Effects of Ephedrine-Containing Products on Weight Loss and Lipid Profiles: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Results
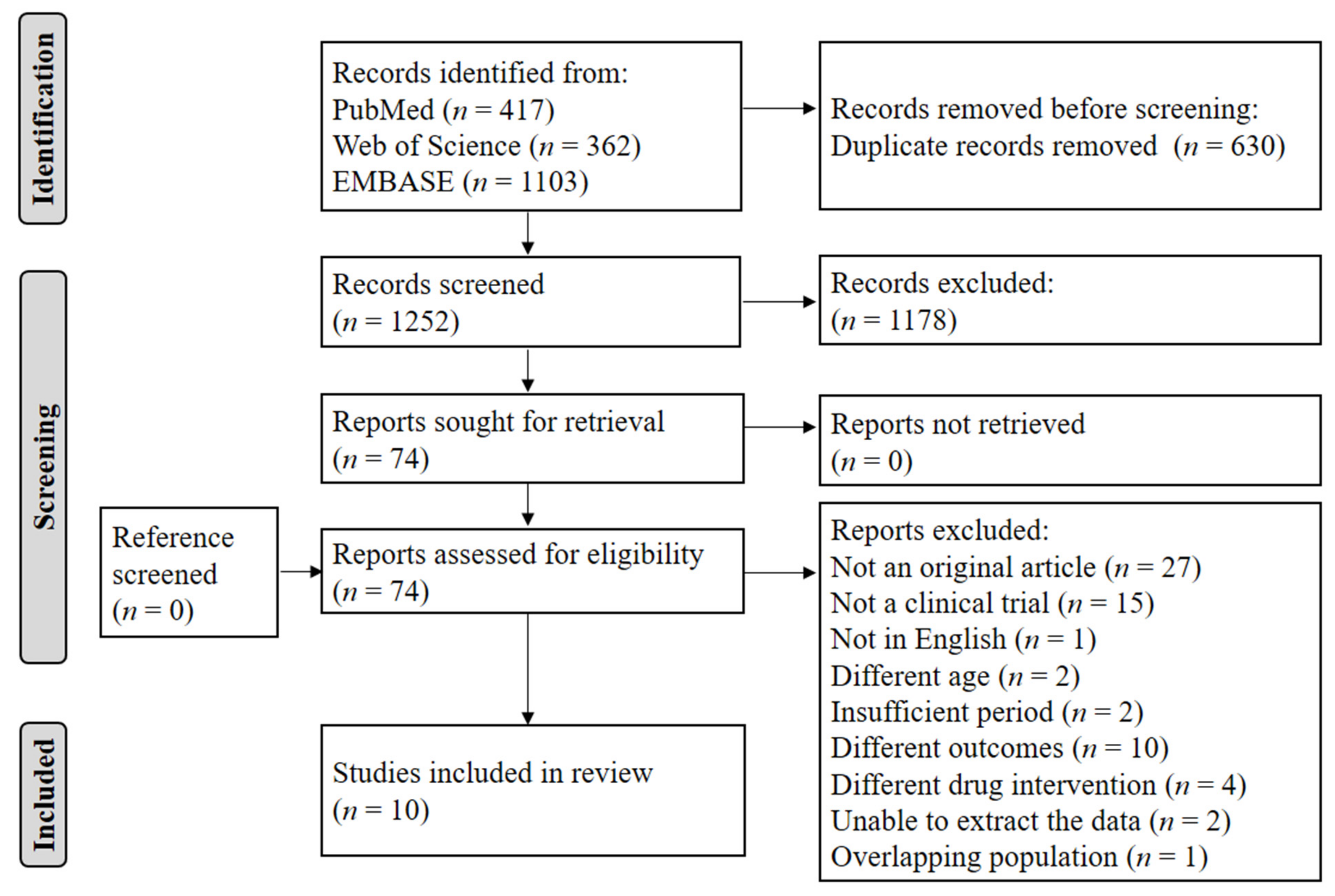
2.1. Literature Search
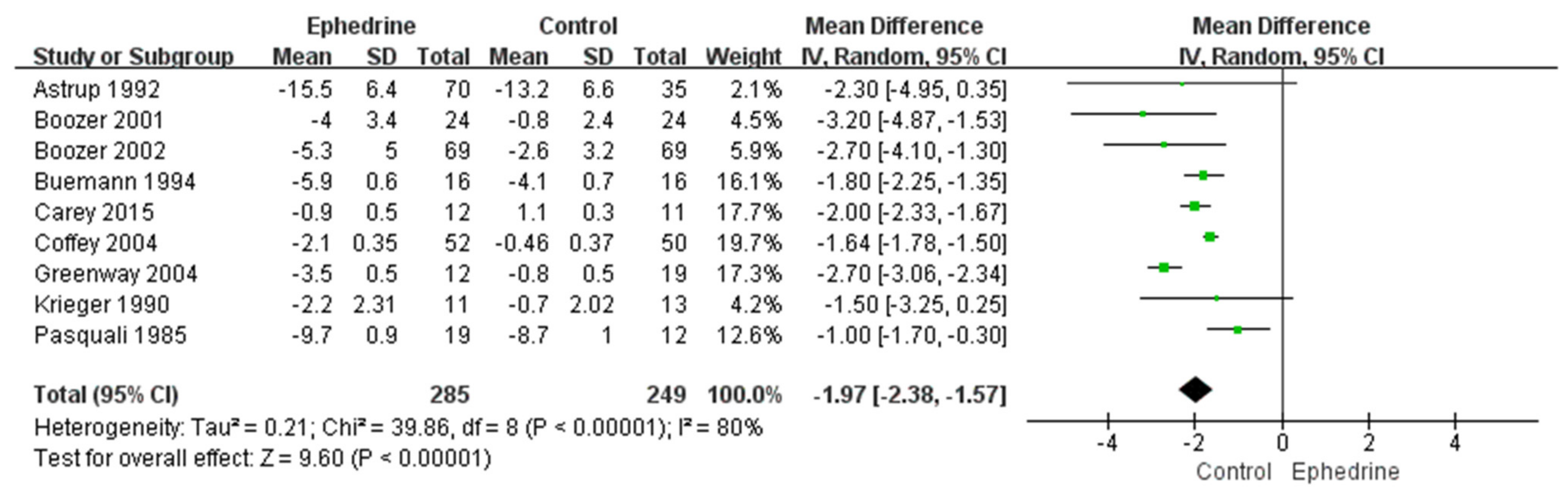
2.2. Weight Loss
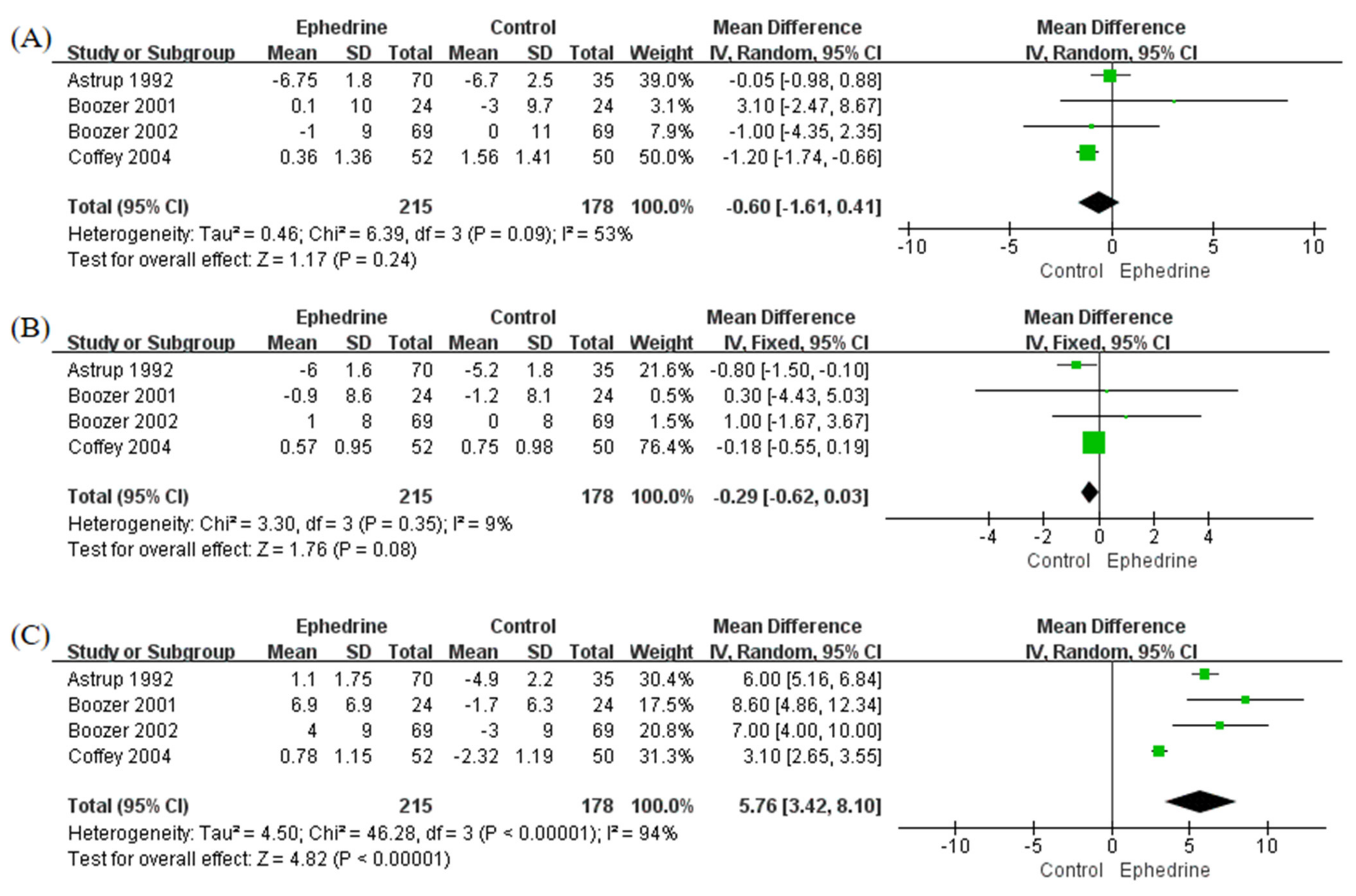
2.3. Blood Pressure and Heart Rate
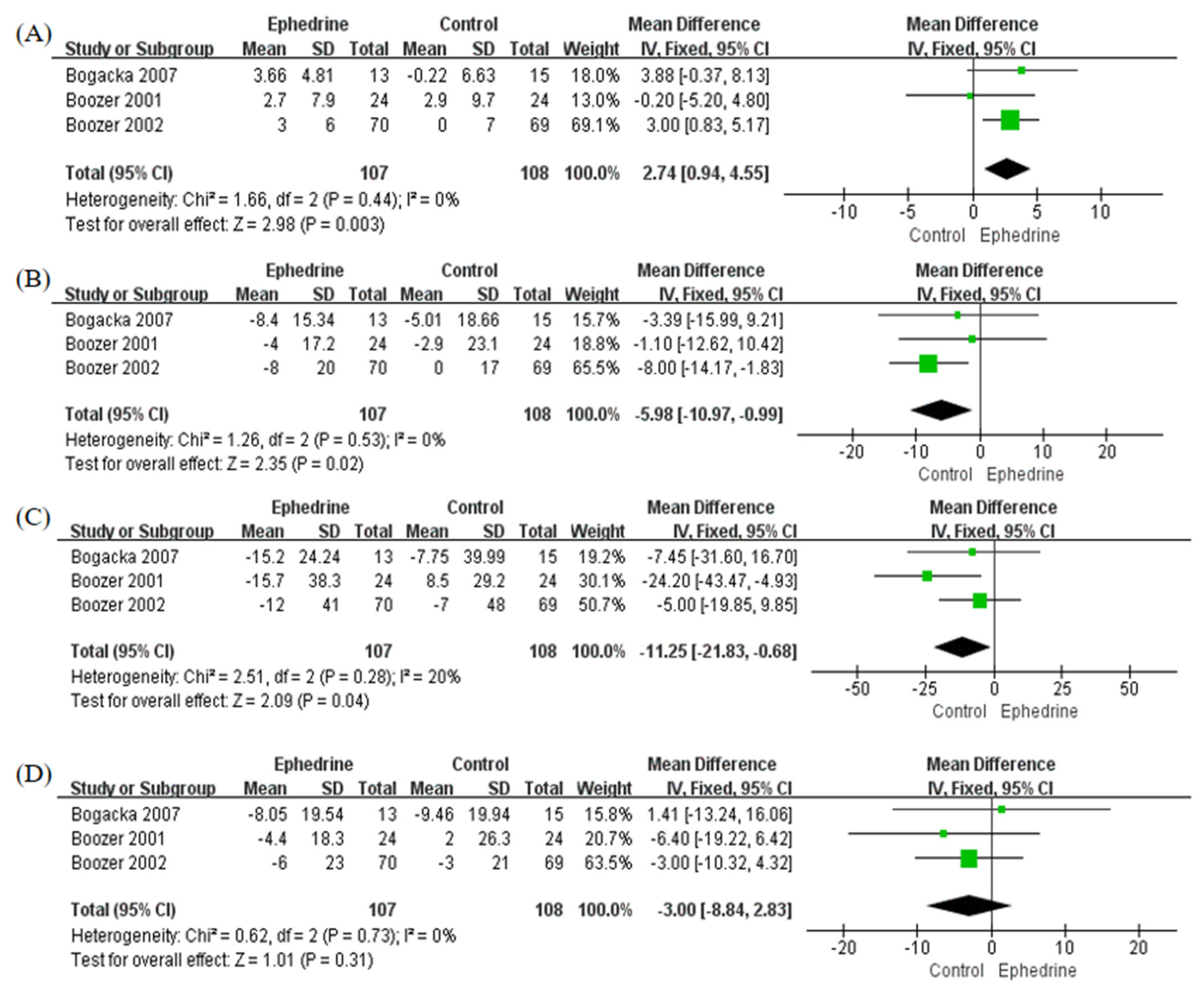
2.4. Lipid Levels
2.5. Subgroup Analysis
3. Discussion
4. Materials and Methods
4.1. Literature Search Strategy and Inclusion Criteria
4.2. Data Extraction and Study Quality Assessment
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ogden, C.L.; Fryar, C.D.; Martin, C.B.; Freedman, D.S.; Carroll, M.D.; Gu, Q.; Hales, C.M. Trends in Obesity Prevalence by Race and Hispanic Origin-1999–2000 to 2017–2018. JAMA 2020, 324, 1208–1210. [Google Scholar] [CrossRef] [PubMed]
- Noncommunicable Diseases. Available online: https://www.who.int/en/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 13 October 2021).
- Freemantle, N.; Holmes, J.; Hockey, A.; Kumar, S. How strong is the association between abdominal obesity and the incidence of type 2 diabetes? Int. J. Clin. Pract. 2008, 62, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, K.; Douglas, I.; Forbes, H.; dos-Santos-Silva, I.; Leon, D.A.; Smeeth, L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5·24 million UK adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef]
- Ni Mhurchu, C.; Rodgers, A.; Pan, W.H.; Gu, D.F.; Woodward, M.; Asia Pacific Cohort Studies Collaboration. Body mass index and cardiovascular disease in the Asia-Pacific Region: An overview of 33 cohorts involving 310,000 participants. Int. J. Epidemiol. 2004, 33, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, P. Health risks associated with overweight and obesity. Obes. Rev. 2007, 8, 13–17. [Google Scholar] [CrossRef]
- Wadden, T.A.; Butryn, M.L.; Byrne, K.J. Efficacy of lifestyle modification for long-term weight control. Obes. Res. 2004, 12, 151S–162S. [Google Scholar] [CrossRef]
- Apovian, C.M.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Ryan, D.; Still, C.D. Pharmacological management of obesity: An endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015, 100, 342–362. [Google Scholar] [CrossRef]
- Daneschvar, H.L.; Aronson, M.D.; Smetana, G.W. FDA-Approved Anti-Obesity Drugs in the United States. Am. J. Med. 2016, 129, e1–e6. [Google Scholar] [CrossRef]
- Saper, R.B.; Eisenberg, D.M.; Phillips, R.S. Common dietary supplements for weight loss. Am. Fam. Physician 2004, 70, 1731–1738. [Google Scholar]
- Persky, A.M.; Berry, N.S.; Pollack, G.M.; Brouwer, K.L. Modelling the cardiovascular effects of ephedrine. Br. J. Clin. Pharmacol. 2004, 57, 552–562. [Google Scholar] [CrossRef][Green Version]
- Diepvens, K.; Westerterp, K.R.; Westerterp-Plantenga, M.S. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R77–R85. [Google Scholar] [CrossRef]
- Vallerand, A.L.; Jacobs, I.; Kavanagh, M.F. Mechanism of enhanced cold tolerance by an ephedrine-caffeine mixture in humans. J. Appl. Physiol. 1989, 67, 438–444. [Google Scholar] [CrossRef]
- Ingerslev, J.; Svendsen, T.L.; Mørk, A. Is an ephedrine caffeine treatment contraindicated in hypertension? Int. J. Obes. Relat. Metab. Disord. 1997, 21, 666–673. [Google Scholar] [CrossRef][Green Version]
- Kalman, D.S.; Colker, C.M.; Shi, Q.; Swain, M.A. Effects of a weight-loss aid in healthy overweight adults: Double-blind. Curr. Ther. Res. 2000, 61, 199–205. [Google Scholar] [CrossRef]
- Hioki, C.; Yoshimoto, K.; Yoshida, T. Efficacy of bofu-tsusho-san, an oriental herbal medicine, in obese Japanese women with impaired glucose tolerance. Clin. Exp. Pharmacol. Physiol. 2004, 31, 614–619. [Google Scholar] [CrossRef]
- Hackman, R.M.; Havel, P.J.; Schwartz, H.J.; Rutledge, J.C.; Watnik, M.R.; Noceti, E.M.; Stohs, S.J.; Stern, J.S.; Keen, C.L. Multinutrient supplement containing ephedra and caffeine causes weight loss and improves metabolic risk factors in obese women: A randomized controlled trial. Int. J. Obes. (Lond.) 2006, 30, 1545–1556. [Google Scholar] [CrossRef]
- Laccourreye, O.; Werner, A.; Giroud, J.P.; Couloigner, V.; Bonfils, P.; Bondon-Guitton, E. Benefits, limits and danger of ephedrine and pseudoephedrine as nasal decongestants. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2015, 132, 31–34. [Google Scholar] [CrossRef]
- Wooltorton, E.; Sibbald, B. Ephedra/ephedrine: Cardiovascular and CNS effects. CMAJ 2002, 166, 633. [Google Scholar]
- Shekelle, P.G.; Hardy, M.L.; Morton, S.C.; Maglione, M.; Mojica, W.A.; Suttorp, M.J.; Rhodes, S.L.; Jungvig, L.; Gagné, J. Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: A meta-analysis. JAMA 2003, 289, 1537–1545. [Google Scholar] [CrossRef]
- Hasani-Ranjbar, S.; Nayebi, N.; Larijani, B.; Abdollahi, M. A systematic review of the efficacy and safety of herbal medicines used in the treatment of obesity. World J. Gastroenterol. 2009, 15, 3073–3085. [Google Scholar] [CrossRef]
- Maunder, A.; Bessell, E.; Lauche, R.; Adams, J.; Sainsbury, A.; Fuller, N.R. Effectiveness of herbal medicines for weight loss: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2020, 22, 891–903. [Google Scholar] [CrossRef]
- Batsis, J.A.; Apolzan, J.W.; Bagley, P.J.; Blunt, H.B.; Divan, V.; Gill, S.; Golden, A.; Gundumraj, S.; Heymsfield, S.B.; Kahan, S.; et al. A Systematic Review of Dietary Supplements and Alternative Therapies for Weight Loss. Obes. (Silver Spring) 2021, 29, 1102–1113. [Google Scholar] [CrossRef]
- Astrup, A.; Breum, L.; Toubro, S.; Hein, P.; Quaade, F. The effect and safety of an ephedrine/caffeine compound compared to ephedrine, caffeine and placebo in obese subjects on an energy restricted diet. A double blind trial. Int. J. Obes. Relat. Metab. Disord. 1992, 16, 269–277. [Google Scholar]
- Bogacka, I.; Gettys, T.W.; de Jonge, L.; Nguyen, T.; Smith, J.M.; Xie, H.; Greenway, F.; Smith, S.R. The effect of beta-adrenergic and peroxisome proliferator-activated receptor-gamma stimulation on target genes related to lipid metabolism in human subcutaneous adipose tissue. Diabetes Care 2007, 30, 1179–1186. [Google Scholar] [CrossRef]
- Boozer, C.N.; Nasser, J.A.; Heymsfield, S.B.; Wang, V.; Chen, G.; Solomon, J.L. An herbal supplement containing Ma Huang-Guarana for weight loss: A randomized, double-blind trial. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 316–324. [Google Scholar] [CrossRef]
- Boozer, C.N.; Daly, P.A.; Homel, P.; Solomon, J.L.; Blanchard, D.; Nasser, J.A.; Strauss, R.; Meredith, T. Herbal ephedra/caffeine for weight loss: A 6-month randomized safety and efficacy trial. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 593–604. [Google Scholar] [CrossRef]
- Buemann, B.; Marckmann, P.; Christensen, N.J.; Astrup, A. The effect of ephedrine plus caffeine on plasma lipids and lipoproteins during a 4.2 MJ/day diet. Int. J. Obes. Relat. Metab. Disord. 1994, 18, 329–332. [Google Scholar]
- Carey, A.L.; Pajtak, R.; Formosa, M.F.; Van Every, B.; Bertovic, D.A.; Anderson, M.J.; Eikelis, N.; Lambert, G.W.; Kalff, V.; Duffy, S.J.; et al. Chronic ephedrine administration decreases brown adipose tissue activity in a randomised controlled human trial: Implications for obesity. Diabetologia 2015, 58, 1045–1054. [Google Scholar] [CrossRef]
- Coffey, C.S.; Steiner, D.; Baker, B.A.; Allison, D.B. A randomized double-blind placebo-controlled clinical trial of a product containing ephedrine, caffeine, and other ingredients from herbal sources for treatment of overweight and obesity in the absence of lifestyle treatment. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1411–1419. [Google Scholar] [CrossRef]
- Greenway, F.L.; De Jonge, L.; Blanchard, D.; Frisard, M.; Smith, S.R. Effect of a dietary herbal supplement containing caffeine and ephedra on weight, metabolic rate, and body composition. Obes. Res. 2004, 12, 1152–1157. [Google Scholar] [CrossRef]
- Krieger, D.R.; Daly, P.A.; Dulloo, A.G.; Ransil, B.J.; Young, J.B.; Landsberg, L. Ephedrine, caffeine and aspirin promote weight loss in obese subjects. Trans. Assoc. Am. Physicians 1990, 103, 307–312. [Google Scholar] [PubMed]
- Pasquali, R.; Baraldi, G.; Cesari, M.P.; Melchionda, N.; Zamboni, M.; Stefanini, C.; Raitano, A. A controlled trial using ephedrine in the treatment of obesity. Int. J. Obes. 1985, 9, 93–98. [Google Scholar] [PubMed]
- Khera, R.; Murad, M.H.; Chandar, A.K.; Dulai, P.S.; Wang, Z.; Prokop, L.J.; Loomba, R.; Camilleri, M.; Singh, S. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA 2016, 315, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Caterson, I.D.; Finer, N.; Coutinho, W.; Van Gaal, L.F.; Maggioni, A.P.; Torp-Pedersen, C.; Sharma, A.M.; Legler, U.F.; Shepherd, G.M.; Rode, R.A.; et al. Maintained intentional weight loss reduces cardiovascular outcomes: Results from the Sibutramine Cardiovascular OUTcomes (SCOUT) trial. Diabetes Obes. Metab. 2012, 14, 523–530. [Google Scholar] [CrossRef]
- Westfall, T.C.; Macarthur, H.; Westfall, D.P. Adrenergic agonists and antagonists. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13th ed.; Brunton, L.L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McGraw Hill: New York, NY, USA, 2017. [Google Scholar]
- Haller, C.A.; Benowitz, N.L. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N. Engl. J. Med. 2000, 343, 1833–1838. [Google Scholar] [CrossRef]
- Food and Drug Administration HHS. Final rule declaring dietary supplements containing ephedrine alkaloids adulterated because they present an unreasonable risk Final rule. Fed. Regist. 2004, 69, 6787–6854. [Google Scholar]
- Tynes, J.R. Performance enhancing substances. Effects, regulations, and the pervasive efforts to control doping in Major League Baseball. J. Leg. Med. 2006, 27, 493–509. [Google Scholar] [CrossRef]
- Song, M.K.; Um, J.Y.; Jang, H.J.; Lee, B.C. Beneficial effect of dietary Ephedra sinica on obesity and glucose intolerance in high-fat diet-fed mice. Exp. Ther. Med. 2012, 3, 707–712. [Google Scholar] [CrossRef]
- De Matteis, R.; Arch, J.R.; Petroni, M.L.; Ferrari, D.; Cinti, S.; Stock, M.J. Immunohistochemical identification of the beta(3)-adrenoceptor in intact human adipocytes and ventricular myocardium: Effect of obesity and treatment with ephedrine and caffeine. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1442–1450. [Google Scholar] [CrossRef]
- Harpaz, E.; Tamir, S.; Weinstein, A.; Weinstein, Y. The effect of caffeine on energy balance. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 1–10. [Google Scholar] [CrossRef]
- Schubert, M.M.; Hall, S.; Leveritt, M.; Grant, G.; Sabapathy, S.; Desbrow, B. Caffeine consumption around an exercise bout: Effects on energy expenditure, energy intake, and exercise enjoyment. J. Appl. Physiol. 2014, 117, 745–754. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
| Study | Country | Population (Male %) | Age (year) | Baseline Body Weight (kg) | Experimental Group | Duration | Risk of Bias |
|---|---|---|---|---|---|---|---|
| Astrup 1992 | Denmark | 135 (16.3) | 28.3 ± 7.0 | 95.1 ± 14.4 | Ephedrine 20 mg and caffeine 200 mg tid, ephedrine 20 mg tid | 24 weeks | Low |
| Bogacka 2007 | USA | 41 (17.1) | 37.5 ± 7.8 | 94.1 ± 11.4 | Ephedrine 25 mg and caffeine 200 mg tid | 16 weeks | Some concerns |
| Boozer 2001 | USA | 67 (14.9) | 41.1 ± 8.7 | 89.4 ± 10.3 | Ephedrine 24 mg and caffeine 80 mg tid | 8 weeks | Low |
| Boozer 2002 | USA | 167 (18.0) | 45.3 ± 12.3 | 88.0 ± 14.3 | Ephedrine 30 mg and caffeine 64 mg tid | 6 months | Low |
| Buemann 1994 | Denmark | 32 (0.0) | N/A | 93.3 ± 2.9 | Ephedrine 20 mg and caffeine 200 mg tid | 8 weeks | Low |
| Carey 2015 | Australia | 23 (100.0) | 22.5 ± 1.6 | 77.1 ± 2.6 | Day 1: ephedrine 2.5 mg/kg, day 2–28: ephedrine 1.5 mg/kg/day | 28 days | Some concerns |
| Coffey 2004 | USA | 102 (13.7) | 43.5 ± 10.0 | 92.8 ± 12.0 | Ephedrine 10 mg, caffeine 60 mg, and salicin 15 mg (N/A) | 12 weeks | Low |
| Greenway 2004 | USA | 40 (17.5) | 46.1 ± 2.4 | 83.0 ± 2.7 | Ephedrine 24 mg and caffeine 60 mg tid | 3 months | Low |
| Krieger 1990 | USA | 24 (8.3) | 35.4 ± 7.5 | N/A | Week 1–4: ephedrine 25 mg, caffeine 50 mg, and aspirin 100 mg tid, week 5–8: ephedrine 50 mg, caffeine 50 mg, and aspirin 100 mg tid | 8 weeks | Low |
| Pasquali 1985 | Italy | 46 (30.4) | 36.3 ± 3.8 | N/A | Ephedrine 25 mg tid, ephedrine 50 mg tid | 3 months | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, H.-J.; Yoon, H.-Y.; Yee, J.; Gwak, H.-S. Effects of Ephedrine-Containing Products on Weight Loss and Lipid Profiles: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmaceuticals 2021, 14, 1198. https://doi.org/10.3390/ph14111198
Yoo H-J, Yoon H-Y, Yee J, Gwak H-S. Effects of Ephedrine-Containing Products on Weight Loss and Lipid Profiles: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmaceuticals. 2021; 14(11):1198. https://doi.org/10.3390/ph14111198
Chicago/Turabian StyleYoo, Hee-Jeong, Ha-Young Yoon, Jeong Yee, and Hye-Sun Gwak. 2021. "Effects of Ephedrine-Containing Products on Weight Loss and Lipid Profiles: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Pharmaceuticals 14, no. 11: 1198. https://doi.org/10.3390/ph14111198
APA StyleYoo, H.-J., Yoon, H.-Y., Yee, J., & Gwak, H.-S. (2021). Effects of Ephedrine-Containing Products on Weight Loss and Lipid Profiles: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmaceuticals, 14(11), 1198. https://doi.org/10.3390/ph14111198







