Effects of Selective Peroxisome Proliferator Activated Receptor Agonists on Corneal Epithelial Wound Healing
Abstract
1. Introduction
2. Results
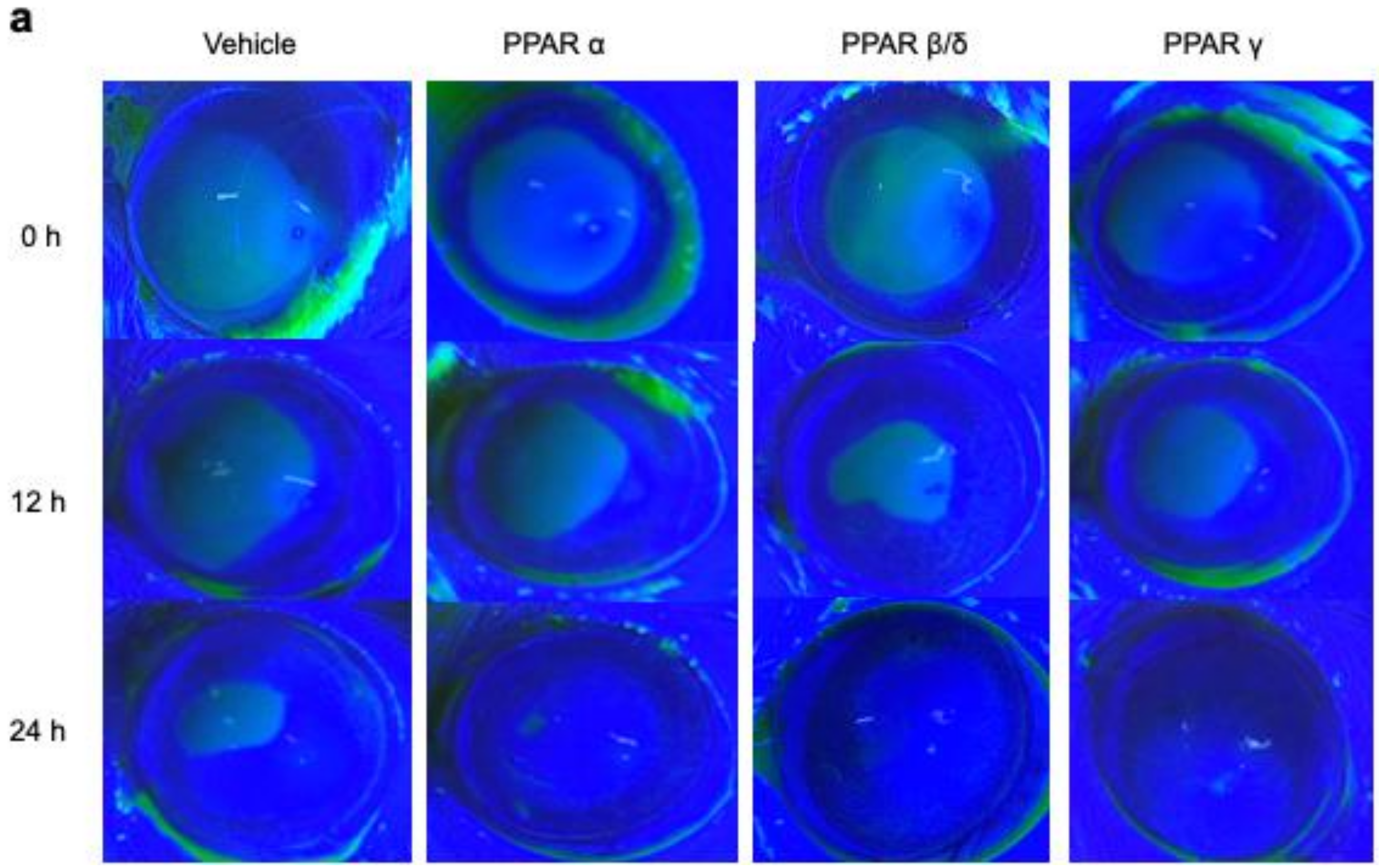
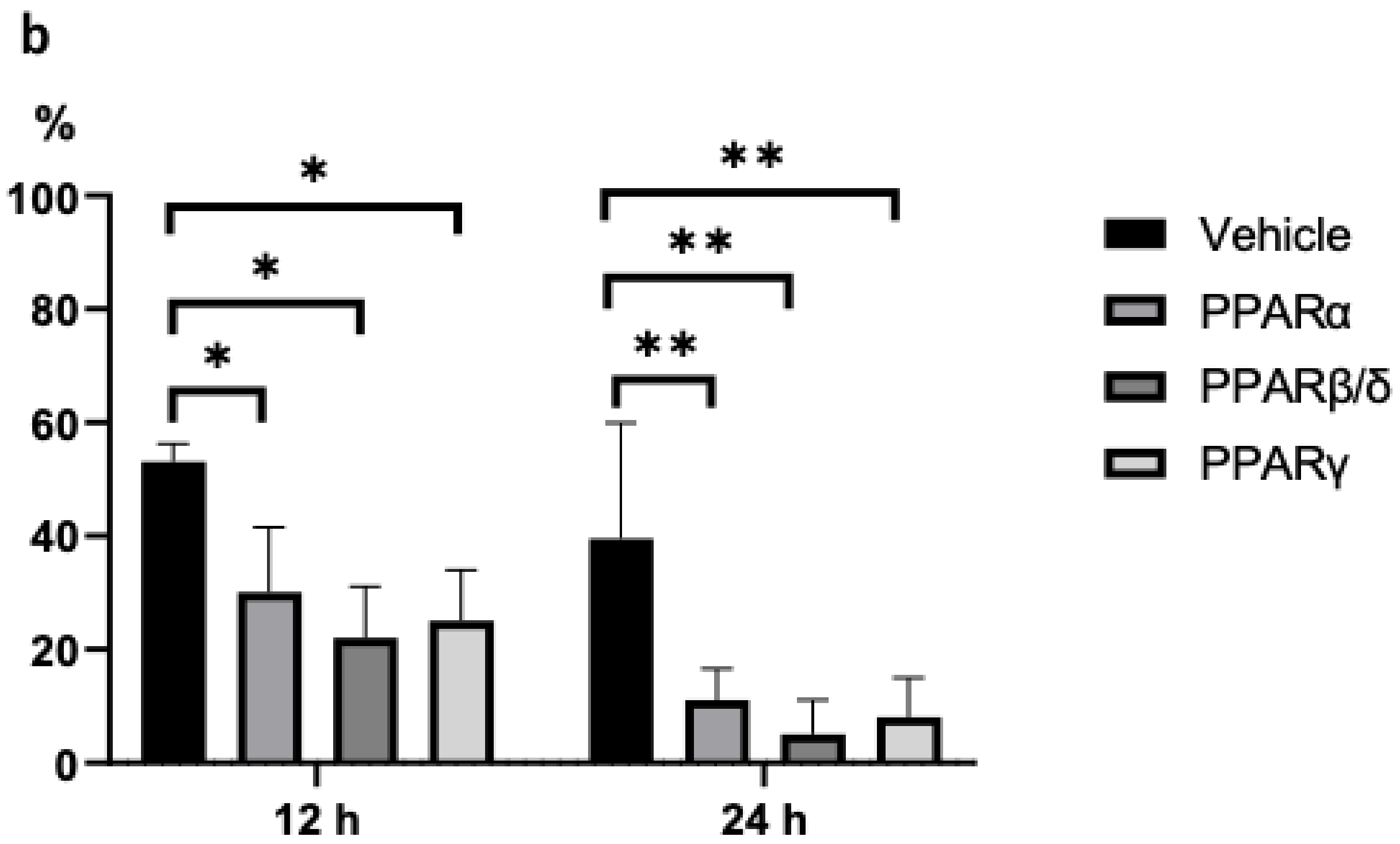
2.1. Corneal Epithelial Wound Healing
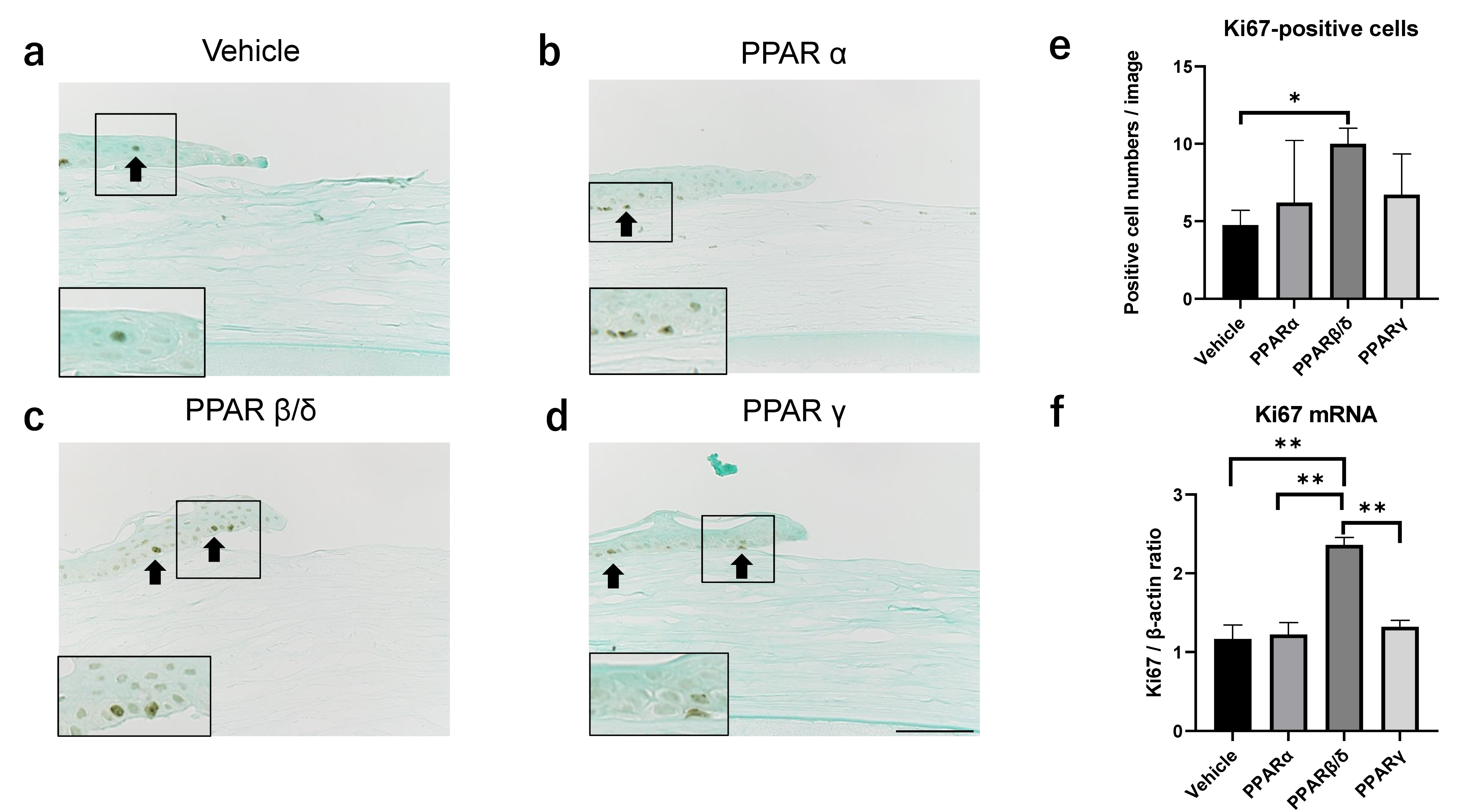
2.2. Ki67 Expression
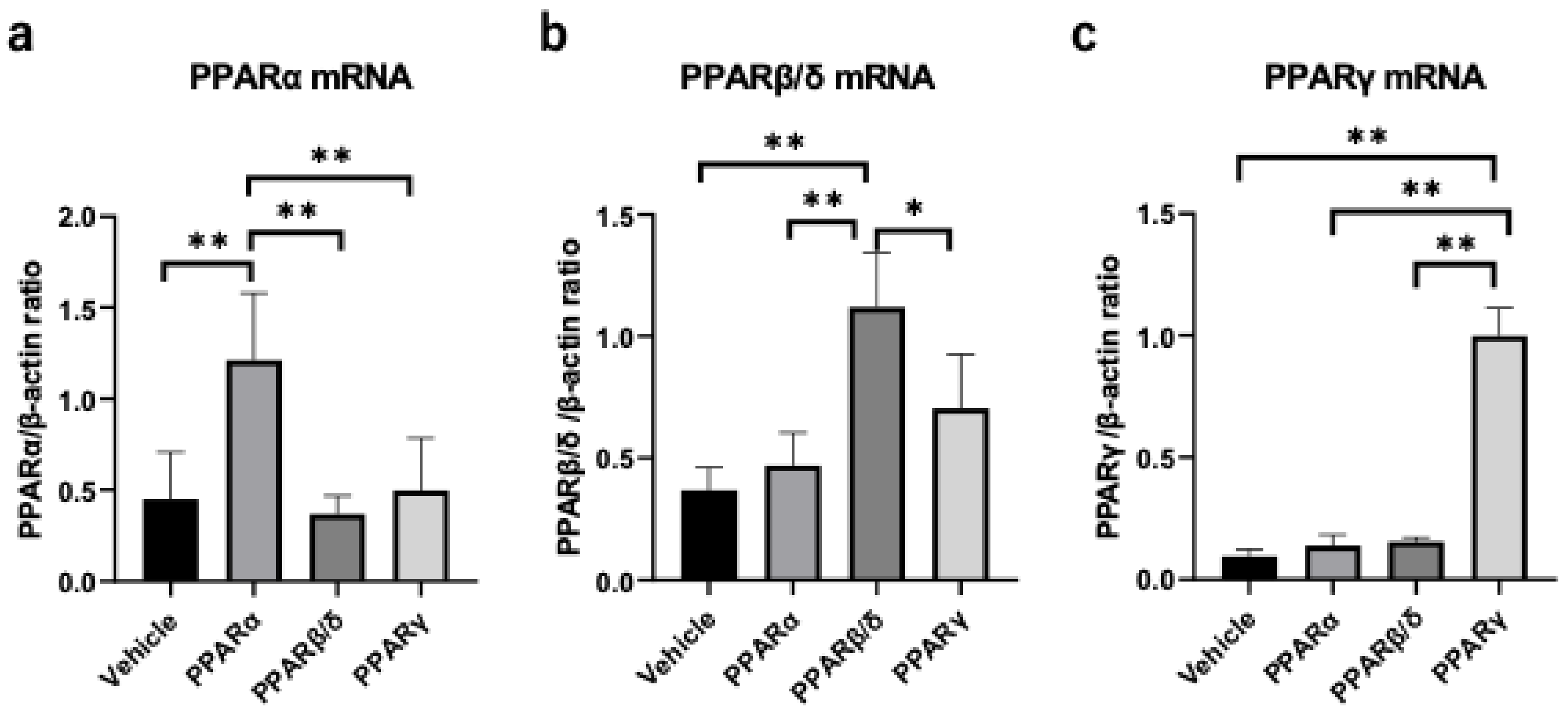
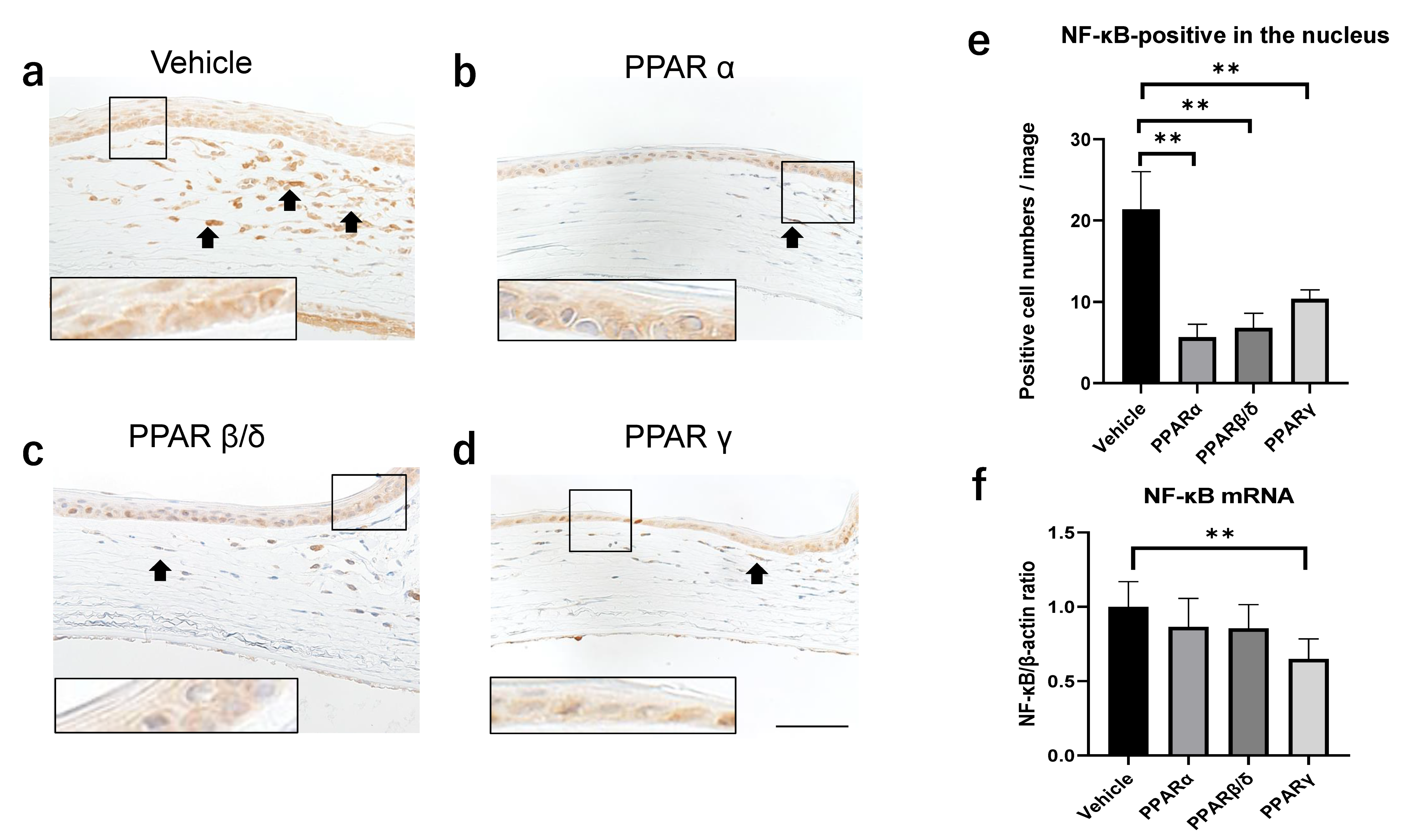
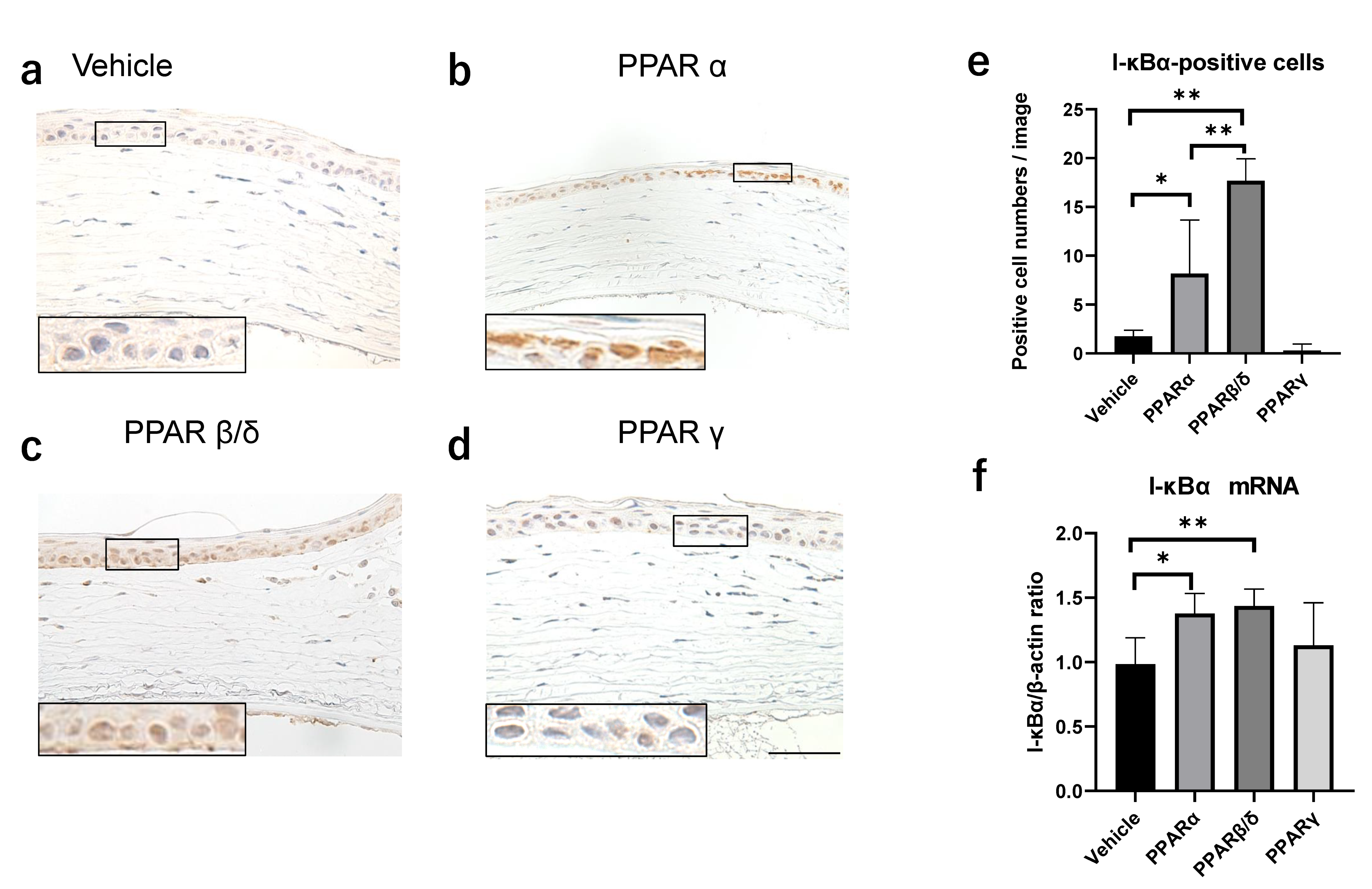
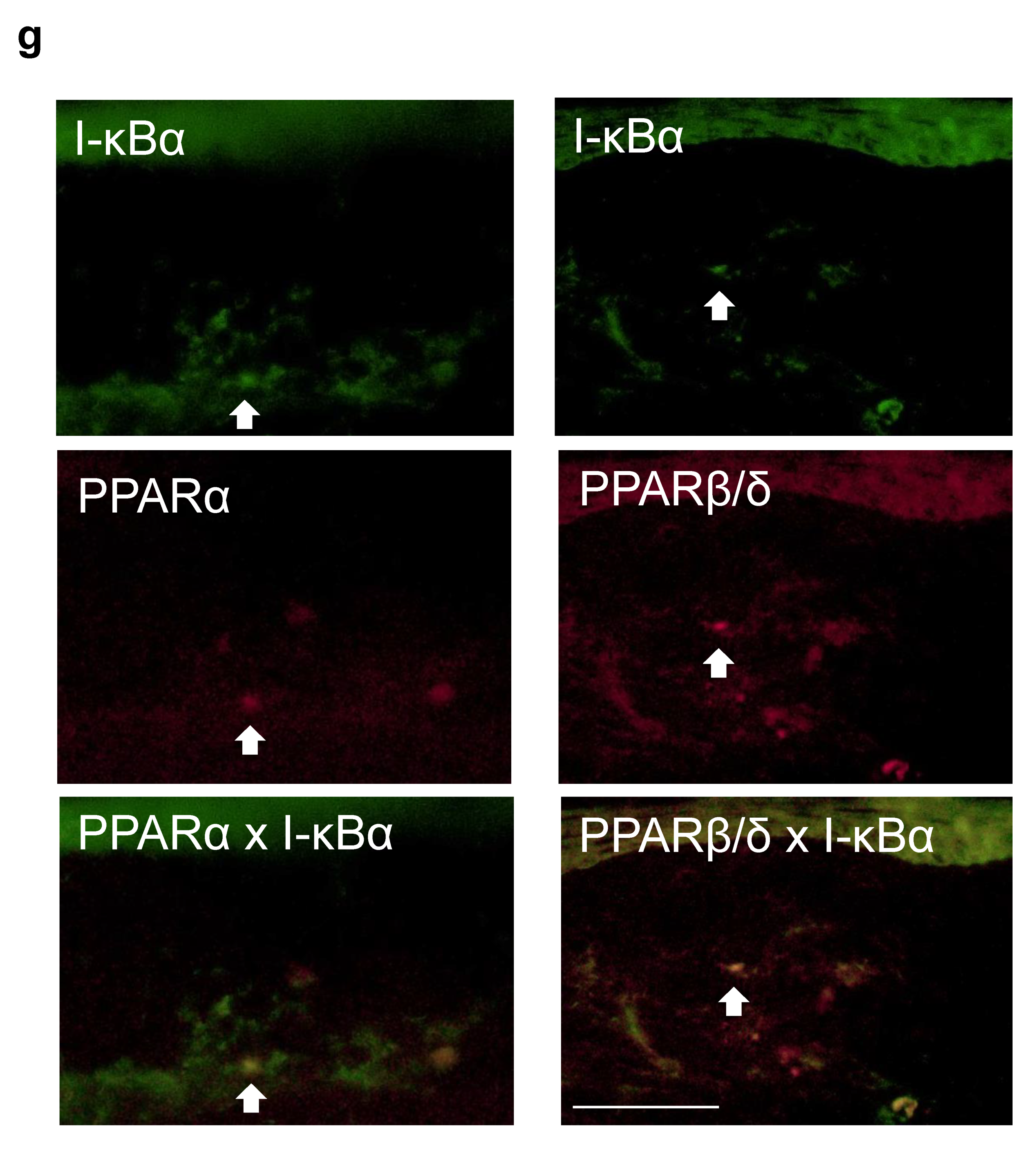
2.3. Nuclear Factor Kappa B (NF-κB) and Kappa Light Polypeptide Gene Enhancer in the B-Cell Inhibitor, Alpha (I-kBα) Expression
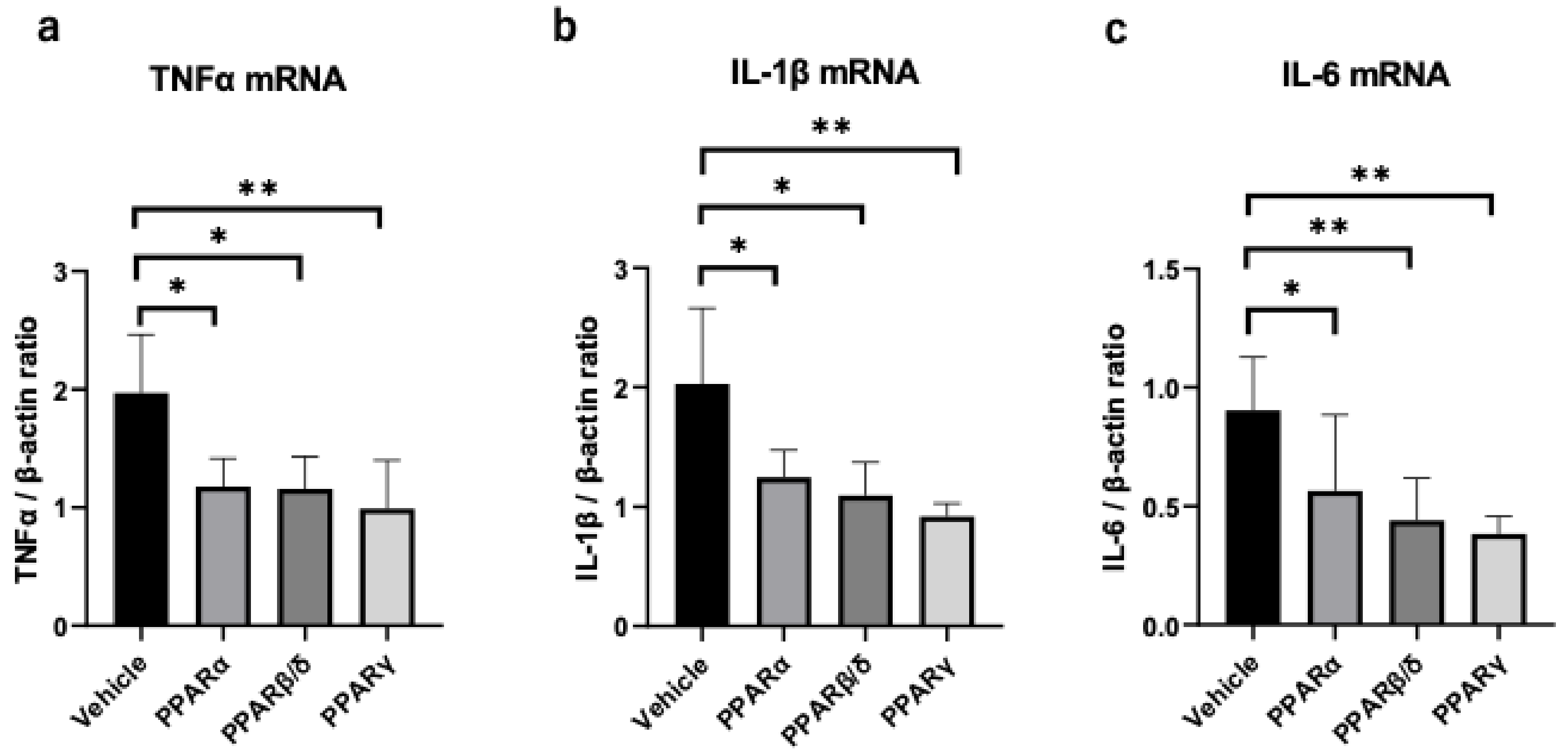
2.4. Inflammatory Cytokines
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Alkali Burn Model
4.3. Treatment with Each PPAR Agonist
4.4. Evaluation of the Corneal Epithelial Defect Area
4.5. Histological and Immunohistochemical Analyses
4.6. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desvergne, B.; Wahli, W. Peroxisome proliferator-activated receptors: Nuclear control of metabolism. Endocr. Rev. 1999, 20, 649–688. [Google Scholar] [CrossRef]
- Berger, J.; Moller, D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef]
- Brown, J.D.; Plutzky, J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation 2007, 115, 518–533. [Google Scholar] [CrossRef]
- Lee, C.H.; Olson, P.; Evans, R.M. Minireview: Lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology 2003, 144, 2201–2207. [Google Scholar] [CrossRef]
- Fruchart, J.C.; Staels, B.; Duriez, P. The role of fibric acids in atherosclerosis. Curr. Atheroscler. Rep. 2001, 3, 83–92. [Google Scholar] [CrossRef]
- Giannini, S.; Serio, M.; Galli, A. Pleiotropic effects of thiazolidinediones: Taking a look beyond antidiabetic activity. J. Endocrinol. Investig. 2004, 27, 982–991. [Google Scholar] [CrossRef]
- Barbier, O.; Torra, I.P.; Duguay, Y.; Blanquart, C.; Fruchart, J.C.; Glineur, C.; Staels, B. Pleiotropic actions of peroxisome proliferator-activated receptors in lipid metabolism and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 717–726. [Google Scholar] [CrossRef]
- Issemann, I.; Green, S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 1990, 347, 645–650. [Google Scholar] [CrossRef]
- Korbecki, J.; Bobiński, R.; Dutka, M. Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflamm. Res. 2019, 68, 443–458. [Google Scholar] [CrossRef]
- Moraes, L.A.; Piqueras, L.; Bishop-Bailey, D. Peroxisome proliferator-activated receptors and inflammation. Pharmacol. Ther. 2006, 110, 371–385. [Google Scholar] [CrossRef]
- Arima, T.; Uchiyama, M.; Nakano, Y.; Nagasaka, S.; Kang, D.; Shimizu, A.; Takahashi, H. Peroxisome proliferator-activated receptor alpha agonist suppresses neovascularization by reducing both vascular endothelial growth factor and angiopoietin-2 in corneal alkali burn. Sci. Rep. 2017, 7, 17763. [Google Scholar] [CrossRef]
- Uchiyama, M.; Shimizu, A.; Masuda, Y.; Nagasaka, S.; Fukuda, Y.; Takahashi, H. An ophthalmic solution of a peroxisome proliferator-activated receptor gamma agonist prevents corneal inflammation in a rat alkali burn model. Mol. Vis. 2013, 19, 2135–2150. [Google Scholar] [PubMed]
- Nakano, Y.; Arima, T.; Tobita, Y.; Uchiyama, M.; Shimizu, A.; Takahashi, H. Combination of Peroxisome Proliferator-Activated Receptor (PPAR) Alpha and Gamma Agonists Prevents Corneal Inflammation and Neovascularization in a Rat Alkali Burn Model. Int. J. Mol. Sci. 2020, 21, 93. [Google Scholar] [CrossRef]
- Tobita, Y.; Arima, T.; Nakano, Y.; Uchiyama, M.; Shimizu, A.; Takahashi, H. Peroxisome Proliferator-Activated Receptor Beta/Delta Agonist Suppresses Inflammation and Promotes Neovascularization. Int. J. Mol. Sci. 2020, 21, 5296. [Google Scholar] [CrossRef]
- Nakano, Y.; Uchiyama, M.; Arima, T.; Nagasaka, S.; Igarashi, T.; Shimizu, A.; Takahashi, H. PPARα Agonist Suppresses Inflammation after Corneal Alkali Burn by Suppressing Proinflammatory Cytokines, MCP-1, and Nuclear Translocation of NF-κB. Molecules 2018, 24, 114. [Google Scholar] [CrossRef]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S.; Rawat, R. Peroxisome proliferator-activated receptors (PPARs) and PPAR agonists: The ‘future’ in dermatology therapeutics? Arch. Dermatol. Res. 2015, 307, 767–780. [Google Scholar] [CrossRef]
- Di-Poï, N.; Michalik, L.; Desvergne, B.; Wahli, W. Functions of peroxisome proliferator-activated receptors (PPAR) in skin homeostasis. Lipids 2004, 39, 1093–1099. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, W.; Bi, M.; Wu, J. The molecular mechanisms of action of PPAR-γ agonists in the treatment of corneal alkali burns (Review). Int. J. Mol. Med. 2016, 38, 1003–1011. [Google Scholar] [CrossRef][Green Version]
- Ho, S.Y.; Kwan, Y.P.; Qiu, B.; Tan, A.; Murray, H.L.; Barathi, V.A.; Tan, N.S.; Cheung, C.M.G.; Wong, T.Y.; Wahli, W.; et al. Investigating the Role of PPARβ/δ in Retinal Vascular Remodeling Using. Int. J. Mol. Sci. 2020, 21, 4403. [Google Scholar] [CrossRef]
- Kuenzli, S.; Saurat, J.H. Peroxisome proliferator-activated receptors in cutaneous biology. Br. J. Dermatol. 2003, 149, 229–236. [Google Scholar] [CrossRef]
- Icre, G.; Wahli, W.; Michalik, L. Functions of the peroxisome proliferator-activated receptor (PPAR) alpha and beta in skin homeostasis, epithelial repair, and morphogenesis. J. Investig. Dermatol. Symp. Proc. 2006, 11, 30–35. [Google Scholar] [CrossRef]
- Michalik, L.; Desvergne, B.; Tan, N.S.; Basu-Modak, S.; Escher, P.; Rieusset, J.; Peters, J.M.; Kaya, G.; Gonzalez, F.J.; Zakany, J.; et al. Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR)alpha and PPARbeta mutant mice. J. Cell Biol. 2001, 154, 799–814. [Google Scholar] [CrossRef]
- Gu, Y.; Li, X.; He, T.; Jiang, Z.; Hao, P.; Tang, X. The Antifibrosis Effects of Peroxisome Proliferator-Activated Receptor δ on Rat Corneal Wound Healing after Excimer Laser Keratectomy. PPAR Res. 2014, 2014, 464935. [Google Scholar] [CrossRef]
- Viatour, P.; Merville, M.P.; Bours, V.; Chariot, A. Phosphorylation of NF-kappaB and IkappaB proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Signaling to NF-kappaB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Bouhlel, M.A.; Derudas, B.; Rigamonti, E.; Dièvart, R.; Brozek, J.; Haulon, S.; Zawadzki, C.; Jude, B.; Torpier, G.; Marx, N.; et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007, 6, 137–143. [Google Scholar] [CrossRef]
- Nakamura, Y.; Nakamura, T.; Tarui, T.; Inoue, J.; Kinoshita, S. Functional role of PPARδ in corneal epithelial wound healing. Am. J. Pathol. 2012, 180, 583–598. [Google Scholar] [CrossRef]
- Choi, H.; Phillips, C.; Oh, J.Y.; Stock, E.M.; Kim, D.K.; Won, J.K.; Fulcher, S. Comprehensive Modeling of Corneal Alkali Injury in the Rat Eye. Curr. Eye Res. 2017, 42, 1348–1357. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobita, Y.; Arima, T.; Nakano, Y.; Uchiyama, M.; Shimizu, A.; Takahashi, H. Effects of Selective Peroxisome Proliferator Activated Receptor Agonists on Corneal Epithelial Wound Healing. Pharmaceuticals 2021, 14, 88. https://doi.org/10.3390/ph14020088
Tobita Y, Arima T, Nakano Y, Uchiyama M, Shimizu A, Takahashi H. Effects of Selective Peroxisome Proliferator Activated Receptor Agonists on Corneal Epithelial Wound Healing. Pharmaceuticals. 2021; 14(2):88. https://doi.org/10.3390/ph14020088
Chicago/Turabian StyleTobita, Yutaro, Takeshi Arima, Yuji Nakano, Masaaki Uchiyama, Akira Shimizu, and Hiroshi Takahashi. 2021. "Effects of Selective Peroxisome Proliferator Activated Receptor Agonists on Corneal Epithelial Wound Healing" Pharmaceuticals 14, no. 2: 88. https://doi.org/10.3390/ph14020088
APA StyleTobita, Y., Arima, T., Nakano, Y., Uchiyama, M., Shimizu, A., & Takahashi, H. (2021). Effects of Selective Peroxisome Proliferator Activated Receptor Agonists on Corneal Epithelial Wound Healing. Pharmaceuticals, 14(2), 88. https://doi.org/10.3390/ph14020088





