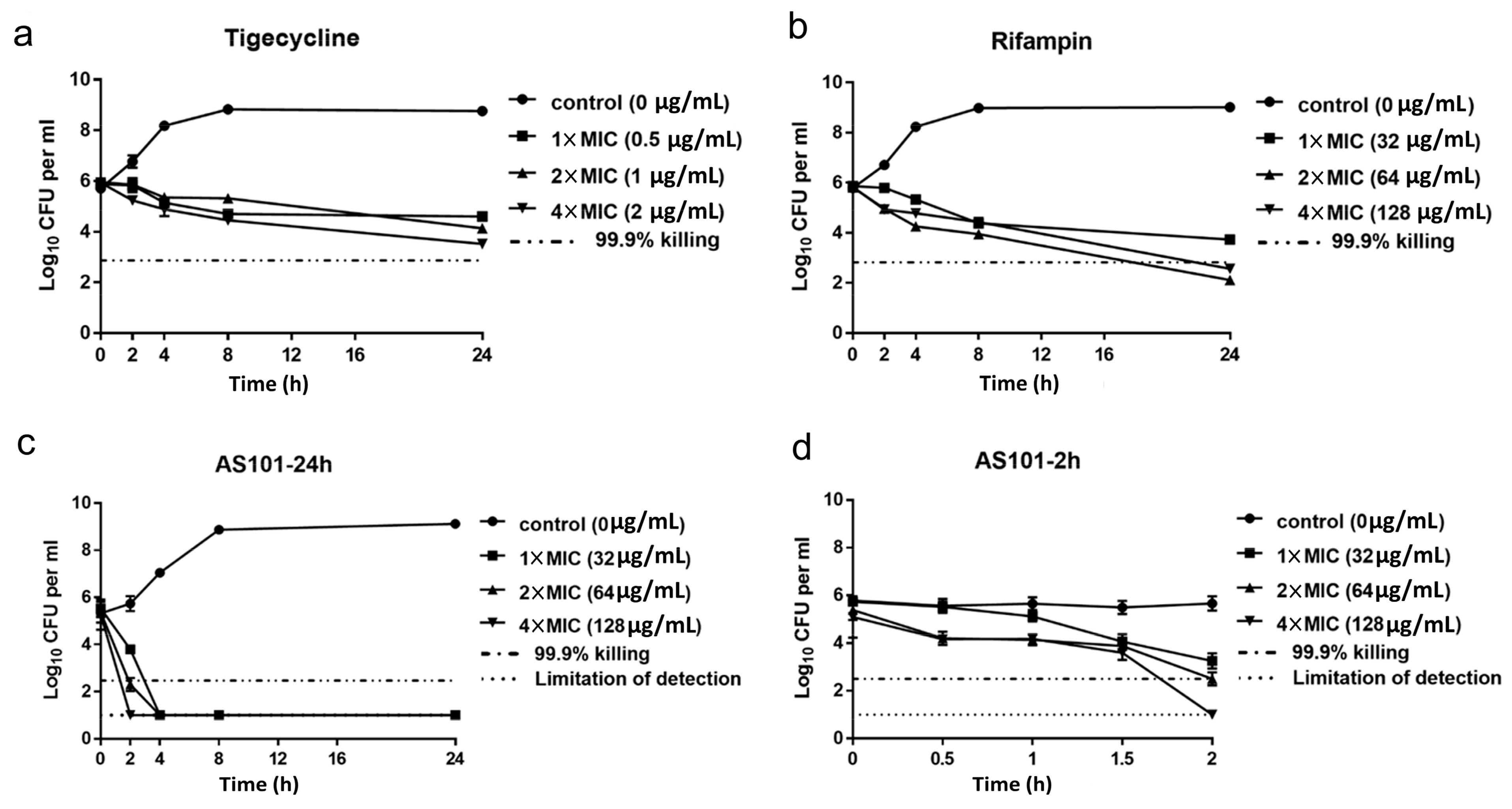
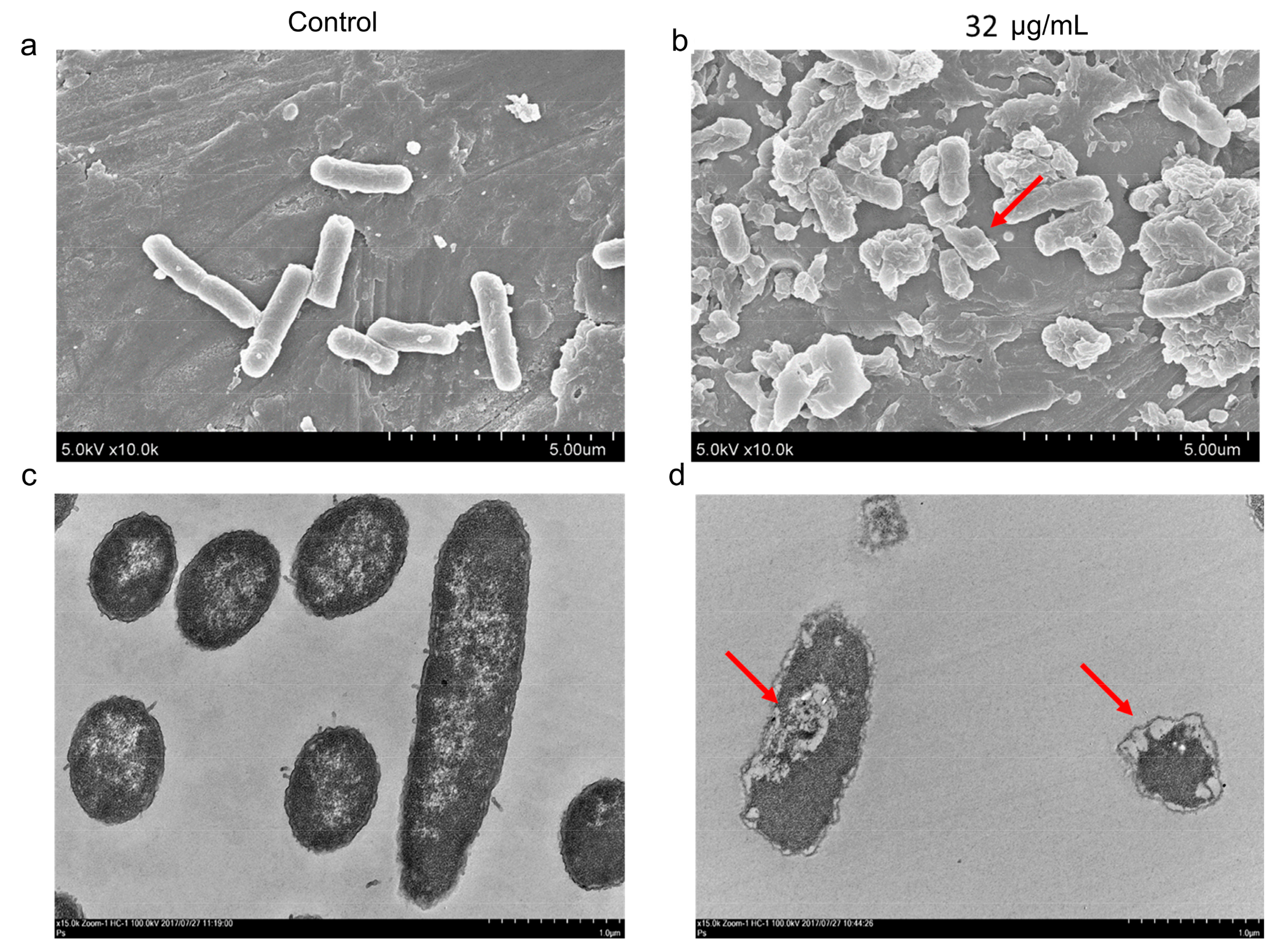
2.2. AS101 Antibacterial Activity Characterization
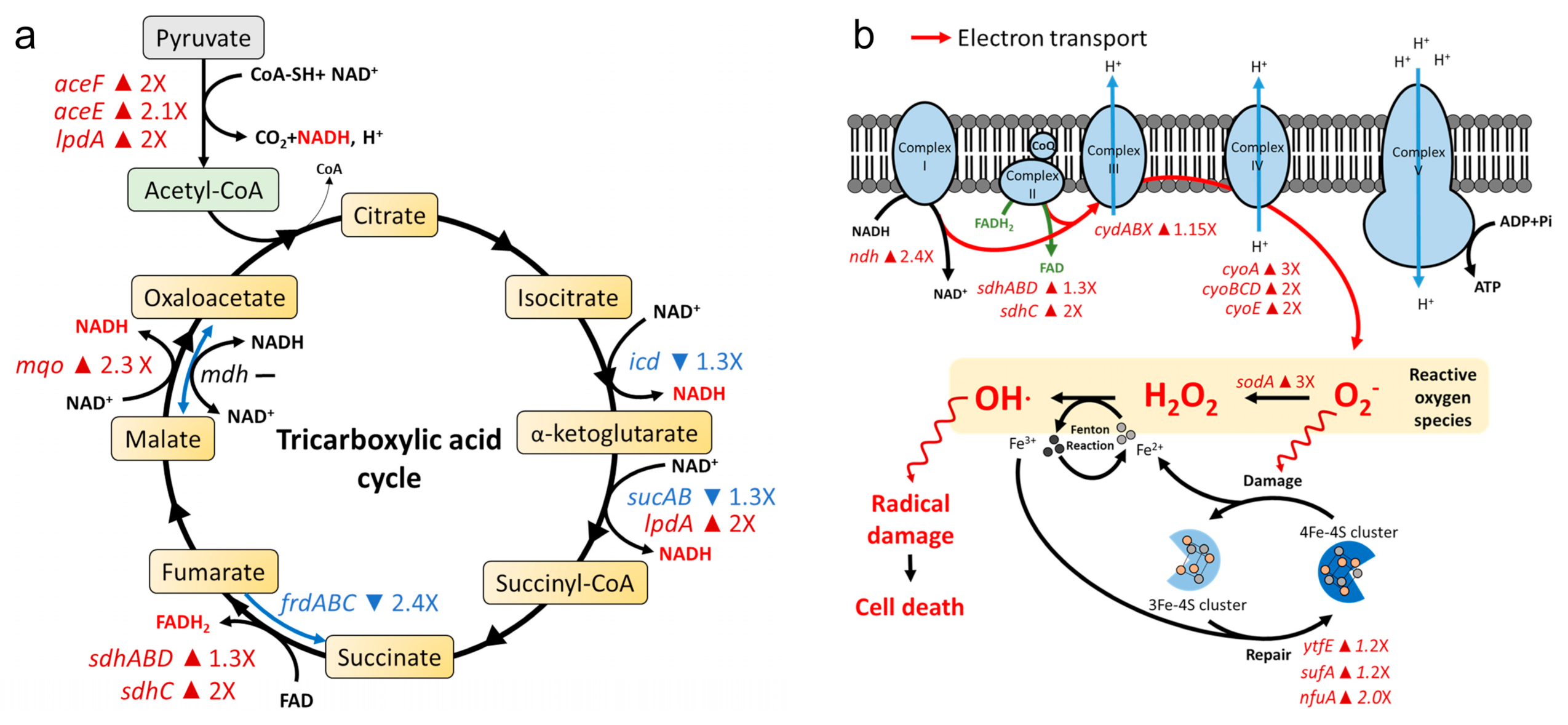
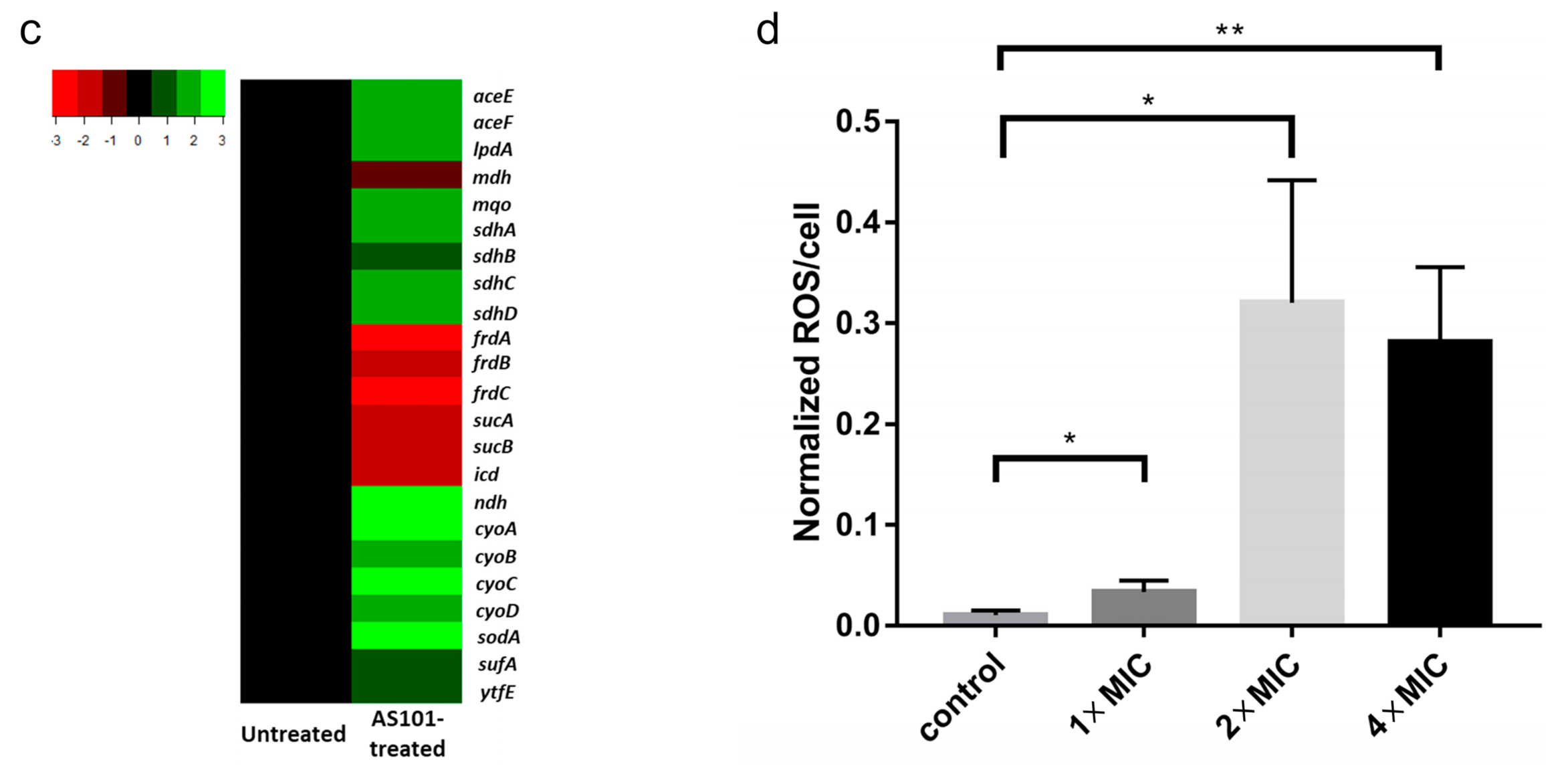
2.3. AS101 Oxidative Damage Leads to Cell Death
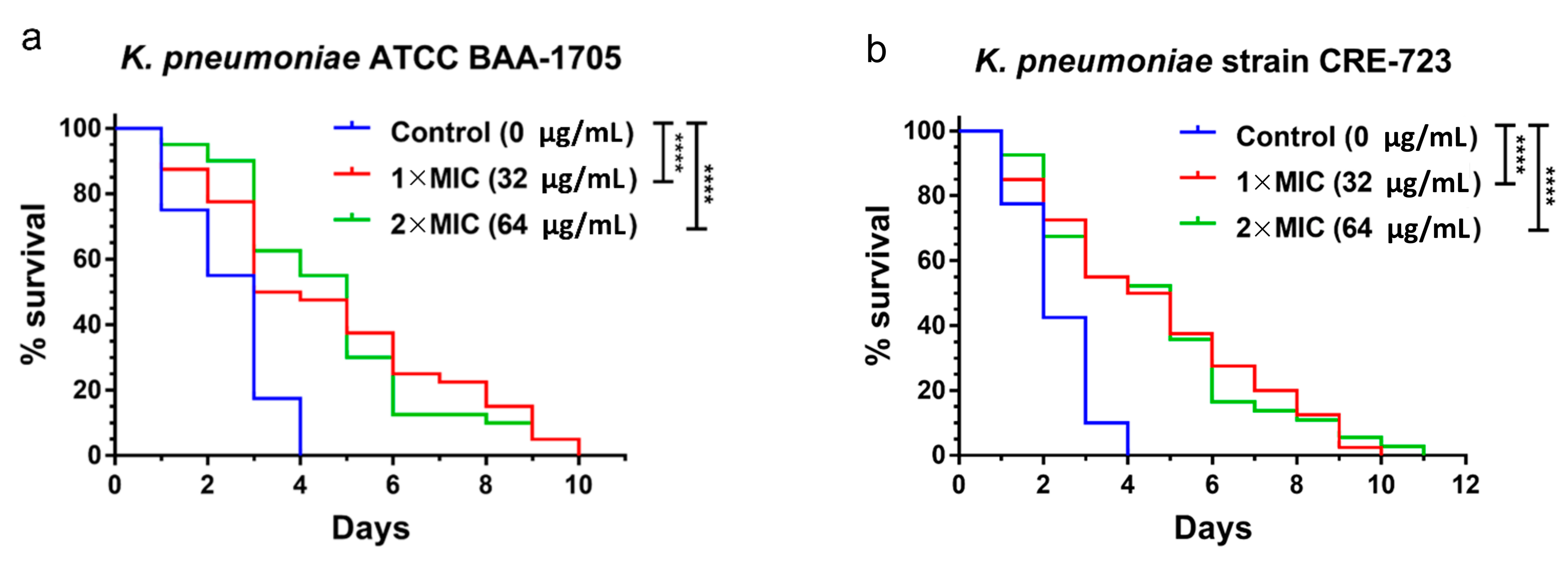
2.4. In Vivo Antibacterial Activity of AS101 in Two Animal Models
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Characterization of Bacterial Strains
4.3. Minimum Inhibitory Concentration (MIC)
4.4. Time-Kill Assays
4.5. Electron Microscopy
4.6. Gene Expression Profiling
4.7. Reactive Oxygen Species (ROS)
4.8. Nematode Survival Assays
4.9. Mouse Model
4.10. Bacterial Infection and Survival
4.11. Organ Bacterial Load
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Couto, R.C.; Carvalho, E.A.; Pedrosa, T.M.; Pedroso, E.R.; Neto, M.C.; Biscione, F.M. A 10-year prospective surveillance of nosocomial infections in neonatal intensive care units. Am. J. Infect. Control 2007, 35, 183–189. [Google Scholar] [CrossRef]
- Ardanuy, C.; Linares, J.; Dominguez, M.A.; Hernandez-Alles, S.; Benedi, V.J.; Martinez-Martinez, L. Outer membrane profiles of clonally related Klebsiella pneumoniae isolates from clinical samples and activities of cephalosporins and carbapenems. Antimicrob. Agents Chemother. 1998, 42, 1636–1640. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, K.Z.; Tansarli, G.S.; Rafailidis, P.I.; Falagas, M.E. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum beta-lactamases: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2012, 67, 2793–2803. [Google Scholar] [CrossRef]
- Chiu, S.K.; Wu, T.L.; Chuang, Y.C.; Lin, J.C.; Fung, C.P.; Lu, P.L.; Wang, J.T.; Wang, L.S.; Siu, L.K.; Yeh, K.M. National surveillance study on carbapenem non-susceptible Klebsiella pneumoniae in Taiwan: The emergence and rapid dissemination of KPC-2 carbapenemase. PLoS ONE 2013, 8, e69428. [Google Scholar]
- Braykov, N.P.; Eber, M.R.; Klein, E.Y.; Morgan, D.J.; Laxminarayan, R. Trends in resistance to carbapenems and third-generation cephalosporins among clinical isolates of Klebsiella pneumoniae in the United States, 1999–2010. Infect. Control Hosp. Epidemiol. 2013, 34, 259–268. [Google Scholar] [CrossRef]
- Biswas, S.; Brunel, J.M.; Dubus, J.C.; Reynaud-Gaubert, M.; Rolain, J.M. Colistin: An update on the antibiotic of the 21st century. Expert Rev. Anti-Infect. Ther. 2012, 10, 917–934. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Lin, T.L.; Pan, Y.J.; Wang, Y.P.; Lin, Y.T.; Wang, J.T. Colistin resistance mechanisms in Klebsiella pneumoniae strains from Taiwan. Antimicrob. Agents Chemother. 2015, 59, 2909–2913. [Google Scholar] [CrossRef]
- Suh, J.Y.; Son, J.S.; Chung, D.R.; Peck, K.R.; Ko, K.S.; Song, J.H. Nonclonal emergence of colistin-resistant Klebsiella pneumoniae isolates from blood samples in South Korea. Antimicrob. Agents Chemother. 2010, 54, 560–562. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Falagas, M.E.; Rafailidis, P.I.; Matthaiou, D.K. Resistance to polymyxins: Mechanisms, frequency and treatment options. Drug Resist. Updates 2010, 13, 132–138. [Google Scholar] [CrossRef]
- Towse, A.; Hoyle, C.K.; Goodall, J.; Hirsch, M.; Mestre-Ferrandiz, J.; Rex, J.H. Time for a change in how new antibiotics are reimbursed: Development of an insurance framework for funding new antibiotics based on a policy of risk mitigation. Health Policy 2017, 121, 1025–1030. [Google Scholar] [CrossRef]
- Luepke, K.H.; Suda, K.J.; Boucher, H.; Russo, R.L.; Bonney, M.W.; Hunt, T.D.; Mohr, J.F., 3rd. Past, present, and future of antibacterial economics: Increasing bacterial resistance, limited antibiotic pipeline, and societal implications. Pharmacotherapy 2017, 37, 71–84. [Google Scholar] [CrossRef]
- Brown, D. Antibiotic resistance breakers: Can repurposed drugs fill the antibiotic discovery void? Nat. Rev. Drug Discov. 2015, 14, 821–832. [Google Scholar] [CrossRef]
- Sun, W.; Weingarten, R.A.; Xu, M.; Southall, N.; Dai, S.; Shinn, P.; Sanderson, P.E.; Williamson, P.R.; Frank, K.M.; Zheng, W. Rapid antimicrobial susceptibility test for identification of new therapeutics and drug combinations against multidrug-resistant bacteria. Emerg. Microbes Infect. 2016, 5, e116. [Google Scholar] [CrossRef]
- Chopra, S.; Torres-Ortiz, M.; Hokama, L.; Madrid, P.; Tanga, M.; Mortelmans, K.; Kodukula, K.; Galande, A.K. Repurposing FDA-approved drugs to combat drug-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 2010, 65, 2598–2601. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.R.; Sullivan, D.J., Jr. New uses for old drugs. Nature 2007, 448, 645–646. [Google Scholar] [CrossRef]
- Sredni, B.; Caspi, R.R.; Klein, A.; Kalechman, Y.; Danziger, Y.; Ben Ya’akov, M.; Tamari, T.; Shalit, F.; Albeck, M. A new immunomodulating compound (AS-101) with potential therapeutic application. Nature 1987, 330, 173–176. [Google Scholar] [CrossRef]
- Strassmann, G.; Kambayashi, T.; Jacob, C.O.; Sredni, D. The immunomodulator AS-101 inhibits IL-10 release and augments TNF alpha and IL-1 alpha release by mouse and human mononuclear phagocytes. Cell Immunol. 1997, 176, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Indenbaum, V.; Bin, H.; Makarovsky, D.; Weil, M.; Shulman, L.M.; Albeck, M.; Sredni, B.; Mendelson, E. In vitro and in vivo activity of AS101 against West Nile virus (WNV). Virus Res. 2012, 166, 68–76. [Google Scholar] [CrossRef]
- Sredni, B.; Albeck, M.; Kazimirsky, G.; Shalit, F. The immunomodulator AS101 administered orally as a chemoprotective and radioprotective agent. Int. J. Immunopharmacol. 1992, 14, 613–619. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef]
- Tehrani, K.; Martin, N.I. beta-lactam/beta-lactamase inhibitor combinations: An update. Medchemcomm 2018, 9, 1439–1456. [Google Scholar] [CrossRef]
- Shirley, M. Ceftazidime-avibactam: A review in the treatment of serious gram-negative bacterial infections. Drugs 2018, 78, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Chen, L.; Cheng, S.; Chavda, K.D.; Press, E.G.; Snyder, A.; Pandey, R.; Doi, Y.; Kreiswirth, B.N.; Nguyen, M.H.; et al. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob. Agents Chemother. 2017, 61, e02097-16. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.A.; Koehler, M.F.T.; Girgis, H.S.; Yan, D.; Chen, Y.; Chen, Y.; Crawford, J.J.; Durk, M.R.; Higuchi, R.I.; Kang, J.; et al. Optimized arylomycins are a new class of gram-negative antibiotics. Nature 2018, 561, 189–194. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2018, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Kalechman, Y.; Gafter, U.; Gal, R.; Rushkin, G.; Yan, D.; Albeck, M.; Sredni, B. Anti-IL-10 therapeutic strategy using the immunomodulator AS101 in protecting mice from sepsis-induced death: Dependence on timing of immunomodulating intervention. J. Immunol. 2002, 169, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Daniel-Hoffmann, M.; Albeck, M.; Sredni, B.; Nitzan, Y. A potential antimicrobial treatment against ESBL-producing Klebsiella pneumoniae using the tellurium compound AS101. Arch. Microbiol. 2009, 191, 631–638. [Google Scholar] [CrossRef]
- Daniel-Hoffmann, M.; Sredni, B.; Nitzan, Y. Bactericidal activity of the organo-tellurium compound AS101 against Enterobacter cloacae. J. Antimicrob. Chemother. 2012, 67, 2165–2172. [Google Scholar] [CrossRef]
- Chiu, S.K.; Ma, L.; Chan, M.C.; Lin, Y.T.; Fung, C.P.; Wu, T.L.; Chuang, Y.C.; Lu, P.L.; Wang, J.T.; Lin, J.C.; et al. Carbapenem Nonsusceptible Klebsiella pneumoniae in Taiwan: Dissemination and increasing resistance of carbapenemase producers during 2012-2015. Sci. Rep. 2018, 8, 8468. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Seventh Informational Supplement; Document M100-S27; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters Version 7.1; Valid from 10 March 2017. 2017. Available online: www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.1_Breakpoint_Tables.pdf (accessed on 11 August 2021).
- Tseng, S.P.; Wang, S.F.; Ma, L.; Wang, T.Y.; Yang, T.Y.; Siu, L.K.; Chuang, Y.C.; Lee, P.S.; Wang, J.T.; Wu, T.L.; et al. The plasmid-mediated fosfomycin resistance determinants and synergy of fosfomycin and meropenem in carbapenem-resistant Klebsiella pneumoniae isolates in Taiwan. J. Microbiol. Immunol. Infect. 2017, 50, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.Y.; Wang, S.F.; Lin, J.E.; Griffith, B.T.S.; Lian, S.H.; Hong, Z.D.; Lin, L.; Lu, P.L.; Tseng, S.P. Contributions of insertion sequences conferring colistin resistance in Klebsiella pneumoniae. Int. J. Antimicrob. Agents 2020, 55, 105894. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Huang, Y.T.; Chen, C.S.; Chen, Y.W.; Huang, Y.T.; Su, J.C.; Teng, L.J.; Shiau, C.W.; Chiu, H.C. In vitro and in vivo activity of a novel sorafenib derivative SC5005 against MRSA. J. Antimicrob. Chemother. 2016, 71, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.P.; Hung, W.C.; Huang, C.Y.; Lin, Y.S.; Chan, M.Y.; Lu, P.L.; Lin, L.; Sheu, J.H. 5-episinuleptolide decreases the expression of the extracellular matrix in early biofilm formation of multi-drug resistant Acinetobacter baumannii. Mar. Drugs 2016, 14, 143. [Google Scholar] [CrossRef]
- Zahller, J.; Stewart, P.S. Transmission electron microscopic study of antibiotic action on Klebsiella pneumoniae biofilm. Antimicrob. Agents Chemother. 2002, 46, 2679–2683. [Google Scholar] [CrossRef]
- Freiberg, C.; Fischer, H.P.; Brunner, N.A. Discovering the mechanism of action of novel antibacterial agents through transcriptional profiling of conditional mutants. Antimicrob. Agents Chemother. 2005, 49, 749–759. [Google Scholar] [CrossRef]
- Tseng, S.P.; Tsai, W.C.; Liang, C.Y.; Lin, Y.S.; Huang, J.W.; Chang, C.Y.; Tyan, Y.C.; Lu, P.L. The contribution of antibiotic resistance mechanisms in clinical Burkholderia cepacia complex isolates: An emphasis on efflux pump activity. PLoS ONE 2014, 9, e104986. [Google Scholar] [CrossRef]
- Pan, C.Y.; Wu, J.L.; Hui, C.F.; Lin, C.H.; Chen, J.Y. Insights into the antibacterial and immunomodulatory functions of the antimicrobial peptide, epinecidin-1, against Vibrio vulnificus infection in zebrafish. Fish Shellfish Immunol. 2011, 31, 1019–1025. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Ong, K.S.; Cheow, Y.L.; Lee, S.M. The role of reactive oxygen species in the antimicrobial activity of pyochelin. J. Adv. Res. 2017, 8, 393–398. [Google Scholar] [CrossRef]
- Chou, T.C.; Chiu, H.C.; Kuo, C.J.; Wu, C.M.; Syu, W.J.; Chiu, W.T.; Chen, C.S. Enterohaemorrhagic Escherichia coli O157:H7 Shiga-like toxin 1 is required for full pathogenicity and activation of the p38 mitogen-activated protein kinase pathway in Caenorhabditis elegans. Cell Microbiol. 2013, 15, 82–97. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.; Reid-Yu, S.A.; Wang, W.; King, D.T.; De Pascale, G.; Strynadka, N.C.; Walsh, T.R.; Coombes, B.K.; Wright, G.D. Aspergillomarasmine A overcomes metallo-beta-lactamase antibiotic resistance. Nature 2014, 510, 503–506. [Google Scholar] [CrossRef]
- Shen, W.C.; Wang, X.; Qin, W.T.; Qiu, X.F.; Sun, B.W. Exogenous carbon monoxide suppresses Escherichia coli vitality and improves survival in an Escherichia coli-induced murine sepsis model. Acta Pharmacol. Sin. 2014, 35, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Powles, M.A.; Galgoci, A.; Misura, A.; Colwell, L.; Dingley, K.H.; Tang, W.; Wu, J.; Blizzard, T.; Motyl, M.; Young, K. In vivo efficacy of relebactam (MK-7655) in combination with imipenem-cilastatin in murine infection models. Antimicrob. Agents Chemother. 2018, 62, e02577-17. [Google Scholar] [CrossRef]
- Band, V.I.; Satola, S.W.; Burd, E.M.; Farley, M.M.; Jacob, J.T.; Weiss, D.S. Carbapenem-resistant Klebsiella pneumoniae exhibiting clinically undetected colistin heteroresistance leads to treatment failure in a murine model of infection. mBio 2018, 9, e02448-17. [Google Scholar] [CrossRef] [PubMed]

| CRKP Isolate (n = 134) | KPC Producer 1 | AS101 MIC (μg/mL) | ||
|---|---|---|---|---|
| MIC Range | MIC50 | MIC90 | ||
| Colistin-susceptible 1 (n = 79) | KPC-2 (+) (n = 35) | 0.5–32 | 16 | 16 |
| KPC-2 (−) (n = 44) | 1–32 | 16 | 32 | |
| Colistin-resistant 1 (n = 55) | KPC-2 (+) (n = 25) | <0.5–16 | 4 | 16 |
| KPC-2 (−) (n = 30) | 1–32 | 16 | 32 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.-Y.; Kao, H.-Y.; Lu, P.-L.; Chen, P.-Y.; Wang, S.-C.; Wang, L.-C.; Hsieh, Y.-J.; Tseng, S.-P. Evaluation of the Organotellurium Compound AS101 for Treating Colistin- and Carbapenem-Resistant Klebsiella pneumoniae. Pharmaceuticals 2021, 14, 795. https://doi.org/10.3390/ph14080795
Yang T-Y, Kao H-Y, Lu P-L, Chen P-Y, Wang S-C, Wang L-C, Hsieh Y-J, Tseng S-P. Evaluation of the Organotellurium Compound AS101 for Treating Colistin- and Carbapenem-Resistant Klebsiella pneumoniae. Pharmaceuticals. 2021; 14(8):795. https://doi.org/10.3390/ph14080795
Chicago/Turabian StyleYang, Tsung-Ying, Hao-Yun Kao, Po-Liang Lu, Pei-Yu Chen, Shu-Chi Wang, Liang-Chun Wang, Ya-Ju Hsieh, and Sung-Pin Tseng. 2021. "Evaluation of the Organotellurium Compound AS101 for Treating Colistin- and Carbapenem-Resistant Klebsiella pneumoniae" Pharmaceuticals 14, no. 8: 795. https://doi.org/10.3390/ph14080795
APA StyleYang, T.-Y., Kao, H.-Y., Lu, P.-L., Chen, P.-Y., Wang, S.-C., Wang, L.-C., Hsieh, Y.-J., & Tseng, S.-P. (2021). Evaluation of the Organotellurium Compound AS101 for Treating Colistin- and Carbapenem-Resistant Klebsiella pneumoniae. Pharmaceuticals, 14(8), 795. https://doi.org/10.3390/ph14080795








