RETRACTED: In Vitro Modulation of P-Glycoprotein Activity by Euphorbia intisy Essential Oil on Acute Myeloid Leukemia Cell Line HL-60R
Abstract
1. Introduction
1.1. P-Glycoprotein Mediated Drug Resistance
1.2. The Genus Euphorbia Uses
2. Results and Discussion
2.1. Chemical Composition
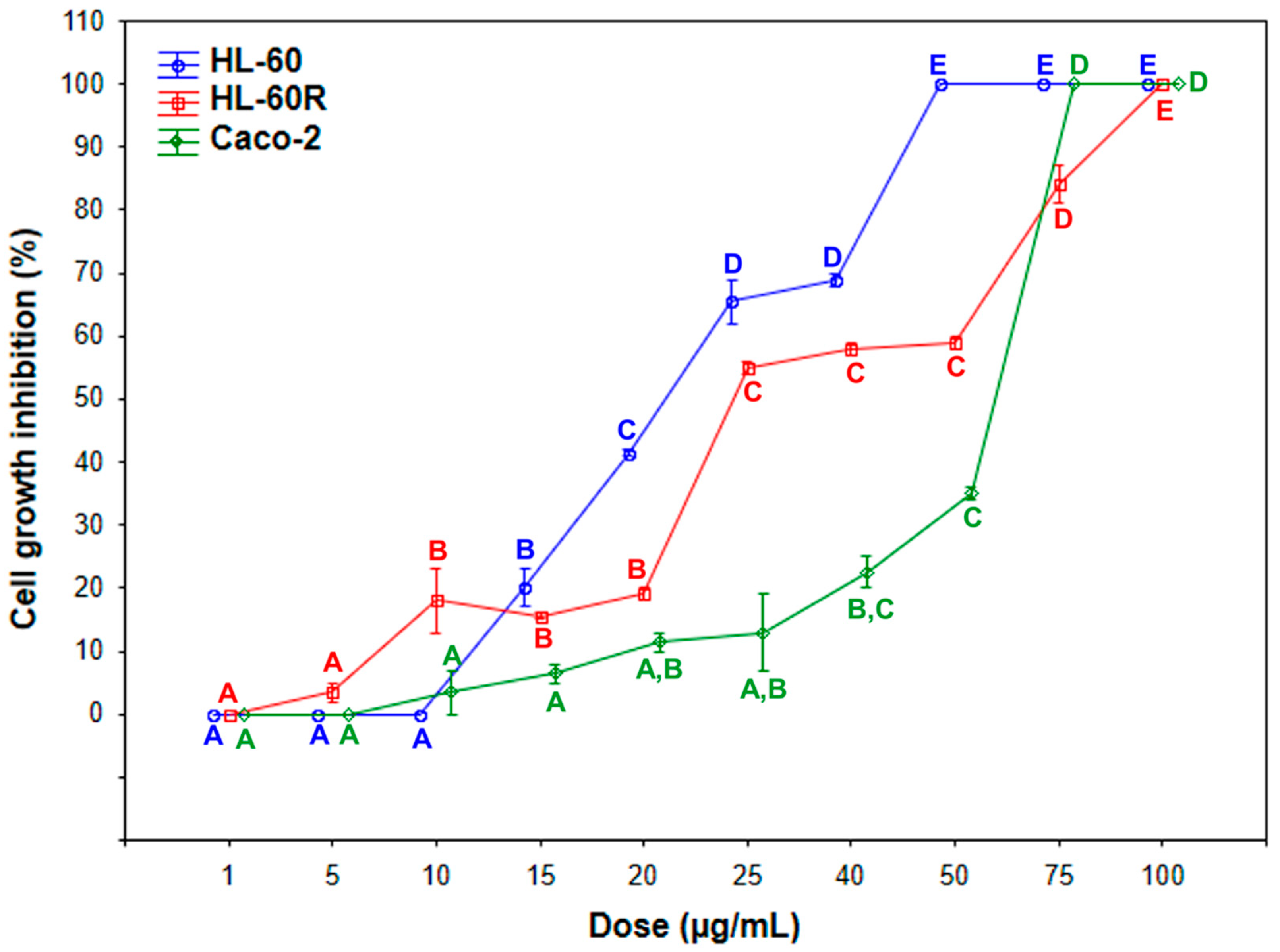
2.2. In Vitro Cytotoxic and Cell Death Effects of E. intisy Essential Oil
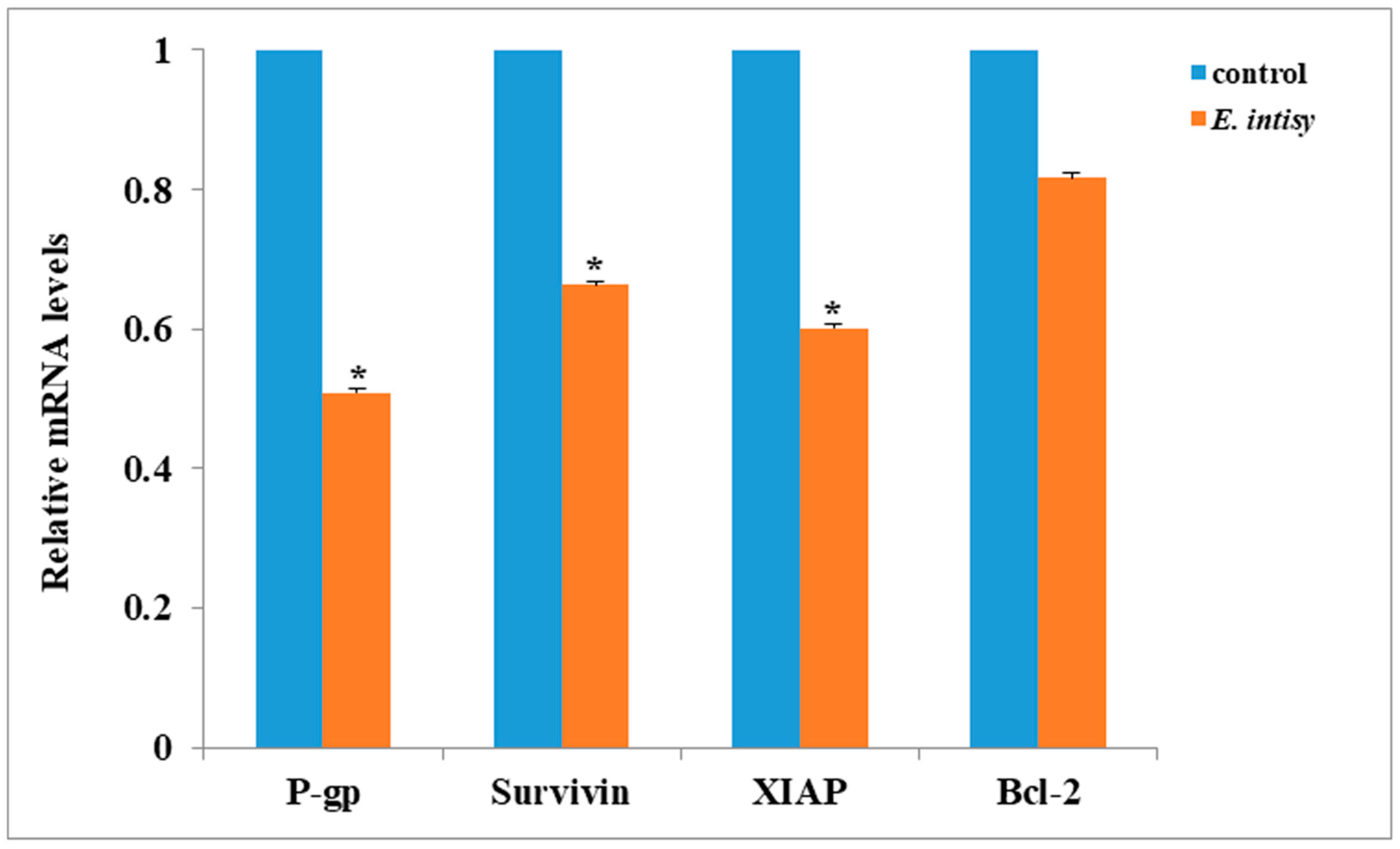
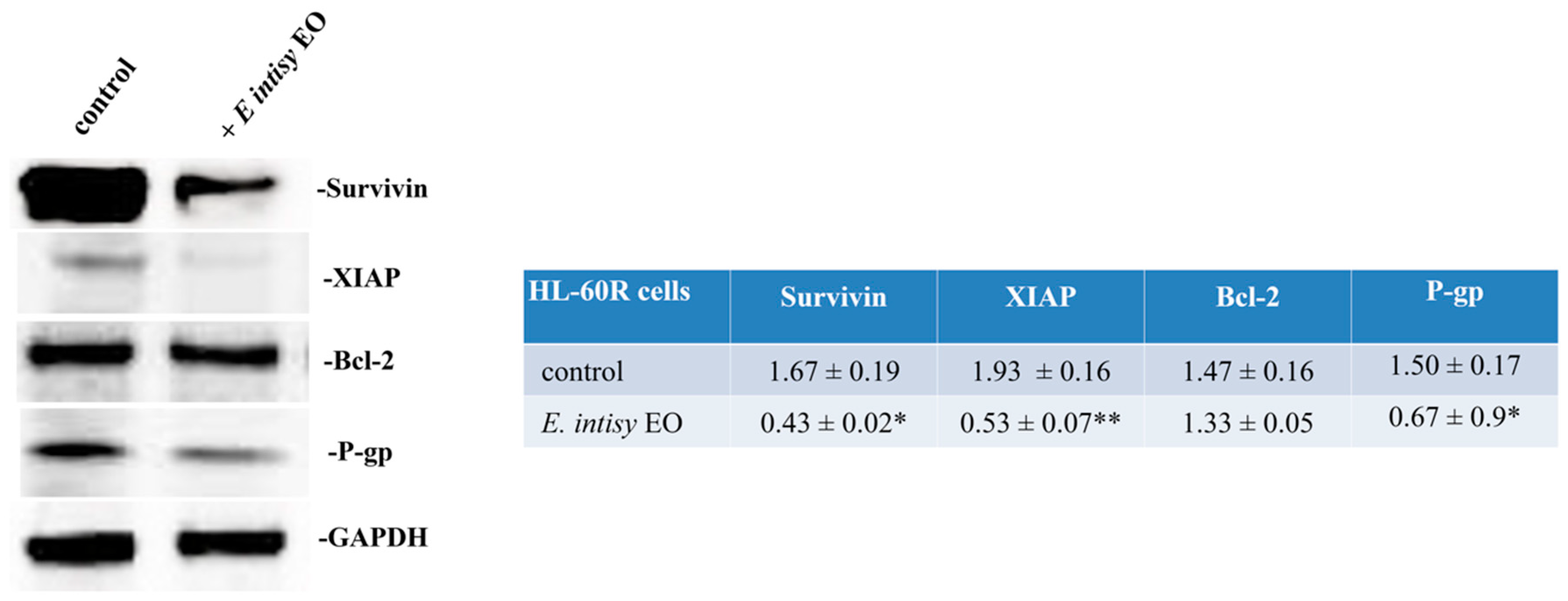
2.3. E. intisy Essential Oil Treatment Inhibits the Activation of The NF-κB Signaling Pathway
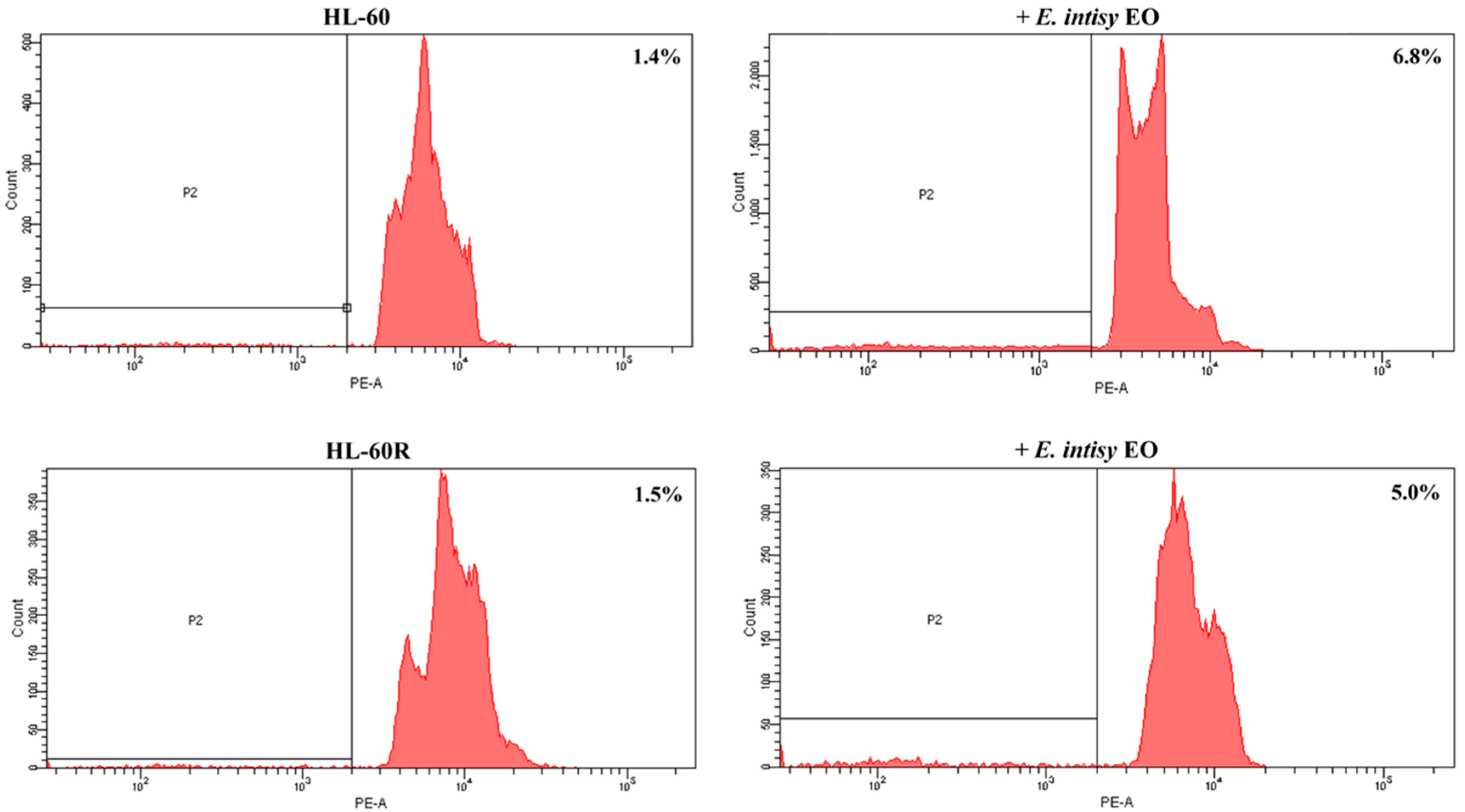
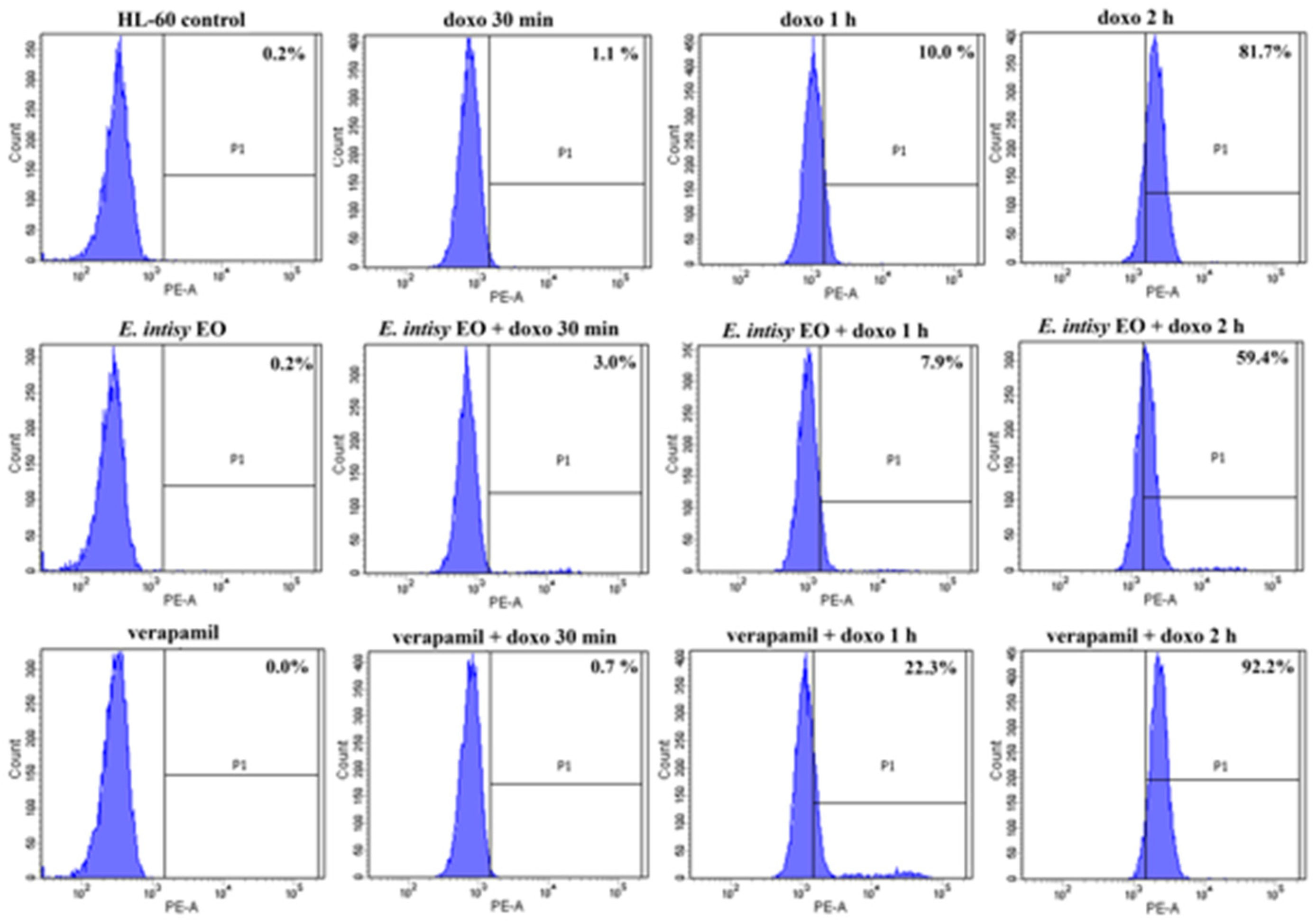
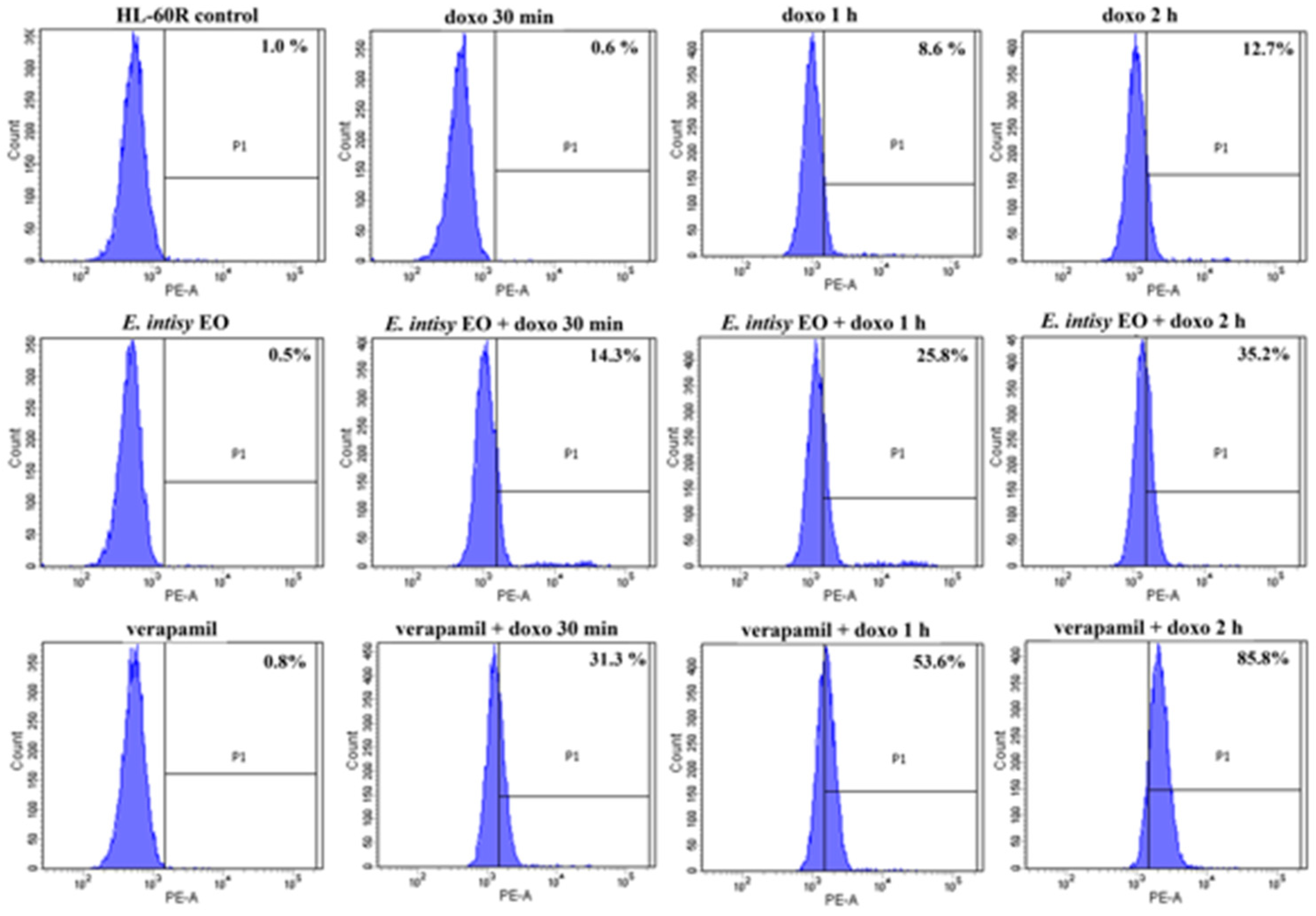
2.4. Effects of E. Intisy EO on Intracellular Accumulation of Doxorubicin in HL-60R Cell Line
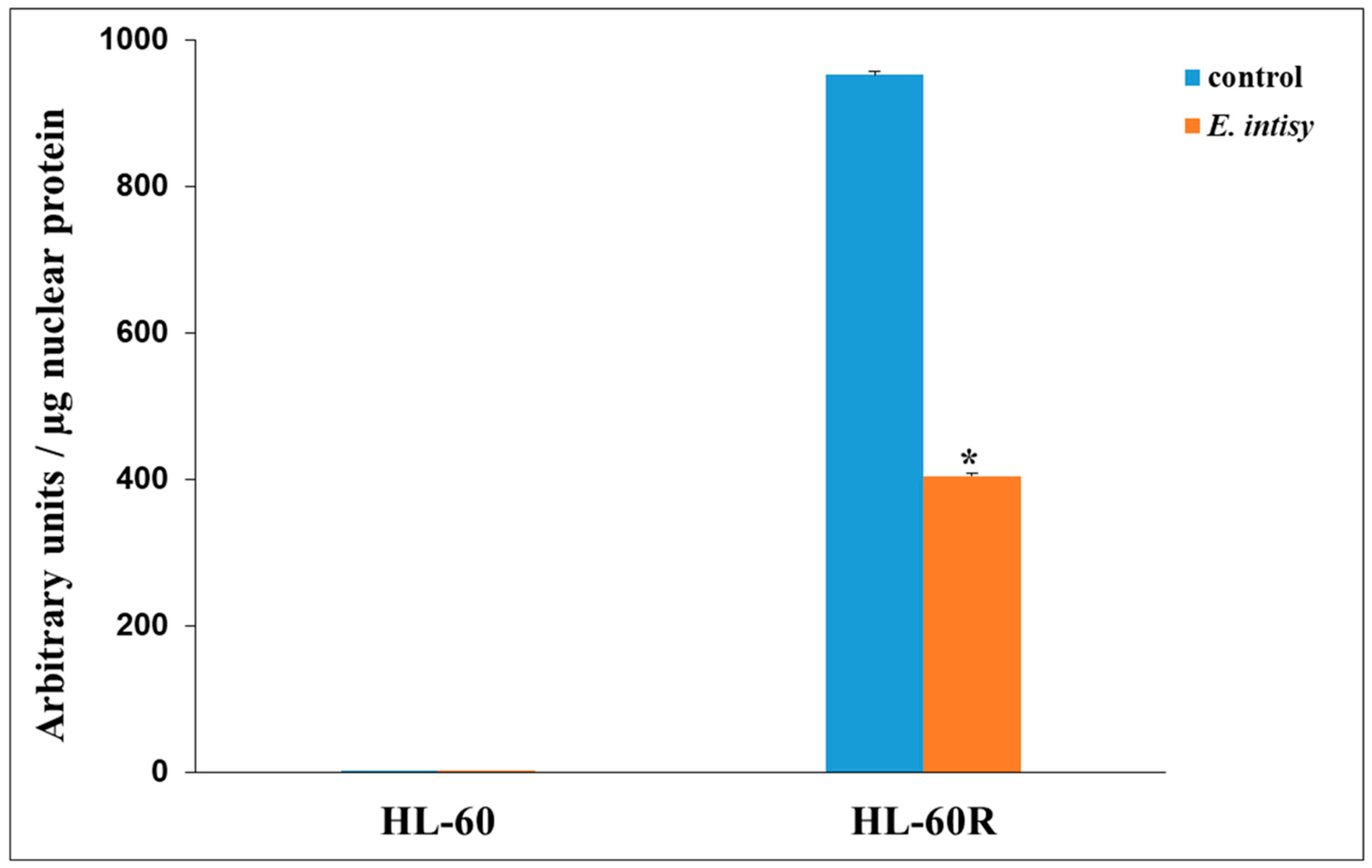
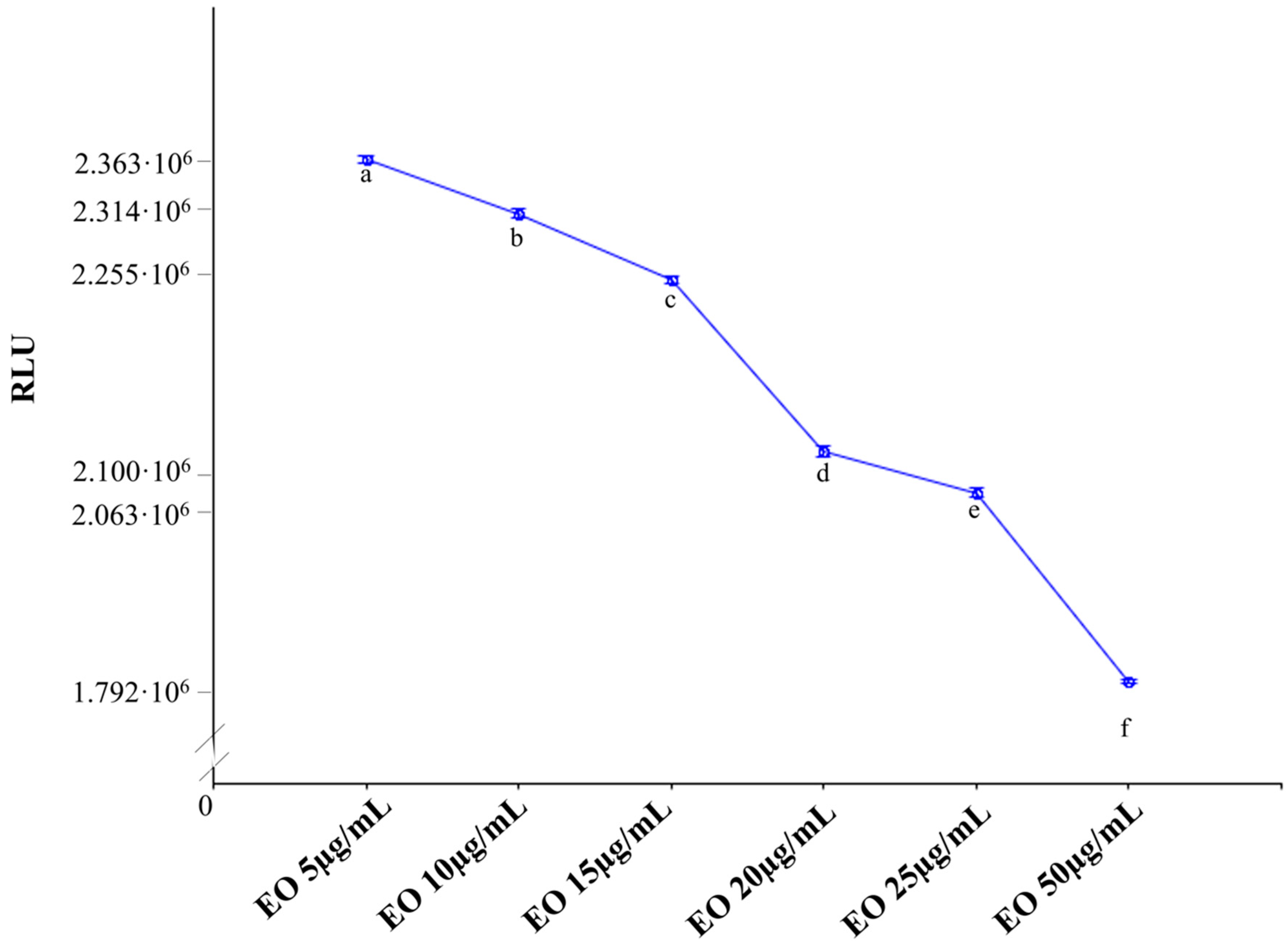
2.5. Effects of E. intisy EO on P-gp Activity
2.6. Cytotoxic and Cell Death Effects of E. intisy EO in Combination with Doxorubicin
3. Material and Methods
3.1. Plant Species
3.2. Plant Material
3.3. Essential Oil
3.4. Gas Chromatography-Mass Spectrometry
3.5. Identification of Compounds
3.6. Cell Lines
3.7. Cell Growth Assays
3.8. Evaluation of Cell Death by Flow Cytometry
3.9. NF-κB Activation
3.10. Extraction of Cellular RNA and Reverse Transcription-Quantitative PCR (RT-qPCR)
3.11. Western Blot Analysis
3.12. Determination of Doxorubicin Accumulation
3.13. P-gp ATPase Activity Determination
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kaye, S.B. The multidrug resistance phenotype. Br. J. Cancer 1988, 58, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.; Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta 1976, 455, 152–162. [Google Scholar] [CrossRef]
- Dewanjee, S.; Dua, T.K.; Bhattacharjee, N.; Das, A.; Gangopadhyay, M.; Khanra, R.; Joardar, S.; Riaz, M.; De Feo, V.; Zia-Ul-Haq, M. Natural Products as Alternative Choices for P-Glycoprotein (P-gp) Inhibition. Molecules 2017, 22, 871. [Google Scholar] [CrossRef] [PubMed]
- Mollazadeha, S.; Sahebkarb, A.; Hadizadeha, F.; Behravanb, J.; Arabzadeh, S. Structural and functional aspects of P-glycoprotein and its inhibitors. Life Sci. 2018, 214, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Zito, P.; Labbozzetta, M.; Notarbartolo, M.; Sajeva, M.; Poma, P. Essential oil of Cyphostemma juttae (Vitaceae): Chemical composition and antitumor mechanism in triple negative breast cancer cells. PLoS ONE 2019, 14, e0214594. [Google Scholar] [CrossRef] [PubMed]
- Poma, P.; Labbozzetta, M.; McCubrey, J.A.; Ramarosandratana, A.V.; Sajeva, M.; Zito, P.; Notarbartolo, M. Antitumor mechanism of the essential oils from two succulent plants in multidrug resistance Leukemia Cell. Pharmaceuticals 2019, 12, 124. [Google Scholar] [CrossRef]
- Poma, P.; Labbozzetta, M.; Zito, P.; Alduina, R.; Ramarosandratana, A.V.; Bruno, M.; Rosselli, S.; Sajeva, M.; Notarbartolo, M. Essential Oil Composition of Alluaudia procera and in Vitro Biological Activity on Two Drug-Resistant Models. Molecules 2019, 24, 2871. [Google Scholar] [CrossRef]
- Borrell, J.S.; Dodsworth, S.; Forest, F.; Pérez-Escobar, O.A.; Lee, M.A.; Mattana, E.; Stevenson, P.C.; Howes, M.-J.R.; Pritchard, H.W.; Ballesteros, D.; et al. The climatic challenge: Which plants will people use in the next century? Environ. Exp. Bot. 2019, 170, 103872. [Google Scholar] [CrossRef]
- Grace, O.M. Succulent plant diversity as natural capital. Plants People Planet 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Harlev, E.; Nevo, E.; Mirsky, N.; Ofir, R. Antidiabetic attributes of desert and steppic plants: A review. Planta Med. 2013, 79, 425–436. [Google Scholar] [CrossRef]
- Rizvi, M.A.; Saeed, A. Medicinal plants of arid zones: Utilization, cultivation and conservation. Hamdard Med. 2013, 56, 53–80. [Google Scholar]
- Dongling, L.; Yinquan, W.; Ling, T. Medicinal Plants in the Northwestern China and Their Medicinal Uses. Aromat. Med. Plants Back Nat. 2017, 215–218. [Google Scholar] [CrossRef]
- Najjaa, H.; Ben Arfa, A.; Máthé, Á.; Neffati, M. Aromatic and medicinal plants of Tunisian arid and desert zone used in traditional medicine, for drug discovery and biotechnological applications. In Medicinal and Aromatic Plants of the World—Africa; Neffati, M., Najjaa, H., Arfa, A.B., Máthé, Á., Eds.; Springer Science + Business Media: Berlin/Heidelberg, Germany, 2017; Volume 3, pp. 157–230. [Google Scholar] [CrossRef]
- Frodin, D.G. History and concepts of big plant genera. Taxon 2004, 53, 753–776. [Google Scholar] [CrossRef]
- Horn, J.W.; van Ee, B.W.; Morawetz, J.J.; Riina, R.; Steinmann, V.W.; Berry, P.E.; Wurdack, K.J. Phylogenetics and the evolution of major structural characters in the giant genus Euphorbia L. (Euphorbiaceae). Mol. Phylogenet. Evol. 2012, 63, 305–326. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Nothias, L.-F.; van der Hooft, J.J.J.; Silva, R.R.; Saslis-Lagoudakis, C.H.; Grace, O.M.; Martinez-Swatson, K.; Hassemer, G.; Funez, L.A.; Simonsen, H.T.; et al. Assessing specialized metabolite diversity in the cosmopolitan plant genus Euphorbia L. Front. Plant. Sci. 2019, 10, 846. [Google Scholar] [CrossRef] [PubMed]
- Kemboi, D.; Peter, X.; Langat, M.; Tembu, J. A Review of the Ethnomedicinal Uses, Biological Activities, and Triterpenoids of Euphorbia Species. Molecules 2020, 25, 4019. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Grace, O.M.; Saslis-Lagoudakis, C.H.; Nilsson, N.; Simonsen, H.T.; Rønsted, N. Global medicinal uses of Euphorbia L. (Euphorbiaceae). J. Ethnopharmacol. 2015, 176, 90–101. [Google Scholar] [CrossRef]
- Shi, Q.-W.; Su, X.-H.; Kiyota, H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [CrossRef]
- Mwine, J.T.; Van Damme, P. Why do Euphorbiaceae tick as medicinal plants? A review of Euphorbiaceae family and its medicinal features. J. Med. Plants Res. 2011, 5, 652–662. [Google Scholar]
- Salehi, B.; Iriti, M.; Vitalini, S.; Antolak, H.; Pawlikowska, E.; Kręgiel, D.; Sharifi-Rad, J.; Oyeleye, S.I.; Ademiluyi, A.O.; Czopek, K.; et al. Euphorbia-derived natural products with potential for use in health maintenance. Biomolecules 2019, 9, 337. [Google Scholar] [CrossRef]
- Duarte, N.; Ramalhete, C.; Varga, A.; Molnár, J.; Ferreira, M.-J.U. Multidrug resistance modulation and apoptosis induction of cancer cells by terpenic compounds isolated from Euphorbia species. Anticancer Res. 2009, 29, 4467–4472. [Google Scholar] [PubMed]
- Aleksandrov, M.; Maksimova, V.; Koleva Gudeva, L. Review of the anticancer and cytotoxic activity of some species from genus Euphorbia. Agric. Conspec. Sci. 2018, 84, 1–5. [Google Scholar]
- Wongrakpanich, A.; Charoensuksai, P. Induction of apoptosis in cancer cells by plants in the genus Euphorbia. Thai Bull. Pharm. Sci. 2018, 13, 1–11. [Google Scholar]
- Kúsz, N. Isolation and Structure Elucidation of Bioactive Compounds from Euphorbia Species. Ph.D. Thesis, University of Szeged, Szeged, Hungary, 2020; 55p. [Google Scholar]
- Fokialakis, N.; Melliou, E.; Magiatis, P.; Harvala, C.; Mitaku, S. Composition of the steam volatiles of six Euphorbia spp. from Greece. Flavour Fragr. J. 2003, 18, 39–42. [Google Scholar] [CrossRef]
- Feizbakhsh, A.; Bighdeli, M.; Tehrani, M.S.; Rustaiyan, A. Chemical constituents of the essential oil of Euphorbia teheranica Boiss., a species endemic to Iran. J. Essent. Oil Res. 2004, 16, 40–41. [Google Scholar] [CrossRef]
- Rojas, J.; Baldovino, S.; Vizcaya, M.; Rojas, L.B.; Morales, A. The chemical composition of the essential oils of Euphorbia caracasana and E. cotinifolia (Euphorbiaceae) from Venezuela. Nat. Prod. Commun. 2009, 4, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Elshamy, A.I.; Abd El-Gawad, A.M.; El Gendy, A.N.G.; Assaeed, A.M. Chemical characterization of Euphorbia heterophylla L. essential oils and their antioxidant activity and allelopathic potential on Cenchrus echinatus L. Chem. Biodivers. 2019, 16, E1900051. [Google Scholar] [CrossRef]
- Haevermans, T. Le genre Euphorbia à Madagascar: Phylogénie Moléculaire et Systématique. Ph.D. Thesis, MNHN, Paris, France, 2003. [Google Scholar]
- Rafidison, V.; Ratsimandresy, F.; Rakotondrajaona, R.; Rasamison, V.; Rakotoarisoa, M.; Rakotondrafara, A.; Rakotonandrasana, S.R. Synthèse et analyse de données sur les inventaires de plantes médicinales de Madagascar. Ethnobot. Res. Appl. 2019, 18, 40. [Google Scholar] [CrossRef]
- Kaufmann, J.C.; Tsirahamba, S. Forests and thorns: Conditions of change affecting Mahafale pastoralists in Southwestern Madagascar. Conserv. Soc. 2006, 4, 231–261. [Google Scholar]
- Swingle, C.F. The anatomy of Euphorbia intisy. J. Agric. Res. 1930, 40, 615–625. [Google Scholar]
- Perrier de la Bathie, H. Les plantes à caoutchouc de Madagascar valeur et possibilité de leur culture. In Revue Internationale de Botanique Appliquée et D’agriculture Tropicale, 29e Année, Bulletin n°315–316; Janvier-Février: Paris, France, 1949; Volume 26, pp. 17–20. Available online: https://www.persee.fr/doc/jatba_0370-5412_1949_num_29_315_6199 (accessed on 22 October 2019). [CrossRef]
- Decary, R. Les emplois des Euphorbiacées malgaches. J. Agric. Trop. Bot. Appl. Août Sept. 1966, 13, 467–473. [Google Scholar] [CrossRef]
- Vacher, C. Le caoutchouc dans l’extrême sud malgache. Bull. Écon. Madag. Dépend. 1907, 7, 128–141. [Google Scholar]
- Haevermans, T. Euphorbia Intisy. The IUCN Red List of Threatened Species 2019: E.T44367A124142437. Available online: https//www.iucnredlist.org (accessed on 19 October 2020).
- Adams, R.P. Identification of Essential oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; p. 456. [Google Scholar]
- El-Sayed, A.M. The Pherobase: Database of Insect Pheromones and Semiochemicals. Available online: http://www. pherobase.com/ (accessed on 30 September 2020).
- Mize, C.E.; Steinberg, D.; Avigan, J.; Fales, H.M. A pathway for oxidative degradation of phytanic acid in mammals. Biochem. Biophys. Res. Commun. 1966, 25, 359–365. [Google Scholar] [CrossRef]
- Steinberg, D.; Avigan, J.; Mize, C.E.; Baxter, J.H.; Cammermeyer, J.; Fales, H.M.; Highet, P.F. Effects of dietary phytol and phytanic acid in animals. J. Lipid Res. 1966, 7, 684–691. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Billah, M.M. Phytol: A review of biomedical activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Robbins, W.E.; Kaplanis, J.N.; Thompson, M.J.; Shortino, T.J.; Cohen, C.F.; Joyner, S.C. Ecdysones and analogs: Effects on development and reproduction of insects. Science 1968, 161, 1158–1160. [Google Scholar] [CrossRef]
- Song, J.E.; Kim, J.M.; Lee, N.H.; Yang, J.Y.; Lee, H.S. Acaricidal and insecticidal activities of essential oils against a stored-food mite and stored-grain insects. J. Food Prot. 2016, 79, 174–178. [Google Scholar] [CrossRef]
- Dąbrowska, P.; Boland, W. iso-OPDA: An Early Precursor of cis-Jasmone in Plants? Chembiochem 2007, 8, 2281–2285. [Google Scholar] [CrossRef]
- Radulović, N.; Stojanović, G.; Palić, R. Composition and antimicrobial activity of Equisetum arvense L. essential oil. Phytother. Res. 2006, 20, 85–88. [Google Scholar] [CrossRef]
- Duru, M.E.; Cakir, A.; Kordali, S.; Zengin, H.; Harmandar, M.; Izumi, S.; Hirata, T. Chemical composition and antifungal properties of essential oils of three Pistacia species. Fitoterapia 2003, 74, 170–176. [Google Scholar] [CrossRef]
- Notarbartolo, M.; Cervello, M.; Dusonchet, L.; Cusimano, A.; D’Alessandro, N. Resistance to diverse apoptotic triggers in multidrug resistant HL60 cells and its possible relationship to the expression of P-glycoprotein, Fas and of the novel anti-apoptosis factors IAP (inhibitory of apoptosis proteins). Cancer Lett. 2002, 180, 91–101. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—Natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Notarbartolo, M.; Cervello, M.; Poma, P.; Dusonchet, L.; Meli, M.; D’Alessandro, N. Expression of the IAPs in multidrug resistant tumor cells. Oncol. Rep. 2004, 11, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.Y.; El-Readi, M.Z.; Eldin, E.E.M.N.; Fatani, S.H.; Wink, M. Influence of combinations of digitonin with selected phenolics, terpenoids, and alkaloids on the expression and activity of P-glycoprotein in leukaemia and colon cancer cells. Phytomedicine 2013, 21, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Wortelboer, H.M.; Usta, M.; van Zanden, J.J.; van Bladeren, P.J.; Rietjens, I.M.; Cnubben, N.H. Inhibition of multidrug resistance proteins MRP1 and MRP2 by a series of α, β-unsaturated carbonyl compounds. Biochem. Pharmacol. 2005, 69, 1879–1890. [Google Scholar] [CrossRef]
- Valente, I.; Reis, M.; Duarte, N.; Serly, J.; Molnár, J.; Ferreira, M.J.U. Jatrophane diterpenes from Euphorbia mellifera and their activity as P-glycoprotein modulators on multidrug-resistant mouse lymphoma and human colon adenocarcinoma cells. J. Nat. Prod. 2012, 75, 1915–1921. [Google Scholar] [CrossRef]
- Madureira, A.M.; Gyémánt, N.; Ascenso, J.R.; Abreu, P.M.; Molnár, J.; Ferreira, M.J.U. Euphoportlandols A and B, Tetracylic Diterpene Polyesters from Euphorbia portlandica and Their Anti-MDR Effects in Cancer Cells. J. Nat. Prod. 2006, 69, 950–953. [Google Scholar] [CrossRef]
- Molnár, J.; Gyémánt, N.; Tanaka, M.; Hohmann, J.; Bergmann-Leitner, E.; Molnár, P.; Deli, J.; Didiziapetris, R.; Ferreira, M.J.U. Inhibition of multidrug resistance of cancer cells by natural diterpenes, triterpenes and carotenoids. Curr. Pharm. Des. 2006, 12, 287–311. [Google Scholar] [CrossRef]
- Neto, S.; Duarte, N.; Pedro, C.; Spengler, G.; Molnár, J.; Ferreira, M.J.U. Effective MDR reversers through phytochemical study of Euphorbia boetica. Phytochem. Anal. 2019, 30, 498–511. [Google Scholar] [CrossRef]
- Reis, M.A.; Ahmed, O.B.; Spengler, G.; Molnár, J.; Lage, H.; Ferreira, M.J.U. Jatrophane diterpenes and cancer multidrug resistance–ABCB1 efflux modulation and selective cell death induction. Phytomedicine 2016, 23, 968–978. [Google Scholar] [CrossRef]
- Zito, P.; Sajeva, M.; Bruno, M.; Rosselli, S.; Maggio, A.; Senatore, F.; Formisano, C. Essential oil composition of Periploca laevigata Aiton subsp. angustifolia (Labill.) Markgraf (Apocynaceae-Periplocoideae). Nat. Prod. Res. 2013, 27, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Analysis of combined drug effects: A new look at a very old problem. Trends Pharmacol. Sci. 1983, 4, 450–454. [Google Scholar] [CrossRef]


| KRI | Compound | Relative Amount % | MS Similarity |
|---|---|---|---|
| Aliphatic Acids | |||
| 2512 | Dodecanoic acid | 0.42 | 93 |
| 2720 | Oleic acid | 1.37 | 90 |
| Aliphatic Alcohols | |||
| 1354 | 1-Hexanol | 0.05 | 92 |
| 1383 | (Z)-3-Hexen-1-ol | 0.05 | 97 |
| 1407 | (E)-2-Hexen-1-ol | 0.02 | 91 |
| 1557 | (E)-3-Octen-1-ol | 0.02 | 92 |
| 1968 | 1-Dodecanol | 0.02 | 85 |
| 1557 | (E)-3-Octen-1-ol | 0.02 | 92 |
| 2447 | 1-Heptacosanol | 0.85 | 96 |
| Aliphatic Aldheydes | |||
| 1081 | Hexanal | 0.08 | 91 |
| 1217 | (E)-2-Hexenal | 0.65 | 96 |
| 1389 | Nonanal | 0.06 | 90 |
| Aliphatic Alkanes | |||
| 1900 | Nonadecane | 0.05 | H |
| 2200 | Docosane | 0.15 | H |
| 2300 | Tricosane | 0.33 | H |
| 2400 | Tetracosane | 0.44 | H |
| 2500 | Pentacosane | 1.53 | H |
| 2600 | Hexacosane | 0.31 | H |
| 2700 | Heptacosane | 15.73 | H |
| Aliphatic Esters | |||
| 2550 | Methyl linolenate | 0.74 | 92 |
| Aromatic Esters | |||
| 2606 | Benzyl benzoate | 0.13 | 96 |
| Diterpene Alcohols | |||
| 2296 | Isophytol | 0.38 | 84 |
| 2615 | Phytol | 7.89 | 97 |
| Irregular Terpene Ketones | |||
| 2124 | Hexahydrofarnesyl acetone | 0.29 | 90 |
| Miscellaneous Ketones | |||
| 1932 | (Z)-Jasmone | 0.19 | 95 |
| Monoterpene Alchols | |||
| 1550 | Linalool | 1.25 | 98 |
| 1612 | Hotrienol | 0.02 | 91 |
| 1849 | Geraniol | 0.22 | 95 |
| Monoterpene Ethers | |||
| 1435 | (Z)-Linalool oxide furanoid | 0.42 | 97 |
| Monoterpene Hydrocarbons | |||
| 1247 | (E)-beta-Ocimene | 1.50 | 97 |
| Monoterpene Ketones | |||
| 1629 | Pulegone | 0.07 | 82 |
| Sesquiterpene Alcohols | |||
| 1876 | epi-Cubebol | 0.01 | 81 |
| 2042 | Nerolidol isomer | 0.21 | 75 |
| 2073 | beta-Elemol | 0.06 | 88 |
| 2156 | gamma-Eudesmol | 3.06 | 95 |
| 2186 | Pogostol | 1.58 | 83 |
| 2206 | alpha-Eudesmol | 1.58 | 80 |
| 2213 | beta-Eudesmol | 1.76 | 82 |
| 2219 | alpha-Cadinol | 0.11 | 89 |
| 2231 | (–)-Spathulenol | 0.28 | 80 |
| 2262 | Valerenol | 0.46 | 84 |
| 2281 | Juniper camphor | 0.48 | 87 |
| 2354 | Farnesol | 0.09 | 90 |
| 2492 | gamma-Costol | 0.09 | 86 |
| Sesquiterpene Ethers | |||
| 1464 | (E)-Linalool oxide furanoid | 0.26 | 92 |
| 1507 | Vitispirane | 0.03 | 93 |
| 1510 | Vitispirane isomer | 0.02 | 93 |
| 2023 | Caryophyllene oxide | 0.02 | 80 |
| Sequiterpene Hydrocarbons | |||
| 1441 | alpha-Cubebene | 0.05 | 93 |
| 1470 | alpha-Copaene | 2.94 | 97 |
| 1495 | beta-Bourbonene | 0.49 | 96 |
| 1571 | beta-Caryophyllene | 1.11 | 96 |
| 1573 | (Z)-beta-Elemene | 0.12 | 84 |
| 1622 | gamma-Elemene | 0.02 | 82 |
| 1644 | alpha-Humulene | 0.08 | 93 |
| 1666 | alpha-Amorphene | 1.85 | 94 |
| 1684 | beta-Guaiene | 2.64 | 91 |
| 1698 | alpha-Selinene | 0.06 | 89 |
| 1704 | alpha-Muurolene | 1.56 | 95 |
| 1736 | delta-Cadinene | 2.07 | 96 |
| 1742 | (E,E)-alpha-Farnesene | 0.14 | 86 |
| 1759 | Cubenene | 0.06 | 91 |
| 1811 | Isocalamenene | 0.10 | 87 |
| 1894 | alpha-Calacorene | 0.34 | 93 |
| 1926 | Neophytadiene | 0.38 | 94 |
| 1939 | beta-Calacorene | 0.03 | 84 |
| 1955 | Neophytadiene isomer | 0.12 | 87 |
| 1981 | Neophytadiene isomer | 0.27 | 96 |
| 1987 | alpha-Gorgonene | 0.40 | 87 |
| Sesquiterpene Ketones | |||
| 2234 | Mustakone | 1.01 | 92 |
| Unidentified | |||
| 2136 | Unknown m/z: 161 (100), 105 (99), 43 (93), 121 (65), 93 (62), 81 (60), 95 (56), 119 (56), 79 (49), 91 (48) | 4.58 | 90 |
| 2255 | Unknown m/z: 107 (100), 93 (78), 105 (74), 119 (63), 69 (60), 41 (56), 81 (53), 91 (49), 79 (44), 161 (43) | 1.35 | NF |
| 2329 | Unknown m/z: 105 (100), 143 (94), 91 (80), 107 (67), 81 (60), 119 (57), 79 (54), 93 (50), 147 (50), 121 (46) | 1.67 | 85 |
| Total | 68.84 | ||
| Cell Line | IC50 (Mean ± SE) |
|---|---|
| HL-60 | 22.0 ± 0.7 µg/mL |
| HL-60R | 24.5 ± 0.07 µg/mL |
| Caco-2 | 62.5 ± 0.35 µg/mL |
| Treatment | Fluorescence % | |
|---|---|---|
| HL-60 | HL-60R | |
| control | 0.2 ± 0.004 | 0.9 ± 0.004 |
| doxo 1 µg/mL 30 min | 1.0 ± 0.05 | 0.6 ± 0.09 |
| doxo 1 µg/mL 1 h | 9.7 ± 0.70 * | 8.8 ± 0.09 * |
| doxo 1 µg/mL 2 h | 80.2 ± 0.65 * | 11.9 ± 0.79 * |
| E. intisy EO 15 µg/mL | 0.12 ± 0.03 | 0.47 ± 0.07 |
| verapamil 10 µM | 0.03 ± 0.03 | 0.73 ± 0.10 |
| E. intisy EO + doxo 30 min | 3.0 ± 0.41 * | 14.7 ± 0.18 *,a |
| E. intisy EO + doxo 1 h | 7.97 ± 0.53 * | 25.6 ± 0.25 *,a |
| E. intisy EO + doxo 2 h | 59.8 ± 0.97 * | 35.5 ± 0.19 *,a |
| verapamil + doxo 30 min | 0.6 ± 0.05 | 31.1 ± 0.05 *,a |
| verapamil + doxo 1 h | 21.8 ± 0.75 *,a | 53.5 ± 0.24 *,a |
| verapamil + doxo 2 h | 91.7 ± 0.70 * | 85.6 ± 0.25 *,a |
| Treatment | Cell Growth Inhibition % |
|---|---|
| E. intisy EO 15 µg/mL | 16.0 ± 1.4 * |
| E. intisy EO 20 µg/mL | 22.0 ± 0.7 * |
| E. intisy EO 30 µg/mL | 56.0 ± 1.4 * |
| doxo 1 µg/mL | 0.5 ± 0.3 |
| doxo 5 µg/mL | 5.7 ± 3.5 |
| doxo 10 µg/mL | 11.3 ± 3.8 * |
| Treatment | Cell Growth Inhibition % | Combination Index |
|---|---|---|
| E. intisy EO (15 µg/mL) + doxo (1 µg/mL) | 17.0 ± 0.5 * | 0.80 |
| E. intisy EO (15 µg/mL) + doxo (5 µg/mL) | 40.0 ± 4.0 * | 0.82 |
| E. intisy EO (15 µg/mL) + doxo (10 µg/mL) | 58.0 ± 3.4 * | 0.83 |
| E. intisy EO (20 µg/mL) + doxo (1 µg/mL) | 24.0 ± 0.7 * | 0.85 |
| E. intisy EO (20 µg/mL) + doxo (5 µg/mL) | 59.3 ± 1.8 * | 0.88 |
| E. intisy EO (20 µg/mL) + doxo (10 µg/mL) | 64.3 ± 3.2 * | 0.88 |
| E. intisy EO (30 µg/mL) + doxo (1µg/mL) | 49.0 ± 0.5 * | 0.90 |
| E. intisy EO (30 µg/mL) + doxo (5 µg/mL) | 69.0 ± 6.3 * | 0.91 |
| E. intisy EO (30 µg/mL) + doxo (10 µg/mL) | 81.6 ± 0.9 * | 0.88 |
| HL-60R | Cell Death % | Expected % |
|---|---|---|
| 4.5 ± 0.25 | ||
| + E.intisy EO 25 µg/mL | 5.1 ± 0.10 | |
| + doxo 2 µg/mL | 4.8 ± 0.20 | |
| + doxo 5 µg/mL | 8.6 ± 0.10 | |
| + doxo 10 µg/mL | 12.7 ± 0.30 | |
| + doxo 25 µg/mL | 15.0 ± 0.15 | |
| + E.intisy EO + doxo 2 µg/mL | 6.6 ± 0.10 | 5.3 ± 0.55 |
| + E.intisy EO + doxo 5 µg/mL | 17.6 ± 0.35 | 9.1 ± 0.45 * |
| + E.intisy EO + doxo 10 µg/mL | 21.6 ± 0.20 | 13.2 ± 0.65 * |
| + E.intisy EO + doxo 25 µg/mL | 36.8 ± 0.15 | 15.6 ± 0.50 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poma, P.; Labbozzetta, M.; Ramarosandratana, A.V.; Rosselli, S.; Tutone, M.; Sajeva, M.; Notarbartolo, M. RETRACTED: In Vitro Modulation of P-Glycoprotein Activity by Euphorbia intisy Essential Oil on Acute Myeloid Leukemia Cell Line HL-60R. Pharmaceuticals 2021, 14, 111. https://doi.org/10.3390/ph14020111
Poma P, Labbozzetta M, Ramarosandratana AV, Rosselli S, Tutone M, Sajeva M, Notarbartolo M. RETRACTED: In Vitro Modulation of P-Glycoprotein Activity by Euphorbia intisy Essential Oil on Acute Myeloid Leukemia Cell Line HL-60R. Pharmaceuticals. 2021; 14(2):111. https://doi.org/10.3390/ph14020111
Chicago/Turabian StylePoma, Paola, Manuela Labbozzetta, Aro Vonjy Ramarosandratana, Sergio Rosselli, Marco Tutone, Maurizio Sajeva, and Monica Notarbartolo. 2021. "RETRACTED: In Vitro Modulation of P-Glycoprotein Activity by Euphorbia intisy Essential Oil on Acute Myeloid Leukemia Cell Line HL-60R" Pharmaceuticals 14, no. 2: 111. https://doi.org/10.3390/ph14020111
APA StylePoma, P., Labbozzetta, M., Ramarosandratana, A. V., Rosselli, S., Tutone, M., Sajeva, M., & Notarbartolo, M. (2021). RETRACTED: In Vitro Modulation of P-Glycoprotein Activity by Euphorbia intisy Essential Oil on Acute Myeloid Leukemia Cell Line HL-60R. Pharmaceuticals, 14(2), 111. https://doi.org/10.3390/ph14020111








