Canine Myocytes Represent a Good Model for Human Ventricular Cells Regarding Their Electrophysiological Properties
Abstract
1. Introduction
2. Significance of the Action Potential Voltage Clamp Technique
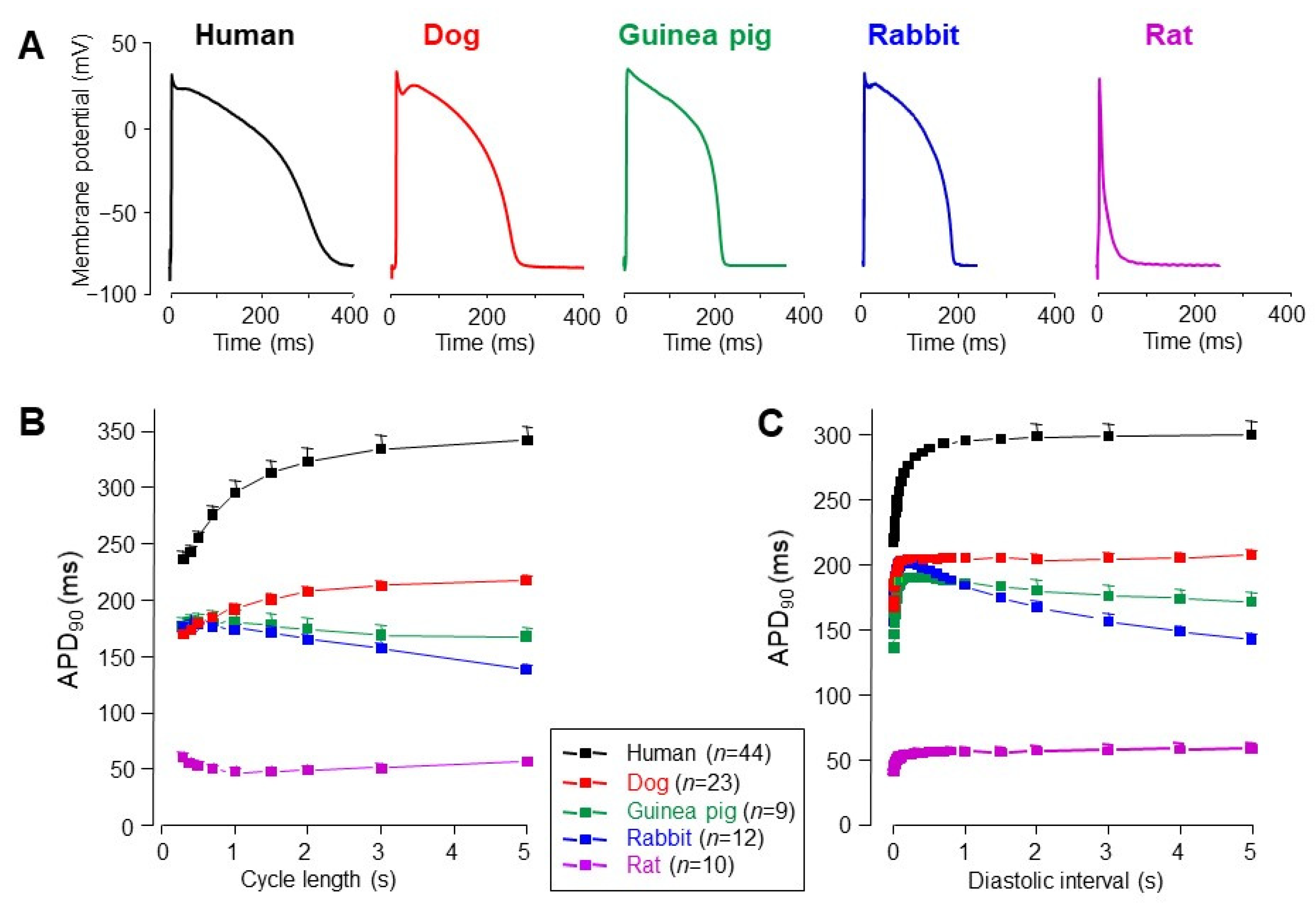
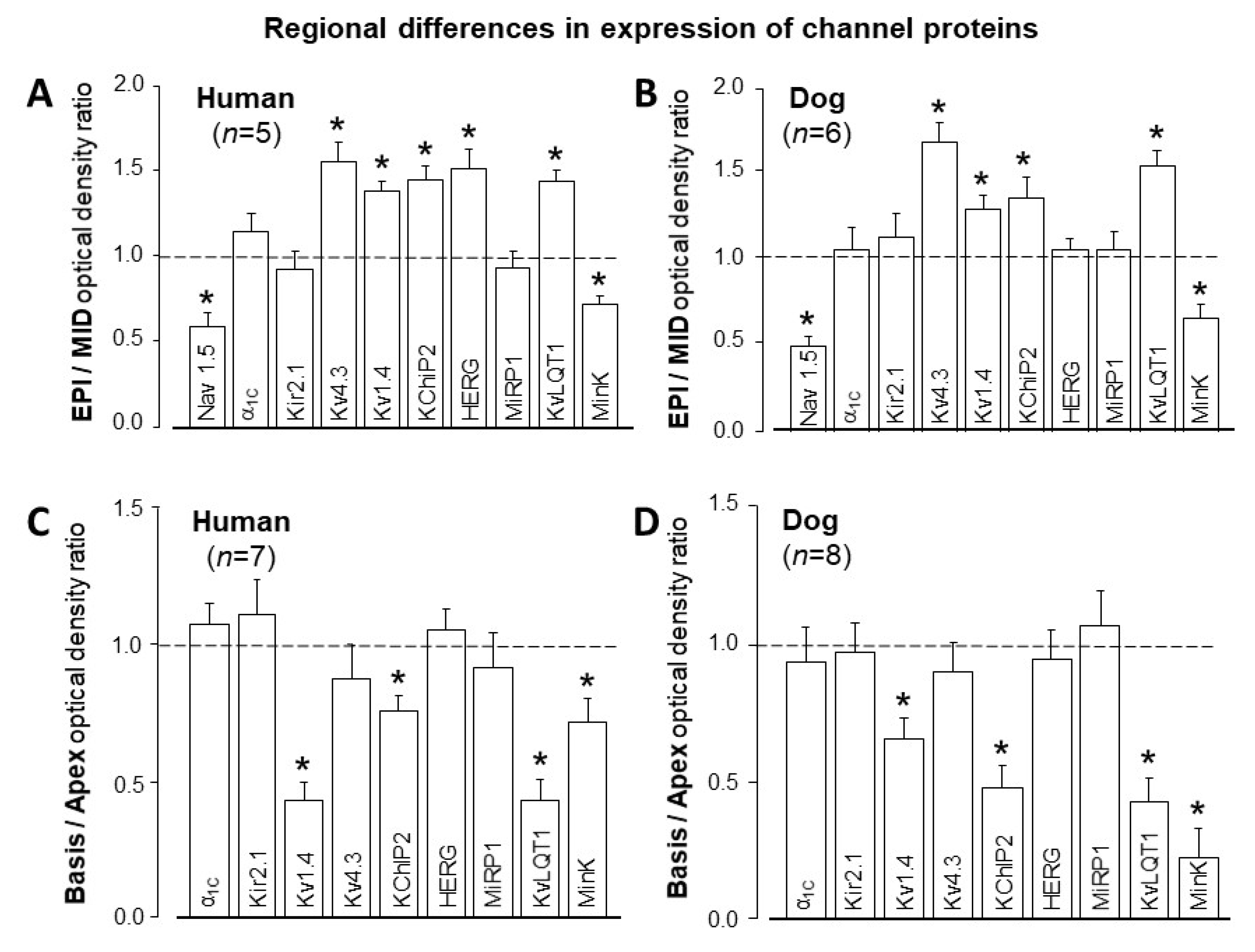
3. Interspecies Differences in Action Potential Morphology and the Underlying Ion Currents
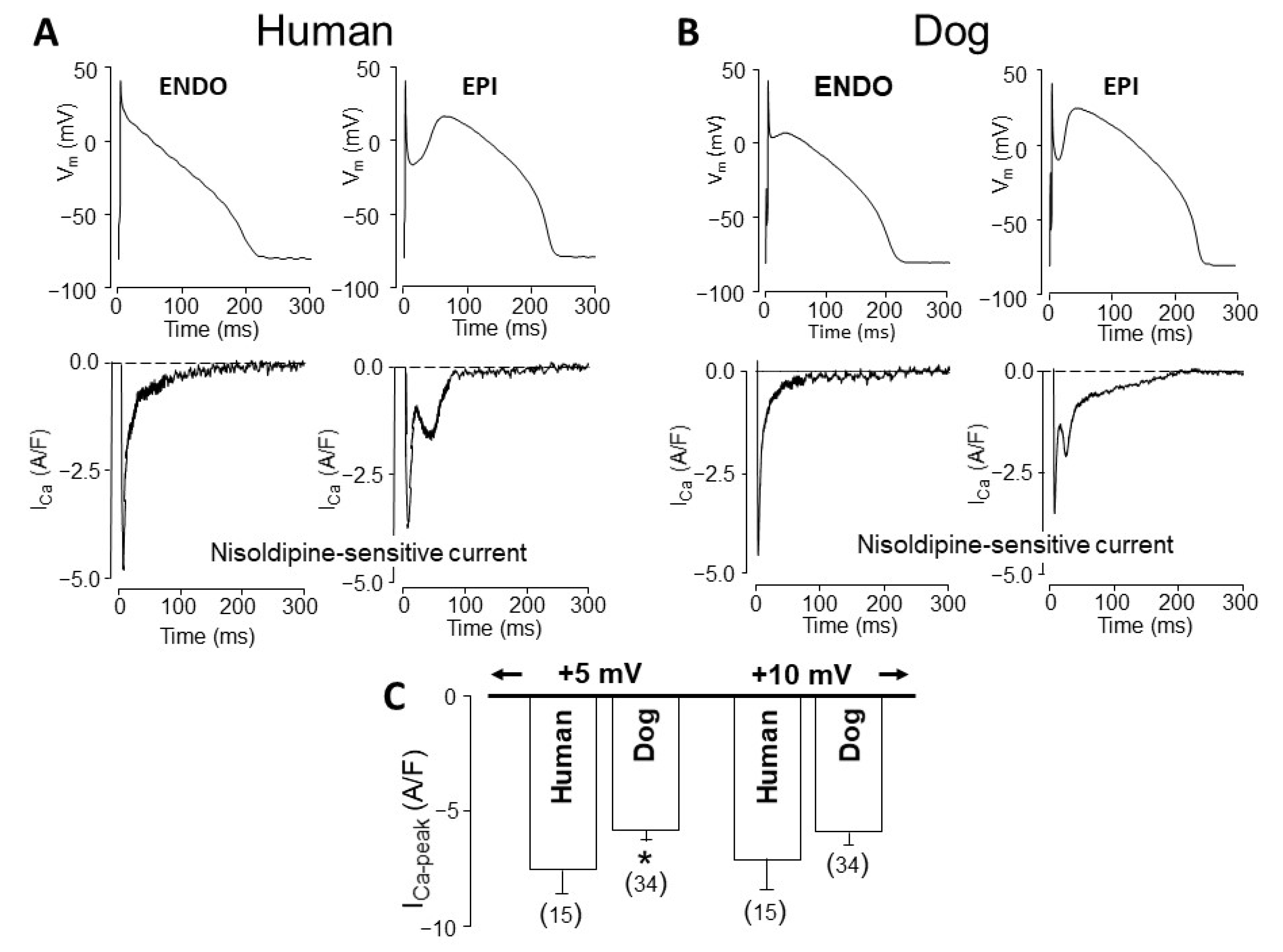
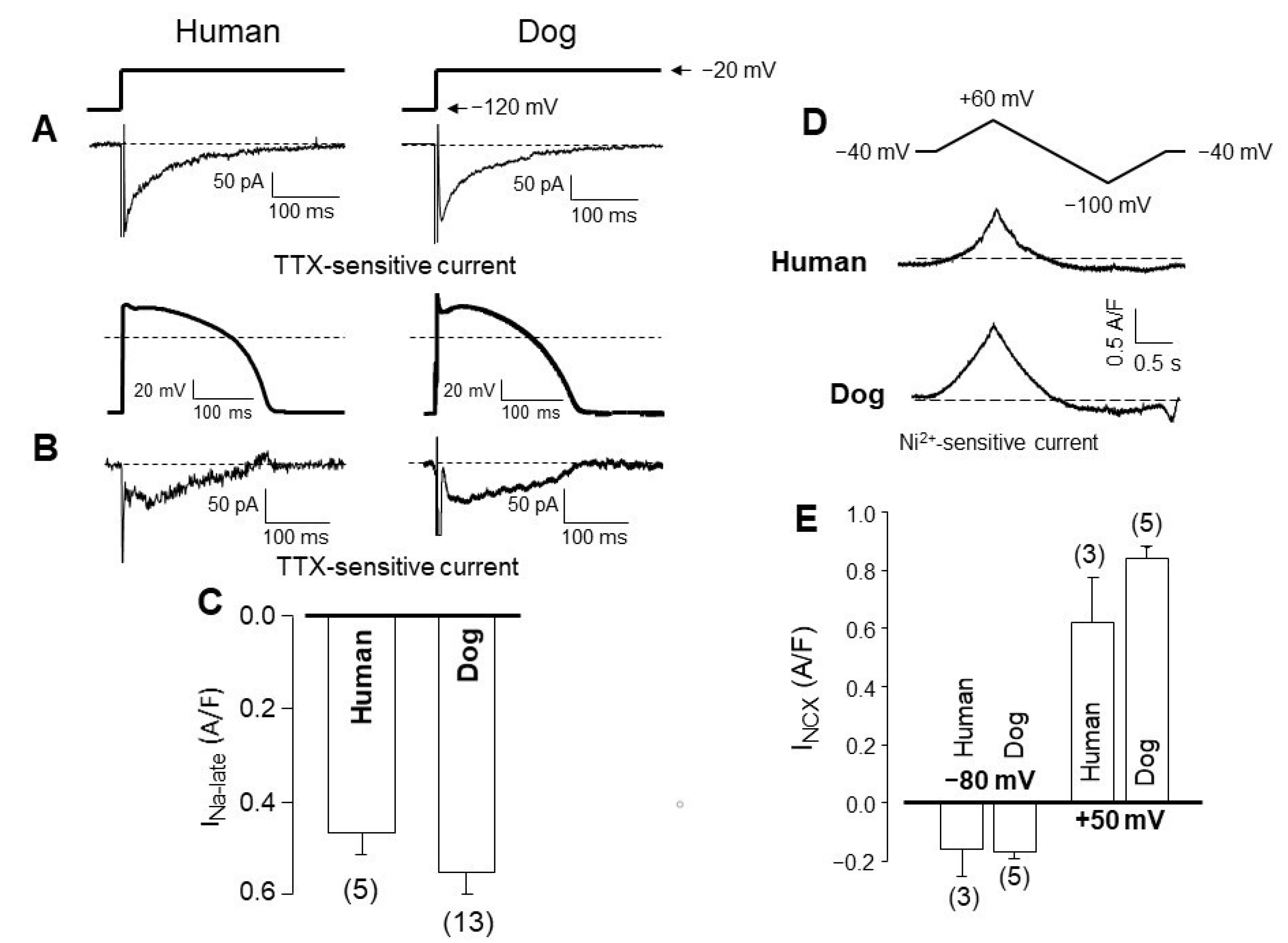
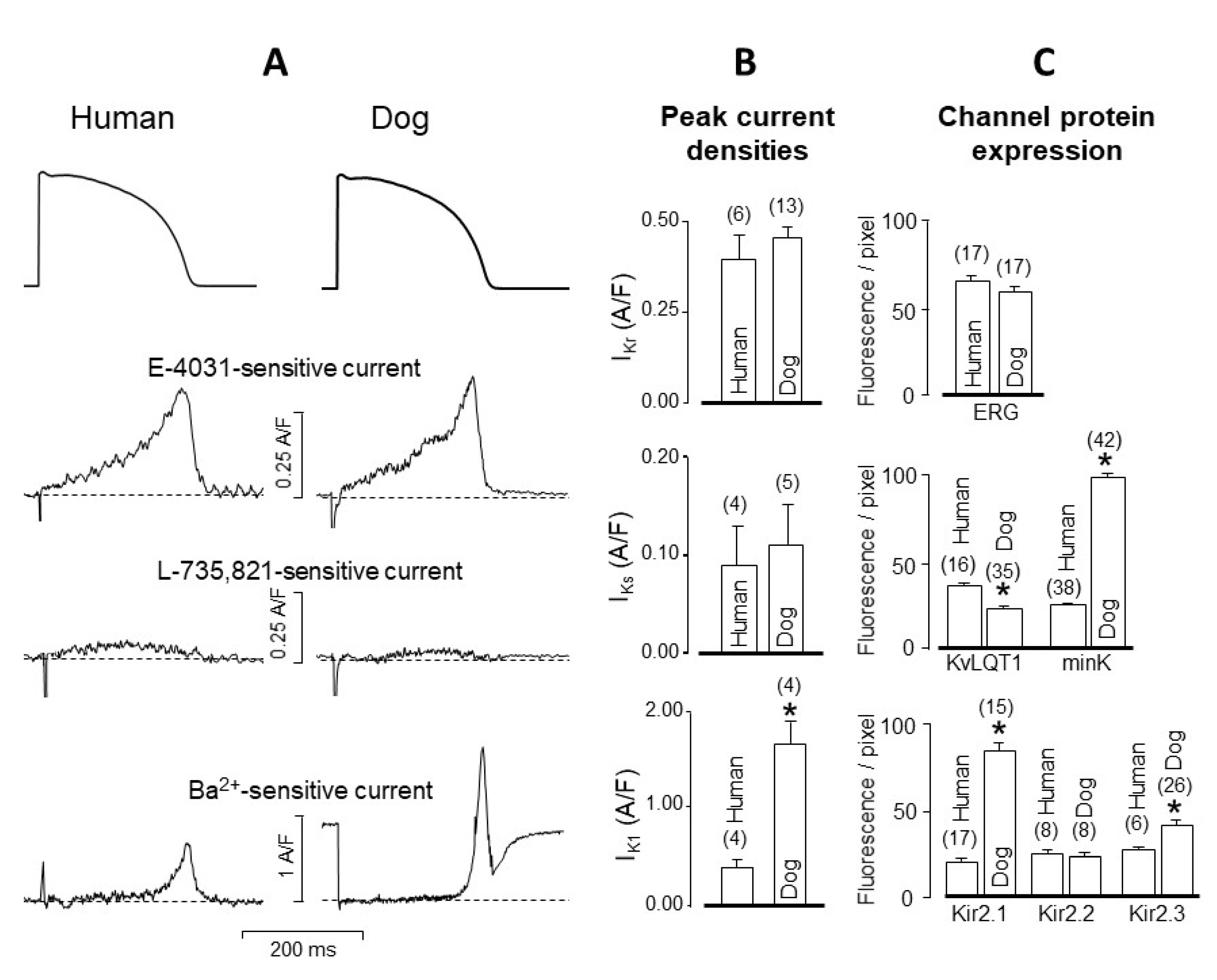
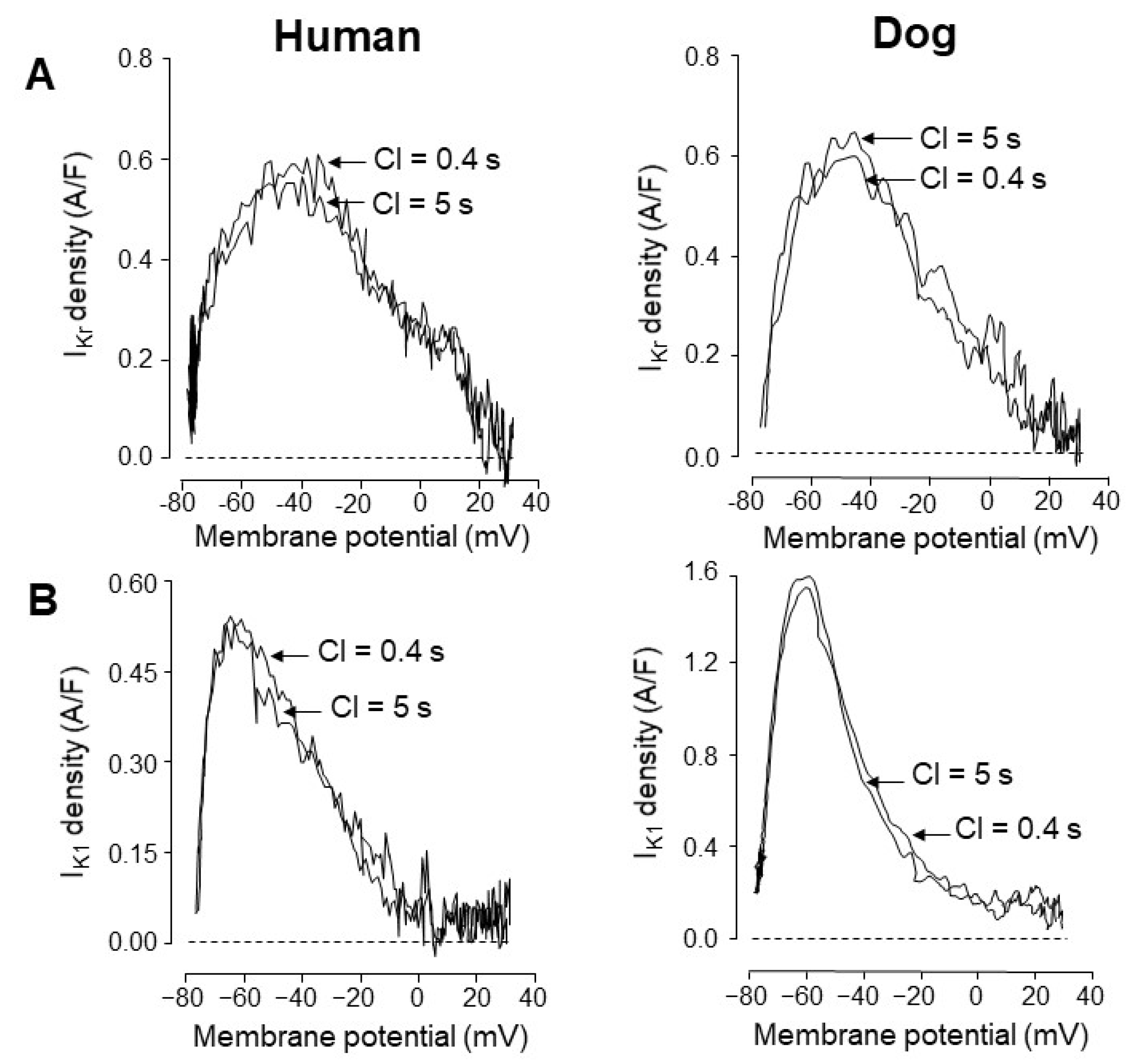
4. Comparison of Human and Canine Ion Currents under Action Potential Voltage Clamp Conditions
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grandi, E.; Pasqualini, F.S.; Bers, D.M. A novel computational model of the human ventricular action potential and Ca transient. J. Mol. Cell Cardiol. 2010, 48, 112–121. [Google Scholar] [CrossRef]
- O’Hara, T.; Virág, L.; Varró, A.; Rudy, Y. Simulation of the undiseased human cardiac ventricular action potential: Model formulation and experimental validation. PLoS Comput Biol. 2011, 7, e1002061. [Google Scholar] [CrossRef] [PubMed]
- Pueyo, E.; Dangerfield, C.E.; Britton, O.J.; Virág, L.; Kistamás, K.; Szentandrássy, N.; Jost, N.; Varró, A.; Nánási, P.P.; Burrage, K.; et al. Experimentally-based computational investigation into beat-to-beat variability in ventricular repolarization and its response to ionic current inhibition. PLoS ONE 2016, 11, e0151461. [Google Scholar] [CrossRef]
- Tomek, J.; Bueno-Orovio, A.; Passini, E.; Zhou, X.; Minchole, A.; Britton, O.; Bartolucci, C.; Severi, S.; Shrier, A.; Virag, L.; et al. Development, calibration, and validation of a novel human ventricular myocyte model in health, disease, and drug block. eLife 2019, 8, e48890. [Google Scholar] [CrossRef] [PubMed]
- Ten Tusscher, K.H.; Noble, D.; Noble, P.J.; Panfilov, A.V. A model for human ventricular tissue. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1573–H1589. [Google Scholar] [CrossRef] [PubMed]
- Beuckehnann, D.J.; Nabauer, M.; Erdmann, E. Characteristics of calcium-current in isolated human ventricular myocytes from patients with terminal heart failure. J. Mol. Cell Cardiol. 1991, 23, 929–937. [Google Scholar] [CrossRef]
- Nánási, P.P.; Varró, A.; Lathrop, D.A. Ionic currents in ventricular myocytes isolated from the heart of a patient with idiopathic cardiomyopathy. Cardioscience 1992, 3, 85–89. [Google Scholar]
- Varró, A.; Nánási, P.P.; Lathrop, D.A. Potassium currents in isolated human atrial and ventricular cardiocytes. Acta Physiol. Scand. 1993, 149, 133–142. [Google Scholar] [CrossRef]
- Mummery, C.L. Perspectives on the use of human induced pluripotent stem cell-derived cardiomyocytes in biomedical research. Stem Cell Rep. 2018, 11, 1306–1311. [Google Scholar] [CrossRef]
- Casini, S.; Verkerk, A.O.; Remme, C.A. Human iPSC-derived cardiomyocytes for investigation of disease mechanisms and therapeutic strategies in inherited arrhythmia syndromes: Strengths and limitations. Cardiovasc. Drugs Ther. 2017, 31, 325–344. [Google Scholar] [CrossRef]
- Horváth, A.; Lemoine, M.D.; Löser, A.; Mannhardt, I.; Flenner, F.; Uzun, A.U.; Neuber, C.; Breckwoldt, K.; Hansen, A.; Girdauskas, E.; et al. Low resting membrane potential and low inward rectifier potassium currents are not inherent features of hiPSC-derived cardiomyocytes. Stem Cell Rep. 2018, 10, 822–833. [Google Scholar] [CrossRef]
- Verkerk, A.O.; Remme, C.A. ‘Mature’ resting membrane potentials in human-induced pluripotent stem cell-derived cardiomyocytes: Fact or artefact? Europace 2019, 21, 1928. [Google Scholar] [CrossRef]
- Varró, A.; Lathrop, D.A.; Hester, S.B.; Nánási, P.P.; Papp, J.G. Ionic currents and action potentials in rabbit, rat and guinea pig ventricular myocytes. Basic Res. Cardiol. 1993, 88, 93–102. [Google Scholar] [PubMed]
- Meyer, R.; Linz, K.W.; Surges, R.; Meinardus, S.; Vees, J.; Hoffmann, A.; Windholz, O.; Grohe, C. Rapid modulation of L-type calcium current by acutely applied oestrogens in isolated cardiac myocytes from human, guinea-pig and rat. Exp. Physiol. 1998, 83, 305–321. [Google Scholar] [CrossRef]
- Sham, J.S.; Hatem, S.N.; Morad, M. Species differences in the activity of the Na+-Ca2+ exchanger in mammalian cardiac myocytes. J. Physiol. 1995, 488, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Linz, K.W.; Meyer, R. Profile and kinetics of the L-type calcium current during the cardiac ventricular action potential compared in guinea-pigs, rats and rabbits. Pflügers Arch. 2000, 439, 588–599. [Google Scholar] [CrossRef]
- Horváth, B.; Hézső, T.; Szentandrássy, N.; Kistamás, K.; árpádffy-Lovas, T.; Varga, R.; Gazdag, P.; Veress, R.; Dienes, C.; Baranyai, D.; et al. Late sodium current in human, canine and guinea pig ventricular myocardium. J. Mol. Cell Cardiol. 2020, 139, 14–23. [Google Scholar] [CrossRef]
- Sala, L.; Hegyi, B.; Bartolucci, C.; Altomare, C.; Rocchetti, M.; Váczi, K.; Mostacciuolo, G.; Szentandrássy, N.; Severi, S.; Nánási, P.P.; et al. Action potential contour contributes to species differences in repolarization response to β-adrenergic-stimulation. Europace 2018, 20, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Szentadrássy, N.; Bányász, T.; Bíró, T.; Szabó, G.; Tóth, I.B.; Magyar, J.; Lázár, J.; Varró, A.; Kovács, L.; Nánási, P.P. Apico-basal inhomogeneity in distribution of ion channels in canine and human ventricular myocardium. Cardiovasc. Res. 2005, 65, 851–860. [Google Scholar] [CrossRef]
- Szabó, G.; Szentandrássy, N.; Bíró, T.; Tóth, I.B.; Czifra, G.; Magyar, J.; Bányász, T.; Varró, A.; Kovács, L.; Nánási, P.P. Asymmetrical distribution of ion channels in canine and human left ventricular wall: Epicardium versus midmyocardium. Pflügers Arch. 2005, 450, 307–316. [Google Scholar] [CrossRef]
- Jost, N.; Virág, L.; Comtois, P.; Ördög, B.; Szűts, V.; Seprényi, G.; Bitay, M.; Kohajda, Z.; Koncz, I.; Nagy, N.; et al. Ionic mechanisms limiting cardiac repolarization reserve in humans compared to dogs. J. Physiol. 2013, 591, 4189–4206. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, L.; Bányász, T.; Magyar, J.; Szentandrássy, N.; Varró, A.; Nánási, P.P. Reopening of L-type calcium channels in human ventricular myocytes during applied epicardial action potentials. Acta Physiol. Scand. 2004, 180, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Jost, N.; Acsai, K.; Horváth, B.; Bányász, T.; Bitay, M.; Bogáts, G.; Nánási, P.P. Contribution of IKr and IK1 to ventricular repolarization in canine and human myocytes. Is there any influence of action potential duration? Basic Res. Cardiol. 2009, 104, 33–41. [Google Scholar] [CrossRef]
- Bányász, T.; Fülöp, L.; Magyar, J.; Szentandrássy, N.; Varró, A.; Nánási, P.P. Endocardial versus epicardial differences in L-type calcium current in canine ventricular myocytes studied by action potential voltage clamp. Cardiovasc. Res. 2003, 58, 66–75. [Google Scholar] [CrossRef]
- Maltsev, V.A.; Silverman, N.; Sabbah, H.N.; Undrovinas, A.I. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: Implications for repolarization variability. Eur. J. Heart Fail 2007, 9, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Varró, A.; Tomek, J.; Nagy, N.; Virag, L.; Passini, E.; Rodriguez, B.; Baczkó, I. Cardiac transmembrane ion channels and action potentials: Cellular physiology and arrhythmogenic behavior. Physiol. Rev. 2021, 101, 1083–1176. [Google Scholar] [CrossRef]
- Clancy, C.E.; Tateyama, M.; Liu, H.; Wehrens, X.H.; Kass, R.S. Non-equilibrium gating in cardiac sodium cahannels: An original mechanism of arrhythmia. Circulation 2003, 107, 2233–2237. [Google Scholar] [CrossRef]
- Fischmeister, R.; DeFelice, L.J.; Ayer, R.K.; Levi, R.; DeHaan, R.L. Channel currents during spontaneous action potentials in embryonic chick heart sells. The action potential patch clamp. Biophys. J. 1984, 46, 267–271. [Google Scholar] [CrossRef]
- Nánási, P.P.; Szentandrássy, N.; Tóth, A.; Bányász, T. Power of the action potential voltage clamp technique. In Advances in Cardiomyocyte Research; Nanasi, P., Ed.; Transworld Research Network: Kerala, India, 2010; pp. 93–119. [Google Scholar]
- Hegyi, B.; Chen-Izu, Y.; Izu, L.T.; Rajamani, S.; Belardinelli, L.; Bers, D.M.; Bányász, T. Balance between rapid delayed rectifier K+ current and late Na+ current on ventricular repolarization. An effective antiarrhythmic target? Circ. Arrhythm. Electrophysiol. 2020, 13, e008130. [Google Scholar] [CrossRef]
- Hegyi, B.; Bossuyt, J.; Griffiths, L.G.; Shimkunas, R.; Coulibalya, Z.; Jiana, Z.; Grimsrudf, K.N.; Sondergaard, C.S.; Ginsburg, K.S.; Chiamvimonvata, N.; et al. Complex electrophysiological remodeling in postinfarction ischemic heart failure. Proc. Natl. Acad. Sci. USA 2018, 115, E3036–E3044. [Google Scholar] [CrossRef]
- Puglisi, J.L.; Yuan, W.; Bassani, J.W.M.; Bers, D.M. Ca2+ influx through Ca2+ channels in rabbit ventricular myocytes during action potential clamp: Influence of temperature. Circ. Res. 1999, 85, e7–e16. [Google Scholar] [CrossRef]
- Shimoni, Y.; Clark, R.B.; Giles, W.R. Role of an inwardly rectifying potassium current in rabbit ventricular action potential. J. Physiol. 1992, 448, 709–727. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, B.; Bányász, T.; Izu, L.T.; Belardinelli, L.; Bers, D.M.; Chen-Izu, Y. β-adrenergic regulation of late Na+ current during cardiac action potential is mediated by both PKA and CaMKII. J. Mol. Cell Cardiol. 2018, 123, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Arreola, J.; Dirksen, R.T.; Shieh, R.C.; Williford, D.J.; Sheu, S.S. Ca2+ current and Ca2+ transients under action potential clamp in guinea pig ventricular myocytes. Am. J. Physiol. 1991, 261, C393–C397. [Google Scholar] [CrossRef]
- Doerr, T.; Dengerm, R.; Doerr, A.; Trautwein, W. Ionic currents contributing to the action potential in single ventricular myocytes of the guinea pig studied with action potential clamp. Pflügers Arch. 1990, 416, 230–237. [Google Scholar] [CrossRef]
- Ibarra, J.; Morley, G.E.; Delmar, M. Dynamics of the inward rectifier K+ current during the action potential of guinea pig ventricular myocytes. Biophys. J. 1991, 60, 1534–1539. [Google Scholar] [CrossRef]
- Rocchetti, M.; Besana, A.; Gurrola, G.B.; Possani, L.D.; Zaza, A. Rate dependency of delayed rectifier currents during the guinea-pig ventricular action potential. J. Physiol. 2001, 534, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Banyasz, T.; Horvath, B.; Jian, Z.; Izu, L.T.; Chen-Izu, Y. Profile of L-type Ca2+ current and Na+/Ca2+ exchange current during the cardiac action potential in ventricular myocytes. Heart Rhythm. 2012, 9, 134–142. [Google Scholar] [CrossRef]
- Horvath, B.; Banyasz, T.; Jian, Z.; Hegyi, B.; Kistamas, K.; Nanasi, P.P.; Izu, L.T.; Chen-Izu, Y. Dynamics of the late Na+ current during cardiac action potential and its contribution to afterdepolarizations. J. Mol. Cell Cardiol. 2013, 64, 59–68. [Google Scholar] [CrossRef]
- Zaza, A.; Rocchetti, M.; Brioschi, A.; Cantadori, A.; Ferroni, A. Dynamic Ca2+-induced inward rectification of K+ current during the ventricular action potential. Circ. Res. 1998, 82, 947–956. [Google Scholar] [CrossRef]
- Bányász, T.; Magyar, J.; Szentandrássy, N.; Horváth, B.; Birinyi, P.; Szentmiklósi, J.; Nánási, P.P. Action potential clamp fingerprints of K+ currents in canine cardiomyocytes: Their role in ventricular repolarization. Acta Physiol. Scand. 2007, 190, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.; Renodin, D.; Antzelevitch, C.; Di Diego, J.M.; Cordeiro, J.M. Extracellular proton depression of peak and late Na+ current in the canine left ventricle. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H936–H944. [Google Scholar] [CrossRef] [PubMed]
- Apkon, M.; Nerbonne, J.M. Characterization of two distinct depolarization-activated K+ currents in isolated adult rat ventricular myocytes. J. Gen. Physiol. 1991, 97, 973–1011. [Google Scholar] [CrossRef] [PubMed]
- Josephson, I.R.; Sanchez-Chapula, J.; Brown, A.M. Early outward current in rat single ventricular cells. Circ. Res. 1984, 54, 157–162. [Google Scholar] [CrossRef]
- Josephson, I.R.; Sanchez-Chapula, J.; Brown, A.M. A comparison of calcium currents in rat and guinea pig single ventricular cells. Circ. Res. 1984, 54, 144–156. [Google Scholar] [CrossRef]
- Benndorf, K.; Nilius, B. Properties of an early outward current in single cells of the mouse ventricle. Gen. Physiol. Biophys. 1988, 7, 449–466. [Google Scholar]
- Giles, W.R.; Imaizumi, Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J. Physiol. 1988, 405, 123–145. [Google Scholar] [CrossRef]
- Hiraoka, M.; Kawano, S. Calcium-sensitive and insensitive transient outward current in rabbit ventricular myocytes. J. Physiol. 1989, 410, 187–212. [Google Scholar] [CrossRef]
- Varró, A.; Nánási, P.P.; Lathrop, D.A. Voltage clamp characteristics of ventricular myocytes in rabbit. Cardioscience 1991, 2, 233–243. [Google Scholar] [PubMed]
- Wang, Z.; Feng, J.; Shi, H.; Pond, A.; Nerbonne, J.M.; Nattel, S. Potential molecular basis of different physiological properties of the transient outward K+ current in rabbit and human atrial myocytes. Circ. Res. 1999, 84, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Akar, F.G.; Wu, R.C.; Deschenes, I.; Armoundas, A.A.; Piacentino, V., 3rd; Houser, S.R.; Tomaselli, G.F. Phenotypic differences in transient outward K+ current of human and canine ventricular myocytes: Insights into molecular composition of ventricular Ito. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H602–H609. [Google Scholar] [CrossRef]
- Calloe, K. Doctoral Dissertation: The transient outward potassium current in healthy and diseased hearts. Acta Physiol. 2019, 225 (Suppl. S717), e13225. [Google Scholar] [CrossRef]
- Johnson, E.K.; Springer, S.J.; Wang, W.; Dranoff, E.J.; Zhang, Y.; Kanter, E.M.; Yamada, K.A.; Nerbonne, J.M. Differential expression and remodeling of transient outward potassium currents in human left ventricles. Circ. Arrhythm. Electrophysiol. 2018, 11, e005914. [Google Scholar] [CrossRef]
- Kukushkin, N.I.; Gainullin, R.Z.; Sosunov, E.A. Transient outward current and rate dependence of action potential duration in rabbit cardiac ventricular muscle. Pflügers Arch. 1983, 399, 87–92. [Google Scholar] [CrossRef]
- Szigligeti, P.; Pankucsi, C.; Bányász, T.; Varró, A.; Nánási, P.P. Action potential duration and force-frequency relationship in isolated rabbit, guinea pig and rat cardiac muscle. J. Comp. Physiol. B 1996, 166, 150–155. [Google Scholar] [CrossRef] [PubMed]
- árpádffy-Lovas, T.; Baczkó, I.; Baláti, B.; Bitay, M.; Jost, N.; Lengyel, C.; Nagy, N.; Takács, J.; Varró, A.; Virág, L. Electrical restitution and its modifications by antiarrhythmic drugs in undiseased human ventricular muscle. Front. Pharmacol. 2020, 11, 479. [Google Scholar] [CrossRef]
- Hume, J.R.; Uehara, A. Ionic basis of the different action potential configurations of single guinea-pig atrial and ventricular myocytes. J. Physiol. 1985, 368, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Litovsky, S.H.; Antzelevitch, C. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ. Res. 1988, 62, 116–126. [Google Scholar] [CrossRef]
- Sanguinetti, M.C.; Jurkiewicz, N.K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J. Gen. Physiol. 1990, 96, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Lambrigts, D.; Sachs, D.H.; Cooper, D.K. Discordant organ xenotransplantation in primates: World experience and current status. Transplantation 1998, 66, 547–561. [Google Scholar] [CrossRef]
- Li, G.-R.; Du, X.-L.; Siow, Y.L.; Karmin, O.; Tse, H.-F.; Lau, C.-P. Calcium-activated transient outward chloride current and phase 1 repolarization of swine ventricular action potential. Cardiovasc. Res. 2003, 58, 89–98. [Google Scholar] [CrossRef]
- Stankovicova, T.; Szilard, M.; De, S.I.; Sipido, K.R. M cells and transmural heterogeneity of action potential configuration in myocytes from the left ventricular wall of the pig heart. Cardiovasc. Res. 2000, 45, 952–960. [Google Scholar] [CrossRef]
- Rodriguez-Sinovas, A.; Cinca, J.; Tapias, A.; Armadans, L.; Tresanchez, M.; Soler-Soler, J. Lack of evidence of M-cells in porcine left ventricular myocardium. Cardiovasc. Res. 1997, 33, 307–313. [Google Scholar] [CrossRef]
- Horváth, B.; Kiss, D.; Dienes, C.; Hézső, T.; Kovács, Z.; Szentandrássy, N.; Almássy, J.; Magyar, J.; Bányász, T.; Nánási, P.P. Ion current profiles in canine ventricular myocytes obtained by the “onion peeling” technique. J. Mol. Cell Cardiol. 2021, 158, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, A.C.; Robitelle, D.C.; Eddlestone, G.T. Ito1 dictates behavior of ICl(Ca) during early repolarization of canine ventricle. Am. J. Physiol. 1997, 273, H1096–H1106. [Google Scholar] [CrossRef]
- Mukherjee, R.; Hewett, K.W.; Walker, J.D.; Basler, C.G.; Spinale, F.G. Changes in L-type calcium channel abundance and function during the transition to pacing-induced congestive heart failure. Cardiovasc. Res. 1998, 37, 432–444. [Google Scholar] [CrossRef][Green Version]
- Antzelevitch, C.; Sicouri, S.; Litovsky, S.H.; Lukas, A.; Krishnan, S.C.; Di Diego, J.M.; Gintant, G.A.; Liu, D.W. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ. Res. 1991, 69, 1427–1449. [Google Scholar] [CrossRef]
- Näbauer, M.; Beuckelmann, D.J.; Uberfuhr, P.; Steinbeck, G. Regional differences in current density and rate-dependent properties of the transient outward current in subepicardial and subendocardial myocytes of human left ventricle. Circulation 1996, 93, 168–177. [Google Scholar] [CrossRef]
- Liu, D.W.; Gintant, G.A.; Antzelevitch, C. Ionic bases for electrophysiological distinctions among epicardial, midmyocardial, and endocardial myocytes from the free wall of the canine left ventricle. Circ. Res. 1993, 72, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Drouin, E.; Charpentier, F.; Gauthier, C.; Laurent, K.; Le Marec, H. Electrophysiologic characteristics of cells spanning the left ventricular wall of human heart: Evidence for the presence of M cells. J. Am. Coll. Cardiol. 1995, 26, 185–192. [Google Scholar] [CrossRef]
- Li, G.-R.; Lau, C.-P.; Ducharme, A.; Tardif, J.-C.; Nattel, S. Transmural action potential and ionic current remodeling in ventricles of failing canine hearts. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1031–H1041. [Google Scholar] [CrossRef]
- Wettwer, E.; Amos, G.J.; Posival, H.; Ravens, U. Transient outward current in human ventricular myocytes of subepicardial and subendocardial origin. Circ. Res. 1994, 75, 473–482. [Google Scholar] [CrossRef]
- Li, G.-R.; Feng, J.; Yue, L.; Carrier, M. Transmural heterogeneity of action potentials and Ito1 in myocytes isolated from the human right ventricle. Am. J. Physiol. 1998, 275, H369–H377. [Google Scholar] [PubMed]
- Niwa, N.; Nerbonne, J.M. Molecular determinants of cardiac transient outward potassium current (Ito) expression and regulation. J. Mol. Cell Cardiol. 2010, 48, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, J.M.; Calloe, K.; Moise, N.S.; Kornreich, B.; Giannandrea, D.; Di Diego, J.M.; Olesen, S.P.; Antzelevitch, C. Physiological consequences of transient outward K+ current activation during heart failure in the canine left ventricle. J. Mol. Cell Cardiol. 2012, 52, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Rosati, B.; Grau, F.; Rodriguez, S.; Li, H.; Nerbonne, J.M.; McKinnon, D. Concordant expression of KChIP2 mRNA, protein and transient outward current throughout the canine ventricle. J. Physiol. 2003, 548, 815–822. [Google Scholar] [CrossRef]
- Virág, L.; Jost, N.; Papp, R.; Koncz, I.; Kristóf, A.; Kohajda, Z.; Harmati, G.; Carbonell-Pascual, B.; Ferrero, J.M., Jr.; Papp, J.G.; et al. Analysis of the contribution of Ito to repolarization in canine ventricular myocardium. Br. J. Pharmacol. 2011, 164, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, V.A.; Sabbah, H.N.; Higgins, R.S.D.; Silverman, N.; Lesch, M.; Undrovinas, A.I. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation 1998, 98, 2545–2552. [Google Scholar] [CrossRef]
- Varró, A.; Baláti, B.; Iost, N.; Takács, J.; Virág, L.; Lathrop, D.A.; Lengyel, C.; Tálosi, L.; Papp, J.G. The role of the delayed rectifier component IKs in dog ventricular muscle and Purkinje fibre repolarization. J. Physiol. 2000, 523, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Lost, N.; Virag, L.; Opincariu, M.; Szecsi, J.; Varro, A.; Papp, J.G. Delayed rectifier potassium current in undiseased human ventricular myocardium. Cardiovasc. Res. 1998, 40, 508–515. [Google Scholar]
- Stengl, M.; Volders, P.G.A.; Thomsen, M.B.; Spätjens, R.L.H.M.G.; Sipido, K.R.; Vos, M.A. Accumulation of slowly activating delayed rectifier potassium current (IKs) in canine ventricular myocytes. J. Physiol. 2003, 551, 777–786. [Google Scholar] [CrossRef]
- Volders, P.G.A.; Stengl, M.; van Opstal, J.M.; Gerlach, U.; Spätjens, R.L.H.M.G.; Beekman, J.D.M.; Sipido, K.R.; Vos, M.A. Probing the contribution of IKs to canine ventricular repolarization. Key role for β-adrenergic receptor stimulation. Circulation 2003, 107, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Imredy, J.P.; Penniman, J.R.; Dech, S.J.; Irving, W.D.; Salata, J.J. Modeling of the adrenergic response of the human IKs current (hKCNQ1/hKCNE1) stably expressed in HEK-293 cells. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1867–H1881. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nánási, P.P.; Horváth, B.; Tar, F.; Almássy, J.; Szentandrássy, N.; Jost, N.; Baczkó, I.; Bányász, T.; Varró, A. Canine Myocytes Represent a Good Model for Human Ventricular Cells Regarding Their Electrophysiological Properties. Pharmaceuticals 2021, 14, 748. https://doi.org/10.3390/ph14080748
Nánási PP, Horváth B, Tar F, Almássy J, Szentandrássy N, Jost N, Baczkó I, Bányász T, Varró A. Canine Myocytes Represent a Good Model for Human Ventricular Cells Regarding Their Electrophysiological Properties. Pharmaceuticals. 2021; 14(8):748. https://doi.org/10.3390/ph14080748
Chicago/Turabian StyleNánási, Péter P., Balázs Horváth, Fábián Tar, János Almássy, Norbert Szentandrássy, Norbert Jost, István Baczkó, Tamás Bányász, and András Varró. 2021. "Canine Myocytes Represent a Good Model for Human Ventricular Cells Regarding Their Electrophysiological Properties" Pharmaceuticals 14, no. 8: 748. https://doi.org/10.3390/ph14080748
APA StyleNánási, P. P., Horváth, B., Tar, F., Almássy, J., Szentandrássy, N., Jost, N., Baczkó, I., Bányász, T., & Varró, A. (2021). Canine Myocytes Represent a Good Model for Human Ventricular Cells Regarding Their Electrophysiological Properties. Pharmaceuticals, 14(8), 748. https://doi.org/10.3390/ph14080748





