CCR5 Antagonist Maraviroc Inhibits Acute Exacerbation of Lung Inflammation Triggered by Influenza Virus in Cigarette Smoke-Exposed Mice
Abstract
:1. Introduction
2. Results
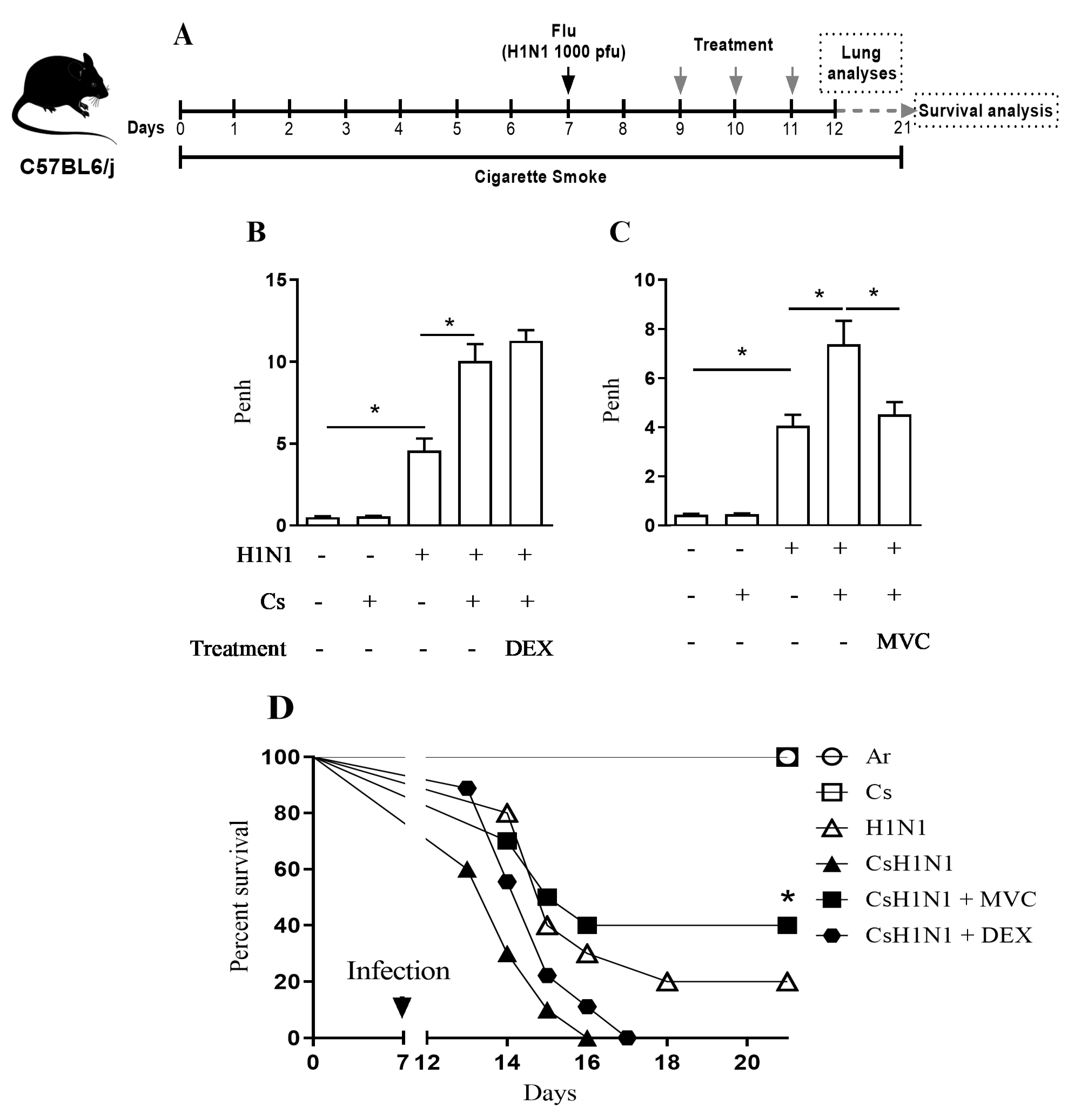
2.1. Maraviroc Improves Lung Function and Survival in Mice Combining Cs Exposure and H1N1 Infection
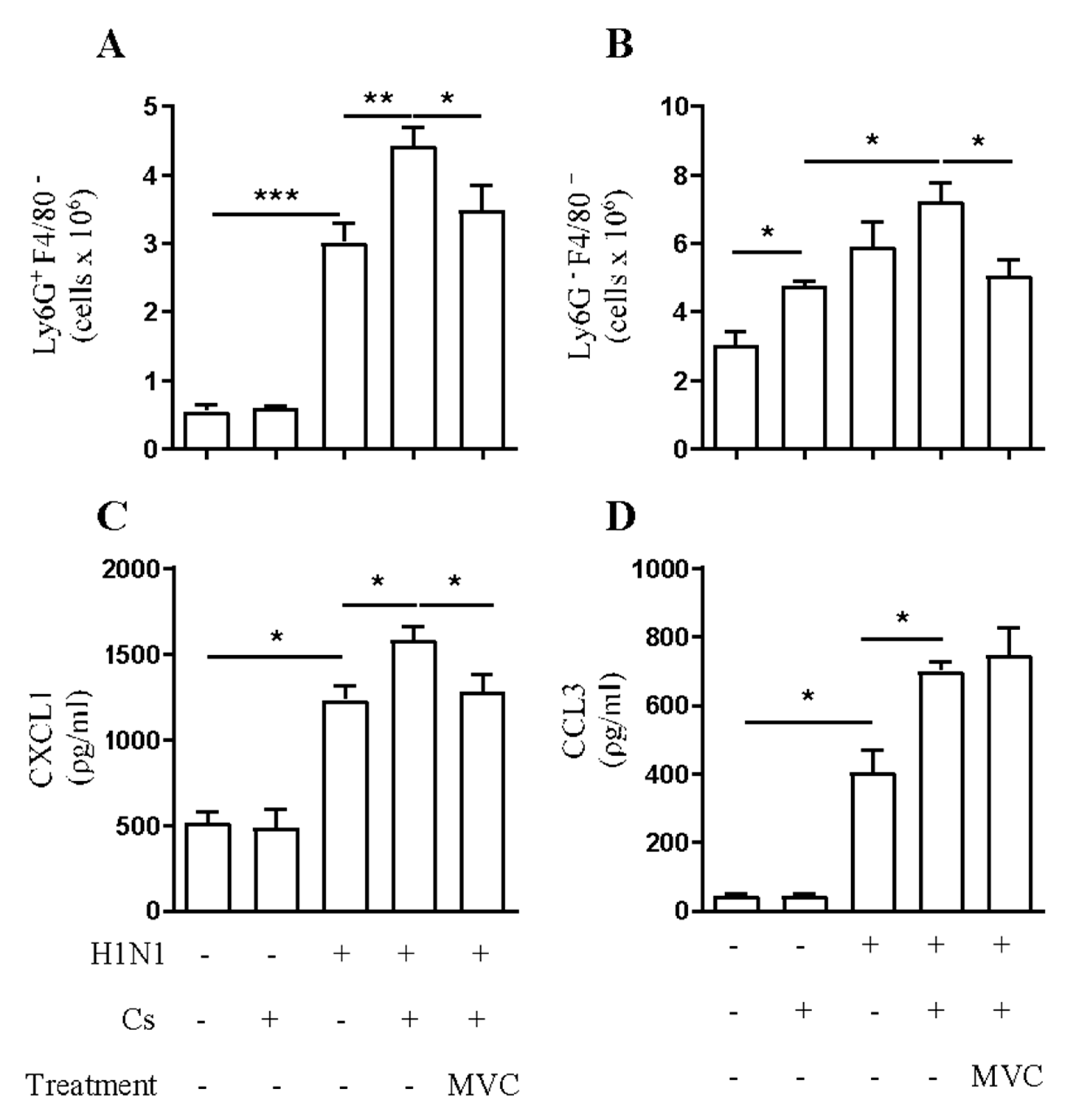
2.2. Maraviroc Reduces Neutrophil and Macrophage Infiltration in the Airways of Mice Experiencing Exacerbated Lung Inflammation by Combining Cs and Infection
2.3. Maraviroc Reduces Increased Macrophage and Neutrophil Infiltration in the Lung Tissue under Combined Insult
2.4. Maraviroc Reduces IL-6 Levels in Mice Serum
2.5. Maraviroc Does Not Alter Viral Load in the Lung Tissue
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Cs-Induced Exacerbation of Influenza-A Infection Model
4.3. Pharmacological Schedule
4.4. Assessment of Pulmonary Mechanics
4.5. Assessment of Leukocyte Content in the Airways
4.6. FACS Analyses of Lung Tissue
4.7. Cytokine Analysis in Lung Tissue and Serum
4.8. Virus Quantification (Plaque Assay)
4.9. Statistical analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Chronic Obstructive Pulmonary Disease (COPD); World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Pauwels, R.A.; Buist, A.S.; Calverley, P.M.; Jenkins, C.R.; Hurd, S.S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am. J. Respir. Crit. Care Med. 2001, 163, 1256–1276. [Google Scholar] [CrossRef]
- Barnes, P.J.; Shapiro, S.D.; Pauwels, R.A. Chronic obstructive pulmonary disease: Molecular and cellular mechanisms. Eur. Respir. J. 2003, 22, 672–688. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2018, 18, 454–466. [Google Scholar] [CrossRef]
- Mackay, A.J.; Hurst, J.R. COPD exacerbations: Causes, prevention, and treatment. Immunol. Allergy Clin. N. Am. 2012, 33, 95–115. [Google Scholar] [CrossRef]
- Barnes, P.J. Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2000, 343, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2013, 131, 636–645. [Google Scholar] [CrossRef]
- Magnussen, H.; Disse, B.; Rodriguez-Roisin, R.; Kirsten, A.; Watz, H.; Tetzlaff, K.; Towse, L.; Finnigan, H.; Dahl, R.; Decramer, M.; et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N. Engl. J. Med. 2014, 371, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Suissa, S.; Drazen, J.M. Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. N. Engl. J. Med. 2018, 378, 1671–1680. [Google Scholar] [CrossRef]
- Guarascio, A.J.; Ray, S.M.; Finch, C.K.; Self, T.H. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clin. Outcomes Res. 2013, 5, 235–245. [Google Scholar] [CrossRef]
- Ravi, A.K.; Khurana, S.; Lemon, J.; Plumb, J.; Booth, G.; Healy, L.; Catley, M.; Vestbo, J.; Singh, D. Increased levels of soluble interleukin-6 receptor and CCL3 in COPD sputum. Respir. Res. 2014, 15, 103. [Google Scholar] [CrossRef]
- Fahey, T.J.; Tracey, K.J.; Cousens, L.S.; Jones, G.; Shires, G.T.; Cerami, A.; Sherry, B.; Iii, T.J.F.; Tracey, K.J.; Tekamp-olson, P.; et al. Macrophage inflammatory protein 1 modulates macrophage function. J. Immunol. 1992, 148, 2764–2769. [Google Scholar]
- Wedzicha, J.A.; Seemungal, T.A.; MacCallum, P.K.; Paul, E.A.; Donaldson, G.C.; Bhowmik, A.; Jeffries, D.J.; Meade, T.W. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb. Haemost. 2000, 84, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Chinnapaiyan, S.; Dutta, R.; Bala, J.; Parira, T.; Agudelo, M.; Nair, M.; Unwalla, H.J. Cigarette smoke promotes HIV infection of primary bronchial epithelium and additively suppresses CFTR function. Sci. Rep. 2018, 8, 7984. [Google Scholar] [CrossRef]
- Costa, C.; Traves, S.L.; Tudhope, S.J.; Fenwick, P.S.; Belchamber, K.B.; Russell, R.E.; Barnes, P.J.; Donnelly, L.E. Enhanced monocyte migration to CXCR3 and CCR5 chemokines in COPD. Eur. Respir. J. 2016, 47, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Grommes, J.; Drechsler, M.; Soehnlein, O. CCR5 and FPR1 mediate neutrophil recruitment in endotoxin-induced lung injury. J. Innate Immun. 2014, 6, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Russkamp, N.F.; Ruemmler, R.; Roewe, J.; Moore, B.B.; Ward, P.A.; Bosmann, M. Experimental design of complement component 5a-induced acute lung injury (C5a-ALI): A role of CC-chemokine receptor type 5 during immune activation by anaphylatoxin. FASEB J. 2015, 29, 3762–3772. [Google Scholar] [CrossRef]
- Karampoor, S.; Zahednasab, H.; Amini, R.; Esghaei, M.; Sholeh, M.; Keyvani, H. Maraviroc attenuates the pathogenesis of experimental autoimmune encephalitis. Int. Immunopharmacol. 2020, 80, 106138. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, A.; Mohammad, T.; Anwar, S.; AlAjmi, M.F.; Hussain, A.; Rehman, M.T.; Islam, A.; Hassan, M.I. Glecaprevir and Maraviroc are high-affinity inhibitors of SARS-CoV-2 main protease: Possible implication in COVID-19 therapy. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef]
- Mattos, M.S.; Ferrero, M.R.; Kraemer, L.; Lopes, G.A.O.; Reis, D.C.; Cassali, G.D.; Oliveira, F.M.S.; Brandolini, L.; Allegretti, M.; Garcia, C.C.; et al. CXCR1 and CXCR2 Inhibition by Ladarixin Improves Neutrophil-Dependent Airway Inflammation in Mice. Front. Immunol. 2020, 11, 566953. [Google Scholar] [CrossRef]
- Ramos, C.D.; Canetti, C.; Souto, J.T.; Silva, J.S.; Hogaboam, C.M.; Ferreira, S.H.; Cunha, F.Q. MIP-1alpha[CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF-alpha and LTB4. J. Leukoc. Biol. 2005, 78, 167–177. [Google Scholar] [CrossRef]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Reid, C.; Haldar, P.; McCormick, M.; Haldar, K.; Kebadze, T.; Duvoix, A.; et al. Acute exacerbations of chronic obstructive pulmonary disease: Identification of biologic clusters and their biomarkers. Am. J. Respir. Crit. Care Med. 2011, 184, 662–671. [Google Scholar] [CrossRef]
- Mathioudakis, A.G.; Janssens, W.; Sivapalan, P.; Singanayagam, A.; Dransfield, M.T.; Jensen, J.S.; Vestbo, J. Acute exacerbations of chronic obstructive pulmonary disease: In search of diagnostic biomarkers and treatable traits. Thorax 2020, 75, 520–527. [Google Scholar] [CrossRef]
- Papi, A.; Bellettato, C.M.; Braccioni, F.; Romagnoli, M.; Casolari, P.; Caramori, G.; Fabbri, L.M.; Johnston, S.L. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am. J. Respir. Crit. Care Med. 2006, 173, 1114–1121. [Google Scholar] [CrossRef]
- Wilkinson, T.M.A.; Aris, E.; Bourne, S.; Clarke, S.C.; Peeters, M.; Pascal, T.G.; Schoonbroodt, S.; Tuck, A.C.; Kim, V.; Ostridge, K.; et al. A prospective, observational cohort study of the seasonal dynamics of airway pathogens in the aetiology of exacerbations in COPD. Thorax 2017, 72, 919–927. [Google Scholar] [CrossRef]
- Bucher, H.; Duechs, M.J.; Tilp, C.; Jung, B.; Erb, K.J. Tiotropium Attenuates Virus-Induced Pulmonary Inflammation in Cigarette Smoke-Exposed Mice. J. Pharmacol. Exp. Ther. 2016, 357, 606–618. [Google Scholar] [CrossRef]
- Bracke, K.R.; D’Hulst, A.I.; Maes, T.; Demedts, I.K.; Moerloose, K.B.; Kuziel, W.A.; Joos, G.F.; Brusselle, G.G. Cigarette smoke-induced pulmonary inflammation, but not airway remodelling, is attenuated in chemokine receptor 5-deficient mice. Clin. Exp. Allergy 2007, 37, 1467–1479. [Google Scholar] [CrossRef]
- Ma, B.; Kang, M.J.; Lee, C.G.; Chapoval, S.; Liu, W.; Chen, Q.; Coyle, A.J.; Lora, J.M.; Picarella, D.; Homer, R.J.; et al. Role of CCR5 in IFN-gamma-induced and cigarette smoke-induced emphysema. J. Clin. Investig. 2005, 115, 3460–3472. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.C.; Beck, M.A.; Kuziel, W.A.; Henderson, F.; Maeda, N. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am. J. Pathol. 2000, 156, 1951–1959. [Google Scholar] [CrossRef]
- Tavares, L.P.; Garcia, C.C.; Goncalves, A.P.F.; Kraemer, L.R.; Melo, E.M.; Oliveira, F.M.S.; Freitas, C.S.; Lopes, G.A.O.; Reis, D.C.; Cassali, G.D.; et al. ACKR2 contributes to pulmonary dysfunction by shaping CCL5:CCR5-dependent recruitment of lymphocytes during influenza A infection in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L655–L670. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.C.; Seemungal, T.A.; Bhowmik, A.; Wedzicha, J.A. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002, 57, 847–852. [Google Scholar] [CrossRef]
- Vestbo, J.; Edwards, L.D.; Scanlon, P.D.; Yates, J.C.; Agusti, A.; Bakke, P.; Calverley, P.M.; Celli, B.; Coxson, H.O.; Crim, C.; et al. Changes in forced expiratory volume in 1 second over time in COPD. N. Engl. J. Med. 2011, 365, 1184–1192. [Google Scholar] [CrossRef]
- Soler-Cataluna, J.J.; Martinez-Garcia, M.A.; Roman Sanchez, P.; Salcedo, E.; Navarro, M.; Ochando, R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005, 60, 925–931. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory endotypes in COPD. Allergy 2019, 74, 1249–1256. [Google Scholar] [CrossRef]
- Hoth, J.J.; Wells, J.D.; Hiltbold, E.M.; McCall, C.E.; Yoza, B.K. Mechanism of neutrophil recruitment to the lung after pulmonary contusion. Shock 2011, 35, 604–609. [Google Scholar] [CrossRef]
- Beckett, E.L.; Stevens, R.L.; Jarnicki, A.G.; Kim, R.Y.; Hanish, I.; Hansbro, N.G.; Deane, A.; Keely, S.; Horvat, J.C.; Yang, M.; et al. A new short-term mouse model of chronic obstructive pulmonary disease identifies a role for mast cell tryptase in pathogenesis. J. Allergy Clin. Immunol. 2013, 131, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Zaynagetdinov, R.; Sherrill, T.P.; Kendall, P.L.; Segal, B.H.; Weller, K.P.; Tighe, R.M.; Blackwell, T.S. Identification of myeloid cell subsets in murine lungs using flow cytometry. Am. J. Respir. Cell Mol. Biol. 2013, 49, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Baggiolini, M. Chemokines and leukocyte traffic. Nature 1998, 392, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Burdon, P.C.; Martin, C.; Rankin, S.M. Migration across the sinusoidal endothelium regulates neutrophil mobilization in response to ELR + CXC chemokines. Br. J. Haematol. 2008, 142, 100–108. [Google Scholar] [CrossRef]
- Rudd, J.M.; Pulavendran, S.; Ashar, H.K.; Ritchey, J.W.; Snider, T.A.; Malayer, J.R.; Marie, M.; Chow, V.T.K.; Narasaraju, T. Neutrophils Induce a Novel Chemokine Receptors Repertoire During Influenza Pneumonia. Front. Cell Infect. Microbiol. 2019, 9, 108. [Google Scholar] [CrossRef]
- Zhao, K.; Dong, R.; Yu, Y.; Tu, C.; Li, Y.; Cui, Y.; Bao, L.; Ling, C. Cigarette smoke-induced lung inflammation in COPD mediated via CCR1/JAK/STAT /NF-kappaB pathway. Aging 2020, 12, 9125–9138. [Google Scholar] [CrossRef]
- Yang, X.; Walton, W.; Cook, D.N.; Hua, X.; Tilley, S.; Haskell, C.A.; Horuk, R.; Blackstock, A.W.; Kirby, S.L. The chemokine, CCL3, and its receptor, CCR1, mediate thoracic radiation-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2011, 45, 127–135. [Google Scholar] [CrossRef]
- Tokuda, A.; Itakura, M.; Onai, N.; Kimura, H.; Kuriyama, T.; Matsushima, K. Pivotal role of CCR1-positive leukocytes in bleomycin-induced lung fibrosis in mice. J. Immunol. 2000, 164, 2745–2751. [Google Scholar] [CrossRef] [PubMed]
- Amat, M.; Benjamim, C.F.; Williams, L.M.; Prats, N.; Terricabras, E.; Beleta, J.; Kunkel, S.L.; Godessart, N. Pharmacological blockade of CCR1 ameliorates murine arthritis and alters cytokine networks in vivo. Br. J. Pharmacol. 2006, 149, 666–675. [Google Scholar] [CrossRef]
- Lam, E.W.; Glassford, J.; Banerji, L.; Thomas, N.S.; Sicinski, P.; Klaus, G.G. Cyclin D3 compensates for loss of cyclin D2 in mouse B-lymphocytes activated via the antigen receptor and CD40. J. Biol. Chem. 2000, 275, 3479–3484. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; Edwards, L.D.; Rennard, S.I.; MacNee, W.; Tal-Singer, R.; Miller, B.E.; Vestbo, J.; Lomas, D.A.; Calverley, P.M.; Wouters, E.; et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: A novel phenotype. PLoS ONE 2012, 7, e37483. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.R.; Locantore, N.; Yates, J.; Tal-Singer, R.; Miller, B.E.; Bakke, P.; Calverley, P.; Coxson, H.; Crim, C.; Edwards, L.D.; et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Oostwoud, L.C.; Gunasinghe, P.; Seow, H.J.; Ye, J.M.; Selemidis, S.; Bozinovski, S.; Vlahos, R. Apocynin and ebselen reduce influenza A virus-induced lung inflammation in cigarette smoke-exposed mice. Sci. Rep. 2016, 6, 20983. [Google Scholar] [CrossRef]
- Pinto, D.P.; Coutinho, D.S.; Carvalho, K.I.M.; Ferrero, M.R.; Silva, L.V.D.; Silveira, G.P.E.; Silva, D.M.D.; Araujo, J.F.G.; Silva, A.C.A.; Pereira, H.M.; et al. Pharmacological profiling of JME-173, a novel mexiletine derivative combining dual anti-inflammatory/anti-spasmodic functions and limited action in Na(+) channels. Eur. J. Pharmacol. 2020, 885, 173367. [Google Scholar] [CrossRef]
- Insuela, D.B.; Daleprane, J.B.; Coelho, L.P.; Silva, A.R.; e Silva, P.M.; Martins, M.A.; Carvalho, V.F. Glucagon induces airway smooth muscle relaxation by nitric oxide and prostaglandin E(2). J. Endocrinol. 2015, 225, 205–217. [Google Scholar] [CrossRef]
- Insuela, D.B.R.; Azevedo, C.T.; Coutinho, D.S.; Magalhaes, N.S.; Ferrero, M.R.; Ferreira, T.P.T.; Cascabulho, C.M.; Henriques-Pons, A.; Olsen, P.C.; Diaz, B.L.; et al. Glucagon reduces airway hyperreactivity, inflammation, and remodeling induced by ovalbumin. Sci. Rep. 2019, 9, 6478. [Google Scholar] [CrossRef]
- Galani, I.E.; Triantafyllia, V.; Eleminiadou, E.E.; Koltsida, O.; Stavropoulos, A.; Manioudaki, M.; Thanos, D.; Doyle, S.E.; Kotenko, S.V.; Thanopoulou, K.; et al. Interferon-λ Mediates Non-redundant Front-Line Antiviral Protection against Influenza Virus Infection without Compromising Host Fitness. Immunity 2017, 46, 875–890.e6. [Google Scholar] [CrossRef] [PubMed]


| H1N1 | CsH1N1 | CsH1N1 + Maraviroc |
|---|---|---|
| 0.989 ± 0.195 | 1.059 ± 0.142 | 0.829 ± 0.129 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrero, M.R.; Garcia, C.C.; Dutra de Almeida, M.; Torres Braz da Silva, J.; Bianchi Reis Insuela, D.; Teixeira Ferreira, T.P.; de Sá Coutinho, D.; Trindade de Azevedo, C.; Machado Rodrigues e Silva, P.; Martins, M.A. CCR5 Antagonist Maraviroc Inhibits Acute Exacerbation of Lung Inflammation Triggered by Influenza Virus in Cigarette Smoke-Exposed Mice. Pharmaceuticals 2021, 14, 620. https://doi.org/10.3390/ph14070620
Ferrero MR, Garcia CC, Dutra de Almeida M, Torres Braz da Silva J, Bianchi Reis Insuela D, Teixeira Ferreira TP, de Sá Coutinho D, Trindade de Azevedo C, Machado Rodrigues e Silva P, Martins MA. CCR5 Antagonist Maraviroc Inhibits Acute Exacerbation of Lung Inflammation Triggered by Influenza Virus in Cigarette Smoke-Exposed Mice. Pharmaceuticals. 2021; 14(7):620. https://doi.org/10.3390/ph14070620
Chicago/Turabian StyleFerrero, Maximiliano Ruben, Cristiana Couto Garcia, Marcella Dutra de Almeida, Jullian Torres Braz da Silva, Daniella Bianchi Reis Insuela, Tatiana Paula Teixeira Ferreira, Diego de Sá Coutinho, Carolina Trindade de Azevedo, Patrícia Machado Rodrigues e Silva, and Marco Aurélio Martins. 2021. "CCR5 Antagonist Maraviroc Inhibits Acute Exacerbation of Lung Inflammation Triggered by Influenza Virus in Cigarette Smoke-Exposed Mice" Pharmaceuticals 14, no. 7: 620. https://doi.org/10.3390/ph14070620
APA StyleFerrero, M. R., Garcia, C. C., Dutra de Almeida, M., Torres Braz da Silva, J., Bianchi Reis Insuela, D., Teixeira Ferreira, T. P., de Sá Coutinho, D., Trindade de Azevedo, C., Machado Rodrigues e Silva, P., & Martins, M. A. (2021). CCR5 Antagonist Maraviroc Inhibits Acute Exacerbation of Lung Inflammation Triggered by Influenza Virus in Cigarette Smoke-Exposed Mice. Pharmaceuticals, 14(7), 620. https://doi.org/10.3390/ph14070620






